Abstract
The detection of extended-spectrum β-lactamase-producing (ESBL) bacteria is of importance for infection control and epidemiological surveillance. We aimed to compare phenotypic methods available in the routine laboratory and to evaluate two-step strategies using these methods for the detection of ESBL-positive Enterobacteriaceae. Two methods used for routine susceptibility testing (Vitek2 and disk diffusion methods) and seven methods designed for the detection of ESBL production (ESBL Etests, combination disks, double-disk synergy [DDS] methods on Mueller-Hinton [MH] agar and cloxacillin-containing MH agar, and the Cica-Beta test) were tested against 107 strains of Enterobacteriaceae not susceptible to extended-spectrum cephalosporins. All strains were screened for the presence of acquired ESBL-encoding genes by PCR, and the PCR result was considered the gold standard for evaluation of the other test methods. Among the 107 strains, 52 (49%) were ESBL positive. With Vitek2, sensitivities were the highest when using extended cards (73% to 79%), but 25% to 31% of the strains yielded indeterminate results. For the disk diffusion method, sensitivities were the highest (96%) when testing at least cefotaxime, cefepime, and a third compound (ceftazidime, cefpodoxime, or aztreonam). For the specific methods, specificities ranged from 62% (ceftazidime ESBL Etest) to 100% (DDS using a disk spacing of 20 mm). When a method designed for ESBL detection was used on strains considered ESBL negative or with an indeterminate result by a first routine susceptibility method, sensitivities reached 100% for a majority of combinations. In conclusion, two-step strategies using phenotypic methods available in most clinical laboratories may reach a sensitivity of 100% for ESBL detection among a large panel of species, including AmpC producers, providing a sensible choice of tests.
INTRODUCTION
The recent international spread of Escherichia coli producing CTX-M extended-spectrum β-lactamases (ESBL) is a major concern because of new microbiological and epidemiological features (2, 29). Indeed, E. coli is a commensal of our digestive tract and it is the most frequent organism isolated from urinary tract infections in the community and in hospitals. Therefore, increases in multiresistance in this species will lead to increased use of the few antibiotics that remain active, such as carbapenems, and possibly to the emergence of carbapenem-resistant organisms (22). Finally, ESBL-producing Enterobacteriaceae are now found in ambulatory patients without recognized risk factors for multidrug-resistant organisms (28). Consequently, recognition of ESBL-producing organisms has become a concern for general hospitals and private practice laboratories. The recent changes in clinical MIC breakpoints for extended-spectrum cephalosporins and for aztreonam against Enterobacteriaceae by EUCAST and CLSI decrease the likelihood of interpreting an ESBL-producing Enterobacteriaceae as susceptible to exended-spectrum cephalosporins (6, 13). Thus, recognition of ESBL production would not be necessary for prediction of clinical outcome. However, it is still of importance for infection control to limit its spread and to measure the evolution of the spread and the impact of control programs.
Several phenotypic methods have been developed to detect or confirm ESBL production by Enterobacteriaceae (8, 10, 15, 19, 33–35). The CLSI in the United States issued national guidelines for laboratory detection of E. coli, Proteus mirabilis, and Klebsiella spp. with ESBL (6), but not for species with inducible AmpC β-lactamases, such as Enterobacter spp. The Health Protection Agency in the United Kingdom released guidelines for ESBL detection regardless of the tested species (14). Most guidelines recommend screening isolates based on decreased susceptibility to extended-spectrum cephalosporins in primary susceptibility testing and to use one of the available tests to confirm ESBL production. However, it is not clear which confirmatory tests are the most sensitive and which extended-spectrum cephalosporins should be tested. In addition, the recent emergence of plasmidic AmpC β-lactamases in E. coli and Klebsiella pneumoniae may lead to changes in recommendations. Guidelines from the Antibiogram Committee of the French Society for Microbiology (CA-SFM) consider all Enterobacteriaceae species and do not recommend use of a confirmatory test if the screening test provides specific evidence of ESBL production (7). Of note, the latter guidelines suggest the use of cloxacillin-containing Mueller-Hinton agar to detect ESBL in AmpC-derepressed mutants of Enterobacteriaceae.
In the era of changing epidemiology of ESBL, we sought to compare nine phenotypic methods that can be routinely applied in most microbiological laboratories for their ability to detect ESBL production. We analyzed the overall characteristics of two combined methods, i.e., a routine antibiotic susceptibility method followed by a second method designed specifically for ESBL identification, as it is routinely applied in many clinical laboratories (12).
MATERIALS AND METHODS
Bacterial strains.
All consecutive nonduplicate strains of Enterobacteriaceae isolated at Pitié-Salpêtrière Hospital, Paris, France, during a 1-month period and fulfilling at least one of the following criteria were included in the study: (i) cefotaxime (CTX), ceftazidime (CAZ), cefepime (FEP), or aztreonam (ATM) MIC of >1 mg/liter (inhibition zone diameter of ≤25 mm with 30-μg disk); (ii) cefpodoxime (CPD) inhibition zone diameter of ≤17 mm (6, 7, 12, 14, 15).
Strains of less frequently isolated species, such as Morganella spp. or Serratia sp., were collected over two additional weeks. In addition, 13 wild-type strains belonging to each species were included as control strains.
VITEK2 system (bioMérieux).
We tested four Vitek2 antimicrobial susceptibility test cards, including two standard cards, AST-N017 and AST-N052, as well as their corresponding extended cards, AST-EXN3 and EXN5. N017 and N052 include β-lactam antibiotics, such as CTX, CAZ, and cefoxitin, and differ only by the replacement of ticarcillin-clavulanic acid (TIM) by ertapenem in N052. EXN3 and EXN5 cards test for susceptibility to ceftriaxone, FEP, and ATM, and, in addition, EXN5 tests for susceptibility to three cephalosporins with or without clavulanic acid (CLA) to detect the production of ESBLs. The EXN5 extended card is currently only validated for detection of ESBLs in E. coli, K. pneumoniae, and Klebsiella oxytoca. An expert system (AES) interprets the results obtained with Vitek2 by using nine different phenotypes relevant to β-lactam antibiotics, including the wild type, and ESBL production. For each card, we considered a strain ESBL positive if the phenotypic interpretation by the AES included ESBL with or without decreased outer membrane permeability (i.e., porin loss) and negative if only the wild type or β-lactamases other than ESBLs were proposed by AES. All other interpretation results were considered indeterminate (ND).
DDS30.
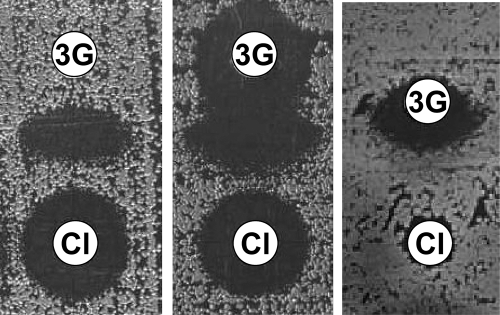
The double-disk synergy method at 30 mm (DDS30) is integrated as an adjunct of the routine susceptibility test by the disk diffusion method, as recommended by the CA-SFM (15). CTX (30 μg), CAZ (30 μg), FEP (30 μg), ATM (30 μg), and CPD (10 μg) disks (Bio-Rad) were placed by an automatic disk dispenser on Mueller-Hinton (MH) agar at a distance of 30 mm, center to center, from either an amoxicillin-clavulanate (AMC; 20 and 10 μg) or a TIM (75 and 10 μg) disk. The presence of ESBL was inferred when the inhibition zone around any of the five antibiotic disks was enhanced on the side of the CLA-containing disk, resulting in a characteristically shaped zone referred to as a “champagne-cork,” “keyhole,” “ellipsis,” or “phantom image” (Fig. 1).
