Abstract
Clostridium difficile is a major cause of nosocomial antibiotic-associated infectious diarrhea and pseudomembranous colitis. Detection of C. difficile by anaerobic bacterial culture and/or cytotoxicity assays has been largely replaced by rapid enzyme immunoassays (EIA). However, due to the lack of sensitivity of stool EIA, we developed a multiplex real-time PCR assay targeting the C. difficile toxin genes tcdA and tcdB. Stool samples from hospitalized pediatric patients suspected of having C. difficile-associated disease were prospectively cultured on cycloserine-cefoxitin-fructose agar following alcohol shock. Six testing modalities were evaluated, including stool EIA, culture EIA, and real-time PCR (tcdA and tcdB) of cultured isolates and stool samples. Real-time PCR detection was performed with tcdA and tcdB gene-specific primers and hydrolysis probes using the LightCycler platforms (Roche Diagnostics, Indianapolis, IN). A total of 157 samples from 96 pediatric patients were analyzed. The sensitivities of stool real-time PCR and stool EIA were 95% and 35%, respectively, with a specificity of 100% for both methods. The lower limit of detection of the stool real-time PCR was 30 CFU/ml of stool sample per reaction for tcdA and tcdB. This study highlights the poor performance of stool toxin EIAs in pediatric settings. Direct detection of C. difficile toxin genes in stool samples by real-time PCR showed sensitivity superior to that of stool and culture EIAs and performance comparable to that of real-time PCR assay of cultured isolates. Real-time PCR of DNA from stool samples is a rapid and cost-effective diagnostic modality for children that should facilitate appropriate patient management and halt the practice of serial testing by EIA.
INTRODUCTION
Clostridium difficile, a Gram-positive spore-forming bacillus, is the most common identifiable etiologic agent of antibiotic-associated diarrhea (19, 26). Initially described as a member of the commensal microbiota of neonates, C. difficile was identified as a causal agent of antibiotic-associated diarrhea in the 1970s (4, 17). The clinical presentation of C. difficile-associated disease (CDAD) can range from asymptomatic carriage in the gastrointestinal tract, mild diarrhea, and potentially fatal pseudomembranous colitis (19, 26). Symptoms occur secondary to the production of two exotoxins, toxin A and toxin B, which disrupt the integrity of the colonic mucosa (44).
Alarming changes in the epidemiology of CDAD, including an increase in both the incidence and severity of the disease, have highlighted concerns about patterns of C. difficile infection (24, 26, 27, 32). Analysis of U.S. hospital discharge data revealed that the national rates of CDAD doubled from 2000 to 2003 (24). In 2004, the Centers for Disease Control and Prevention reported that the mortality rate related to CDAD increased from 5.7 deaths per million individuals in 1999 to 23.7 deaths per million individuals (32). In addition to the profound morbidity and mortality, CDAD is also generating a substantial economic burden, with estimates ranging from $1.3 million to more than $3 billion annually (11, 22, 29). Due to the formidable impact of CDAD on the U.S. health care system, rapid and accurate diagnosis is essential for the timely enactment of infection control and treatment measures.
The changing epidemiology of C. difficile infections in the pediatric population is a serious concern. While benign neonatal colonization with toxigenic C. difficile is a well-documented phenomenon, recent studies have suggested an increased incidence of CDAD in children (3, 20, 34, 45). A large study encompassing data collected from 22 children's hospitals in the United States reported an increased prevalence of CDAD in children, including infants (increased by 53% from 2001 to 2006, with 26% of patients with CDAD ≤1 year of age) (20). Utilizing CDAD data from the Agency for Healthcare and Research Quality, a similar study noted that the highest number of CDAD hospitalizations occurred in patients ≤1 year of age (45).
Initial strategies to detect C. difficile consisted of anaerobic stool sample culture, usually with cycloserine-cefoxitin-fructose agar (CCFA) or a similar medium with or without a pretreatment alcohol shock step (9). Although this modality was quite sensitive and specific for detecting C. difficile, it took up to 5 days to confirm a negative culture and it did not discriminate between toxigenic and nontoxigenic isolates without further testing strategies. Furthermore, colonies with indeterminate colony characteristics were tested with l-proline-aminopeptidase (PRO Disc) or other biochemical tests to ensure the accurate identification of C. difficile (12, 14). The development of the cell culture cytotoxicity assay circumvented stool sample culture by observing cytopathic effects of toxin B directly on cultured cells (5, 8). The cell culture cytotoxicity assay requires a neutralization step for specificity and maintenance of toxin-susceptible mammalian cell lines, and it takes 48 to 72 h to perform the assay (2, 6). Rapid antigen detection assays, consisting of common antigen testing (glutamate dehydrogenase) and toxin immunoassays, have largely replaced culture and the cytotoxic assay; however, neither type has the desired sensitivity or specificity to reliably confirm or rule out CDAD without the need for either serial testing or subsequent testing modalities. Therefore, real-time PCR is being investigated as the preferred diagnostic modality due to its rapid turnaround time and track record of superior sensitivity and specificity. Concerns regarding lighter organism loads in pediatric patients also highlight the issue of the sensitivity of enzyme immunoassays (EIAs).
