Abstract
The aim of the present study was to assess the diagnostic efficacy of a combination of two quantitative Aspergillus PCR assays, targeting a mitochondrial and a ribosomal target, in patients with risk factors for invasive aspergillosis (IA) and positive galactomannan (GM) antigen. Forty-four patients with hematological malignancies and risk factors for IA according to revised European Organization for Research on Treatment of Cancer and the Mycoses Study Group criteria (EORTC/MSG) criteria and presenting at least two sequential GM-positive sera were included in the study. Mitochondrial PCR was carried out prospectively on all GM-positive serum samples. Ribosomal PCR was carried out retrospectively on frozen stored sera. The sensitivities of mitochondrial and ribosomal PCRs were 58% and 50%, respectively. The diagnostic test performance was improved by using a combination of both PCR assays and by considering a patient PCR positive when at least two positive results were obtained. The sensitivity, specificity, and positive and negative likelihood ratios were 65%, 94%, and 11.8 and 0.37, respectively. A significant association between fatal outcome at 90 days and positive results of ribosomal PCR assays was observed (adjusted hazard ratio = 8.2; 95% confidence interval [CI] = 1.0 to 65.8; P = 0.048). Our results showed that the combination of two PCR assays targeting mitochondrial and ribosomal Aspergillus DNA improves the sensitivity of PCR in the diagnosis of IA in hematological patients with risk factors and positive GM results. This study also confirms that a positive PCR result is associated with a poor prognosis in these patients and should lead to specific antifungal therapy being introduced immediately.
INTRODUCTION
Invasive aspergillosis (IA) is a life-threatening infection whose incidence is still increasing in immunocompromised patients. There has been a significant decrease in mortality in patients diagnosed with IA following hematopoietic cell transplantation in recent years, but the average death rate continues to exceed 50% (26). Early diagnosis and treatment are essential to improve the prognosis. Serial testing for detection of galactomannan (GM) in serum using the Platelia Aspergillus enzyme immunoassay (Bio-Rad Laboratories) has been proposed as a screening tool for diagnosis of IA, as a positive GM result has been reported to appear before clinical signs, especially when a cutoff value of 0.5 is used (19, 20). However, false-positive GM results frequently occur (21, 27). A combination of GM detection with other types of biomarker detection, including circulating DNA, could be a good way to improve early diagnosis of IA (12, 15, 29).
Although hundreds of publications have reported the use of PCR for diagnosing IA, PCR is still not included in the European Organization for Research on Treatment of Cancer and the Mycoses Study Group (EORTC/MSG) criteria (10). The reason for not including PCR is mainly the absence of a commercially available system and the lack of standardization. Up to now, only methodological recommendations concerning DNA extraction from whole-blood specimens have been determined by the European Aspergillus PCR Initiative (EAPCRI) working group (28). There is a major consensus in favor of real-time PCR technology due to the sensitivity and rapidity of the techniques, as well as the absence of contamination by PCR products. However, a range of chemistry types are currently used (SYBR green, hybridization probes, and hydrolysis probes), and another highly debated area is the choice of the target DNA. Several multicopy genes, such as ribosomal genes—5.8S (24), 28S (2, 5), and, most frequently, 18S (4, 15, 16, 18), and the mitochondrial gene (3, 7, 25)—have been investigated for the assessment of real-time PCR assay in the diagnosis of IA. However, the designs of these studies are very diverse, making it impossible to compare the diagnostic performance with each type of DNA target.
In a previous study, we showed that detecting circulating Aspergillus DNA on the first GM-positive serum sample could help diagnose IA and assist in the decision to start antifungal therapy (22). Consequently, a strategy was implemented in our hospital in 2005 that consists of carrying out the detection of Aspergillus DNA by a real-time PCR assay targeting mitochondrial DNA on each GM-positive sample. The aim of the present study was to evaluate retrospectively a real-time PCR assay targeting 18S ribosomal DNA on the same sample and to assess the diagnostic efficacy of a combination of the two PCR assays, focusing on the ability to manage the initiation of specific antifungal therapy in high-risk patients with an initial positive GM result.
MATERIALS AND METHODS
Strains.
Fourteen strains from species most frequently isolated in human infection, either from an international collection (Belgian Coordinated Collections of Microorganisms/Institute of Hygiene and Epidemiology—Mycology section [BCCM/IHEM], Brussels, Belgium; Centraalbureau voor Schimmelcultures [CBS], Utrecht, Netherlands; Institut Pasteur [IP] collection, Paris, France) or from our own laboratory collection (Mycology laboratory—Univ-hospital Besançon [BES]), were used in this study: Candida albicans IHEM 9559, Candida glabrata CBS 138, Candida tropicalis CBS 192, Saccharomyces cerevisiae IHEM 6036, Aspergillus fumigatus IP 2279, Aspergillus nidulans BES 1022, Aspergillus terreus BES 1429, Aspergillus niger BES 1798, Aspergillus flavus BES 1561, Lichtheimia corymbifera BES 227, Rhizopus oryzae BES 1798, Scedosporium prolificans IHEM 23387, Scedosporium apiospermum BES 328, and Fusarium solani complex BES 308.