Fig. 1.
Examples of positive double-disk synergy tests between a disk containing clavulanic acid (Cl) and a disk containing aztreonam or an extended-spectrum cephalosporin (3G). The inhibition zone around the 3G disk is enhanced, highly suggesting the production of ESBL.
DDS20.
An amoxicillin-clavulanate disk was manually placed at 20 mm, center to center, of CTX, CAZ, FEP, and ATM disks on MH agar. Interpretation criteria for ESBL production were similar as those described above (12, 33, 34).
DDS30 on cloxacillin MH agar.
Because cloxacillin is known to inhibit AmpC-type β-lactamases, the DDS30 method was carried out on MH agar containing 250 mg/ml of cloxacillin (AES Chemunex, Combourg, France) as previously described (12). Interpretation criteria were similar as those of the DDS30 test.
ESBL Etest.
Three ESBL Etest strips CT/CTL, TZ/TZL, and PM/PML (bioMérieux, France; kindly provided by AES Chemunex) for testing the synergy between a gradient of concentrations of either CTX, CAZ, or FEP, respectively, and a fixed concentration of CLA (4 mg/liter) were tested against each strain on MH agar. The respective concentrations ranges were as follows: 0.25 to 16 mg/liter and 0.016 to 1 mg/liter for CT-CTL; 0.5 to 32 mg/liter and 0.064 to 4 mg/liter for TZ/TZL; 0.25 to 16 mg/liter and 0.064 to 4 mg/liter for PM/PML. Interpretation criteria followed the manufacturer's recommendations, and strains were considered ESBL positive when there was (i) a reduction of the MIC by three doubling dilutions in the presence of CLA (i.e., MIC ratio of ≥8) for any of the three cephalosporins and the CTX MIC was ≥0.5 mg/liter or the CAZ MIC was ≥1 mg/liter (currently, there is no cutoff for FEP), or (ii) a rounded zone (“phantom” zone) below the lowest concentration of CTL, TZL, or PML, or (iii) a deformation of the CTX, CAZ, or FEP inhibition ellipse at the tapering end regardless of MIC ratios. A result was considered indeterminate when MICs were higher than the predefined range (making it impossible to calculate the MIC ratio) or when one of the tested strips displayed an indeterminate result and the others produced a negative result.
ESBL Etest on cloxacillin MH agar.
To inhibit cephalosporinases, the three ESBL Etests described above were carried out on cloxacillin MH agar, and interpretation of the results was similar.
Combination disk method.
Disks containing 30 μg of CTX, CAZ, or FEP and disks containing a combination of the three drugs plus 10 μg of CLA (CCTX, CCAZ, CFEP, respectively; all kindly provided by Oxoid, Dardilly, France) were placed on MH agar. Isolates were considered ESBL positive if the inhibition zone measured around one of the combination disks after overnight incubation was at least 5 mm larger than that of the corresponding cephalosporin disk, as recommended by the manufacturer and CLSI (5).
Combination disk method on cloxacillin MH agar.
The combination disk method was carried out on cloxacillin-containing MH agar to inhibit cephalosporinases, and interpretation of the results was similar to that described above.
Cica-Beta test.
The Cica-Beta test method (MAST Diagnostic, Amiens, France) is a technically simple and fast (15 min maximum) method to detect ESBLs, but also overexpressed AmpC and metallo-β-lactamases (MBL). The method is based on the hydrolysis of a chromogenic cephalosporin, HMRZ-86, on paper strips. Four strips are available: a control strip with no inhibitor, to detect hydrolysis of extended-spectrum cephalosporins, one with CLA to detect ESBL, one with boronic acid to detect overproduction of AmpC, and one with sodium mercapto-acetic acid to detect metallo-β-lactamases. The four tests can be performed within 15 min using colonies isolated on solid media (18). We tested the four strips (kindly provided by the manufacturer) on each strain. Isolates were considered possibly ESBL positive in cases with a result in favor of ESBL production or of multiresistance (i.e., ESBL plus another mechanism of resistance). Isolates were considered ESBL negative when there was no cephalosporin hydrolysis (first strip was negative) or when the results suggested only AmpC or MBL production. All other results were considered indeterminate.
Molecular characterization of ESBL β-lactamases.
Strains were screened for the presence of acquired ESBL-encoding genes by PCR using primers already described (Table 1) (11). The DNA amplifications were performed on 50-μl samples containing DNA (5 μl), deoxynucleoside triphosphate (250 M), primers (0.4 M each), Taq DNA polymerase (1 U), and its buffer. The following cycles were used: 10 min of denaturation at 94°C (1 cycle), 35 cycles of 1 min of denaturation at 94°C, 1 min of annealing (see temperatures in Table 1), and 1 min of polymerization at 72°C, and then a 10-min extension at 72°C.
Table 1.
Primers used for ESBL-encoding gene detection in Enterobacteriaceae
| Gene | Primer name | Sequence | Annealing temp (°C) |
|---|---|---|---|
| blaSHV | SHV-bis | 5′-ATGCGTTATATTCGCCTGTGTATT-3′ | 64 |
| SHV-rev | 5′-GCGTTGCCAGTGCTCGATCAGCGC-3′ | ||
| blaTEM | TEM-A | 5′-GAGTATTCAACATTTCCGTGTC-3′ | 57 |
| TEM-B | 5′-TAATCAGTGAGGCACCTATCTC-3′ | ||
| blaCTX-M-1 | M13-upper | 5′-GGTTAAAAAATCACTGCGTC-3′ | 53 |
| M13-lower | 5′-TTGGTGACGATTTTAGCCGC- 3′ | ||
| blaCTX-M-2 | M25-upper | 5′-ATGATGACTCAGAGCATTCG-3′ | 53 |
| M25-lower | 5′-TGGGTT ACGATTTTCGCCGC-3′ | ||
| blaCTX-M-9 | M9-upper | 5′-ATGGTGACAAAGAGAGTGCA-3′ | 53 |
| M9-lower | 5′-CCCTTCGGCGATGATTCTC-3′ | ||
| blaCTX-M-25 | CTX25bis | 5′ ATGATGAGAAAAAGCGTA AG-3′ | 47 |
| CTX25rev | 5′ ATAACCGTCGGTGACAATTC 3′ | ||
| blaPER-1 | PER1-A | 5′ ATGAATGTCATTATAAAAGC 3′ | 49 |
| PER1-B | 5′ AATTTGGGCTTAGGGCAGAA 3′ | ||
| blaPER-2 | PER2-F | 5′ TGTGTTTTCACCGCTTCTGCTCTG 3′ | 64 |
| PER2-R | 5′ AGCTCAAACTGATAAGCCGCTTG 3′ | ||
| blaVEB | VEB-1A | 5′ CGACTTCCATTTCCCGATGC 3′ | 55 |
| VEB-1B | 5′ GGACTCTGCAACAAATACGC 3′ | ||
| blaGES | GES-1A | 5′ ATGCGCTTCATTCACGCAC 3′ | 53 |
| GES-1B | 5′ CTATTTGTCCGTGCTCAGG 3′ | ||
| blaKPC | KPCs | 5′ ATGTCACTGTATCGCCGT 3′ | 50 |
| KPCas | 5′ CCTTACTGCCCGTTGACG 3′ | ||
| blaOXA-2-group | OXA2A | 5′ ATGGCAATCCGAATCTTCGC 3′ | 53 |
| OXA2-2 | 5′ ATAGAGCGAAGGATTGCCCG 3′ | ||
| blaOXA-10- group | OXA10A | 5′ TTTCGAGTACGGCATTAGCT 3′ | 51 |
| OXA10-2 | 5′ GAATGGATTTTCTTAGCGGC 3′ |
Amplicons were sequenced, except for blaCTX-M genes, which were all considered ESBL-encoding genes, by using the BigDye Terminator cycle sequencing ready reaction kit (Applied Biosystems Inc., Foster City, CA) in an ABI Prism 310 DNA sequencer (Applied Biosystems). Sequences were compared with those available in the GenBank database (www.ncbi.nlm.nih.gov/GenBank). Using a probabilistic strategy based on the epidemiology of ESBL-encoding genes in Enterobacteriaceae, E. coli (21, 24) and Klebsiella spp. isolates (23) were first screened for the presence of blaCTX-M genes and secondarily for the presence of a blaTEM or blaSHV gene in cases of an absence of a CTX-M-encoding gene. For Enterobacter spp. isolates, SHV-specific PCR was performed first (4), and in the case of a negative result, the presence of blaCTX-M or blaTEM was determined. For other Enterobacteriaceae, or in case of the absence of CTX-M-, TEM-, and SHV-encoding genes, isolates were screened for the presence of the following bla genes: blaPER-1, blaPER-2, blaVEB, blaGES, blaKPC, blaOXA-2, blaOXA-10.