Toxigenic strains of C. difficile contain a 19.6-kb pathogenicity locus (PaLoc) that includes five contiguous chromosomal genes responsible for the development of CDAD—tcdABCDE (see Fig. S1 in the supplemental material) (44). tcdA and tcdB encode exotoxins A (enterotoxin) and B (cytotoxin), respectively; tcdC and tcdD encode negative and positive regulators, respectively, that control the level of toxin production; and tcdE is purported to encode a holin-like protein thought to facilitate toxin release from the bacterial cell wall (44). Because toxins A and/or B are implicated in CDAD and genetic diversity of the PaLoc has been reported (37), we developed and clinically validated two separate hydrolysis probe real-time PCR assays targeting the tcdA and tcdB genes on the Roche LightCycler 1.0 platform (18, 21, 44). We subsequently multiplexed the tcdA and tcdB PCRs on the Roche LightCycler 2.0 platform. The in-house tcdA primers and internal hydrolysis probe developed at Texas Children's Hospital (Houston, TX) generate a fluorescently labeled 201-bp amplicon within a highly conserved region of the tcdA gene. The tcdB primers and internal hydrolysis probe generate a fluorescently labeled 177-bp amplicon within the nonrepeat region of the tcdB gene as previously described (41). While the molecular methods utilized by this assay were not novel, the application of molecular testing for C. difficile infection in children, as well as the dual-toxin (tcdA and tcdB) target approach, is unique. In addition, the multiyear analysis of CDAD allowed the evaluation of the clinical utility of C. difficile testing by real-time PCR in a pediatric hospital setting.
MATERIALS AND METHODS
Setting, specimen acquisition, and identification of C. difficile.
Texas Children's Hospital is a pediatric tertiary health care facility located within the Texas Medical Center (Houston, TX). During an 8-week period (August and September 2006), stool samples (n = 157) from hospitalized patients (age range, 15 days to 25 years; mean, 6.2 years; median, 4 years) suspected of having CDAD were prospectively cultured on CCFA following alcohol shock. Stool sample consistency was predominantly watery or unformed. Alcohol shock consisted of mixing an aliquot of a stool sample with an equal volume of 95% ethanol, followed by thorough mixing and incubation (room temperature for 1 h). After incubation, each specimen was thoroughly mixed and 100 μl of the homogeneous solution was plated to prereduced CCFA (Anaerobe Systems, Morgan Hill, CA) and incubated anaerobically at 35°C for up to 5 days. CCFA plates were read on day 3 and day 5. Definitive identification of all C. difficile isolates was confirmed by DNA pyrosequencing and/or 16S rRNA Sanger sequencing (25).
Detection of toxin production (EIA).
Patient stool samples and all cultured C. difficile isolates were tested for toxin production by the C. difficile Tox A/B II immunoassay (Wampole Laboratories, Princeton, NJ) according to the manufacturer's instructions. With regard to cultured isolates, the amount of culture inoculum applied to each swab (provided by the manufacturer) was equivalent to the amount of fecal material applied to the swab for routine testing in our laboratory as recommended by the manufacturer.
Molecular detection of toxin genes. (i) Stool specimen preparation.
For each specimen, 500 μl of stool sample and 1,500 μl of S.T.A.R. Buffer (Roche Diagnostics, Indianapolis, IN) were added to a sterile 2.5-ml screw-top Eppendorf tube, thoroughly mixed, and centrifuged (4,000 × g for 1 min) in order to sediment the particulate matter. An aliquot of the stool sample supernatant (100 μl) was placed into a sterile screw-top Eppendorf tube along with proteinase K buffer (130 μl) and proteinase K (20 μl), followed by thorough mixing and incubation (65°C for 10 min and then 95°C for 10 min). Proteinase K-treated stool sample supernatant (100 μl) underwent DNA extraction using the DNA Kit III (Bacteria/Fungi) on the MagNA Pure LC extraction platform (Roche Diagnostics, Indianapolis, IN) in accordance with the manufacturer's instructions, with a final extraction volume of 200 μl. DNA purity and quantity were measured by absorbance spectrophotometry (Nanodrop-1000; NanoDrop Technologies, Wilmington, DE). During the clinical validation period, each specimen that tested negative by real-time PCR with DNA taken directly from a stool sample was subsequently spiked with toxigenic C. difficile and retested by real-time PCR to control for inhibition.
