Abstract
In the present population-based study, we determined the prevalences of the most common human-pathogenic microsporidia, Encephalitozoon spp. and Enterocytozoon bieneusi, in asymptomatic healthy people living in the Czech Republic. A total of 382 males and females (ages, 1 to 84 years) living in the Czech Republic, of whom 265 were Czech nationals and 117 were foreign students, were included in a study testing for the presence of microsporidia by use of coprology and molecular methods. Single-species infections with Enterocytozoon bieneusi or an Encephalitozoon sp. were detected for 9 and 136 individuals, respectively. Moreover, coinfections were detected for 14 individuals. Four genotypes of 3 human-pathogenic Encephalitozoon spp. and 7 E. bieneusi genotypes, including 3 novel genotypes, were detected. Some of these were reported in humans for the first time. The highest prevalence was recorded for individuals older than 50 years and for loose, unformed stool samples. These findings clearly show that exposure to microsporidia is common among immunocompetent people and that microsporidiosis is not linked to any clinical manifestation in healthy populations.
INTRODUCTION
Microsporidia have been recognized as opportunistic pathogens of immunocompromised patients, but microsporidiosis is also becoming increasingly common in immunocompetent individuals (21). Most human microsporidial infections are caused by Enterocytozoon bieneusi or members of the genus Encephalitozoon (Encephalitozoon cuniculi, Encephalitozoon hellem, and Encephalitozoon intestinalis). Enterocytozoon bieneusi is found mainly in the upper gastrointestinal tract and is associated with chronic diarrhea and weight loss, although a couple of reports have identified E. bieneusi in respiratory samples (19). Encephalitozoon intestinalis, the second most frequently identified microsporidian, causes disseminated microsporidiosis, affecting the gastrointestinal tract (19). The two other species of Encephalitozoon, E. cuniculi and E. hellem, are also known to cause disseminated infections, affecting the urogenital, respiratory, and ocular organs. There are, however, two reports of enteric localization of E. cuniculi (12), and E. hellem has been recognized in stools (26).
Based on sequence differences at the internal transcribed spacer (ITS) region, E. bieneusi consists of multiple genotypes. To date, 81 distinct genotypes of E. bieneusi have been described on the basis of nucleotide sequence polymorphisms in this 243-bp region; 26 of those genotypes have been found in humans only, and 8 different genotypes have been reported in both humans and animals by several research groups (30). Also, E. cuniculi has been considered a zoonotic pathogen and has been classified into three different strains according to phenotypic and genotypic differences based on the number of repeats of a 4-base sequence (5′-GTTT-3′) in the ITS region of the rRNA gene. Encephalitozoon cuniculi type I (rabbit strain) has been isolated from rabbits and humans, while E. cuniculi type II (mouse strain) has been identified only in mice and blue foxes so far, and E. cuniculi type III (dog strain) has been isolated from dogs and humans (22, 24). The intraspecies variability of E. hellem is based on differences in the ITS and polar tube protein (PTP) gene sequences, which have led to 5 different genotypes designated 1A, 1C, 1D, 2B, and 2C (14). All these genotypes were originally identified in humans. To date, no molecular differences in the markers used have been observed among E. intestinalis isolates originating from infected humans versus infected animals, implying the absence of a transmission barrier between the different host species (15).
Studies examining the prevalence of human microsporidiosis have been limited to patients who are positive for human immunodeficiency virus (HIV) or who have AIDS. However, recent molecular epidemiological studies have shown that organ transplant recipients and other immunocompromised patients, as well as immunocompetent individuals, are at risk for infections that are mostly asymptomatic (4, 29, 37). In contrast to the publication of hundred of records about immunocompromised individuals, only a few reports concerning microsporidial infections of immunocompetent humans have been published (1, 5, 28, 35).
The purpose of this study was to investigate the occurrence and prevalences of the microsporidial species Enterocytozoon bieneusi and Encephalitozoon spp. in the immunocompetent human population in the Czech Republic and to determine which species and genotypes are the most prevalent by using microscopy, PCR, and subsequent analysis of ITS sequences.
MATERIALS AND METHODS
Stool specimens.
Between October 2008 and March 2009, a total of 672 individual stool samples from 382 individuals were provided by the Regional Institute of Public Health, Department of Parasitology, Prague, Czech Republic. The samples originated from 265 non-HIV-infected Czech nationals, who provided 1 to 3 stool samples within a week (n = 555), and from 117 healthy, HIV-negative foreign students living in the Czech Republic who were screened once during immigration. The nationalities of these people are listed in Table 1. The study was approved by the ethics committee of the Hospital České Budějovice, a.s. (protocol 202/07). The stool samples were placed at 4°C in the dark without any preservative and were examined immediately. The age structure of the population sampled is shown in Table 2. The majority of individuals belonged to the 20- to 49-year age group. The majority of foreigners were 12 to 19 (62 individuals) or 20 to 49 (54 individuals) years old. The number of females was slightly higher (55%) for all individuals and was equal to the number of males (50%) among foreigners. Stool consistency was evaluated as hard and formed (referred to below as “formed”), loose and unformed (“paste”), or liquid (diarrhea). All samples were examined previously for the presence of other gastrointestinal protozoan parasites, such as Giardia spp. and Cryptosporidium spp., with negative results.