Fungal DNA was extracted using the High Pure PCR Template Preparation Kit (Roche Diagnostics, Meylan, France), according to the manufacturer's recommendations, after the mechanical disruption of mycelia in liquid nitrogen using a pestle for molds. A. fumigatus DNA extract was serially diluted from 3 × 105 fg/10 μl to 0.03 fg/10 μl for sensitivity testing. For all other species, DNA concentrations were adjusted to 3 × 103 fg/10 μl for real-time PCR specificity testing.
Patients.
Four hundred eighty-nine patients followed in the hematology unit in Besançon University Hospital were screened for GM antigenemia from September 2005 to January 2009. Forty-four of them presented at least two sequential GM-positive sera over a period of less than 30 days and a risk factor for IA according to EORTC/MSG criteria (10) and were included in this study. The mean (minimum-maximum) age of patients was 46 (12 to 74) years. Hematological malignancies were as follows: acute myeloblastic leukemia (n = 21), acute lymphoblastic leukemia (n = 6), chronic lymphocytic leukemia (n = 5), lymphoma (n = 4), chronic myelocytic leukemia (n = 2), multiple myeloma (n = 1), aplastic anemia (n = 1), sickle-cell anemia (n = 1), granulocytic sarcoma (n = 1), acute plasmablastic leukemia (n = 1), and myelodysplastic syndrome (n = 1). Sixteen of them received a hematopoietic stem cell transplant (HSCT).
Clinical, radiological, and biological data were prospectively recorded using the information sheet from the French network for surveillance of IA. Antibiotic and antifungal therapies were collected from the nominative prescription system of anti-infective agents used in our hospital.
Cases of IA were classified as proven, probable IA, or no IA according to EORTC/MSG criteria revised in 2008 (10) (Table 1). There were 4 proven cases, 22 probable cases, and 18 no-IA patients. The 18 patients classified as no IA had host criteria and positive GM, but no clinical criteria; 3/18 received piperacillin-tazobactam at the time of the positive GM test.
Table 1.
Radiological findings, results of GM and PCR assays, antifungal treatment, and outcomes for 26 patients with proven IA and probable IA
| Patient no. | Primary disease(s)a | Sexb | Age (yr) | Radiologic sign | No. of samples tested/positivef |
Mycological culture | I.v. antifungal(s)c | Outcome at day 90 after diagnosis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| GM (index minimum-maximum) | PCRm (Cq minimum-maximum) | PCRr (Cq minimum-maximum) | ||||||||
| Proven IA (n = 4) | ||||||||||
| 1 | AML | F | 13 | Halo | 42/28 (0.5–5.5) | 28/2 (43–41) | 28/2 (40–41) | VOR, CAS, AMB | Survival | |
| 2 | AML, HSCT | F | 22 | Nodules | 30/21 (0.5–15.3) | 21/6 (34–40) | 21/none | A. flavus (palate biopsy) | AMB | Survival |
| 3 | AML | M | 38 | 26/7 (0.6–3.7) | 7/3 (41–43) | 7/none | A. flavus (palate biopsy) | AMB, CAS | Survival | |
| 4 | Aplastic anemia | M | 74 | Not done | 4/2 (0.4–0.5) | 2/2 (35–37) | 2/2 (34–34) | A. fumigatus, F. oxysporum (lung biopsy) | AMB, CAS | Death |
| Probable IA (n = 22) | ||||||||||
| 1 | AML | M | 50 | Nodules | 34/6 (0.5–0.8) | 6/none | 6/none | CAS, AMB | Survival | |
| 2 | AML, HSCT | F | 34 | Nodules | 7/4 (0.5–1) | 6/none | 6/none | Survival | ||
| 3 | CLL | M | 72 | Halo | 10/7d (1.1–6) | 7/2 (40–40) | 7/2 (38–38) | VOR, AMB | Survival | |
| 4 | Lymphoma | M | 46 | Nodules, halo | 3/3d(1.6–4.4) | 3/2(40–41) | 3/2(38–38) | AMB, VOR | Death | |
| 5 | AML | F | 54 | Nodules | 9/3d (1.6–5.4) | 3/2 (42–46) | 3/2 (40–42) | CAS, VOR | Survival | |
| 6 | CLL | M | 56 | Cerebral lesion | 3/3 (1.2–4.9) | 3/3 (38–45) | 3/3 (33–45) | CAS, AMB | Death | |
| 7 | AML, HSCT | M | 54 | Nodules | 19/4 (0.5–1.7) | 4/none | 4/none | VOR | Survival | |
| 8 | AML | F | 16 | Nodules, halo | 14/4 (0.5–0.6) | 4/none | 4/none | AMB | Survival | |
| 9 | CLL | M | 62 | Nodules | 8/3 (0.6–0.7) | 3/none | 3/none | VOR | Survival | |
| 10 | Sickle-cell anemia, HSCT | M | 16 | Air crescent sign | 16/5d (0.6–0.8) | 5/3 (41–45) | 5/none | VOR. CAS | Survival | |