(ii) Specimen preparation from CCFA culture.
DNA was extracted from C. difficile isolates using the UltraClean microbial DNA isolation kit (MoBio Laboratories, Inc., Carlsbad, CA) in accordance with the manufacturer's instructions. Briefly, C. difficile isolates from each patient specimen were resuspended in a proprietary bead-containing solution and mixed with a lysis solution, which facilitated cytolysis via a combination of heat, detergent, and bead-assisted mechanical disruption. The released DNA was captured on a silica spin filter, washed, and subsequently resuspended in DNA-free Tris buffer. DNA purity and quantity were measured by absorbance spectrophotometry (Nanodrop-1000; NanoDrop Technologies, Wilmington, DE).
(iii) Singleplex real-time PCR.
The tcdA and tcdB real-time PCR singleplex assays were performed on the LightCycler 1.0 (Roche Diagnostics, Indianapolis, IN) under identical conditions with a total reaction volume of 20 μl. Each amplification reaction mixture using the LightCycler TaqMan Master kit (Roche Diagnostics, Indianapolis, IN) consisted of TaqMan Master Mix (4.0 μl 5×), 0.6 μM forward primer, 0.6 μM reverse primer, 0.1 μM hydrolysis probe, PCR grade water, and a 5-μl DNA sample. Cycling parameters were as follows: program 1, 1 cycle of 95°C for 10 min; program 2, 45 cycles of 95°C for 10 min, 57°C for 20 s, and 72°C for 10 s; program 3, hold at 4°C. For the tcdA assay, the following in-house-designed primers and internal probe were utilized: tcdAF, 5′GGTAATAATTCAAAAGCGGCT; tcdAR, 5′AGCATCCGTATTAGCAGGTG; tcdATM, 5′FAM (6-carboxyfluorescein)-AGCCTAATACAGCTATGGGTGCGAA-TAMRA (6-carboxytetramethylrhodamine). For the tcdB assay, the following previously described (41) primers and internal probe were utilized: tcdBF, 5′GAAAGTCCAAGTTTACGCTCAAT; tcdBR, 5′GCTGCACCTAAACTTACACCA; tcdBTM, 5′FAM-ACAGATGCAGCCAAAGTTGTTGAATT-TAMRA.
(iv) Multiplex real-time PCR.
The tcdA and tcdB gene-specific multiplex assay was performed on the LightCycler 2.0 platform (Roche Diagnostics, Indianapolis, IN) with a total reaction volume of 20 μl (with 10 μl input DNA compared to the 5 μl DNA required for the singleplex reactions). The amplification reaction using the LightCycler TaqMan Master kit (Roche Diagnostics, Indianapolis, IN) consisted of TaqMan Master Mix (4.0 μl 5×), 0.48 μM tcdA forward primer, 0.6 μM tcdB forward primer, 0.48 μM tcdA reverse primer, 0.6 μM tcdB reverse primer, 0.08 μM tcdA hydrolysis probe, 0.1 μl of tcdB hydrolysis probe, PCR grade water, 0.4 μl AmpErase (Applied Biosystems, Inc., Foster City, CA), and 10 μl extracted DNA. Cycling parameters were as follows: program 1, 1 cycle of 50°C for 2 min; program 2, 1 cycle of 95°C for 15 min; program 3, 40 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 10 s; program 4, hold for 4°C.
The tcdA and tcdB primers and probes were identical to those utilized in the singleplex reactions, with the exception that the 5′-end reporter dyes for the tcdA and tcdB internal probes were switched to FAM and hexachloro-6-carboxyfluorescein, respectively. In addition, the 3′ quencher was transitioned to Black Hole Quencher 1 for both probes. All primers and probes were obtained from TIB-MolBiol (Adelphia, NJ). AmpErase was added to the reaction to preserve specificity. Regarding changes in cycling parameters, the initial denaturation step was extended by 5 min, the annealing temperature was increased to 60°C, and the total number of cycles was decreased to 40 cycles.
Analytical sensitivity.