Detection by two-step strategies.
We assessed the performance of two-step strategies, based on a first routine method used for global susceptibility testing (“triage test”), i.e., routine disk diffusion method or Vitek2, and a second method specifically designed to detect ESBL production in strains negative or with an indeterminate result with the first method. Characteristics of the two-steps strategies were computed by using the test which yielded the highest sensitivity for each of the nine methods described above.
Statistical analysis.
All test results were blindly read by two persons before ESBL-encoding gene detection. They were performed once per strain, except in cases of the absence of growth on cloxacillin-containing agar or in cases of doubtful interpretation for both readers.
Statistical analysis was performed by using Stata 10 (StataCorp, College Station, TX) and SAS (SAS Institute, Cary, NC) software. Sensitivity (Se) and specificity (Sp) of each phenotypic test were computed by using ESBL-encoding gene detection by PCR as the gold standard. However, one strain with none of the tested ESBL genes but which yielded a typical ESBL pattern with all methods was considered ESBL positive. Indeterminate results were grouped with negative results for sensitivity computations and with positive results for specificity computations.
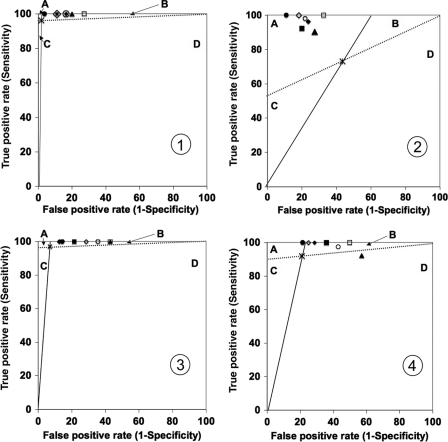
Statistical comparisons of characteristics of the tests (Se and Sp) were performed by using McNemar's test with exact significance probability. In addition, to evaluate the interest of each two-step strategy, we computed likelihood ratios (LR) of a positive and a negative test for each strategy. The LR of a positive test (LR+) is the ratio of the true-positive rate to the false-positive rate, and the LR of a negative test (LR−) is the ratio of the false-negative rate to the true-negative rate. LR are not dependent on the pretest probability of the event in the tested population, in contrast with positive and negative predictive values, and are therefore preferred for assessment of performances of diagnostic tests. The gain of adding a second test (II) to a first “screening test” (I) was assessed by using LR graphs as previously described (1) and derived from standard receiver operating characteristics curves analysis. In brief, the false-positive rate (1 − specificity) of the “screening test” (I) is plotted against its true-positive rate (sensitivity). The slope of a solid line connecting this defined point to the point (0,0) represents the LR of a positive test, and the slope of a dashed line connecting this point to the point (1,1) represents the LR of a negative test (see Fig. 3, below). Consequently, four regions are defined: region A, where the addition of a second test (II) yields a result superior overall to the single test (LR+ II > LR+ I and LR− II < LR− I); region B, where the addition of test II is superior to test I for confirming the absence of ESBL (LR+ II < LR+ I and LR− II < LR− I); region C, where the addition of test II is superior for confirming the presence of ESBL (LR+ II > LR+ I and LR− II > LR− I); region D, where the addition of test II is inferior overall (LR+ II < LR+ I and LR− II > LR− I).
Fig. 3.
Assessment of gains from each two-step strategy for all 107 Enterobacteriaceae isolates (panels 1 and 2) and only the group 1 and 2 Enterobacteriaceae (panels 3 and 4) by using likelihood ratio graphs. Symbols: ×, the result with the first routine test, i.e., double-disk synergy test for graphs 1 and 3 or Vitek2 for graphs 2 and 4; ●, double-disk synergy test on MH agar; ○, double-disk synergy test on MH agar plus cloxacillin; ■, ESBL Etest on MH agar; □, ESBL Etest on MH agar plus cloxacillin; ◆, combined disk on MH agar; ◇, combined disk on MH agar plus cloxacillin; ▴, Cica-Beta test.
RESULTS
Bacterial strains.
Among the 416 strains of Enterobacteriaceae isolated during the study period, a total of 107 strains (25%) fulfilled the inclusion criteria. The strains were classified into three groups according to their natural susceptibility to β-lactams (Table 2).
Table 2.
Distribution of the 107 Enterobacteriaceae isolates included in the study and their ESBL genes
| Enterobacteriaceae group and species | No. of isolates (% of total) | No. of ESBL producersa (% of total) | No. of isolates with indicated ESBL type |
||
|---|---|---|---|---|---|
| CTX-M | SHV | TEM | |||
| Group 1 | 28 (26) | 21 (40) | |||
| Escherichia coli | 27 | 20 | 18 | 2 | |
| Proteus mirabilis | 1 | 1 | 1 | ||
| Group 2 | 25 (23) | 18 (35) | |||
| Klebsiella pneumoniae | 21 | 17 | 10 | 2 | 5 |
| Klebsiella oxytoca | 2 | 0 | |||
| Citrobacter koseri | 2 | 1 | 1 | ||
| Group 3 | 54 (51) | 13 (25) | |||
| Enterobacter cloacae | 26 | 10 | 10 | ||
| Enterobacter aerogenes | 10 | 2 | 2 | ||
| Citrobacter freundii | 10 | 0 | |||
| Morganella morganii | 4 | 0 | |||
| Serratia marcescens | 2 | 1* | |||
| Hafnia alvei | 2 | 0 | |||
| Total | 107 (100) | 52 (49) | 31 (60) | 12 (23) | 8 (15) |
ESBL production was defined as positive based on the PCR results for ESBL genes, except for one strain (indicated by the asterisk) that was ESBL negative after PCR amplification but displayed a typical ESBL pattern with all tests.