| 11 | AML, HSCT | F | 36 | Nodules | 17/6 (0.6–4.7) | 6/2 (45–45) | 6/6 (39–41) | AMB, VOR, CAS | Death | |
| 12 | Lymphoma | M | 47 | Nodules | 4/2 (0.9–1) | 2/none | 2/none | Survival | ||
| 13 | ALL, HSCT | M | 22 | Halo | 4/2d (0.6–1.3) | 2/none | 2/none | VOR | Death | |
| 14 | AML, HSCT | F | 28 | Halo | 11/5d (0.5–3.2) | 5/none | 5/none | VOR, CAS, AMB | Death | |
| 15 | CLL | F | 52 | Nodules | 27/16d (0.6–6.8) | 16/3 (42–44) | 16/3 (41–42) | AMB, CAS | Survival | |
| 16 | AML | F | 57 | Nodules | 27/9d (0.5–1.3) | 9/3 (39–44) | 9/5 (40–45) | AMB, CAS | Survival | |
| 17 | CLL | M | 61 | Halo | 8/4 (0.5–1.5) | 4/none | 4/none | VOR | Survival | |
| 18 | AML | F | 59 | Nodules, halo | 8/6d (0.6–2.6) | 6/3 (39–43) | 6/none | AMB, VOR, CAS | Survival | |
| 19 | ALL | F | 39 | Halo | 11/3d (0.6–2) | 3/2 (41–43) | 2/2 (38–40) | VOR, AMB | Death | |
| 20 | AML | F | 28 | Nodules | 9/2 (0.5–3.4) | 2/1 (41) | 2/1 (40) | AMB | Survival | |
| 21 | CML, HSCT | F | 48 | Nodules | 13/2 (0.7–2.1) | 2/none | 2/2 (37–41) | Scopulariopsis sp. (BALe) | VOR | Death |
| 22 | AML, HSCT | M | 56 | Cerebral lesion | 6/6d (0.8–4.9) | 6/none | 6/3 (38–41) | Phanerochaete chrysosporium (sputum) | CAS, VOR, AMB | Death |
AML, acute myeloblastic leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; HSCT, hematopoietic stem cell transplantation.
M, male; F, female.
AMB, liposomal amphotericine B; CAS, caspofungin; VOR, voriconazole. According to the guidelines for antifungal therapies used in our institution (which are quite similar to those of the Infectious Diseases Society of America [IDSA]), caspofungin or l-Amb was used as a first-line treatment for febrile neutropenia and l-Amb was used when physicians suspected mold infection. Voriconazole was the first-line treatment for invasive aspergillosis. A combination of antifungals was used for cerebral and/or sinusal aspergillosis, invasive aspergillosis with extrapulmonary foci, and bilateral IA with multiple nodules in highly immunocompromised patients.
Patient receiving piperacillin-tazobactam.
BAL, bronchoalveolar lavage.
PCRm, mitochondrial PCR; PCRr, ribosomal PCR.
Patients with possible IA (i.e., radiological signs but no positive biomarker) were not included in this study because the strategy implemented in 2005 in our hospital was to perform mitochondrial PCR only when patients had positive GM results.
Patients with only one positive GM result were also excluded from the study, because from 2005 to 2008, according to previous recommendations for classification of IA (1), two sequential positive tests were necessary to consider GM a microbiological criterion for probable cases of IA, and thus, clinical data were not recorded prospectively and sera were not stored for all these patients.
GM testing.
The GM test was performed as part of twice-a-week systematic surveillance of all the patients presenting with host factors of IA monitored in our hematology unit. GM antigenemia was determined using the Platelia Aspergillus sandwich enzyme-linked immunosorbent assay (Bio-Rad, Marnes la Coquette, France), using an index of 0.5 as a positive threshold.
Real-time PCR assays.
Mitochondrial real-time PCR was carried out immediately on each GM-positive serum sample. Automatic DNA extraction was performed using the MagNa Pure Compact Nucleic Acid Isolation Kit I on a MagNa Pure Compact apparatus (Roche Diagnostics, Meylan, France). Two independent DNA extractions were performed for each serum. Detection of mitochondrial Aspergillus DNA was performed using 10 μl of each of the two extracts and a dual hybridization probe and primers, as previously described (7). Quantitative results were expressed by determining the detection threshold, or quantification cycle (Cq), which marked the cycle at which fluorescence of the sample became significantly different from the baseline signal. Thus, the higher the Cq, the smaller the amount of DNA in the sample.