The analytical sensitivity of our assay was defined by two methods. First, DNA was extracted from a tcdA+ tcdB+ C. difficile isolate (ATCC 9689), followed by absorbance spectrophotometric quantitation, and serial 10-fold dilutions (range, 10 ng to 1 ag). Second, 1 g of a semisolid or 1 ml of a liquid stool sample was spiked with a large inoculum of tcdA+ tcdB+ C. difficile, followed by serial 10-fold dilutions into a liquid stool sample that was previously confirmed negative for both the tcdA and tcdB targets. For each specimen (undiluted and each serial dilution), the following steps occurred. (i) A 100-μl volume was plated in triplicate to prereduced CCFA medium and incubated anaerobically at 35°C for 3 days, and colonies were counted, and (ii) a 500-μl volume was added to 1,500 μl of S.T.A.R. Buffer, and then stool specimen processing and real-time PCR were performed.
Analytical specificity.
A total of 34 enteric bacterial organisms, consisting of ATCC organisms (Alcaligenes faecalis ATCC 35655; Aeromonas hydrophilia ATCC 35654; Bacteroides fragilis ATCC 23745; Parabacteroides distasonis ATCC 8503; Bacteroides uniformis ATCC 8492; Campylobacter jejuni ATCC 33291; Clostridium perfringens ATCC 13124; Clostridium sordelii ATCC 9714; Escherichia coli ATCC 35218, ATCC 25922, and O157:H7 ATCC 35150; Enterococcus faecalis ATCC 29212 and ATCC 51299; Enterobacter aerogenes ATCC 13048; Enterobacter cloacae ATCC 13047; Klebsiella pneumoniae ATCC 700603, ATCC 35657, and ATCC 13883; Peptostreptococcus anaerobius ATCC 27337; Proteus mirabilis ATCC 35659; Proteus vulgaris ATCC 13315; Pseudomonas aeruginosa ATCC 27853; and Salmonella enterica serovar Typhimurium ATCC 14028), Clostridium (non-difficile) spp., C. difficile (nontoxigenic), and Shigella sonnei, were individually analyzed by each singleplex real-time PCR assay. In brief, each organism was cultured and DNA was extracted via the UltraClean microbial DNA isolation kit (MoBio Laboratories, Inc., Carlsbad, CA) in accordance with the manufacturer's instructions. DNA purity and quantity were measured by absorbance spectrophotometry (Nanodrop-1000; NanoDrop Technologies, Wilmington, DE).
Determination of assay sensitivity and specificity.
While cell culture cytotoxicity assays have been considered the “gold standard” historically, there is no currently universally agreed upon gold standard for toxigenic C. difficile detection. Commercially available EIAs are the most commonly utilized testing methodologies, and toxin EIA was the method previously employed at Texas Children's Hospital. For this study, we defined our “reference standard” as any stool specimen with positive results by at least four of the following six tests: (i) stool EIA, (ii) postculture EIA, (iii) postculture real-time PCR (tcdA), (iv) postculture real-time PCR (tcdB), (v) stool real-time PCR (tcdA), and (vi) stool real-time PCR (tcdB). A similar approach was previously used by Peterson et al. (31). The performance characteristics of each individual testing method were subsequently compared to those of this reference standard (Table 1).
Table 1.
Performance characteristics of various testing modalities compared to those of our defined reference standarda for the detection of toxigenic C. difficile in patientsb clinically suspected of having CDAD
| Result or parameter | EIA |
Real-time PCR |
||||||
|---|---|---|---|---|---|---|---|---|
| Culture |
Stool sample |
|||||||
| Stool samplee | Culture | tcdA | tcdB | tcdABf | tcdA | tcdB | tcdABf | |
| No. of samples: | ||||||||
| True positive | 7 | 15 | 17 | 18 | 18 | 18 | 18 | 19 |
| True negative | 118 | 118 | 118 | 118 | 118 | 118 | 118 | 118 |
| False positive | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| False negative | 13 | 5 | 3 | 2 | 2 | 2 | 2 | 1 |
| % Sensitivity | 35 | 75 | 85 | 90 | 90 | 90 | 90 | 95 |
| % Specificity | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PPV (%)c | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| NPV (%)d | 90 | 96 | 98 | 98 | 98 | 98 | 98 | 99 |
| TATe | <2 h | 3–5 days | 3–5 days | 3–5 days | 3–5 days | <4 h | <4 h | <4 h |
The reference standard was any stool specimen positive by at least four of the following six testing modalities: (i) stool EIA, (ii) culture EIA, (iii) culture real-time PCR (tcdA), (iv) culture real-time PCR (tcdB), (v) stool real-time PCR (tcdA), and (vi) stool real-time PCR (tcdB). The performance characteristics of each individual testing modality were subsequently compared to those of this reference standard.
Total n = 138.
PPV, positive predictive value.
NPV, negative predictive value.
TAT, turnaround time.