Among the 107 strains, 52 were ESBL producers, including 51 for which an ESBL-encoding gene was detected (Table 2) and one S. marcescens strain that was ESBL positive by all tests but for which PCRs for ESBL genes remained negative despite several attempts. A majority of strains harbored CTX-M-encoding genes (n = 31; mainly E. coli and Klebsiella), or SHV genes (n = 12; mainly Enterobacter cloacae), and TEM genes (n = 8). ESBL-encoding genes were not detected in the remaining 55 strains.
Vitek2 system.
The Vitek2 method yielded indeterminate results for 25% to 31% of the strains, depending on the card used (Table 3). The N017 and the N052 cards gave similar sensitivity results. Combination with an extended card increased Se by about 10% for both cards. Se was higher in group 1 and 2 Enterobacteriaceae than in group 3. The Vitek2 correctly identified the two K. oxytoca strains as not ESBL producers. With N052 and EXN5, the current most commonly used cards, the false-negative results (n = 5) involved SHV-12 E. cloacae (n = 3), CTX-M Enterobacter aerogenes (n = 1), CTX-M P. mirabilis (n = 1), and the indeterminate results (n = 9) involved SHV-12 E. cloacae (n = 6), CTX-M E. aerogenes (n = 1), SHV-2 K. pneumoniae (n = 1), and TEM-21 K. pneumoniae (n = 1). Overall, 11 of the 14 false-negative or indeterminate results involved group 3 Enterobacteriaceae.
Table 3.
Summary of resultsa with the six different methods
| Method | All isolates (n = 107) |
Groups 1 and 2 (n = 53) |
Group 3 (n = 54) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| In (%) | Se (%) | Sp (%) | In (%) | Se (%) | Sp (%) | In (%) | Se (%) | Sp (%) | |
| Disk diffusion (30 mm, MH agar) | |||||||||
| CTX | 0 | 75 | 98 | 0 | 90 | 93 | 0 | 31 | 100 |
| CAZ | 0 | 69 | 100 | 0 | 85 | 100 | 0 | 23 | 100 |
| FEP | 0 | 89 | 98 | 0 | 90 | 93 | 0 | 85 | 100 |
| CPD | 0 | 35 | 100 | 0 | 46 | 100 | 0 | 0b | |
| ATM | 0 | 90 | 100 | 0 | 90 | 100 | 0 | 93 | 100 |
| CTX + CAZ | 0 | 77 | 98 | 0 | 90 | 93 | 0 | 39 | 100 |
| CTX + FEP | 0 | 94 | 98 | 0 | 97 | 93 | 0 | 85 | 100 |
| CTX + CPD | 0 | 75 | 98 | 0 | 90 | 93 | 0 | 31 | 100 |
| CTX + ATM | 0 | 94 | 98 | 0 | 95 | 93 | 0 | 92 | 100 |
| CAZ + FEP | 0 | 92 | 98 | 0 | 95 | 93 | 0 | 85 | 100 |
| CAZ + CPD | 0 | 69 | 100 | 0 | 85 | 100 | 0 | 23 | 100 |
| CAZ + ATM | 0 | 92 | 100 | 0 | 92 | 100 | 0 | 92 | 100 |
| FEP + CPD | 0 | 90 | 98 | 0 | 92 | 93 | 0 | 85 | 100 |
| FEP + ATM | 0 | 92 | 98 | 0 | 92 | 93 | 0 | 92 | 100 |
| CPD + ATM | 0 | 92 | 100 | 0 | 92 | 100 | 0 | 92 | 100 |
| CTX + CAZ + CPD | 0 | 79 | 98 | 0 | 90 | 93 | 0 | 46 | 100 |
| CAZ + CPD + ATM | 0 | 92 | 100 | 0 | 92 | 100 | 0 | 92 | 100 |
| CTX + FEP + (CAZ or CPD or ATM) | 0 | 96 | 98 | 0 | 97 | 93 | 0 | 92 | 100 |
| Any other association of three antibiotics | 0 | 94 | 98 | 0 | 95 | 93 | 0 | 92 | 100 |
| CAZ + CPD + ATM + (CTX or FEP) | 0 | 94 | 98 | 0 | 95 | 93 | 0 | 92 | 100 |
| Any other association of four or five antibiotics | 0 | 96 | 98 | 0 | 97 | 93 | 0 | 92 | 100 |
| Vitek2 card | |||||||||
| N017 | 31 | 64 | 56 | 23 | 77 | 50 | 39 | 23 | 59 |
| N017 + extended card | 25 | 79 | 53 | 9 | 95 | 43 | 41 | 31 | 56 |
| N052 | 27 | 65 | 60 | 15 | 80 | 71 | 39 | 23 | 56 |
| N052 + extended card | 26 | 73 | 56 | 6 | 92 | 79 | 46 | 15 | 49 |
| Cica-Beta test | |||||||||
| ESBL or multidrug resistance | 14 | 75 | 80 | 15 | 74 | 57 | 13 | 77 | 88 |
| ESBL Etest strip (MH agar) | |||||||||
| CTX | 49 | 71 | 29 | 19 | 87 | 50 | 78 | 23 | 22 |
| CAZ | 49 | 62 | 35 | 21 | 74 | 64 | 76 | 23 | 24 |
| FEP | 11 | 90 | 89 | 2 | 97 | 79 | 6 | 69 | 93 |
| CTX + CAZ | 49 | 73 | 27 | 19 | 87 | 50 | 78 | 31 | 20 |
| CTX + FEP | 39 | 90 | 29 | 11 | 97 | 50 | 67 | 69 | 22 |
| CAZ + FEP | 38 | 90 | 31 | 11 | 97 | 50 | 65 | 69 | 24 |
| CTX + CAZ + FEP | 40 | 90 | 27 | 11 | 97 | 50 | 69 | 69 | 20 |
| Combined disk (MH agar) | |||||||||
| CCTX | 0 | 85 | 89 | 0 | 97 | 86 | 0 | 46 | 90 |
| CCAZ | 0 | 75 | 91 | 0 | 85 | 93 | 0 | 46 | 90 |
| CFEP | 0 | 89 | 89 | 0 | 90 | 93 | 0 | 85 | 88 |
| CCTX + CCAZ | 0 | 85 | 86 | 0 | 97 | 86 | 0 | 46 | 85 |
| CCTX + CFEP | 0 | 96 | 84 | 0 | 100 | 86 | 0 | 85 | 83 |
| CCAZ + CFEP | 0 | 92 | 82 | 0 | 95 | 86 | 0 | 85 | 81 |
| CCTX + CCAZ + CFEP | 0 | 96 | 80 | 0 | 100 | 86 | 0 | 85 | 78 |
| Double disk diffusion (20 mm, MH agar) | |||||||||
| CTX | 0 | 87 | 98 | 0 | 100 | 93 | 0 | 46 | 100 |
| CAZ | 0 | 85 | 100 | 0 | 97 | 100 | 0 | 46 | 100 |
| FEP | 0 | 100 | 98 | 0 | 100 | 93 | 0 | 100 | 100 |
| CPD | 0 | 83 | 96 | 0 | 95 | 86 | 0 | 46 | 100 |
| ATM | 0 | 100 | 96 | 0 | 100 | 86 | 0 | 100 | 100 |
| CTX + CAZ | 0 | 87 | 98 | 0 | 100 | 93 | 0 | 46 | 100 |
| CTX + CPD or CAZ + CPD | 0 | 87 | 96 | 0 | 100 | 86 | 0 | 46 | 100 |
| CTX + FEP or CAZ + FEP | 0 | 100 | 98 | 0 | 100 | 93 | 0 | 100 | 100 |
| Any other association of two antibiotics | 0 | 100 | 96 | 0 | 100 | 86 | 0 | 100 | 100 |
| CTX + CAZ + CPD | 0 | 87 | 96 | 0 | 100 | 86 | 0 | 46 | 100 |
| CTX + CAZ + FEP | 0 | 100 | 98 | 0 | 100 | 93 | 0 | 100 | 100 |
| Any other association of three, four, or five antibiotics | 0 | 100 | 96 | 0 | 100 | 86 | 0 | 100 | 100 |
In, percentage of indeterminate results.
