Abstract
Seventy-eight Salmonella enterica serovar Heidelberg isolates from humans were tested for antimicrobial susceptibility, resistance genes, and plasmids and genotyped by pulsed-field gel electrophoresis (PFGE). Most (88%) contained plasmids, and 47% were resistant to antimicrobials. The overall results were compared to those of previous S. Heidelberg studies of food- and animal-related sources, and multiple similarities were observed.
Salmonella enterica serovar Heidelberg (S. Heidelberg) is among the most commonly detected serovars from retail meats and food animals and ranks fourth among serovars associated with human infections, causing an estimated 84,000 illnesses in the United States annually (2, 7, 11). While most Salmonella infections are self-limiting and resolve within a few days, S. Heidelberg tends to cause a disproportionately high percentage of invasive infections (21) for which antimicrobial therapy is often warranted, making antimicrobial resistance a significant concern. Antimicrobial-resistant S. Heidelberg strains have been isolated from humans, retail meats, and food animals (13–15, 17, 18, 24); thus, resistant organisms in the food supply may contribute to human disease. Multiple studies have examined the genetics of antimicrobial resistance in S. Heidelberg isolates from food animals; however, information on those isolated from human patients is limited (6, 19). The goal of this study was to characterize antimicrobial resistance and associated genetic factors in S. Heidelberg from humans and compare these results to those obtained with previously characterized isolates from food and food animal sources (13, 15).
For this study, a convenience sample of 78 S. Heidelberg isolates from human patients was obtained from state health departments in Arkansas (n = 30), New York (n = 18), and Wisconsin (n = 30). Antimicrobial susceptibility testing (AST) was performed by broth microdilution using CMV1AGNF Sensititre panels (Trek Diagnostics, Cleveland, OH), and the results were interpreted according to the CLSI guidelines (4). Overall, 37 (47%) isolates were resistant to at least one antimicrobial, contributing to 21 different susceptibility profiles (Table 1). Resistance phenotypes were similar to those reported from the National Antimicrobial Resistance Monitoring System program and previous studies examining S. Heidelberg from foods and animal sources (8, 13, 15, 23, 24). These similarities included observed susceptibility to ciprofloxacin and amikacin and, most commonly, resistance to tetracycline, ampicillin, kanamycin, and streptomycin (Table 2). Across the studies, the overall percentage of resistance was generally lower in isolates from human versus veterinary sources (8).
Table 1.
Antimicrobial resistance profiles of S. Heidelberg isolates collected from human patients
| Resistance profilea | No. of isolates from: |
Total no. of isolates | % of isolates | ||
|---|---|---|---|---|---|
| ARb | NYc | WId | |||
| No resistance (pansusceptible) | 11 | 14 | 16 | 41 | 52.6 |
| Amp | 3 | 1 | 4 | 5.1 | |
| Tio | 1 | 1 | 1.3 | ||
| Tet | 4 | 4 | 5.1 | ||
| Amp, Str | 2 | 2 | 2.6 | ||
| Tet, Amp | 1 | 1 | 1.3 | ||
| Tet, Kan | 1 | 1 | 1.3 | ||
| Gen, Str, Sul | 3 | 3 | 3.9 | ||
| Tet, Kan, Str | 1 | 2 | 2 | 5 | 6.4 |
| Tet, Amp, Kan | 1 | 1 | 1.3 | ||
| Tio, Fox, Amp, Amc | 2 | 2 | 2.6 | ||
| Tet, Kan, Str, Sul | 1 | 1 | 1.3 | ||
| Tet, Amp, Kan, Str | 2 | 2 | 2.6 | ||
| Tet, Amp, Amc, Kan, Str | 1 | 1 | 1.3 | ||
| Tet, Amp, Gen, Kan, Str | 1 | 1 | 1.3 | ||
| Tet, Gen, Kan, Str, Sul | 1 | 1 | 2 | 2.6 | |
| Tet, Tio, Fox, Amp, Amc | 1 | 1 | 1.3 | ||
| Tet, Tio, Fox, Amp, Amc, Kan, Str | 2 | 2 | 2.6 | ||
| Tet, Tio, Fox, Amp, Amc, Kan, Str, Sul | 1 | 1 | 1.3 | ||
| Chl, Tet, Amp, Amc, Gen, Kan, Str, Sul | 1 | 1 | 1.3 | ||
| Chl, Tet, Tio, Fox, Amp, Amc, Gen, Str, Sul, Sxt | 1 | 1 | 1.3 | ||
| Total | 30 | 18 | 30 | 78 | 100 |
Chl, chloramphenicol; Tet, tetracycline; Tio, ceftiofur; Fox, cefoxitin; Amp, ampicillin; Amc, amoxicillin-clavulanic acid; Gen, gentamicin; Kan, kanamycin; Str, streptomycin, Sul, sulfonamides; Sxt, trimethoprim-sulfamethoxazole.