Combination of singleplex assay results for tcdA and tcdB.
RESULTS
Of the 157 stool specimens (from 96 patients) tested, 19 specimens (from 10 patients) were excluded because they could not be classified according to our defined reference standard (e.g., they produced a positive test result in only one or two of the six testing modalities) and were deemed inconclusive. For the 138 analyzable stool specimens (from 86 patients), the performance characteristics of each testing modality compared to those of our reference standard (as described in Materials and Methods) are depicted in Table 1. Results were compared based on methodology (EIA or real-time PCR) and specimen type (primary stool specimen or cultured organism). True positives were defined by our reference standard approach, and true negatives were defined as negative by all methodologies. The prevalence of CDAD in our institution during a 4-year period (2003 to 2006), as defined by the results of the stool EIA, was approximately 8%. The positivity rate, based on the reference standard, of the stool samples included in this validation was 15% (20 out of 138 samples), with a total of 12 out of 86 patients (14%) positive for C. difficile.
Infection by additional enteric bacterial and viral pathogens was ruled out in 11 of the 12 patients considered true positives for C. difficile infection. Aeromonas hydrophila was isolated from the stool sample culture of the remaining patient, an immunocompromised child in the bone marrow transplant unit. This patient tested positive for C. difficile by EIA of a stool sample on three occasions, with each sample producing subsequent positive results by real-time PCR.
Assay sensitivity.
Direct real-time PCR of stool specimens was superior to all other testing methods, with a sensitivity of 90% for either tcdA or tcdB and a sensitivity of 95% when singleplex assay results were combined. The ability to detect toxigenic strains of C. difficile by EIA was relatively inadequate, with a sensitivity of only 35% with direct stool specimens. Presumably, this result reflects the reduced numbers of organisms or lower levels of toxin production in children with the disease. Even with culture prior to EIA, the sensitivity of toxin detection was only 75%. All patients were tested under the suspicion of C. difficile infection, and while the real-time PCR assay detects only the presence of the toxin genes, C. difficile-specific culture and subsequent real-time PCR confirmed the presence of toxigenic C. difficile organism in the patient specimens. The five false-negative results obtained by EIA performed with cultured bacteria reflect only two patients, one of whom subsequently tested positive by EIA of a cultured organism isolated from a sample obtained hours after the previous samples. While it is possible to identify cases in which toxin production is questionable due to the increased sensitivity of DNA detection, it is evident that many cases were missed by EIA. The effective doubling of the C. difficile detection rate at our institution when comparing EIA positivity to real-time PCR positivity supports this conclusion.
The analytical sensitivity of the real-time PCR assay as determined by serial 10-fold dilutions of tcdA+ tcdB+ C. difficile (ATCC 9689) DNA was 10 fg for the tcdA and tcdB singleplex assays. For each singleplex assay, the analytical sensitivity at 100% detection was initially determined to be 250 CFU/ml of stool sample for tcdA and 500 CFU/ml of stool sample for tcdB.
For the multiplex configuration of the assay, probit analysis determined the lower limits of detection (95% confidence level) to be 1,347 and 2,066 CFU/ml for tcdA and tcdB, respectively, with an absolute lower limit of detection of 250 CFU/ml.
Potential PCR inhibition was addressed by spiking cultured toxigenic C. difficile into aliquots of stool specimens initially reported as negative by real-time PCR. All 118 samples were subsequently shown to be positive by real-time PCR after spiking, suggesting that PCR inhibition by the stool sample matrix was not a factor in assay performance.
Assay specificity.
No cross-reactivity was observed with the tcdA, tcdB, or multiplexed target approach. A total of 34 additional enteric bacteria described previously, including several Clostridium spp., E. coli, and Enterobacter sp., were tested by real-time PCR, and the results yielded 100% specificity.
Validation of the multiplex assay.
Optimization of the multiplex assay included several changes in both the reaction and cycling conditions, i.e., change in the fluorescence quenchers, increased denaturation time, increased annealing temperature, decreased cycle number, and the addition of AmpErase. The multiplex assay including both the tcdA and tcdB targets was validated by testing 28 of the samples included in the original singleplex validation, as well as 45 current patient samples tested in parallel with the singleplex assays. The 73 samples included 28 true positives and 45 true negatives; the multiplex assay exhibited 100% sensitivity and 98% specificity compared to the singleplex assay. In reference to the decreased specificity, one patient sample that originally tested negative by the singleplex assay tested positive by the multiplex assay. The multiplex assay may be more sensitive than the singleplex assay. It should be noted that a greater sample volume (10 μl) is added to the multiplex assay (versus the 5 μl in the singleplex assay). Further supporting this claim, an additional sample positive for only the tcdA target in singleplex was subsequently found to be positive for both tcdA and tcdB by the multiplex assay.
