Abstract
Phaeoacremonium parasiticum is an environmental fungus usually associated with subcutaneous infections. We report the first documented case of central nervous system involvement with brain abscess formation in a patient with chronic granulomatous disease and review the literature on Phaeoacremonium parasiticum infections.
CASE REPORT
A 24-year-old man was transferred to our hospital with complaints of productive cough, right-sided chest pain, and 2 weeks of left arm weakness. His past medical history was notable for chronic granulomatous disease (CGD), seizure disorder, disseminated aspergillosis affecting his lungs, spine, and skin, as well as end-stage kidney disease secondary to focal segmental glomerulosclerosis. His aspergillosis was diagnosed during his early teenage years with no known central nervous system (CNS) involvement. Over the years he had experienced numerous infectious complications from his CGD, including multiple episodes of skin and soft tissue infections, catheter-associated bacteremia, pneumonia, and Clostridium difficile colitis. The patient was on voriconazole and trimethoprim-sulfamethoxazole prophylactically; however, he had a history of medication noncompliance. His social history was remarkable for occasional marijuana use, and he had no reported history of recent outdoor activity, travel, or known sick contacts.
On admission, a chest X-ray and computed tomography (CT) scan identified consolidative opacities in the upper lung zones as well as bilateral pleural effusions, which represented an interval increase from previous imaging 2 months earlier. Antimicrobial coverage for presumed bacterial pneumonia was initiated with levofloxacin. The patient's voriconazole and trimethoprim-sulfamethoxazole treatment were also continued. On hospital day (HD) 2, the patient had a tonic clonic seizure, followed by a right-sided facial droop and pronounced left arm weakness. Magnetic resonance imaging (MRI) of the head demonstrated focal lesions in the right temporal lobe and putamen measuring 0.9 cm by 0.8 cm and 2.3 cm by 1.5 cm, respectively, with associated vasogenic edema. Vancomycin and meropenem were started empirically. Caspofungin was added to his antifungal therapy, followed by liposomal amphotericin B due to undetectable voriconazole levels assayed by high-performance liquid chromatography at the Fungus Testing Laboratory at the University of Texas, San Antonio, TX. Stereotactic brain biopsies were attempted twice on HD 4 and HD 12 with no diagnostic yield from microscopy or from bacterial and fungal cultures, although there was a high clinical suspicion for CNS aspergillosis.
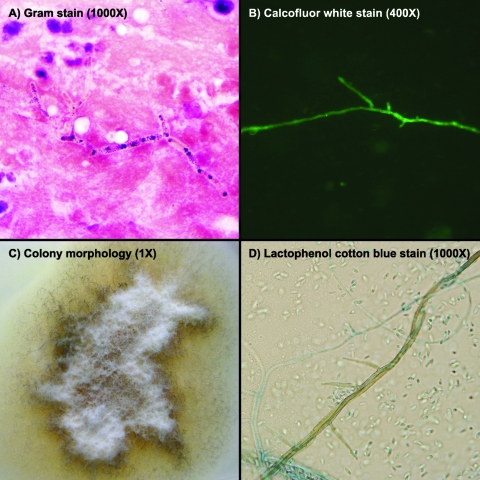
Worsened neurologic findings and expanding CNS lesions, along with vasogenic edema and some midline shifting, prompted a craniotomy with core biopsy of the right temporal lobe lesion on HD 18. The biopsy specimen yielded approximately 8 ml of tissue and green fluid. Gram and calcofluor white stains of the biopsy fluid displayed rare hyphal structures with septation and branching (Fig. 1A and B). Hematoxylin-and-eosin-stained sections of formalin-fixed, paraffin-embedded tissue showed reactive gliosis with acute inflammation. A periodic acid Schiff with diastase stain showed rare hyphal forms.
Fig. 1.
Microbiological findings of Phaeoacremonium parasiticum. Gram stain (A) and calcofluor white stain (B) on brain biopsy fluid demonstrated rare branched, septated hyphae. Colony morphology (C) is shown on potato dextrose agar. Fungal culture (D) revealed pigmented conidiophores with thin tapering phialides and allantoid conidia.