Isolates provided from the Arkansas Public Health Laboratories in 2009 in compliance with the request that multiple isolates from a single outbreak were not included.
Isolates provided from the New York State Department of Health in 2009 in compliance with the request that multiple isolates from a single outbreak were not included.
Isolates provided from the Wisconsin State Laboratory of Hygiene in 2008 in compliance with the request that multiple isolates from a single outbreak were not included. Fifteen isolates from invasive (blood culture) and 15 from gastrointestinal (fecal culture) infections were provided.
Table 2.
Antimicrobial resistance rates of S. Heidelberg isolates examined in the present study and of those reported from previous studies
| Antimicrobial(s) | Rate of resistancea (%) |
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| Chloramphenicol | 2.6 | 3.1 | 2.1 | 27.5 | 2.8 | 1.0 |
| Tetracycline | 32 | 22.4 | 19.1 | 70.7 | 28.9 | 39.9 |
| Ceftriaxone | 0 | 0 | 2.1 | 1.7 | 0 | 0 |
| Ceftiofur | 10 | 7.1 | NDb | 18.9 | 3.3 | 9.0 |
| Cefoxitin | 9 | 7.1 | 4.2 | 25.8 | 3.3 | 9.0 |
| Ampicillin | 25.6 | 18.4 | 8.5 | 32.7 | 15.6 | 19.8 |
| Amoxicillin/clavulanate | 11.5 | 7.1 | 2.1 | 27.5 | 5 | 10.4 |
| Amikacin | 0 | 0 | 0 | 0 | 0 | 0 |
| Gentamicin | 10.3 | 16.3 | 4.2 | 17.2 | 24.4 | 25.7 |
| Kanamycin | 23.1 | 11.2 | 12.7 | 51.7 | 21.1 | 21.5 |
| Streptomycin | 28.2 | 12.2 | 17 | 62.1 | 30.6 | 37.8 |
| Sulfonamides | 11.5 | 18.4 | 6.3 | 29.3 | 21.7 | 27.7 |
| Trimethoprim/sulfamethoxazole | 1.3 | 0 | ND | 24.1 | 0.6 | 0.7 |
| Ciprofloxacin | 0 | 0 | 0 | 0 | 0 | 0 |
| Nalidixic acid | 0 | 0 | ND | 0 | 1.1 | 1.0 |
A, antimicrobial resistance rates of the isolates examined in the present study; B, antimicrobial resistance rates of S. Heidelberg human isolates presented in the 2007 Executive Report from the National Antimicrobial Resistance Monitoring System (NARMS) program (8); C, antimicrobial resistance rates reported by Patchanee et al. for S. Heidelberg isolates from human patients (19); D, overall antimicrobial resistance rates reported by Lynne et al. (2009) for S. Heidelberg isolates from food animals (cattle, swine, chicken, and turkey) (15); E, antimicrobial resistance rates reported by Kaldhone et al. (2008) for S. Heidelberg isolates from turkey production and processing sources and retail ground turkey (13); F, antimicrobial resistance rates reported by Zhao et al. (2008) for S. Heidelberg isolates from retail meats (24).