Performance of C. difficile real-time PCR in a pediatric setting.
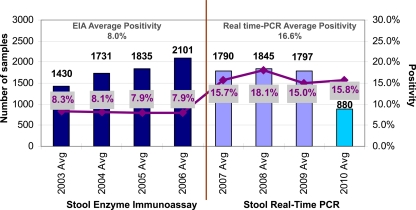
The assay described in this study was implemented at Texas Children's Hospital in December 2006. Previous rates of C. difficile positivity by EIA ranged from 7.9 to 8.3% during the years 2003 to 2006. Overall positivity rates in our pediatric population effectively doubled following the introduction of real-time PCR, and the increased detection rates have remained constant (Fig. 1). In the 3.5 years since molecular testing for C. difficile was implemented (December 2006 to May 2010), a total of 6,417 stool samples have been tested and 1,037 samples have yielded positive results (16.2% positivity). Average yearly positivity rates were 15.7% in 2007, 18.1% in 2008, 14.9% in 2009, and 15.8% through the first half of 2010. The singleplex configuration of the assay yielded a positivity rate of 16.6% (155 out of 933 samples) over the first 6 months of testing, while the multiplex configuration of the assay yielded a positivity rate of 16.1% (882 out of 5,484 samples) over the subsequent 3 years. The sustained improvement in the detection of toxigenic C. difficile by real-time PCR highlights the limitations of the toxin EIA. The difference between the positivity rates obtained by the toxin EIA and real-time PCR testing for C. difficile was statistically significant (P value of <0.0001 by t-test analysis).
Fig. 1.
Annual volumes and positivity rates from 2003 to 2010. While the overall C. difficile test volume has remained constant, positivity rates following the implementation of real-time PCR testing have doubled compared to prior positivity results obtained by EIA. The vertical line in the center denotes the transition to real-time PCR testing. Note that the volumes for 2010 refer to testing through 31 May 2010.
Due to the insufficient detection level of EIA in years past, it had become common practice for physicians to submit multiple stool samples during the same episode of suspected C. difficile infection. This degree of repeat testing resulted in duplicate (and in some cases triplicate) samples being received before the result from the previous sample was ever reported. A comparison of all of the samples tested from January through March in 2007 (singleplex assay) and January through March in 2010 (multiplex assay) was performed. A decrease in the overall positivity rate was observed based on sample positivity (18.06% in 2007 compared to 14.36% in 2010), patient positivity (21.23% in 2007 compared to 17.01% in 2010), and episode positivity (22.81% in 2007 compared to 16.27% in 2010).
A decrease in the number of repeat samples collected per patient episode was realized. Samples were defined as being part of the same episode of care if less than 1 week had passed between sample collections. While 27.78% of the samples represented repeat testing within the same episode in 2007, only 23.52% of the samples were due to repeat testing in 2010. In terms of repeat samples collected before the initial test result from the first sample submitted was ever reported, a decline was also seen (18.58% in 2007 compared to 16.52% in 2010). For additional comparison, the mean numbers of samples collected per episode were 1.68 and 1.33 samples in 2007 and 2010, respectively. With regard to differences in repeat samples, only 1.04% in 2007 and 0.54% in 2010 of all repeat samples collected within a 72-h time frame provided results different from those obtained with the previous sample, further supporting the fact that multiple repeat testing within the same episode is unnecessary.
DISCUSSION
A real-time PCR assay was implemented in order to facilitate the rapid detection of toxigenic C. difficile in stool samples collected from hospitalized patients with diarrhea in a pediatric tertiary health care facility. A total of 157 stool samples were collected during an 8-week period from 96 hospitalized patients with a mean age of 6.2 years (range, 15 days to 25 years; median, 4 years). From a diagnostic perspective, direct real-time PCR performed with stool specimens demonstrated optimal test performance, with 95% sensitivity, 100% specificity, a 100% positive predictive value, and a 99% negative predictive value for the combined tcdA and tcdB singleplex target approach. In addition, the tcdA-tcdB multiplex assay performed similarly to the singleplex assays. The turnaround time of real-time PCR of stool specimens was roughly 4 h. Stool toxin EIA, despite widespread utilization in the United States, demonstrated poor test performance, with 35% sensitivity, 100% specificity, a 100% positive predictive value, and a 90% negative predictive value.