Table 1.
Nationalities and numbers of foreign students included in the study
| Country of origin | No. of students |
|---|---|
| Angola | 6 |
| Belarus | 5 |
| Bolivia | 1 |
| Bosnia and Herzegovina | 4 |
| Cambodia | 2 |
| China | 8 |
| Cyprus | 3 |
| Democratic Republic of Congo | 1 |
| Egypt | 2 |
| Ethiopia | 3 |
| Georgia | 4 |
| India | 1 |
| Japan | 1 |
| Kazakhstan | 3 |
| Korea | 1 |
| Macedonia | 1 |
| Moldova | 14 |
| Mongolia | 6 |
| Montenegro | 1 |
| Namibia | 2 |
| Nepal | 1 |
| Panama | 2 |
| Peru | 7 |
| Russia | 26 |
| Serbia | 1 |
| Ukraine | 7 |
| Uzbekistan | 2 |
| Vietnam | 2 |
Table 2.
Molecular detection of microsporidian species/genotypes by age and gender
| Age category | Stool consistency | No. examined/no. positive |
Organisms(s) (no. of positive individuals) detected by PCRa |
||
|---|---|---|---|---|---|
| Czechs | Foreigners | Czechs | Foreigners | ||
| 0–5 | Formed | 19/6 | Encephalitozoon cuniculi II (3), Encephalitozoon hellem 1A (1), Encephalitozoon intestinalis (1), Enterocytozoon bieneusi BEB4 (1) | ||
| Paste | 17/6 | Encephalitozoon cuniculi II (3), Encephalitozoon hellem 1A (1), Encephalitozoon intestinalis (1), Enterocytozoon bieneusi EpbA + Encephalitozoon cuniculi II (1) | |||
| Diarrhea | 2/1 | Enterocytozoon bieneusi EpbA + Encephalitozoon cuniculi II (1) | |||
| 6–11 | Formed | 35/14 | 1/0 | Encephalitozoon cuniculi II (10), Encephalitozoon hellem 1A (2), Enterocytozoon bieneusi EpbA (1), Enterocytozoon bieneusi CZ3 + Encephalitozoon hellem 1A (1) | |
| Paste | 23/12 | Encephalitozoon cuniculi I (1), Encephalitozoon cuniculi II (10), Encephalitozoon hellem 1A (1) | |||
| Diarrhea | 3/1 | Encephalitozoon cuniculi II (1) | |||
| 12–19 | Formed | 17/6 | 43/21 | Encephalitozoon cuniculi II (6) | Encephalitozoon cuniculi II (13), Encephalitozoon hellem 1A (2), Enterocytozoon bieneusi EbpA (1), Enterocytozoon bieneusi CZ1 (1), Enterocytozoon bieneusi CZ3 (1), Enterocytozoon bieneusi EbpA + Encephalitozoon hellem 1A (1), Enterocytozoon bieneusi BFRmr2 + Encephalitozoon cuniculi II (1), Enterocytozoon bieneusi CZ3 + Encephalitozoon hellem 1A (1) |
| Paste | 5/2 | 17/7 | Encephalitozoon cuniculi II (2) | Encephalitozoon cuniculi II (5), Enterocytozoon bieneusi EbpA + Encephalitozoon cuniculi II (2) | |
| Diarrhea | 2/1 | Encephalitozoon cuniculi II (1) | |||
| 20–49 | Formed | 63/26 | 30/10 | Encephalitozoon cuniculi I (2), Encephalitozoon cuniculi II (16), Encephalitozoon hellem 1A (5), Enterocytozoon bieneusi CZ2 (1), Enterocytozoon bieneusi CZ3 (1), Enterocytozoon bieneusi EpbA + Encephalitozoon cuniculi II (1) | Encephalitozoon cuniculi II (6), Encephalitozoon hellem 1A (2), Enterocytozoon bieneusi EbpA + Encephalitozoon cuniculi II (1), Enterocytozoon bieneusi BEB4 + Encephalitozoon cuniculi II (1) |
| Paste | 39/19 | 22/7 | Encephalitozoon cuniculi I (1), Encephalitozoon cuniculi II (12), Encephalitozoon hellem 1A (3), Enterocytozoon bieneusi PigEBITS5 (1), Enterocytozoon bieneusi BFRmr2 (1), Enterocytozoon bieneusi PigEBITS5 + Encephalitozoon hellem 1A (1) | Encephalitozoon cuniculi II (5), Enterocytozoon bieneusi EbpA + Encephalitozoon cuniculi II (1), Enterocytozoon bieneusi PigEBITS5 + Encephalitozoon hellem 1A (1) | |
| Diarrhea | 10/2 | 2/0 | Encephalitozoon cuniculi II (2) | ||
| 50+ | Formed | 11/4 | Encephalitozoon cuniculi II (3), Encephalitozoon hellem 1A (1) | ||
| Paste | 13/10 | Encephalitozoon cuniculi II (9), Encephalitozoon hellem 1A (1) | |||
| Diarrhea | 8/4 | Encephalitozoon cuniculi II (4) | |||
| Total | 265/113 | 117/46 | 113 | 46 | |
I, II, and 1A are genotypes of Encephalitozoon spp.; EbpA, BEB4, BFRmr2, PigEBITS5, CZ1, CZ2, and CZ3 are genotypes of Enterocytozoon bieneusi.