ND, not determined.
To further understand genetic factors contributing to the observed antimicrobial resistance, PCR was used to detect the presence of class 1 integrons and 22 resistance genes (15, 16). When resistance was observed, a corresponding resistance gene was detected 96.1% (n = 124/129 isolates) of the time (Table 3; see also Fig. S1 in the supplemental material). There were seven instances where resistance genes were detected without a corresponding phenotype. In these cases, the genes may not have been expressed or their products may not have been fully active (15). Resistance gene profiles from this study were similar to those previously described for S. Heidelberg isolates from food animals (3, 13, 15). For example, the most commonly detected tetracycline resistance gene was tetB, the majority of streptomycin-resistant isolates contained strA, and floR was associated with chloramphenicol resistance (3, 15, 16).
Table 3.
Comparison of antimicrobial resistance phenotype with corresponding resistance genes detected
| Resistance phenotype [no. (%) of isolates resistant to the indicated antimicrobial(s)] | Resistance genotype [no. (%) of resistant isolates with the indicated genes] | Phenotypic-genotypic agreementa | |||
|---|---|---|---|---|---|
| Chloramphenicol [2 (2.6)] | floR [2 (100)] | cat1 [0 (0.0)] | cat2 [0 (0.0)] | 100 | |
| Tetracycline [25 (32.1)] | tetA [4 (16.0)] | tetB [22 (80.0)] | tetC [1 (4.0)] | NDb [1 (4.0)] | 96 |
| Ceftiofur [8 (10.3)] | blaCMY [7 (87.5)] | blaDHA-1 [2 (25.0)] | ND [1 (12.5)] | 87.5 | |
| Cefoxitin [7 (9.0)] | blaCMY [7 (100)] | blaDHA-1 [2 (77.8)] | 100 | ||
| Ampicillin [20 (25.6)] | blaCMY [8 (40.0)] | blaDHA-1 [3 (15.0)] | blaTEM [12 (60.0)] | ND [1 (5.0)] | 95 |
| Amoxicillin/clavulanate [9 (11.5)] | blaCMY [8 (88.9)] | blaDHA-1 [3 (33.3)] | blaTEM [2 (22.2)] | 100 | |
| Gentamicin [8 (10.3)] | aadB [0 (0.0)] | aacC [7 (87.5)] | ND [1 (12.5)] | 87.5 | |
| Kanamycin [18 (23.1)] | Kn [17 (94.4)] | aphAI-IAB [1 (5.6)] | 100 | ||
| Streptomycin [22 (28.2)] | aadA1 [7 (28.2)] | aadA2 [1 (4.5)] | strA [22 (100)] | strB [8 (36.4)] | 100 |
| Sulfonamides [9 (11.5)] | sul1 [6 (31.8)] | sul2 [4 (44.4)] | sul3 [1 (11.1)] | 100 | |
| Trimethoprim/sulfamethoxazole [1 (1.3)] | dhfrI [0 (0.0)] | dhfrXII [0 (0.0)] | ND [1 (100)] | 0.0 | |
| Total | 96.1 | ||||
Percentages of isolates with a resistance phenotype that had at least one corresponding resistance gene present.
ND, isolate with a resistance phenotype for which a corresponding resistance gene was not detected.
Eight isolates contained class 1 integron amplicons of ∼1.1 kb, which is characteristic of Salmonella-associated integrons carrying aadA gene cassettes (19). Isolate 1025 was PCR positive for a class 1 integron and aadA1 but susceptible to all of the antimicrobials tested. The isolate was sul1 negative, suggesting that part of the integron was absent (5), which would explain the lack of resistance.