Approximately 0.5 ml of abscess fluid was inoculated onto Sabouraud dextrose agar (BBL, Sparks, MD) and potato dextrose agar (prepared at our clinical lab facility) (7) with 50 μg/ml chloramphenicol. Plates were incubated at 35°C for 2 days, followed by incubation at 30°C. After 3 days, greater than 50 colonies of a slow-growing mold appeared, which initially were white, flat, and felty and subsequently became brown-gray on potato dextrose agar (Fig. 1C). A cellophane tape mount was done from a potato dextrose agar plate by using lactophenol cotton blue. Microscopic examination of 2-day-old colonies showed unpigmented mycelia and oval conidia (2 to 3 μm in diameter). After 14 days of incubation, microscopic examination showed faintly pigmented conidiophores with thin, tapering phialides 15 to 30 μm in length and allantoid conidia approximately 3 μm in length (Fig. 1D). Occasional conidiophores also produced small exudates, which were 2 to 3 μm in size. Growth at 35 and 42°C demonstrated that the mold was thermotolerant.
Because of nonspecific findings from microscopy and the gravity of the patient's infection, gene sequencing was performed on HD 37 to facilitate microbiological identification of the mold. Fungal colonies were inoculated into 1 ml nuclease-free water and boiled for 10 min, and sterile glass beads were added to lyse the cells. The mixture was shaken for 1 min and centrifuged for 2 min at 12,000 rpm. Three microliters of the supernatant was amplified in a 50-μl reaction mixture consisting of 0.5 μM GCATATCAATAAGCGGAGGA and GGTCCGTGTTTCAAGACGG universal primers to the D1D2 rRNA gene and 1× HotStarTaq Plus master mix (Eurofins MWG Operon, Huntsville, AL). The PCR analysis was performed on a DNA engine thermal cycler (Bio-Rad Laboratories, Hercules, CA). The PCR conditions included a 95°C activation step for 5 min, followed by 35 cycles of 95°C for 40 s, 60°C for 30 s, and 72°C for 2 min and a final 72°C elongation step for 10 min. Eight microliters of the PCR mix was visualized on a 1% agarose gel for the presence of a 0.5-kb amplicon. Cycle sequencing was performed in separate reaction mixture volumes consisting of 2 pM of primers GCATATCAATAAGCGGAGGA and GGTCCGTGTTTCAAGACGG plus 2 μl BigDye Terminator mix, 3 μl 5× BigDye Terminator buffer (PerkinElmer Applied Biosystems, Foster City, CA), and 10 μl diluted (1:6 in water) PCR mix. Cycling conditions included 25 cycles at 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min. Sequencing products were purified with a BigDye XTerminator purification kit and separated on an ABI 3730 genetic analyzer (Applied Biosystems). DNA sequences were assembled with Lasergene software (DNAStar, Madison, WI) and compared to those in the NCBI GenBank (http://www.ncbi.nlm.nih.gov/genbank/). A distance score of 0% to less than 1% was used as the criterion for species identification. The same method was employed to sequence the beta-tubulin gene, using universal primers GGTAACCAAATCGGTGCTGCTTTC and ACCCTCAGTGTAGTGACCCTTGGC and sequencing primers GGTAACCAAATCGGTGCTGCTTTC and ACCCTCAGTGTAGTGACCCTTGGC.
Sequence analysis of the D1D2 rRNA and beta-tubulin genes showed over 99% similarity to that of Phaeoacremonium parasiticum, based on NCBI GenBank reference sequence AB100629 for D1D2 (1) and AY579307 for beta-tubulin (15), respectively. The isolate has been deposited in the American Type Culture Collection (MYA-4758).
Antifungal susceptibility testing was done at the Fungus Testing Laboratory at the University of Texas, San Antonio, TX, using the reference broth dilution method (8). Testing demonstrated the following MICs at 24 h: amphotericin, 2 μg/ml; caspofungin, greater than 8 μg/ml; posaconazole, 0.5 μg/ml; voriconazole, 0.25 μg/ml; and terbinafine, 0.5 μg/ml. The patient's serum voriconazole levels remained undetectable on HD 21 and 22, with 4 mg/kg intravenous dosing twice daily. The voriconazole dose was subsequently increased to 7 mg/kg intravenously twice daily, and the serum level was 2.18 μg/ml on HD 28. Over the course of his hospital stay, the patient underwent three additional dose adjustments in response to fluctuating voriconazole serum levels of >19.4 to 1.06 μg/ml.