No synergy was observed with cefpodoxime among group 3 Enterobacteriaceae isolates.
DDS30.
By using the routine DDS30 method (Table 3), FEP or ATM disks used alone yielded the highest performance among the five β-lactams with, respectively, Se of 89% and 90% and Sp of 98%, and 100%. The Se was improved when taking into account the results obtained with two or three of the five β-lactams, with a higher Se obtained when testing CTX, FEP, and a third β-lactam (Se, 96%; Sp, 98%). The latter combination yielded a false-positive result with a K. oxytoca strain overproducing its chromosomic beta-lactamase and false-negative results for two strains (one E. coli and one E. aerogenes strain) producing CTX-M and highly resistant to extended-spectrum cephalosporins (MIC > 128 mg/liter). The combination of the results of >3 of the 5 β-lactams did not improve the Se. As for Vitek2, DDS30 Se was lower for group 3 Enterobacteriaceae than for other groups for a majority of combinations of β-lactams.
DDS20.
Se reached 100% for FEP or ATM alone, whatever the group of Enterobacteriaceae, but was lower for CTX, CAZ, and CPD (Table 3). The combination of two or three disks with CTX, CAZ, or CPD allowed Se to reach 100%.
DDS30 on cloxacillin MH agar.
Compared to the routine DDS30 test on MH agar, there was no increase in Se or Sp when using cloxacillin-containing MH agar (data not shown), and six strains did not give any growth on this medium, yielding a decrease in the Sp compared to DDS30 MH agar results.
ESBL Etests.
The ESBL Etest method yielded 11% to 49% indeterminate results, mainly because MICs were above the level of detection (Table 3). The highest Se and Sp were obtained when testing FEP alone (Se, 90%; Sp, 89%), but there were five false-negative results (five SHV-12 E. cloacae) and one indeterminate result (one CTX-M K. pneumoniae). Using more than one ESBL Etest strip did not increase the Se.
ESBL Etests on cloxacillin MH agar.
On cloxacillin-containing agar, indeterminate results (5.6% to 14.0%) with the ESBL Etest were globally less frequent than on MH agar, and the Se and Sp were higher than for the respective test on MH agar (data not shown). Se was the highest (100%) when the results of both CTX and FEP strips or the results of the three strips (CTX, CAZ, and FEP) were combined for all 107 strains or for only the subset of group 1 and 2 Enterobacteriaceae. For group 3 Enterobacteriaceae, FEP alone provided the highest Se (100%).
Combination disk method.
For the combination disk method on MH agar (Table 3), CFEP provided the highest Se (89%) and CCAZ the highest Sp (91%). By combining the results of CCTX and CFEP, Se increased to 96% but Sp was only 84%. With the latter combination, there were two false-negative results (two SHV-12 E. cloacae). Addition of CCAZ did not increase the intrinsic qualities of the test. Of note, Se was 100% when considering only groups 1 and 2, compared to 85% for group 3.
Combination disk method on cloxacillin MH agar.
For the combination disk method on cloxacillin-containing MH agar, Se and Sp were higher than the respective test on MH agar for all 107 Enterobacteriaceae isolates and for group 3, but not for groups 1 and 2 (data not shown). Se was the highest (100%) when the results of at least two combined disks were analyzed together for all 107 strains, and for groups 1and 2, whereas Se reached 100% whatever disks were tested for group 3.
Cica-Beta test.
The Cica-Beta test method yielded indeterminate results for 15 strains (14%) and identified correctly 36 out of 52 ESBL producers, resulting in a Se of 75% when the three strains interpreted as multiresistant were considered true positives (Table 3). The test yielded seven false-negative results and six indeterminate results. Of interest, the Se was similar for all groups of Enterobacteriaceae, but Sp was higher for group 3 Enterobacteriaceae (88%) than for the other groups (57%).
Statistical comparisons.
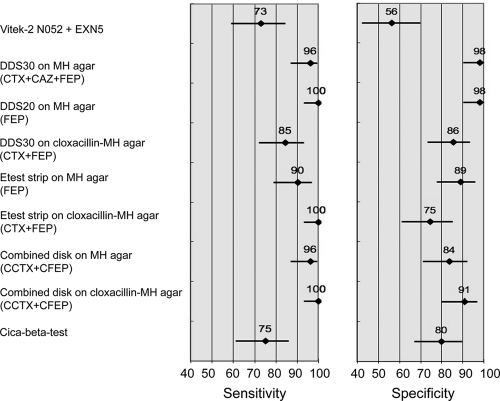
Because the main goal of ESBL detection is to reach high sensitivity, i.e., to detect the highest number of ESBL-positive strains, we performed statistical comparisons among the nine tests with the highest Se for each of the nine methods (Fig. 2 and Table 4). The Vitek2 and Cica-Beta test had significantly lower sensitivities than most other tests, and Vitek2 had a significantly lower specificity than most other tests. The DDS30 test combining the results of CTX, CAZ, and FEP disks and the DDS20 test with FEP alone had significantly higher specificities than other selected tests. Other comparisons did not reach the level of statistical significance.
Fig. 2.
Sensitivity and specificity of tests selected on the basis of the highest sensitivity obtained among each of the nine methods.
Table 4.
Significance levels for sensitivity and specificity comparisons of selected tests for all 107 isolatesa
| Comparison test |
P value for comparison of Se and Sp for indicated tests |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vitek2 (N052 + EXN5) | DDS30 MH (CTX + FEP + ATM) | DDS20 MH (FEP) | DDS30 with cloxacillin (CTX + FEP) | Etest |
Combined disk |
Cica-Beta test | |||
| MH | Cloxacillin | MH | Cloxacillin | ||||||
| Vitek2 N052 + EXN5 | — | 0.002 | <0.001 | 0.26 | 0.01 | <0.001 | <0.001 | <0.001 | 1.0 |
| DDS30 (CTX +FEP + ATM) | <0.001 | — | 0.50 | 0.10 | 0.45 | 0.50 | 1.0 | 0.50 | 0.007 |
| DDS20 MH (FEP) | <0.001 | 1.0 | — | 0.008 | 0.06 | 1.0 | 0.50 | 1.0 | <0.001 |
| DDS30 with cloxacillin (CTX+FEP) | 0.003 | 0.04 | 0.22 | — | 0.58 | 0.008 | 0.11 | 0.008 | 0.33 |
| Etest MH (FEP) | 0.001 | 0.06 | 0.06 | 0.77 | — | 0.06 | 0.37 | 0.06 | 0.08 |
| Etest with cloxacillin (CTX+FEP) | 0.11 | 0.001 | <0.001 | 0.11 | 0.06 | — | 0.50 | 1.0 | <0.001 |
| Combined disk MH (CTX+FEP) | 0.008 | 0.008 | 0.02 | 1.0 | 0.55 | 0.30 | — | 0.50 | 0.003 |
| Combined disk with cloxacillin (CTX+FEP) | <0.001 | 0.22 | 0.22 | 0.25 | 1.0 | 0.04 | 0.34 | — | <0.001 |
| Cica-beta test | 0.03 | 0.002 | 0.002 | 0.51 | 0.19 | 0.58 | 0.79 | 0.11 | — |
P values were calculated using using the McNemar test. The upper part of the table (above the dashes) reports the P values for comparisons of sensitivities, and the lower part reports P values for comparisons of specificities for each pair of the nine selected tests.