Plasmid analysis was carried out using a plasmid Minikit (Qiagen, Valencia, CA) to determine the number and sizes of plasmids (13) and by incompatibility (Inc) (replicon) typing using PCR-based methods previously described (12). Plasmids ranging from <2 to >165 kb in size were detected in 88% (n = 69) of 78 isolates (see Fig. S1 in the supplemental material). Large (>95 kb) plasmids were detected in all isolates (n = 15) resistant to at least four antimicrobials. Only 45% (n = 31/69) of the isolates containing plasmids had identifiable Inc groups. Current replicon typing schemes do not identify all Inc types; thus, a number of plasmids were untypeable. Of those identified, the two predominant Inc groups were IncI1 and IncHI2 (see Fig. S1 in the supplemental material). IncA/C plasmids were detected in two isolates, both resistant to at least 8 antimicrobials.
Resistance to expanded-spectrum cephalosporins such as ceftriaxone is important because of their importance in treating severe Salmonella infections (10). While no isolates were fully resistant to ceftriaxone, eight exhibited intermediate susceptibility to ceftriaxone. Many of the genes whose activity results in this reduced susceptibility to ceftriaxone are located on large conjugative plasmids (13, 22). Each of these strains carried the blaCMY-2 gene, plasmids ≥95 kb in size, and IncA/C or IncI1 plasmids. Plasmids of these replicon types have been found to carry multiple Salmonella resistance genes (1, 9). It is likely that plasmids play key roles in the dissemination of antimicrobial resistance among S. Heidelberg strains (13). This resistance, coupled with the propensity of the serovar to cause invasive infections requiring antimicrobial therapy, make S. Heidelberg a major public health concern.
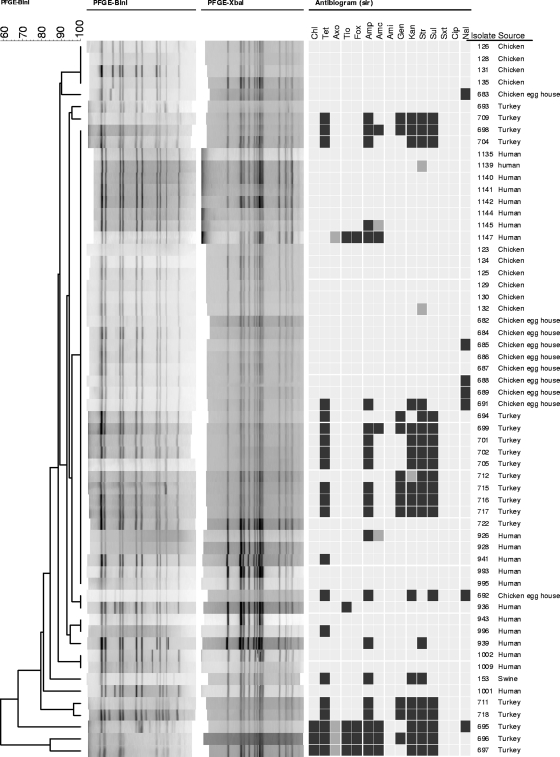
Pulsed-field gel electrophoresis (PFGE) was carried out as previously described (20) to assess relatedness among isolates from human patients and the isolates from foods and animal-associated sources (13, 15). Based on 90% similarity, XbaI patterns for isolates from human patients were broken down into seven clusters (A to G) with at least 2 isolates in each cluster (see Fig. S1 in the supplemental material). Isolates in the largest cluster, cluster A, shared an XbaI pattern with the most common S. Heidelberg profile (JF6X01.0022) in the PulseNet database (13). When PFGE results of isolates from human patients were compared to those from foods, food animals, and related sources, there was considerable overlap. Isolates from chickens, turkeys, swine, and egg houses shared common XbaI profiles with cluster A isolates. These isolates were further discriminated by BlnI PFGE. Isolates from turkeys, chickens, and egg production houses shared BlnI profiles with those from human infections, with the majority sharing an XbaI cluster A-BlnI profile (Fig. 1). These commonalities are consistent with the results of a case-control study in which eggs were the primary vehicle for S. Heidelberg infections (11). The finding of common XbaI/BlnI profiles in isolates from chickens and turkey-related sources (13, 15) may also indicate a potential risk of infection from improperly handled poultry products. It is not known whether isolates of this particular genotype are more apt to cause human infection or whether they are better able to persist in eggs and birds, making it more likely for humans to be exposed to this strain. Interestingly, when PFGE results from human patients were compared to antimicrobial susceptibility profiles, they often did not correlate (Fig. 1). Outside of pansusceptibility (the most common susceptibility phenotype), there were limited instances where susceptibility profiles of food- and animal-associated isolates were identical to those from humans with common PFGE profiles. In general, isolates in particular clusters demonstrated highly variable antimicrobial susceptibility profiles. Among the isolates displaying resistance to two or more antimicrobial agents, the lack of PFGE-susceptibility commonality was likely due to the diversity of plasmids among the isolates (see Fig. S1 in the supplemental material).