All DNA extracts were stored at −20°C. Ribosomal real-time PCR was retrospectively performed on stored DNA extracts, using primers AfumiR1/AfumiF1 and a hydrolysis probe (AfumiP1) targeting the 18S gene, as described by Haugland et al. (13; http://www.epa.gov/microbes/moldtech.htm). The PCR mixture was prepared in a 20-μl final volume using the LightCycler FastStart DNA Master HybProbe (Roche Diagnostics, Meylan, France): 2 μl of Master mix 1a/1b, 2.4 μl of MgCl2 (25 mM, stock solution), 1.6 μl of probe (P1; 1 pM), 4 μl primers (F1/R1; 5 pM) (Pharmacia Amersham, Uppsala, Sweden) and 10 μl of DNA. PCRs were run on a LightCycler 2.0 (Roche Diagnostics). The thermal-cycling conditions were as follows: an initial denaturation step at 95°C for 10 min, followed by 50 cycles of 15 s at 95°C and 1 min at 60°C.
For both PCR assays, samples were considered positive when at least one replicate had a Cq of ≤45 cycles. Results were expressed as the mean Cq of the two extracts or the Cq of the positive replicate if only one replicate was ≤45 cyles. Indeed, a very small amount of fungal DNA is found in blood specimens from patients suffering from invasive aspergillosis (7, 18). The DNA concentration calculated after the positive control never exceeded a few femtograms per microliter. According to Poisson's law, the PCR result cannot be consistently positive at these very low concentrations. However, for decision making concerning hematology patients, we previously suggested considering a sample to be positive even if only one replicate is positive (22).
DNA sequencing.
Real-time PCR products were subsequently sequenced when discordant results between the 2 PCR assays were observed. Sequencing was performed using a BigDye Terminator v3.1 kit (Applied Biosystems, Foster City, CA) with the same primers used in the PCR step on an ABI Prism 3130 DNA analyzer (Applied Biosystems). Sequences were compared to those available in the GenBank database using BLAST software (http://www.ncbi.nlm.nih.gov/blast).
Statistical analysis.
Data were entered in an electronic Excel database. We evaluated the ribosomal and mitochondrial PCR assays for IA diagnosis by determining various indicators of diagnostic test performance, including sensitivity (Se), specificity (Sp), and positive and negative likelihood ratios (LR+ and LR−), with a 95% confidence interval (95% CI). LR+ and LR− describe the discriminatory abilities of positive and negative test results, respectively. An LR+ above 10 and an LR− below 0.1 are considered to provide strong evidence in favor of or against diagnoses, respectively, in most circumstances (9). A receiver operating characteristic (ROC) analysis was performed to establish a decision threshold in terms of the number of positive PCR results. Indeed, four positivity thresholds were assessed for the combination of both PCR tests. These threshold values were level 1 (≤1 positive PCR result), level 2 (a single positive result in each PCR), level 3 (≥2 positive results in the same PCR and none in the other), and level 4 (≥2 positive results in each PCR). We assessed the significance of differences between groups by carrying out the Wilcoxon signed-rank test for continuous paired data and the Pearson χ2 test or Fisher's exact test for categorical variables, as appropriate.
Kaplan-Meier survival curves, the log rank test, and Cox proportional-hazards models were performed to compare survival patterns. All analyses were two tailed, and a P value of less than 0.05 was considered significant. The software package Stata, version 10.0 (Stata Corp., College Station, TX), was used for the analysis.
RESULTS
Cross-reactivity and detection limits of ribosomal and mitochondrial PCRs.
The ribosomal PCR assay was specific for A. fumigatus. The mitochondrial PCR assay was less specific, as DNA extracts from 4 other species of Aspergillus (A. flavus, A. nidulans, A. niger, and A. terreus) and from one species of zygomycete (L. corymbifera) were also amplified. The Cq values were similar, about 30 cycles, for A. flavus and A. niger (100% identity between A. fumigatus, A. flavus, and A. niger mitochondrial sequences). The Cq was higher for A. nidulans (37 cycles), showing that amplification was possible, but with less efficacy due to primer and/or probe mismatches with the target sequence (5 mismatches in primer sequences for A. nidulans [GenBank J01390.1]). The Cq value was also higher with A. terreus (42 cycles) and L. corymbifera (36 cycles), probably also due to primer and/or probe mismatches, but the mitochondrial sequences of these species are unknown. No amplification was obtained with DNA extracts from other species.