Calcofluor M2R staining (38).
Smears made from all stool samples were fixed with methanol for 2 min and were allowed to air dry. Subsequently, the slides were stained with 0.1% calcofluor M2R stain (Sigma) in phosphate-buffered saline (PBS) for 10 min, rinsed gently with PBS, stained with 0.5% Evans blue stain (Sigma) in distilled water for 30 s, rinsed again in PBS, and allowed to air dry. Stained slides were examined for the presence of spores by fluorescence microscopy using UV light at a wavelength of 490 nm and a magnification of ×1,000.
As positive controls, spores of E. intestinalis originally isolated from an AIDS patient (8) and grown in vitro in Vero E6 cells in the Laboratory of Veterinary and Medical Protistology at the Institute of Parasitology ASCR, v.v.i., and spores of E. bieneusi originally isolated from the stool of an HIV-positive patient from Peru (kindly provided by G. S. Visvesvara, CDC, Atlanta, GA) were used.
DNA isolation.
One sample from each individual was selected for DNA isolation on the basis of microscopic examination results. Either a microscopically positive sample was selected or, if microscopy was negative, one sample was randomly selected. Stool samples were homogenized by bead disruption using a FastPrep 24 instrument (MP Biomedicals, CA), and DNA was extracted by using a commercially available isolation kit (QIAamp DNA Stool Mini Kit; Qiagen, Hilden, Germany) according to the manufacturer's instructions. Acquired DNA was stored at −20°C.
PCR amplification.
Microsporidian-specific primers described by Katzwinkel-Wladarsch et al. were used (18). The forward primers MSP-1 [TGA ATG(G/T)GT CCC TGT] and MSP-3 [GGA ATT CACACC GCC CGT C(A/G)(C/T) TAT] were targeted to the 3′ region of the small-subunit (SSU) coding segment of Enterocytozoon bieneusi and all three Encephalitozoon spp. The reverse primers MSP-2B (GTT CAT TCG CAC TAC T) and MSP-4B (CCA AGC TTA TGC TTA AGT CCA GGG AG) were targeted to the 5′ region of the large-subunit (LSU) coding segment of E. bieneusi. The reverse primers MSP-2A (TCA CTC GCC GCT ACT) and MSP-4A [CCA AGC TTA TGC TTA AGT (C/T)(A/C)A A(A/G)G GGT] were targeted to the 5′ region of the coding segment of Encephalitozoon spp. For the primary step of the nested protocol, the PCR mixture contained 1× PCR buffer, 3 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate (dNTP), 1 U Taq, 1 μl bovine serum albumin (BSA) (10 mg/ml), and 200 nM each primer. For the secondary PCR step, the PCR mixture was identical except that BSA was excluded. For both PCR steps, a total of 35 cycles, each consisting of 94°C for 45 s, 54°C for 45 s, and 72°C for 60 s, were performed. An initial incubation at 94°C for 3 min and a final extension at 72°C for 7 min were included. PCR products were visualized on a 2% agarose gel containing 0.2 μg/ml ethidium bromide.
PCR products were directly sequenced on the ABI 3730XL sequence analyzer (Applied Biosystems, Foster City, CA). Each sample was sequenced in both directions. Sequences were aligned and completed using the ChromasPro (Technelysium, Pty, Ltd.), BioEdit, and Clustal X (version 2.0.6) programs and were compared with sequences in GenBank.
Positive controls included DNA isolated from spores of E. intestinalis (3 × 107/ml) of the same origin as that mentioned under “Calcofluor M2R staining” above and from spores of E. bieneusi genotype D originally isolated from a pig.
Phylogenetic analyses of results.