Fig. 1.
Comparison of BlnI PFGE and AST of isolates from human patients that share the predominant XbaI profile (Fig. 1, cluster A) with isolates from food and food animal sources that have the same pattern. The columns at the right side of the figure provide the isolate number and isolate source. The dendrogram at the left of the figure is based on the BlnI profiles of isolates. To the right of the BlnI patterns are the XbaI profiles and AST results for chloramphenicol (Chl), tetracycline (Tet), ceftriaxone (Axo), ceftiofur (Tio), cefoxitin (Fox), ampicillin (Amp), amoxicillin-clavulanic acid (Amc), amikacin (Ami), gentamicin (Gen), kanamycin (Kan), streptomycin (Str), sulfonamides (Sul), trimethoprim-sulfamethoxazole (Sxt), ciprofloxacin (Cip), and nalidixic acid (Nal). For the AST results, the light gray box indicates susceptibility, the dark gray box indicates intermediate susceptibility, and the black box indicates resistance to the corresponding antimicrobial.
Overall, this report shows that antimicrobial resistance was commonly detected among the S. Heidelberg isolated from human patients and that isolates that demonstrated resistance to multiple antimicrobial had large plasmids and were positive for IncA/C or IncI1 types. The similarity in PFGE profiles between isolates from humans, animals, and food indicates the potential of food to serve as a source for human infections. The genotypic and phenotypic information provided by this report helps fill some data gaps associated with S. Heidelberg infections.
Supplementary Material
Acknowledgments
We thank Timothy Musser from the New York Department of Health and Tim Monson from the Wisconsin Department of Health and Alessandra Carattoli for providing isolates and controls for the study and Carl Cerniglia, Huizhong Chen, and Mark Hart for their critical reviewing of the manuscript.
We thank the Marshfield Clinic Research Foundation and the U.S. Food and Drug Administration for financial support of the research. Jing Han is supported through the Oak Ridge Institute for Science and Education.
The views presented in the manuscript do not necessarily reflect those of the U.S. Food and Drug Administration.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 22 December 2010.