An experiment with a serial dilution of A. fumigatus extract showed that for a low concentration of DNA (≤300 fg/10 μl), the Cq was lower with ribosomal PCR than with the mitochondrial PCR assay: the differences in the mean Cq values were 0.7, 2, and 1.6 with concentrations of 300 fg/10 μl, 30 fg/10 μl, and 3 fg/10 μl, respectively (Table 2). When the concentration was ≤3 fg/10 μl, the ribosomal PCR assay was positive for 3/6 extracts while the mitochondrial PCR assay was positive for only 1 of the 6 extracts. When clinical samples were tested, 11 sera from 11 different patients were positive for both mitochondrial and ribosomal PCR assays. The Cq of the ribosomal PCR assay was significantly lower than the Cq of the mitochondrial PCR assay (mean difference ± standard deviation, 1.77 cycles ± 2.18; P = 0.022).
Table 2.
PCR results of calibrated dilution of DNA extract from A. fumigatus (IP 2279)
| Amt of A. fumigatus DNA in vol tested (10 μl) | Quantification cycle (Cq) |
|||
|---|---|---|---|---|
| Mitochondrial PCR |
Ribosomal PCR |
|||
| Extract 1 | Extract 2 | Extract 1 | Extract 2 | |
| 300 pg | 22.3 | 22.3 | 22.9 | 22.8 |
| 30 pg | 25.0 | 26.0 | 25.9 | 25.8 |
| 3 pg | 29.9 | 29.8 | 29.4 | 29.8 |
| 300 fg | 33.7 | 33.9 | 33.4 | 32.9 |
| 30 fg | 38.2 | 38.6 | 36.1 | 36.7 |
| 3 fg | 41.8 | >46 | 39.8 | 40.6 |
| 0.3 fg | >46 | >46 | 41.8 | >46 |
| 0.03 fg | >46 | >46 | >46 | >46 |
Ribosomal and mitochondrial PCR for IA diagnosis and prognosis. (i) ROC analysis and indicators of diagnostic performance.
The ROC analysis revealed that the optimal threshold was level 3 (Se = 61.5%; Sp = 100%). However, a threshold of level 2 (Se = 65.4%; Sp = 94.4%) gave the same result as a threshold level of 3 in terms of patients correctly classified (77.3%). For the diagnosis of IA (highly lethal), high sensitivity is the most important element. Thus, the positivity threshold retained was level 2. The area under the curve was equal to 0.82 (95% CI = 0.67 to 0.92; P < 0.001).
The diagnostic efficacy of each PCR and the association of both PCR assays, when performed in patients with at least 2 positive GM results, were evaluated by comparing the results in probable and proven cases versus no-IA cases. The Se, Sp, LR+, and LR− of the PCR diagnosis were improved by using the association of both PCR assays and by considering a patient PCR positive when at least two positive results were obtained (i.e., at least one positive mitochondrial PCR test and one positive ribosomal PCR test, or at least two positive ribosomal PCR tests, or at least two positive mitochondrial PCR tests). Under these conditions, the Se, Sp, LR+, and LR− were 65%, 94%, 11.8, and 0.37, respectively (Table 3).
Table 3.
Indicators of diagnostic test performance
| Testa | Resultb |
|||||
|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ | LR− | |
| ≥1 positive result | ||||||
| PCRm | 57.7 (37.2–76) | 94.4 (70.6–99.7) | 93.8 (67.7–99.7) | 60.7 (40.7–77.9) | 10.4 (1.33–81.1) | 0.45 (0.37–0.54) |
| PCRr | 50 (30.4–69.6) | 66.7 (41.2–85.6) | 68.4 (43.5–86.4) | 48 (28.3–68.2) | 1.50 (0.93–2.42) | 0.75 (0.59–0.95) |
| PCRm-PCRr | 65.4 (44.4–82.1) | 66.7 (41.2–85.6) | 73.9 (51.3–88.9) | 57.1 (34.4–77.4) | 1.96 (1.33–2.89) | 0.52 (0.38–0.70) |
| ≥2 positive results | ||||||
| PCRm | 53.8 (33.7–72.9) | 100 (78.1–100) | 100 (73.2–100) | 60 (40.7–76.8) | 0.46 (0.39–0.54) | |
| PCRr | 46.2 (27.1–66.3) | 100 (78.1–100) | 100 (69.9–100) | 56.3 (37.9–73.2) | 0.54 (0.47–0.62) | |
| PCRm-PCRr | 65.4 (44.4–82.1) | 94.4 (70.6–99.7) | 94.4 (70.6–99.7) | 65.4 (44.4–82.1) | 11.8 (1.56–88.8) | 0.37 (0.29–0.46) |
PCRm, mitochondrial PCR; PCRr, ribosomal PCR; PCRm-PCRr, combination of both PCR assays.
PPV, positive predictive value; NPV, negative predictive value; LR+, likelihood ratio of a positive test; LR−, likelihood ratio of a negative test. The 95% CI is in parentheses.