The sequences included in phylogenetic analysis were aligned in the Clustal X (version 2.0.6) program. Only complete ITS sequences were included in the analysis. In the case of multiple names for identical sequences, the first name published was used. The alignment was checked in BioEdit for ambiguously aligned positions, which were manually deleted. The resulting alignment contained 256 positions. Neighbor-joining (NJ) analysis of the data set was performed with the PAUP* 4.0b10 program. The number of bootstrap replicates was 1,000. The most distant sequences of E. bieneusi originated from humans, marmosets, raccoons, and cattle (GenBank accession no. DQ683749, DQ885588, AY237209, AY237210, AY237211, and DQ885585) and were used as an outgroup.
Statistical analysis.
Data were analyzed using the R 2.10.0 programming environment and SAS, version 9.0. The associations between age, gender, and nationality variables and the presence of microsporidia in stool samples were tested using the classical chi-square test without Yates' continuity correction. The risk was evaluated through odds ratios (OR). We provide 95% confidence intervals for OR and P values. In case of k × 2 contingency tables, we used the Cochran-Armitage test for trend, which tests for trends in binomial proportions across levels of covariates. Significance was evaluated at a P value of <0.05.
Nucleotide sequence accession numbers.
The GenBank accession numbers assigned to the newly identified genotypes of E. bieneusi determined in this study are GU198949 (CZ1), GU198950 (CZ2), and GU198951 (CZ3).
RESULTS
Of the 672 stool specimens obtained from 382 individuals, 75 microscopically positive samples originating from 58 people were detected (15%) (data not shown). On the basis of the consistency of the specimen, microsporidial spores were most often identified in formed stool samples (47/430). However, no significant associations were found between diarrhea and microsporidial detection when the proportions of positive paste samples (24/197) and diarrheal samples (4/45) were compared.
The highest prevalence was found for individuals 6 to 11 years old (15/62); the lowest, for individuals 12 to 19 years old (5/84). Microsporidia were microscopically identified for 26 men and 32 women, of whom 49 were Czechs and 9 were foreigners (data not shown).
Amplification of DNA from stool samples with primers specific for the most common microsporidia infecting humans allowed us to confirm the presence of microsporidial DNA in 159 of the 382 people screened (42%) (Table 2). Single-species infections were detected in 9 individuals with E. bieneusi (6%) and in 136 people with Encephalitozoon spp. (86%). Moreover, coinfections were detected in 14 individuals (9%). All microscopically positive samples tested positive by PCR.
The sequence analyses of E. bieneusi-positive samples revealed 7 different genotypes (Table 2). In addition to the previously described genotypes from cattle (BEB4 and EbpA) and pigs (PigEBITS5, EbpA, and BFRmr2), three new genotypes were detected (E. bieneusi CZ1 to CZ3). The most prevalent E. bieneusi genotypes were EbpA (in 10 individuals), CZ3 (in 4 individuals), and PigEBITS5 (in 3 individuals), followed by BFRmr2 and BEB4 (each in 2 individuals). The CZ1 and CZ2 genotypes were identified only in single individuals. All ITS sequences from the study samples matched the reference genotypes from GenBank 100% as follows: E. bieneusi EbpA, AF076040; E. bieneusi PigEBITS5, AF348473; E. bieneusi BFRmr2, EU849132; E. bieneusi BEB4, AY331008.
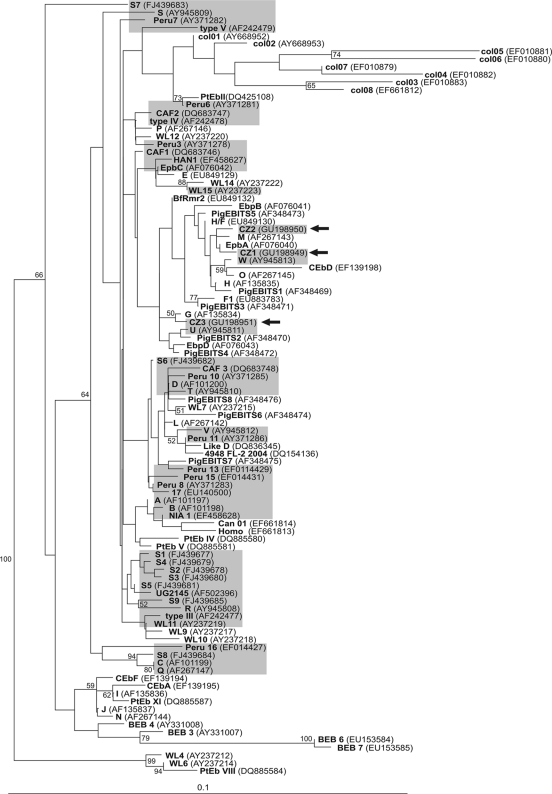
To further characterize the new genotypes found, phylogenetic analysis was carried out on all E. bieneusi ITS sequences available at GenBank. As shown in Fig. 1, phylogeny segregated the sequences into several major groups and an outlier group, all of which were supported by bootstrap values higher than 50%. All of the genotypes detected, including the novel genotypes, were identical or related to genotypes reported from pigs and cattle.