Two-step strategies.
When using the DDS30 with CTX, FEP, and CAZ on MH agar as the first routine method and then applying one of the specific tests (DDS20, DDS30 on cloxacillin agar, Etest strips, or combination disk methods) on ESBL-negative strains and strains with an indeterminate result with DDS30, the global Se of all two-step strategies reached 100% (Table 5). Global Sp ranged from 73% to 96%, the highest value being obtained with the modified double-disk synergy test DDS20 and using FEP on MH agar. False-positive results were due to two K. oxytoca isolates after the latter two-step strategy. To assess the gain of a second test against the DDS30 method used alone as a first-line test, we plotted the global characteristics of each two-step strategy on an LR graph, in which the characteristics of the DDS30 method delineated four zones. All strategies fell in the B zone, indicating that the addition of an ESBL-specific second test was always superior to DDS30 alone for confirming the absence of ESBL in all the strains (Fig. 3, panel 1) and in the group 1 and 2 subset (panel 2).
Table 5.
Summary of results with two-step strategies combining either DDS30 (CTX + FEP + ATM) or Vitek2 (N052 + EXN5) with a second method specifically designed to detect ESBL
| Test(s) | Value for the indicated comparisona |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All strains (n = 107) |
Group 1 and 2 Enterobacteriaceae (n = 53) |
Group 3 Enterobacteriaceae (n = 54) |
|||||||||||||
| In | Se | Sp | LR+ | LR− | In | Se | Sp | LR+ | LR− | In | Se | Sp | LR+ | LR− | |
| Routine DDS30 alone | 0.0 | 96 | 98 | 52.9 | 0.04 | 0.0 | 97 | 93 | 9.6 | 0.04 | 0.0 | 92 | 100 | 75 | 0.11 |
| Routine DDS30 with: | |||||||||||||||
| DDS20 on MH (FEP) | 0.0 | 100 | 96 | 22.2 | 0.01 | 0 | 100 | 86 | 5.9 | 0.02 | 0.0 | 100 | 100 | 81.0 | 0.04 |
| DDS30 on MH + cloxacillin (CTX+FEP) | 5.6 | 100 | 84 | 15.8 | 0.01 | 5.7 | 100 | 64 | 5.9 | 0.02 | 5.6 | 100 | 90 | 27.0 | 0.04 |
| Etest strip on MH (FEP) | 3.7 | 100 | 89 | 22.2 | 0.01 | 1.9 | 100 | 79 | 5.9 | 0.02 | 5.6 | 100 | 93 | 81.0 | 0.04 |
| Etest strips on MH + cloxacillin (CTX+FEP) | 12.1 | 100 | 72 | 22.2 | 0.01 | 7.5 | 100 | 57 | 5.9 | 0.02 | 6.7 | 100 | 78 | 81.0 | 0.04 |
| Combined disk on MH (CTX+FEP) | 0.0 | 100 | 84 | 5.8 | 0.01 | 0 | 100 | 88 | 5.9 | 0.02 | 0.0 | 100 | 83 | 5.4 | 0.04 |
| Combined disk on MH + cloxacillin (CTX+FEP) | 4.7 | 100 | 89 | 37.0 | 0.01 | 5.7 | 100 | 71 | 9.9 | 0.02 | 3.7 | 100 | 95 | 81.0 | 0.04 |
| Cica-Beta test | 8.4 | 100 | 80 | 22.2 | 0.01 | 7.5 | 100 | 57 | 5.9 | 0.02 | 9.3 | 100 | 88 | 81.0 | 0.04 |
| Vitek2 alone | 26 | 73 | 56 | 8.0 | 0.17 | 6 | 92 | 79 | 6.5 | 0.09 | 46 | 15 | 49 | 2.1 | 0.91 |
| Vitek2 with: | |||||||||||||||
| DDS20 on MH (FEP) | 0.0 | 100 | 89 | 8.5 | 0.01 | 0.0 | 100 | 79 | 4.2 | 0.02 | 0.0 | 100 | 93 | 11.6 | 0.04 |
| DDS30 on MH with cloxacillin (CTX + FEP) | 5.6 | 98 | 78 | 9.0 | 0.01 | 5.7 | 97 | 57 | 4.6 | 0.04 | 5.6 | 100 | 85 | 11.6 | 0.04 |
| Etest strip on MH (FEP) | 3.7 | 92 | 80 | 7.3 | 0.10 | 1.9 | 100 | 64 | 3.3 | 0.02 | 5.6 | 69 | 85 | 9.5 | 0.38 |
| Etest strip on MH with cloxacillin (CTX + FEP) | 11.2 | 100 | 67 | 8.5 | 0.01 | 7.5 | 100 | 50 | 4.2 | 0.03 | 14.8 | 100 | 73 | 11.6 | 0.05 |
| Combined disk on MH (CTX + FEP) | 0.0 | 96 | 76 | 4.1 | 0.05 | 0.0 | 100 | 71 | 3.3 | 0.02 | 0.0 | 85 | 78 | 3.6 | 0.23 |
| Combined disk on MH with cloxacillin (CTX + FEP) | 4.7 | 100 | 82 | 10.1 | 0.01 | 5.7 | 100 | 75 | 5.9 | 0.02 | 3.7 | 100 | 88 | 11.6 | 0.04 |
| Cica-Beta test | 9.3 | 90 | 73 | 7.1 | 0.13 | 7.5 | 92 | 43 | 3.2 | 0.18 | 11.1 | 85 | 83 | 11.6 | 0.19 |
Results shown are the percent indeterminate results (In), Se, and Sp, as well as positive and negative likelihood ratios (LR+ and LR−, respectively).