(ii) Analysis of discordant PCR results.
For 2 probable cases (patients 21 and 22), mitochondrial PCR was negative while ribosomal PCR was positive. The ribosomal PCR product was sequenced, and in the two cases, the presence of A. fumigatus DNA was confirmed (100% identity with part of the A. fumigatus ribosomal DNA available in the GenBank database).
For 2 proven cases (patients 2 and 3) and 2 probable cases (patients 10 and 18), mitochondrial PCR was positive while ribosomal DNA was negative. In the 2 proven cases, A. flavus had been isolated by mycological culture of a palate biopsy specimen. We failed to sequence mitochondrial PCR products.
(iii) PCR results and outcomes for the 26 patients with proven or probable IA.
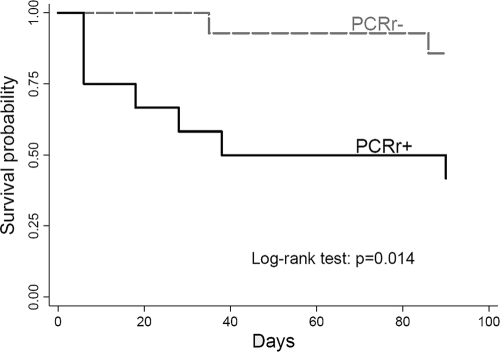
Kaplan-Meier survival curves (Fig. 1) revealed significantly shorter survival (outcome at 90 days) among patients with positive ribosomal PCR than those with negative ribosomal PCR (log rank test, P = 0.014) results. Cox proportional-hazards models showed a significant association between fatal outcome at 90 days and positive results of a ribosomal PCR assay (after adjusting for “primary disease,” categorized as follows: 1, acute myeloblastic leukemia; 2, lymphoma and chronic lymphocytic leukemia; 3, hematopoietic stem cell transplantation; 4, other; adjusted hazard ratio = 8.2; 95% CI = 1.0 to 65.8; P = 0.048). No significant association was found between the mitochondrial PCR result or the combination of both ribosomal and mitochondrial PCR results and patient outcome. In addition, the Cox regression analysis did not show a significant difference in patient survival, according to the number of antifungal agents received (≤1 versus >1).
Fig. 1.
Kaplan-Meier survival curves of patients with positive and negative ribosomal PCR results (PCRr+ and PCRr−) among 26 patients with proven or probable invasive aspergillosis.
(iv) PCR results and outcomes for the 18 patients classified as no IA.
One of 18 patients had two positive PCR results (ribosomal PCR and mitochondrial PCR); he had no symptoms of respiratory tract infection. However, he received intravenous (i.v.) antifungal therapy and piperacillin-tazobactam at the time of the positive results and was alive at day 90.
Five of 18 patients had only one positive PCR result (ribosomal PCR only). Two of them died within 90 days: one patient had a Rhizomucor pusillus sinusal infection and died despite antifungal treatment with liposomal amphotericin B and posaconazole; the other patient who died had symptoms of respiratory tract infection but nonspecific radiological signs and received i.v. voriconazole treatment. The other 3 patients with only one positive PCR result had nonspecific clinical or radiological signs: one patient had a Streptococcus pneumoniae infection and received adapted antibiotherapy, the second had oral voriconazole, and the last one had no antifungal treatment.
Twelve of 18 patients had negative PCR results; 2 patients had nonspecific clinical or radiological signs, received i.v. antifungal therapy, and were alive at day 90; the last 10 patients, who presented 2 to 8 sequential GM-positive sera, had no clinical signs of invasive fungal infection and were alive in the absence of antifungal treatment, reinforcing the idea that the GM test result was false positive. Of these 10 patients, only 1 received piperacillin-tazobactam; the causes of false positivity for the other 9 patients remain unknown.
DISCUSSION
Our results showed that the combination of two PCR assays targeting mitochondrial and ribosomal Aspergillus DNA improves the sensitivity of PCR in the diagnosis of IA in patients with risk factors and positive GM results. This study also showed that, in such patients, a positive ribosomal PCR result is associated with a poor prognosis.
Serum samples allow free-circulating Aspergillus DNA to be detected, while the use of EDTA–whole-blood samples allows conidia, hyphal fragments, and/or free-circulating DNA to be detected (17). We chose serum samples because serum has been shown to be an appropriate source of Aspergillus DNA amplification (7, 8), and also for several practical reasons: the use of the same sample as for GM detection and the fact that it is easy to store frozen samples and that standardized DNA extraction is straightforward using an automated commercial kit.