Despite extensive antifungal coverage with liposomal amphotericin B, voriconazole, and caspofungin, by HD 30 the patient developed superior vena cava syndrome due to a compressive upper lung lesion along with venous thromboses of the right subclavian, brachiocephalic, and internal jugular veins. In the setting of clinical decline he underwent a diagnostic fine-needle biopsy of the right lung on HD 33. The biopsy specimen grew P. parasiticum. In consultation with the immunology service, a decision was made to commence gamma interferon (IFN-γ) therapy. His antimicrobial regimen was once again modified to optimize treatment for documented ventriculitis, and terbinafine was added to his antifungal regimen. There was marginal improvement in his pulmonary and neurological status, but despite continued therapy he succumbed to his disease on HD 50.
Discussion.
Phaeoacremonium parasiticum was first described in 1974 as the first species of the genus Phaeoacremonium to cause phaeohyphomycosis (2). Phaeoacremonium species are found in the environment and can either cause plant diseases or grow as symbionts in woody plants (15). Most cases of Phaeoacremonium infection in humans involve traumatic inoculation and present as subcutaneous infections, such as abscesses and cysts or osteoarthritis. P. parasiticum is a rare cause of human disease and has been reported in cases of arthritis (13), subcutaneous infections (2), eumycetoma (11), onychomycosis (19), endophthalmitis (12), endocarditis (10), and disseminated disease, including fungemia and multiorgan involvement (5). This report is the first documented case of disseminated disease with central nervous system involvement and brain abscess formation secondary to P. parasiticum infection.
The most common cause of CNS phaeohyphomycosis is Cladophialophora bantiana, with an even distribution among immunocompetent and immunocompromised patients. In general, mortality in cases of CNS phaeohyphomycosis treated with both surgical debridement and chemotherapy is high, nearing 70% (14, 17). The optimal antifungal regimen to treat P. parasiticum infections, particularly those with CNS involvement, is unclear. Immunocompetent patients with localized infections such as subcutaneous or joint-based disease are likely to survive regardless of treatment choice; however, no treatment has been shown to be effective for immunocompromised patients with disseminated infection (5).
Further complicating this case was the patient's underlying CGD, which gives way to abnormal host defense against opportunistic fungi. In this patient population, aspergillosis is one of the most commonly diagnosed fungal infections. When there is involvement of the CNS with Aspergillus infection, mortality may be as high as 95%. Though the mechanism of action is not clearly understood, IFN-γ has proven to be useful in the prophylaxis of patients with CGD. Moreover, there have been reports of successful treatment of filamentous fungus infection when IFN-γ is combined with appropriate antifungal therapy (3).
Amphotericin appears to be the most commonly used agent in cases of CNS phaeohyphomycosis, at times combined with azoles such as itraconazole. Lipid formulations of amphotericin may have a role in decreasing toxicity, therefore allowing higher doses and longer courses of treatment (17). An animal study has shown greater brain tissue penetration with lipid formulations of amphotericin than with other preparations of amphotericin B in candidal CNS infection (9); however, use of lipid formulations in reported cases of CNS phaeohyphomycosis has been limited. 5FC has been found to have excellent CSF penetration, and in vitro and animal models confirm its activity in cases of systemic and CNS phaeohyphomycosis (17). Terbinafine is typically not used in cases of CNS phaeohyphomycosis; however, it has been used successfully in the treatment of systemic or CNS mold infections in immunocompromised hosts (6, 16, 18). More recently, studies have supported the use of posaconazole in CNS phaeohyphomycosis, such as that with C. bantiana (4); however, this drug is available only in oral formulation and requires nutrient intake to ensure absorption. In our case, we obtained susceptibility testing with MICs and directed our therapy accordingly; however, there is no standard interpretation for MICs of molds, and serum levels of antifungal drugs may not correlate with levels achieved in the brain.
With the availability of molecular methods such as gene sequencing for accurate species identification, we anticipate that future cases of P. parasiticum infection will be diagnosed more rapidly and lead to earlier directed antifungal therapy and management. Such improvements may ultimately lead to better outcomes in immunocompromised hosts where the infection would have been uniformly fatal.
Nucleotide sequence accession numbers.
Sequences of the D1D2 rRNA and beta-tubulin genes of the Phaeoacremonium parasiticum isolate reported in this article have been deposited in GenBank (HM486494 and HM486495).
Acknowledgments
We thank Michelle Ho of the Pharmacy Division at Stanford University Hospital for her assistance with antimicrobial and antifungal history. Additionally, we thank Indre Budvytiene, Diane Getsinger, and Laleh Ghafghaichi for their technical assistance.
There was no financial support for the production of this paper. We have no conflict of interest.