When using Vitek2 (i.e., AST-N052 card combined with EXN5 extended card) as the first routine method and then applying one of the specific tests to ESBL-negative strains and strains with an indeterminate result with Vitek2, the global Se increased to over 90% with all two-step strategies (Table 5). Of note, the global Se reached 100% after the use of Vitek2 followed by either Etest strips, combined disks on cloxacillin-containing MH agar, or the modified DDS20 test using FEP on MH agar. However, only the combined disk test and DDS20 using FEP provided no indeterminate result when used as the second test after Vitek2. The global Sp of the two-step strategy was the highest when using DDS20 (89%) as specific test, with false-positive results being mainly (five out of six cases) due to false-positive results obtained after Vitek2 testing (two E. coli, one E. cloacae, one M. morganii, and one C. freundii), while a K. oxytoca strain displayed a false-positive result after DDS20. To assess the gain of a second test against the Vitek2 used alone, we used LR graphs, in which characteristics of the Vitek2 method delineated four zones. All two-step strategies fell in the A zone when considering all 107 strains, indicating that the addition of a specific test was always superior to Vitek2 alone to confirm the absence of ESBL as well as to confirm the presence of ESBL (Fig. 3, panel 2). When considering the subset of group 1 and 2 Enterobacteriaceae, the Cica-Beta test fell in the D zone, indicating that addition of the latter test gave inferior results than the Vitek2 alone. In contrast, most of the other strategies provided an added gain for confirming the absence of ESBL among this subset (B zone), and DDS20 was superior to confirm the presence and absence of ESBL (A zone) (Fig. 3, panel 4).
DISCUSSION
Detection of ESBL production by Enterobacteriaceae remains a challenge for microbiologists. Although recent changes in the breakpoints of extended-spectrum cephalosporins have decreased the likelihood of reporting ESBL producers as susceptible to these compounds, ESBL detection is of interest for prevention of dissemination of ESBL producers by cross-transmission and for epidemiological purposes. We compared nine phenotypic methods, including two methods widely used for routine antibiotic susceptibility testing and seven methods specifically developed to detect ESBL production (32). We showed that the combination of a routine method followed by a specific test might achieve almost 100% sensitivity for ESBL detection in a large panel of Enterobacteriaceae species.
Numerous studies have tested the ability of phenotypic methods to detect ESBL production by Enterobacteriaceae (8–10, 15, 17–19, 26, 27, 30, 33–35). Our study is original, because it involved a set of consecutive nonduplicated and nonselected strains and because 50% of all tested strains were chromosomal AmpC producers not covered by some ESBL detection guidelines (3, 6). In addition, we compared numerous phenotypic methods, including some modified methods that have not been systematically evaluated. However, the selection process of the set of strains relied on the disk diffusion method, and the use of another selection method may have led to a somewhat different set of species and ESBL. Consequently, the global results may have been slightly modified. In addition, some rare types of ESBL have not been tested (20), and further confirmation of our results with isolates producing these enzymes may be of interest. Finally, our results may not apply to laboratories that use other phenotypic methods for routine susceptibility testing.
The high sensitivity of the disk diffusion method when using three or more extended-spectrum cephalosporins has been reported by others, despite different settings and ESBL being tested (9, 35). However, all combinations of two or three compounds are not equivalent. To our knowledge, this is the first attempt to evaluate systematically the sensitivities of different combinations of extended-spectrum cephalosporins or monobactam for detection of ESBL. The Health Protection Agency of the United Kingdom recommends testing cefpodoxime or both cefotaxime and ceftazidime as a first screening test (14). In the present study, the combination of the two latter drugs achieves 77% sensitivity to adequately detect ESBL production, meaning that only 33% of the isolates would need further testing. Including cefepime in the primary routine method will result in 96% sensitivity and therefore reduce the need for further testing to as few as 4% of the isolates. When using cefpodoxime alone, the results imply that almost two-thirds of isolates would need further testing, and all of the group 3 Enterobacteriaceae would have to go through a second test.
The ability of the Vitek2 system as a routine method to detect ESBL production was rather low, as it remained below 80% when considering all species. As expected and previously reported, sensitivity peaked to 92% to 95% for ESBL detection in E. coli and K. pneumoniae (17, 30, 35). Of note, the Vitek2 specificity was low (50% to 79%) because of a rather high frequency of indeterminate results. Microbiologists should bear in mind that this method has been validated only for group 1 and 2 Enterobacteriaceae and that it is not reliable to detect ESBL among group 3 Enterobacteriaceae, although some authors have reported a high sensitivity, albeit combined with very low specificity (35).
Recommendations of the manufacturer are to test cefotaxime and ceftazidime as the first-line method with ESBL Etest strips and to complete testing with the cefepime ESBL Etest in cases with an inconclusive result from the first two strips. Our results do not support this strategy. First, the sensitivity of the cefotaxime ESBL Etest strip was not significantly improved by concomitant testing of the ceftazidime strip (71% versus 73%). Second, the sensitivity and specificity of the cefepime ESBL Etest strip used alone were significantly higher than those obtained with both cefotaxime and ceftazidime ESBL Etest strips (90% versus 73%). Such a high sensitivity of the cefepime test has previously been reported for group 1 and 2 Enterobacteriaceae (31, 35).
As previously reported, the ability of the combined disk method to detect ESBL is very satisfactory, and sensitivity can reach 100% when testing both cefotaxime and cefepime against group 1 and 2 Enterobacteriaceae (16). However, in the present study, sensitivity after testing the two latter drugs was not different from that of cefotaxime alone.
The modified double-disk synergy method has been previously reported to increase the sensitivity of the double-disk method (15, 33, 34), but usually the distance between disks is left to the judgment of the microbiologist (14, 15). Some ESBL producers are missed by using the 25- to 30-mm distance recommended elsewhere (14, 15). We demonstrated that a standard distance of 20 mm between disks displayed the highest sensitivity and specificity among all methods.
The present report is the first to compare the characteristics of three widely used phenotypic methods for ESBL detection on cloxacillin-containing MH agar. Overall, sensitivities were improved by using cloxacillin compared to the same method on MH agar. However, and as expected, the gain in global sensitivity was mainly due to a better detection of ESBL among group 3 Enterobacteriaceae, as shown previously for Acinetobacter baumannii (25). The increased prevalence of plasmidic AmpC β-lactamases among E. coli and K. pneumoniae species may lead to a wider interest for this type of agar. The fact that some strains did not grow on this medium may limit its use.
The recommended approach for ESBL detection is to use a screening test, usually the routine susceptibility method used in the clinical laboratory, and to apply a confirmatory method dedicated to ESBL detection on all strains selected by the screening test (6, 14). However, screening tests are also used as confirmatory tests when results are characteristics of ESBL production. A second test appears, therefore, necessary only for the subset of isolates that are deemed ESBL negative or indeterminate by the first routine method although fulfilling the screening criteria. The intrinsic characteristics (Se and Sp) of the second test evaluated on the entire population of strains are in fact of no direct use, because this test is constantly part of a two-step strategy. It is necessary to compare the characteristics of strategies including two or more tests but not of individualized tests. We demonstrated that a combined strategy based on this approach yielded a very high sensitivity and specificity. We also demonstrated that, when using a primary method that is very sensitive (i.e., the disk diffusion method), the gain from a second confirmatory test was almost exclusively in confirming the absence of ESBL production. In contrast, the second confirmatory test also is a gain to confirm the presence of ESBL when the primary method has a low sensitivity. When considering a patient's treatment, most methods for antibiotic susceptibility testing will yield similar results because of the recent changes in breakpoints of extended-spectrum cephalosporins, i.e., isolates will be considered resistant whatever the mechanism of resistance. ESBL will be identified in almost 100% of the cases by using a second test. However, rapid identification of ESBL producers is of interest to implement hygiene precautions. In that case, use of a very sensitive primary test is of major interest.
ACKNOWLEDGMENTS
The excellent technical assistance of Salima Chalabi and Gérald Millot is gratefully acknowledged. We thank also Karine Grenet for technical help during the Vitek2 analysis.