Fig. 1.
NJ tree based on SSU rDNA sequences of 106 Enterocytozoon bieneusi isolates, including our three new sequences CZ1, CZ2, and CZ3 (arrows). Genotypes previously found in humans are shaded. The six most divergent sequences (DQ683749, DQ885588, AY237209, AY237210, AY237211, and DQ885585) are not shown in the tree and were used as an outgroup to root the branch of all other isolates. Bootstrap values are given at the nodes.
All three human-pathogenic Encephalitozoon spp. were detected (Table 2). E. cuniculi genotype II and E. hellem genotype 1A were the most prevalent, found in 120 and 24 individuals, respectively. E. cuniculi genotype I was detected in 4 samples; E. intestinalis, in 2 individuals. All ITS sequences from the study samples exhibited 100% matches to the reference genotypes from GenBank as follows: E. hellem 1A, AF338367; E. cuniculi I, AF338410; E. cuniculi II, GQ422153; E. intestinalis, Y11611. Moreover, among these infections, coinfections with E. bieneusi EbpA and E. cuniculi II (in 7 individuals), E. bieneusi CZ3 and E. hellem 1A, and E. bieneusi PigEBITS5 and E. hellem 1A (each in 2 individuals) were found. In single cases, coinfections with E. bieneusi BFRmr2 and E. cuniculi II, E. bieneusi BEB4 and E. cuniculi II, and E. bieneusi EbpA and E. hellem 1A were identified.
To our knowledge, our study is the first report of identification of the four animal-derived E. bieneusi genotypes and E. cuniculi genotype II in humans.
The highest prevalence of PCR-positive samples was recorded for individuals older than 50 years (18/32); the lowest, for children younger than 5 years (13/38) (Table 2). In total, microsporidia were identified in 66 men and 93 women, of whom 34 were foreigners PCR positive for Encephalitozoon spp., 3 were foreigners positive for E. bieneusi, and 9 were foreigners who had coinfections. The total prevalence of PCR-positive people was 42%, and E. bieneusi and/or Encephalitozoon spp. were most often identified in paste stool samples (69/136), followed by formed (94/219) and diarrheal (10/27) samples. However, statistical analysis revealed no relation between the factors analyzed and the acquisition of infection in different categories (Tables 3 and 4).
Table 3.
Results of univariate analysis of risk factors for the acquisition of microsporidiosis
| Variable and characteristic | No. of individuals examined | % of individuals infected | OR (95% CI)a | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 173 | 38.15 | 0.769 (0.51–1.15) | 0.2103 |
| Female | 209 | 44.50 | ||
| Nationality | ||||
| Czech | 265 | 42.64 | 1.147 (0.74–1.79) | 0.543 |
| Foreigner | 117 | 39.32 |
OR, odds ratio; CI, confidence interval.
Table 4.
Risk factors for the acquisition of microsporidiosis by the Cochran-Armitage trend test
| Variable | No. of individuals examined | % of individuals infected | Z | One-sided Pr < Za | Two-sided Pr > |Z|b |
|---|---|---|---|---|---|
| Stool consistency | |||||
| Formed | 219 | 39.73 | −0.3179 | 0.3753 | 0.751 |
| Paste | 136 | 46.32 | |||
| Diarrhea | 27 | 33.33 | |||
| Age category (yr)c | |||||
| 0–5 (3.08) | 38 | 34.21 | −1.202 | 0.1148 | 0.2295 |
| 6–11 (8.11) | 62 | 43.55 | |||
| 12–19 (17.39) | 84 | 44.05 | |||
| 20–50 (29.72) | 166 | 38.55 | |||
| 50+ (62.00) | 32 | 56.25 |
P value for one-sided test.
P value for two-sided test.
The mean age in each category is given in parentheses.
DISCUSSION
Enterocytozoon bieneusi and Encephalitozoon species are known to cause intestinal microsporidiosis in both humans and animals. The epidemiology of microsporidial infection is still being determined, and the prevalence differs greatly in different parts of the world. Microsporidial infections in humans are detected mainly by serology, light microscopic stain techniques, electron microscopy, and PCR-based methods. Infection rates are difficult to compare because of the considerable differences in the diagnostic methods employed, the specimens analyzed, the geographical locations, the patient groups, and patient characteristics (sex, age, sociodemographic data, immune status, and clinical features). Moreover, although all microscopically positive samples were confirmed to be positive by PCR, in accordance with the study reported previously (27), light microscopy was less sensitive than PCR. Whereas surveys for antibodies to microsporidia in human sera have focused exclusively on Encephalitozoon species, with the frequency of positive specimens ranging from 0 to 38% in various population groups (16, 33), the absence of serological surveys for E. bieneusi is attributed to the lack of a standardized method of in vitro cultivation and animal model hosts. The majority of prevalence data are based on surveys using molecular methods performed predominantly with immunodeficient individuals, mostly in developing countries. Limited data about the prevalence of microsporidial infections among immunocompetent people are available. Therefore, this study investigated the occurrence and prevalences of the microsporidial species Enterocytozoon bieneusi and Encephalitozoon spp. in immunocompetent humans in the Czech Republic.