REFERENCES
- 1. Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 53:2227–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention 2008. Salmonella surveillance: annual summary. 2006 Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 3. Chen S., et al. 2004. Characterization of multiple-antimicrobial-resistant Salmonella serovars isolated from retail meats. Appl. Environ. Microbiol. 70:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement (M100-S18). Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Foley S. L., Lynne A. M. 2007. Food animal-associated Salmonella challenges: pathogenicity and antimicrobial resistance. J. Anim. Sci. 86:E173–E187 [DOI] [PubMed] [Google Scholar]
- 6. Folster J. P., et al. 2010. Characterization of extended-spectrum cephalosporin-resistant Salmonella enterica serovar Heidelberg isolated from humans in the United States. Foodborne Pathog. Dis. 7:181–187 [DOI] [PubMed] [Google Scholar]
- 7. Food and Drug Administration 2009. National antimicrobial resistance monitoring system—enteric bacteria (NARMS): 2006 executive report. U.S. Department of Health and Human Services, U.S. FDA, Silver Spring, MD [Google Scholar]
- 8. Food and Drug Administration 2010. National antimicrobial resistance monitoring system—enteric bacteria (NARMS): 2007 executive report. U.S. Department of Health and Human Services, U.S. FDA, Silver Spring, MD [Google Scholar]
- 9. Fricke W. F., et al. 2009. Comparative genomics of the IncA/C multidrug resistance plasmid family. J. Bacteriol. 191:4750–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilbert D. N., Moellering R. C., Eliopoulos G. M., Sande M. A. 2004. The Sanford guide to antimicrobial therapy, 34th ed Antimicrobial Therapy, Inc., Hyde Park, VT [Google Scholar]
- 11. Hennessy T. W., et al. 2004. Egg consumption is the principal risk factor for sporadic Salmonella serotype Heidelberg infections: a case-control study in FoodNet sites. Clin. Infect. Dis. 38(Suppl. 3):S237–S243 [DOI] [PubMed] [Google Scholar]
- 12. Johnson T. J., et al. 2007. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl. Environ. Microbiol. 73:1976–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaldhone P., et al. 2008. Characterization of Salmonella enterica serovar Heidelberg from turkey-associated sources. Appl. Environ. Microbiol. 74:5038–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Logue C. M., Sherwood J. S., Olah P. A., Elijah L. M., Dockter M. R. 2003. The incidence of antimicrobial-resistant Salmonella spp. on freshly processed poultry from US midwestern processing plants. J. Appl. Microbiol. 94:16–24 [DOI] [PubMed] [Google Scholar]
- 15. Lynne A. M., Kaldhone P., David D., White D. G., Foley S. L. 2009. Characterization of antimicrobial resistance in Salmonella enterica serotype Heidelberg isolated from food animals. Foodborne Pathog. Dis. 6:207–215 [DOI] [PubMed] [Google Scholar]
- 16. Lynne A. M., Rhodes-Clark B. S., Bliven K., Zhao S., Foley S. L. 2008. Antimicrobial resistance genes associated with Salmonella enterica serovar Newport isolates from food animals. Antimicrob. Agents Chemother. 52:353–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nayak R., et al. 2004. Genetic diversity and virulence gene determinants of antibiotic-resistant Salmonella isolated from preharvest turkey production sources. Int. J. Food Microbiol. 91:51–62 [DOI] [PubMed] [Google Scholar]
- 18. Oloya J., Doetkott D., Khaitsa M. L. 2009. Antimicrobial drug resistance and molecular characterization of Salmonella isolated from domestic animals, humans, and meat products. Foodborne Pathog. Dis. 6:273–284 [DOI] [PubMed] [Google Scholar]
- 19. Patchanee P., Zewde B. M., Tadesse D. A., Hoet A., Gebreyes W. A. 2008. Characterization of multidrug-resistant Salmonella enterica serovar Heidelberg isolated from humans and animals. Foodborne Pathog. Dis. 5:839–851 [DOI] [PubMed] [Google Scholar]
- 20. Ribot E. M., et al. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59–67 [DOI] [PubMed] [Google Scholar]
- 21. Vugia D. J., et al. 2004. Invasive Salmonella infections in the United States, FoodNet, 1996–1999: incidence, serotype distribution, and outcome. Clin. Infect. Dis. 38(Suppl. 3):S149–S156 [DOI] [PubMed] [Google Scholar]
- 22. Welch T. J., et al. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao S., et al. 2006. Antimicrobial resistance and genetic relatedness among Salmonella from retail foods of animal origin: NARMS retail meat surveillance. Foodborne Pathog. Dis. 3:106–117 [DOI] [PubMed] [Google Scholar]
- 24. Zhao S., et al. 2008. Antimicrobial resistance in Salmonella enterica serovar Heidelberg isolates from retail meats, including poultry, from 2002 to 2006. Appl. Environ. Microbiol. 74:6656–6662 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.