With regard to toxigenic C. difficile, selecting a testing strategy has been a major diagnostic dilemma facing clinical laboratories as they try to balance the need for rapid results with a test that can produce accurate results in order to facilitate appropriate patient management and the timely enactment of infection control measures. Although anaerobic culture was considered the most sensitive assay for the detection of C. difficile, it lacks specificity for toxigenic strains unless coupled with other testing modalities (e.g., “toxigenic culture”) and is limited by a poor turnaround time, usually 3 to 5 days (7, 10, 31, 33, 35, 36). The cell culture cytotoxicity assay, once considered by many to be the gold standard, also has significant limitations. This assay takes 48 to 72 h to complete, is labor-intensive, and requires a toxin B neutralization step to ensure assay specificity. In addition, the sensitivity of the cytotoxicity assay ranges from 67 to 71% (30, 38). The poorest-performing tests on the clinical market are commercially available toxin (versus glutamate dehydrogenase) EIAs, where the reported sensitivity and specificity may be as low as 50% and 70%, respectively (10, 15, 28, 31, 39, 40). Due to poor assay performance, serial EIA is commonly performed to effectively rule out CDAD as the etiology of persistent diarrhea (13). Our findings support the ineffectiveness of EIA as a preferred diagnostic testing strategy, especially in pediatric settings, with an observed sensitivity of 35% in our study. Since the development of this test, several commercial real-time PCR assays have become available, including Cepheid GeneXpert C. difficile (Cepheid, Sunnyvale, CA), Prodesse proGastro Cd (Gen-Probe Prodesse, Waukesha, WI), and BD GeneOhm Cdiff (BD, Franklin Lakes, NJ). The introduction of these assays into the clinical market has further supported the important role of molecular testing in the diagnosis of C. difficile infection.
The idea of diagnosing CDAD by real-time PCR testing of stool specimens began entering the clinical realm shortly after the initial works of Kato et al. (18) and Bélanger et al. (6). Numerous studies during the last 10 years have clearly demonstrated the clinical benefit of real-time PCR-based testing to diagnose CDAD due to its superior test performance and optimal turnaround time (1, 6, 16, 23, 31, 36, 38, 41–43). Despite different DNA extraction protocols and variations in the real-time PCR instrumentation and reagents, the clinical performance of real-time PCR testing of stool samples has been excellent, with sensitivities and specificities ranging from 83.6 to 93.3% and 96 to 98.2%, respectively (1, 31, 38, 41, 43). We were able to obtain favorable test performance when using real-time PCR testing of stool specimens, with our observed 95% sensitivity and 100% specificity for the combined tcdA-tcdB singleplex approach (listed in the rightmost column of Table 1). During our study of the tcdA and tcdB genes in direct stool specimens, three isolates were positive for only one of the gene targets. When testing DNA extracted from stool specimens, missed detection of either the tcdA or the tcdB gene may be an uncommon phenomenon. This phenomenon was observed in our clinical validation and is depicted in the three rightmost columns of Table 1, whereby the individual sensitivities of the tcdA and tcdB singleplex assays were less than the combined sensitivity of both singleplex reactions. Primer and/or hydrolysis probe binding site mutations within the tcdA and/or tcdB genes of C. difficile could account for this situation; however, this possibility was not supported based upon our detection of the tcdA and tcdB genes from the respective cultured C. difficile isolates. For the two isolates that were not positive for both targets directly from a stool sample, a real-time PCR assay of the cultured isolate was, in fact, positive for both tcdA and tcdB, indicating the ability to detect the presence of both toxin genes only in the more concentrated sample.
While molecular detection of C. difficile toxin genes may detect DNA in the absence of toxin production, the increasing rate of CDAD in the pediatric population supports the use of this sensitive testing strategy in the pediatric setting so that cases are not missed. CDAD should not be summarily ruled out in patients ≤1 year of age, and a highly sensitive approach to C. difficile detection appears to be beneficial for pediatric patients. As always, physicians must utilize proper judgment when evaluating results for patients ≤1 year of age, but the mounting literature pertaining to CDAD in this age group suggests that children symptomatic for C. difficile infection should continue to be tested (20, 45).
In conclusion, this report summarizes the most comprehensive evaluation of real-time PCR testing for toxigenic C. difficile in a pediatric setting to date. We have demonstrated that our assay has test performance superior to that of a commercially available EIA and proved comparable to real-time PCR testing of cultivated C. difficile isolates. The assay has been performed clinically at Texas Children's Hospital since 2006 and confirms an ongoing C. difficile positivity rate of roughly twice the number of pediatric patients historically diagnosed by EIA. The rapid turnaround time and cost-effectiveness of this laboratory-developed assay have made it the ideal solution for the diagnosis of CDAD in pediatric patients at our institution.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the assistance of Shaunte C. Jones, Trang T. Ton, and Kyle Menne in the Division of Molecular Pathology of the Department of Pathology at Texas Children's Hospital. We also thank the medical technologists in the Clinical Microbiology Laboratory at Texas Children's Hospital.