The first documented case of E. bieneusi infection in the Czech Republic was reported for an HIV-positive patient in 1994 (9). The serological study of Kučerová-Pospíšilová and Ditrich (20) included female prostitutes and alcohol and intravenous drug abusers as groups at risk for HIV/AIDS infections. Of 132 individuals, 12% revealed positive results by an enzyme-linked immunosorbent assay (ELISA) with E. cuniculi antigen, and of 125 people, 11% reacted with E. hellem antigen. However, no cases of microsporidial infection were found by parasitological examination, suggesting that the patients examined could have shown residual antibodies from past or latent infections. In a study by Sak et al. (28) in which 115 sera were tested for anti-E. bieneusi antibodies by an immunofluorescence assay (IFA), 20% of HIV-positive patients, 33% of persons with occupational exposure to animals, and 10% of people representing a healthy population were IFA positive.
In developed countries in North America and Europe, and in South Africa, studies involving HIV-seropositive persons with diarrhea reported microsporidian prevalence rates between 2% and 78% by use of different detection methods (3, 4, 11, 29, 32, 34). In these studies, E. bieneusi was the species detected most often, followed by E. intestinalis; these findings are not in accordance with our results, where E. bieneusi was detected four times less frequently than E. cuniculi, and E. intestinalis was identified only sporadically. Moreover, while the association between microsporidian occurrence and diarrhea was statistically supported in many of these studies, and infection rates as low as 1.5% to 4.3% were reported among HIV-seropositive persons without diarrhea (4, 32, 34), in our study, the majority of microsporidial infections (reaching 94%) were reported for nondiarrheal specimens.
In studies conducted in developing countries, E. bieneusi prevalence rates of 2.5% to 51% for HIV-seropositive adult patients with diarrhea (13, 25, 29, 31, 40) and 4.6% for patients without diarrhea (31) have been reported. In contrast, Encephalitozoon sp. infections have been reported in single case reports or at a very low prevalence, reaching a maximum of 10% (10).
As mentioned above, most of what is now known about human microsporidiosis can be attributed to experience with HIV-infected patients. Immunodeficiency, especially that associated with HIV/AIDS, low CD4+ T cell counts (≤50 cells per μl of blood), and younger age are all considered risk factors for microsporidiosis (6, 37). However, cases of intestinal microsporidiosis have been increasingly reported for other immunocompromised individuals, such as organ transplant recipients, as well as travelers, children, and the elderly, mostly suffering from self-limiting or acute diarrhea (21, 26, 39). On the other hand, in our study, only a minority of diarrheal samples were microsporidian positive, and microsporidia were present mainly in formed or paste stool specimens. This could be explained by the lower infection intensity reported for immunocompetent individuals than for immunodeficient individuals, who often suffer from chronic profuse diarrhea.
Some reports of studies with humans had suggested that microsporidia can produce asymptomatic infections in immunocompetent individuals (29, 37). It would be unrealistic to expect that only immunocompromised individuals become infected, since microsporidia have been detected in groundwater and surface water sources (2, 36). In addition, several microsporidian species that infect humans have been identified in animals, especially mammals (7, 21), raising public health concerns about zoonotic and waterborne transmission of microsporidia (2, 23). Also, our sequence analysis results show clearly that the E. bieneusi genotypes detected, including three new sequences, belong to a group of isolates that mostly originate from pigs and cattle. These data suggest that isolates of this group are able to switch hosts from animals to humans and should be regarded as isolates with high zoonotic potential. Moreover, the finding of E. cuniculi genotype II, which has been detected in mice and birds previously (17, 22, 27), suggested the role of wild and captive animals as important sources of human-pathogenic microsporidia in the Czech Republic.
As far as we know, this is the largest study of microsporidial infection in a healthy human population carried out so far. The data obtained during our study show surprisingly high prevalences in different populations of immunocompetent humans, raising the question of whether the findings represent true infection resulting in shedding of parasites or ingested parasites that just passed through the gastrointestinal tract without establishing an infection. However, the chance of detecting the temporary passage of spores is quite low in the number of individuals sampled in the present study. Moreover, the fact that microsporidian spores were detected microscopically in 47% of PCR-positive samples implies actual infection. Although the ability of microsporidia to survive in immunocompetent hosts has not been fully elucidated, on the basis of our results it is obvious that immunocompetent individuals are able to shed detectable amounts of spores in their excretions. In addition, the data we obtained by microscopic screening in this study suggest the necessity of multiple samplings of the same individual, because in some cases, only one of triplicate samples was positive for microsporidia.