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1. Biggerstaff B. J. 2000. Comparing diagnostic tests: a simple graphic using likelihood ratios. Stat. Med. 19:649–663 [DOI] [PubMed] [Google Scholar]
- 2. Bonnet R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brasme L., et al. 2007. Incidence of class A extended-spectrum beta-lactamases in Champagne-Ardenne (France): a 1 year prospective study. J. Antimicrob. Chemother. 60:956–964 [DOI] [PubMed] [Google Scholar]
- 4. Cambau E., et al. 2006. Occurrence of qnrA-positive clinical isolates in French teaching hospitals during 2002–2005. Clin. Microbiol. Infect. 12:1013–1020 [DOI] [PubMed] [Google Scholar]
- 5. Carter M. W., Oakton K. J., Warner M., Livermore D. M. 2000. Detection of extended-spectrum beta-lactamases in Klebsiellae with the Oxoid combination disk method. J. Clin. Microbiol. 38:4228–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing. M100-S20 CLSI, Wayne, PA [Google Scholar]
- 7. Comité de l'Antibiogramme de la Société Française de Microbiologie 2010. Recommendations 2010. Société Française de Microbiologie, Paris, France [Google Scholar]
- 8. Cormican M. G., Marshall S. A., Jones R. N. 1996. Detection of extended-spectrum beta-lactamase (ESBL)-producing strains by the Etest ESBL screen. J. Clin. Microbiol. 34:1880–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Gheldre Y., Avesani V., Berhin C., Delmee M., Glupczynski Y. 2003. Evaluation of Oxoid combination discs for detection of extended-spectrum beta-lactamases. J. Antimicrob. Chemother. 52:591–597 [DOI] [PubMed] [Google Scholar]
- 10. Donaldson H., et al. 2008. Evaluation of the VITEK 2 AST N-054 test card for the detection of extended-spectrum beta-lactamase production in Escherichia coli with CTX-M phenotypes. J. Antimicrob. Chemother. 62:1015–1017 [DOI] [PubMed] [Google Scholar]
- 11. Drieux L., et al. 2009. Increase in hospital-acquired bloodstream infections caused by extended spectrum beta-lactamase-producing Escherichia coli in a large French teaching hospital. Eur. J. Clin. Microbiol. Infect. Dis. 28:491–498 [DOI] [PubMed] [Google Scholar]
- 12. Drieux L., Brossier F., Sougakoff W., Jarlier V. 2008. Phenotypic detection of extended-spectrum β-lactamase production in Enterobacteriaceae: review and bench guide. Clin. Microbiol. Infect. 14(Suppl. 1):90–103 [DOI] [PubMed] [Google Scholar]
- 13. European Committee on Antimicrobial Susceptibility Testing 2010. Breakpoint tables for interpretation of MICs and zone diameters. European Committee on Antimicrobial Susceptibility Testing, Vaxjo, Sweden [Google Scholar]
- 14. Health Protection Agency 2008. Laboratory detection and reporting of bacteria with extended spectrum β-lactamases. National standard method QSOP 51. Issue 2.2. Health Protection Agency, London, United Kingdom [Google Scholar]
- 15. Jarlier V., Nicolas M. H., Fournier G., Philippon A. 1988. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867–878 [DOI] [PubMed] [Google Scholar]
- 16. Linscott A. J., Brown W. J. 2005. Evaluation of four commercially available extended-spectrum beta-lactamase phenotypic confirmation tests. J. Clin. Microbiol. 43:1081–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Livermore D. M., et al. 2002. Multicentre evaluation of the VITEK 2 Advanced Expert System for interpretive reading of antimicrobial resistance tests. J. Antimicrob. Chemother. 49:289–300 [DOI] [PubMed] [Google Scholar]
- 18. Livermore D. M., Warner M., Mushtaq S. 2007. Evaluation of the chromogenic Cica-Beta-Test for detecting extended-spectrum, AmpC and metallo-beta-lactamases. J. Antimicrob. Chemother. 60:300–311 [DOI] [PubMed] [Google Scholar]
- 19. M'Zali F. H., Chanawong A., Kerr K. G., Birkenhead D., Hawkey P. M. 2000. Detection of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae: comparison of the MAST DD test, the double disc and the Etest ESBL. J. Antimicrob. Chemother. 45:881–885 [DOI] [PubMed] [Google Scholar]
- 20. Naas T., Poirel L., Nordmann P. 2008. Minor extended-spectrum beta-lactamases. Clin. Microbiol. Infect. 14(Suppl. 1):42–52 [DOI] [PubMed] [Google Scholar]
- 21. Nicolas-Chanoine M. H., et al. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273–281 [DOI] [PubMed] [Google Scholar]
- 22. Nordmann P., Cuzon G., Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236 [DOI] [PubMed] [Google Scholar]
- 23. Paterson D. L., Bonomo R. A. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pitout J. D., Nordmann P., Laupland K. B., Poirel L. 2005. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J. Antimicrob. Chemother. 56:52–59 [DOI] [PubMed] [Google Scholar]
- 25. Poirel L., Menuteau O., Agoli N., Cattoen C., Nordmann P. 2003. Outbreak of extended-spectrum beta-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 41:3542–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robberts F. J., Kohner P. C., Patel R. 2009. Unreliable extended-spectrum beta-lactamase detection in the presence of plasmid-mediated AmpC in Escherichia coli clinical isolates. J. Clin. Microbiol. 47:358–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robin F., Delmas J., Schweitzer C., Bonnet R. 2008. Evaluation of the Vitek-2 extended-spectrum beta-lactamase test against non-duplicate strains of Enterobacteriaceae producing a broad diversity of well-characterised beta-lactamases. Clin. Microbiol. Infect. 14:148–154 [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez-Bano J., et al. 2008. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch. Intern. Med. 168:1897–1902 [DOI] [PubMed] [Google Scholar]
- 29. Rossolini G. M., D'Andrea M. M., Mugnaioli C. 2008. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin. Microbiol. Infect. 14(Suppl. 1):33–41 [DOI] [PubMed] [Google Scholar]
- 30. Spanu T., et al. 2006. Evaluation of the new VITEK 2 extended-spectrum beta-lactamase (ESBL) test for rapid detection of ESBL production in Enterobacteriaceae isolates. J. Clin. Microbiol. 44:3257–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stürenburg E., Sobottka I., Noor D., Laufs R., Mack D. 2004. Evaluation of a new cefepime-clavulanate ESBL Etest to detect extended-spectrum β-lactamases in an Enterobacteriaceae strain collection. J. Antimicrob. Chemother. 54:134–138 [DOI] [PubMed] [Google Scholar]
- 32. Thomson K. S., et al. 2007. Comparison of Phoenix and VITEK 2 extended-spectrum beta-lactamase detection tests for analysis of Escherichia coli and Klebsiella isolates with well-characterized beta-lactamases. J. Clin. Microbiol. 45:2380–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomson K. S., Sanders C. C. 1992. Detection of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae: comparison of the double-disk and three-dimensional tests. Antimicrob. Agents Chemother. 36:1877–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tzelepi E., et al. 2000. Detection of extended-spectrum beta-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 38:542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wiegand I., Geiss H. K., Mack D., Sturenburg E., Seifert H. 2007. Detection of extended-spectrum beta-lactamases among Enterobacteriaceae by use of semiautomated microbiology systems and manual detection procedures. J. Clin. Microbiol. 45:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]