The selection of an appropriate target for amplification is another key point for standardizing a PCR-based detection method, and multicopy genes, such as ribosomal or mitochondrial genes, are the best candidates. Our study demonstrates that the combination of the two targets increased the diagnostic efficacy. Two positive results of one or both PCR assays were strongly predictive of a diagnosis of IA, as the LR+ was higher than 10. On the other hand, negative results for both PCR assays had a moderate informative value for ruling out the diagnosis of IA, as the LR− was between 0.2 and 0.5. Unlike the predictive values, which are used more frequently, likelihood ratios are not dependent on the prevalence of disease in the study sample. Thus, findings can be generalized beyond the study and across different contexts.
The size of the A. fumigatus genome is about 29 Mb, and it is organized in 8 chromosomes with 9,631 (23) to 9,906 (11) genes. The ribosomal DNA gene (5.8S, 18S, and 28S) is located on chromosome 4, and the copy number was first reported to be about 35 copies (23). The copy numbers of the 18S RNA were recently determined for a variety of isolates and were found to vary with the strain from 38 to 91 copies per genome (14). Mitochondrial DNA is made up of 16 genes and is 32 kb in size (23). The copy number of mitochondrial genes used as targets in this study was estimated at between 9 and 10 mitochondrial copies per single-copy gene (8). Thus, ribosomal DNA, with a number of copies at least three times higher than that of mitochondrial DNA, can theoretically generate a higher signal when the same initial DNA concentration is amplified. We actually observed better detection of low concentrations of DNA by using ribosomal targets in experiments with calibrated DNA extracts and significantly lower Cq for ribosomal PCR when both PCRs were positive in clinical samples. We also observed 2 probable IA cases with positive ribosomal PCR and negative mitochondrial PCR results. In these 2 cases, it was demonstrated, using sequencing, that A. fumigatus DNA was present; the amounts of DNA in serum were probably very small and were detected using a ribosomal target only.
Experiments with calibrated DNA extracts showed that the ribosomal PCR assay was very specific for A. fumigatus, while other opportunistic Aspergillus species were detected using the mitochondrial DNA target, notably, A. flavus. This is particularly interesting because A. flavus has been increasingly recognized as an opportunistic pathogen in several cancer centers (6, 30). In the 2 proven cases with positive mitochondrial PCR and negative ribosomal PCR results, the implication of A. flavus was demonstrated by culture.
Thus, sensitivity is increased by using both PCRs, because of the decreasing detection limit using a ribosomal target and the enhancement of detected species using a mitochondrial target. This should be balanced by the fact that repetition of the same PCR assay could also lead to increased sensitivity. Indeed, in a sample with very small amounts of DNA, according to the Poisson law, the probability of having a copy of the target in the volume of sample tested, and thus of having a positive PCR result, would have been increased if triplicates or more had been tested, regardless of the mitochondrial or ribosomal probe.
The ribosomal PCR positive result, when obtained in patients at risk of IA with positive GM, was associated with a poor prognosis. Indeed, survival was significantly shorter among patients with positive ribosomal PCR results after adjusting for primary disease. The association between a positive mitochondrial PCR result and a fatal outcome was not found in the present study, while it was significant in our previous study (22). The small number of patients in each of the two studies probably explains this discordance. However, this second study confirms that a positive PCR result in patients at risk of IA with a positive GM could reinforce the decision to initiate specific anti-Aspergillus antifungal therapy, with the understanding that the therapy may be stopped if the diagnosis of IA is not confirmed.
In this retrospective study, we showed that the combination of mitochondrial and ribosomal targets increased the sensitivity of PCR for the diagnosis of IA and could help in decision making for initiating specific antifungal therapy, but this should be checked in a prospective multicenter study on a larger cohort. Determining the most efficient sequence of testing (including both types of PCR, and GM and beta-glucan testing) and the cost-effectiveness of diagnostic strategies using such a combination should also be included in further investigations.
ACKNOWLEDGMENTS
We are grateful to Florence Skana, Alice Barraquin, and Fabrice Poncet for their excellent technical support. We thank Frances Sheppard (Clinical Investigation Center [Inserm], Besançon, France) for her editorial assistance. We thank Stephane Bretagne for his critical reading of the manuscript and helpful discussions.
Footnotes
Published ahead of print on 12 January 2011.