ACKNOWLEDGMENTS
This study was funded by grants from the Academy of Sciences of the Czech Republic (KJB500960701), the Grant Agency of the Czech Republic (project 206/09/0927), and a research project of the Institute of Parasitology, Academy of Sciences of the Czech Republic (Z60220518).
Footnotes
Published ahead of print on 29 December 2010.
REFERENCES
- 1. Abreu-Acosta N., et al. 2005. Enterocytozoon bieneusi (microsporidia) in clinical samples from immunocompetent individuals in Tenerife, Canary Islands, Spain. Trans. R. Soc. Trop. Med. Hyg. 99:848–855 [DOI] [PubMed] [Google Scholar]
- 2. Cotte L., et al. 1999. Waterborne outbreak of intestinal microsporidiosis in persons with and without human immunodeficiency virus infection. J. Infect. Dis. 180:2003–2008 [DOI] [PubMed] [Google Scholar]
- 3. Cotte L., et al. 1993. Prevalence of intestinal protozoans in French patients infected with HIV. J. Acquir. Immune Defic. Syndr. 6:1024–1029 [PubMed] [Google Scholar]
- 4. Coyle C. M., et al. 1996. Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clin. Infect. Dis. 23:1002–1006 [DOI] [PubMed] [Google Scholar]
- 5. Croppo G. P., Gomez Morales M. A., Pozio E. 1991. Microsporidia infections in immunocompetent and immunosuppressed subjects. Parassitologia 33:209–218 (In Italian.) [PubMed] [Google Scholar]
- 6. Dascomb K., Frazer T., Clark R. A., Kissinger P., Didier E. S. 2000. Microsporidiosis and HIV. J. Acquir. Immune Defic. Syndr. 24:290–292 [DOI] [PubMed] [Google Scholar]
- 7. Deplazes P., Mathis A., Weber R. 2000. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 6:236–260 [DOI] [PubMed] [Google Scholar]
- 8. Didier E. S., et al. 1996. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J. Eukaryot. Microbiol. 43:34–43 [DOI] [PubMed] [Google Scholar]
- 9. Ditrich O., Lom J., Dyková I., Vávra J. 1994. First case of Enterocytozoon bieneusi infection in the Czech Republic: comments on the ultrastructure and teratoid sporogenesis of the parasite. J. Eukaryot. Microbiol. 41:35S–36S [PubMed] [Google Scholar]
- 10. Endeshaw T., et al. 2006. Intestinal microsporidiosis in diarrheal patients infected with human immunodeficiency virus-1 in Addis Ababa, Ethiopia. Jpn. J. Infect. Dis. 59:306–310 [PubMed] [Google Scholar]
- 11. Ferreira F. M., et al. 2001. Intestinal microsporidiosis: a current infection in HIV-seropositive patients in Portugal. Microbes Infect. 3:1015–1019 [DOI] [PubMed] [Google Scholar]
- 12. Fournier S., et al. 2000. Disseminated infection due to Encephalitozoon cuniculi in a patient with AIDS: case report and review. HIV Med. 1:155–161 [DOI] [PubMed] [Google Scholar]
- 13. Gumbo T., et al. 1999. Intestinal parasites in patients with diarrhea and human immunodeficiency virus infection in Zimbabwe. AIDS 13:819–821 [DOI] [PubMed] [Google Scholar]
- 14. Haro M., del Águila C., Fenoy S., Henriques-Gil N. 2003. Intraspecies genotype variability of the microsporidian parasite Encephalitozoon hellem. J. Clin. Microbiol. 41:4166–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haro M., et al. 2005. First detection and genotyping of human-associated microsporidia in pigeons from urban parks. Appl. Environ. Microbiol. 71:3153–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollister W. S., Canning E. U. 1987. An enzyme-linked immunosorbent assay (ELISA) for detection of antibodies to Encephalitozoon cuniculi and its use in determination of infections in man. Parasitology 94:209–219 [DOI] [PubMed] [Google Scholar]
- 17. Kašičková D., Sak B., Kváč M., Ditrich O. 2009. Sources of potentially infectious human microsporidia: molecular characterisation of microsporidia isolates from exotic birds in the Czech Republic, prevalence study and importance of birds in epidemiology of the human microsporidial infections. Vet. Parasitol. 165:125–130 [DOI] [PubMed] [Google Scholar]
- 18. Katzwinkel-Wladarsch S., Lieb M., Helse W., Loscher T., Rinder H. 1996. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop. Med. Int. Health 1:373–378 [DOI] [PubMed] [Google Scholar]
- 19. Kotler D. P., Orenstein J. M. 1998. Clinical syndromes associated with microsporidiosis. Adv. Parasitol. 40:321–349 [DOI] [PubMed] [Google Scholar]