Footnotes
Published ahead of print on 29 December 2010.
REFERENCES
- 1. Abliz P., Fukushima K., Takizawa K., Nishimura K. 2004. Identification of pathogenic dematiaceous fungi and related taxa based on large subunit ribosomal DNA D1/D2 domain sequence analysis. FEMS Immunol. Med. Microbiol. 40:41–49 [DOI] [PubMed] [Google Scholar]
- 2. Ajello L., Georg L. K., Steigbigel R. T., Wang C. J. 1974. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia 66:490–498 [PubMed] [Google Scholar]
- 3. Alsultan A., Williams M. S., Lubner S., Goldman F. D. 2006. Chronic granulomatous disease presenting with disseminated intracranial aspergillosis. Pediatr. Blood Cancer 47:107–110 [DOI] [PubMed] [Google Scholar]
- 4. Badali H., de Hoog G. S., Curfs-Breuker I., Klaassen C. H., Meis J. F. 2010. Use of amplified fragment length polymorphism to identify 42 Cladophialophora strains related to cerebral phaeohyphomycosis with in vitro antifungal susceptibility. J. Clin. Microbiol. 48:2350–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baddley J. W., Mostert L., Summerbell R. C., Moser S. A. 2006. Phaeoacremonium parasiticum infections confirmed by beta-tubulin sequence analysis of case isolates. J. Clin. Microbiol. 44:2207–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhat S. V., Paterson D. L., Rinaldi M. G., Veldkamp P. J. 2007. Scedosporium prolificans brain abscess in a patient with chronic granulomatous disease: successful combination therapy with voriconazole and terbinafine. Scand. J. Infect. Dis. 39:87–90 [DOI] [PubMed] [Google Scholar]
- 7. Burtelow M., Merker J. D., Baron E. J. 2009. Growth of Histoplasma capsulatum Isolates is better on potato dextrose agar with chloramphenicol than on brain heart infusion agar. J. Mycologie Medicale 19:197–199 [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 2nd ed Approved standard M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Groll A. H., et al. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274–282 [DOI] [PubMed] [Google Scholar]
- 10. Heath C. H., Lendrum J. L., Wetherall B. L., Wesselingh S. L., Gordon D. L. 1997. Phaeoacremonium parasiticum infective endocarditis following liver transplantation. Clin. Infect. Dis. 25:1251–1252 [DOI] [PubMed] [Google Scholar]
- 11. Hood S. V., Moore C. B., Cheesbrough J. S., Mene A., Denning D. W. 1997. Atypical eumycetoma caused by Phialophora parasitica successfully treated with itraconazole and flucytosine. Br. J. Dermatol. 136:953–956 [PubMed] [Google Scholar]
- 12. Huynh T. K., Lee L. R., Ellis D. 2007. Late-onset post-traumatic Phaeoacremonium parasiticum endophthalmitis. Clin. Experiment. Ophthalmol. 35:366–368 [DOI] [PubMed] [Google Scholar]
- 13. Kaell A. T., Weitzman I. 1983. Acute monoarticular arthritis due to Phialophora parasitica. Am. J. Med. 74:519–522 [DOI] [PubMed] [Google Scholar]
- 14. Li D. M., de Hoog G. S. 2009. Cerebral phaeohyphomycosis—a cure at what lengths? Lancet Infect. Dis. 9:376–383 [DOI] [PubMed] [Google Scholar]
- 15. Mostert L., et al. 2005. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. J. Clin. Microbiol. 43:1752–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neuburger S., et al. 2008. Successful salvage treatment of disseminated cutaneous fusariosis with liposomal amphotericin B and terbinafine after allogeneic stem cell transplantation. Transpl. Infect. Dis. 10:290–293 [DOI] [PubMed] [Google Scholar]
- 17. Revankar S. G., Sutton D. A., Rinaldi M. G. 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 38:206–216 [DOI] [PubMed] [Google Scholar]
- 18. Rothe A., et al. 2004. Combination therapy of disseminated Fusarium oxysporum infection with terbinafine and amphotericin B. Ann. Hematol. 83:394–397 [DOI] [PubMed] [Google Scholar]
- 19. Sun P. L., Ju Y. M. 27 September 2009, posting date Onychomycosis caused by Phaeoacremonium parasiticum: first case report. Mycoses doi:10.1111/j.1439-0507.2009.01789.x [DOI] [PubMed] [Google Scholar]