REFERENCES
- 1. Ascioglu S., et al. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7–14 [DOI] [PubMed] [Google Scholar]
- 2. Basková L., Landlinger C., Preuner S., Lion T. 2007. The Pan-AC assay: a single-reaction real-time PCR test for quantitative detection of a broad range of Aspergillus and Candida species. J. Med. Microbiol. 56:1167–1173 [DOI] [PubMed] [Google Scholar]
- 3. Buchheidt D., et al. 2002. Clinical evaluation of a polymerase chain reaction assay to detect Aspergillus species in bronchoalveolar lavage samples of neutropenic patients. Br. J. Haematol. 116:803–811 [DOI] [PubMed] [Google Scholar]
- 4. Cesaro S., et al. 2008. Assessment of the lightcycler PCR assay for diagnosis of invasive aspergillosis in paediatric patients with onco-haematological diseases. Mycoses 51:497–504 [DOI] [PubMed] [Google Scholar]
- 5. Challier S., Boyer S., Abachin E., Berche P. 2004. Development of a serum-based Taqman real-time PCR assay for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 42:844–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chamilos G., et al. 2006. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003). Haematologica 91:986–989 [PubMed] [Google Scholar]
- 7. Costa C., et al. 2002. Real-time PCR coupled with automated DNA extraction and detection of galactomannan antigen in serum by enzyme-linked immunosorbent assay for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 40:2224–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Costa C., et al. 2001. Development of two real-time quantitative TaqMan PCR assays to detect circulating Aspergillus fumigatus DNA in serum. J. Microbiol. Methods 44:263–269 [DOI] [PubMed] [Google Scholar]
- 9. Deeks J. J., Altman D. G. 2004. Diagnostic tests 4: likelihood ratios. BMJ 329:168–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Pauw B., et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fedorova N. D., et al. 2008. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 4:e1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Florent M., et al. 2006. Prospective evaluation of a polymerase chain reaction-ELISA targeted to Aspergillus fumigatus and Aspergillus flavus for the early diagnosis of invasive aspergillosis in patients with hematological malignancies. J. Infect. Dis. 193:741–747 [DOI] [PubMed] [Google Scholar]
- 13. Haugland R. A., Varma M., Wymer L. J., Vesper S. J. 2004. Quantitative PCR analysis of selected Aspergillus, Penicillium and Paecilomyces species. Syst. Appl. Microbiol. 27:198–210 [DOI] [PubMed] [Google Scholar]
- 14. Herrera M. L., Vallor A. C., Gelfond J. A., Patterson T. F., Wickes B. L. 2009. Strain-dependent variation of 18S ribosomal DNA copy number in Aspergillus fumigatus. J. Clin. Microbiol. 47:1325–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kami M., et al. 2001. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin. Infect. Dis. 33:1504–1512 [DOI] [PubMed] [Google Scholar]
- 16. Kawazu M., et al. 2004. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1→3)-beta-D-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J. Clin. Microbiol. 42:2733–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klingspor L., Loeffler J. 2009. Aspergillus PCR formidable challenges and progress. Med. Mycol. 47:S241–S247 [DOI] [PubMed] [Google Scholar]
- 18. Loeffler J., et al. 2000. Quantification of fungal DNA by using fluorescence resonance energy transfer and the light cycler system. J. Clin. Microbiol. 38:586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maertens J., et al. 1999. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive Aspergillosis. J. Clin. Microbiol. 37:3223–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maertens J. A., et al. 2007. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin. Infect. Dis. 44:1329–1336 [DOI] [PubMed] [Google Scholar]
- 21. Mennink-Kersten M. A., Donnelly J. P., Verweij P. E. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4:349–357 [DOI] [PubMed] [Google Scholar]
- 22. Millon L., et al. 2005. Use of real-time PCR to process the first galactomannan-positive serum sample in diagnosing invasive aspergillosis. J. Clin. Microbiol. 43:5097–5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nierman W. C., et al. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151–1156 [DOI] [PubMed] [Google Scholar]
- 24. Pham A. S., et al. 2003. Diagnosis of invasive mold infection by real-time quantitative PCR. Am. J. Clin. Pathol. 119:38–44 [DOI] [PubMed] [Google Scholar]
- 25. Spiess B., et al. 2003. Development of a LightCycler PCR assay for detection and quantification of Aspergillus fumigatus DNA in clinical samples from neutropenic patients. J. Clin. Microbiol. 41:1811–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Upton A., Kirby K. A., Carpenter P., Boeckh M., Marr K. A. 2007. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin. Infect. Dis. 44:531–540 [DOI] [PubMed] [Google Scholar]
- 27. Walsh T. J., et al. 2004. Detection of galactomannan antigenemia in patients receiving piperacillin-tazobactam and correlations between in vitro, in vivo, and clinical properties of the drug-antigen interaction. J. Clin. Microbiol. 42:4744–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White P. L., et al. 2010. Aspergillus PCR: one step closer to standardization. J. Clin. Microbiol. 48:1231–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. White P. L., Linton C. J., Perry M. D., Johnson E. M., Barnes R. A. 2006. The evolution and evaluation of a whole blood polymerase chain reaction assay for the detection of invasive aspergillosis in hematology patients in a routine clinical setting. Clin. Infect. Dis. 42:479–486 [DOI] [PubMed] [Google Scholar]
- 30. Zmeili O. S., Soubani A. O. 2007. Pulmonary aspergillosis: a clinical update. QJM 100:317–334 [DOI] [PubMed] [Google Scholar]