- 20. Kučerová-Pospíšilová Z., Ditrich O. 1998. The serological surveillance of several groups of patients using antigens of Encephalitozoon hellem and E. cuniculi antibodies to microsporidia in patients. Folia Parasitol. 45:108–112 [PubMed] [Google Scholar]
- 21. Lores B., et al. 2002. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus-negative patients from Vigo, Spain. Clin. Infect. Dis. 34:918–921 [DOI] [PubMed] [Google Scholar]
- 22. Mathis A., et al. 1997. Two Encephalitozoon cuniculi strains of human origin are infectious to rabbits. Parasitology 114:29–35 [DOI] [PubMed] [Google Scholar]
- 23. Mathis A., Weber R., Deplazes P. 2005. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 18:423–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mertens R. B., et al. 1997. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod. Pathol. 10:68–77 [PubMed] [Google Scholar]
- 25. Mohandas S. R., Sud A., Malla N. 2002. Prevalence of intestinal parasitic pathogens in HIV-seropositive individuals in Northern India. Jpn. J. Infect. Dis. 55:83–84 [PubMed] [Google Scholar]
- 26. Müller A., et al. 2001. Detection of microsporidia in travelers with diarrhea. J. Clin. Microbiol. 39:1630–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sak B., Kašičková D., Kváč M., Květoňová D., Ditrich O. 2010. Microsporidia in exotic birds: intermittent spore excretion of Encephalitozoon spp. in naturally infected budgerigars (Melopsittacus undulatus). Vet. Parasitol. 168:196–200 [DOI] [PubMed] [Google Scholar]
- 28. Sak B., et al. 2010. Seropositivity for Enterocytozoon bieneusi, Czech Republic. Emerg. Infect. Dis. 16:335–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samie A., Obi C. L., Tzipori S., Weiss L. M., Guerrant R. L. 2007. Microsporidiosis in South Africa: PCR detection in stool samples of HIV-positive and HIV-negative individuals and school children in Vhembe district, Limpopo Province. Trans. R. Soc. Trop. Med. Hyg. 101:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santín M., Fayer R. 2009. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. J. Eukaryot. Microbiol. 56:34–38 [DOI] [PubMed] [Google Scholar]
- 31. Sarfati C., et al. 2006. Prevalence of intestinal parasites including microsporidia in human immunodeficiency virus-infected adults in Cameroon: a cross-sectional study. Am. J. Trop. Med. Hyg. 74:162–164 [PubMed] [Google Scholar]
- 32. Schmidt W., et al. 1997. Mucosal abnormalities in microsporidiosis. AIDS 11:1589–1594 [DOI] [PubMed] [Google Scholar]
- 33. Singh M., et al. 1982. Detection of antibodies to Nosema cuniculi (Protozoa: Microscoporidia) in human and animal sera by the indirect fluorescent antibody technique. Southeast Asian J. Trop. Med. Public Health 13:110–113 [PubMed] [Google Scholar]
- 34. Sobottka I., et al. 1998. Prevalence and clinical significance of intestinal microsporidiosis in human immunodeficiency virus-infected patients with and without diarrhea in Germany: a prospective coprodiagnostic study. Clin. Infect. Dis. 26:475–480 [DOI] [PubMed] [Google Scholar]
- 35. Svenungsson B., Capraru T., Evengård B., Larsson R., Lebbad M. 1998. Intestinal microsporidiosis in a HIV-seronegative patient. Scand. J. Infect. Dis. 30:314–316 [DOI] [PubMed] [Google Scholar]
- 36. Thurston-Enriquez J. A., et al. 2002. Detection of protozoan parasites and microsporidia in irrigation waters used for crop production. J. Food Prot. 65:378–382 [DOI] [PubMed] [Google Scholar]
- 37. Tumwine J. K., et al. 2002. Enterocytozoon bieneusi among children with diarrhea attending Mulago Hospital in Uganda. Am. J. Trop. Med. Hyg. 67:299–303 [DOI] [PubMed] [Google Scholar]
- 38. Vávra J., Chalupský J. 1982. Fluorescence staining of microsporidian spores with the brightener “Calcofluor White M2R.” J. Protozool. 29:503 [Google Scholar]
- 39. Wanke C. A., DeGirolami P., Federman M. 1996. Enterocytozoon bieneusi infection and diarrheal disease in patients who were not infected with human immunodeficiency virus: case report and review. Clin. Infect. Dis. 23:816–818 [DOI] [PubMed] [Google Scholar]
- 40. Waywa D., et al. 2001. Protozoan enteric infection in AIDS related diarrhea in Thailand. Southeast Asian J. Trop. Med. Public Health 32:151–155 [PubMed] [Google Scholar]