Financial support for this study was provided by the Department of Pathology at Texas Children's Hospital and the Department of Pathology and Immunology at Baylor College of Medicine.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 5 January 2011.
REFERENCES
- 1. Aichinger E., Schleck C. D., Harmsen W. S., Nyre L. M., Patel R. 2008. Nonutility of repeat laboratory testing for detection of Clostridium difficile by use of PCR or enzyme immunoassay. J. Clin. Microbiol. 46:3795–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aldeen W. E., et al. 2000. Comparison of the TOX A/B test to a cell culture cytotoxicity assay for the detection of Clostridium difficile in stools. Diagn. Microbiol. Infect. Dis. 36:211–213 [DOI] [PubMed] [Google Scholar]
- 3. Baker S. S., Faden H., Sayej W., Patel R., Baker R. D. 2010. Increasing incidence of community-associated atypical Clostridium difficile disease in children. Clin. Pediatr. (Phila.) 49:644–647 [DOI] [PubMed] [Google Scholar]
- 4. Bartlett J. G., Chang T. W., Taylor N. S., Onderdonk A. B. 1978. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 298:531–534 [DOI] [PubMed] [Google Scholar]
- 5. Bartlett J. G., Onderdonk A. B., Cisneros R. L., Kasper D. L. 1977. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J. Infect. Dis. 136:701–705 [DOI] [PubMed] [Google Scholar]
- 6. Bélanger S. D., Boissinot M., Clairoux N., Picard F. J., Bergeron M. G. 2003. Rapid detection of Clostridium difficile in feces by real-time PCR. J. Clin. Microbiol. 41:730–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouza E., et al. 2001. “Second-look” cytotoxicity: an evaluation of culture plus cytotoxin assay of Clostridium difficile isolates in the laboratory diagnosis of CDAD. J. Hosp. Infect. 48:233–237 [DOI] [PubMed] [Google Scholar]
- 8. Chang T. W., Bartlett J. G., Gorbach S. L., Onderdonk A. B. 1978. Clindamycin-induced enterocolitis in hamsters as a model of pseudomembranous colitis in patients. Infect. Immun. 20:526–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clabots C. R., Gerding S. J., Olson M. M., Peterson L. R., Gerding D. N. 1989. Detection of asymptomatic Clostridium difficile carriage by an alcohol shock procedure. J. Clin. Microbiol. 27:2386–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delmée M., Van Broeck J., Simon A., Janssens M., Avesani V. 2005. Laboratory diagnosis of Clostridium difficile-associated diarrhoea: a plea for culture. J. Med. Microbiol. 54:187–191 [DOI] [PubMed] [Google Scholar]
- 11. Dubberke E. R., Reske K. A., Olsen M. A., McDonald L. C., Fraser V. J. 2008. Short- and long-term attributable costs of Clostridium difficile-associated disease in nonsurgical inpatients. Clin. Infect. Dis. 46:497–504 [DOI] [PubMed] [Google Scholar]
- 12. Fedorko D. P., Williams E. C. 1997. Use of cycloserine-cefoxitin-fructose agar and l-proline-aminopeptidase (PRO Discs) in the rapid identification of Clostridium difficile. J. Clin. Microbiol. 35:1258–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fekety R. 1997. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 92:739–750 [PubMed] [Google Scholar]
- 14. García A., García T., Perez J. L. 1997. Proline-aminopeptidase test for rapid screening of Clostridium difficile. J. Clin. Microbiol. 35:3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gerding D. N., Johnson S., Peterson L. R., Mulligan M. E., Silva J., Jr 1995. Clostridium difficile-associated diarrhea and colitis. Infect. Control Hosp. Epidemiol. 16:459–477 [DOI] [PubMed] [Google Scholar]
- 16. Guilbault C., et al. 2002. Development and evaluation of a PCR method for detection of the Clostridium difficile toxin B gene in stool specimens. J. Clin. Microbiol. 40:2288–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hall I. C., O'Toole E. 1935. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am. J. Dis. Child. 49:390–402 [Google Scholar]
- 18. Kato H., et al. 1998. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J. Clin. Microbiol. 36:2178–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelly C. P., Pothoulakis C., LaMont J. T. 1994. Clostridium difficile colitis. N. Engl. J. Med. 330:257–262 [DOI] [PubMed] [Google Scholar]
- 20. Kim J., et al. 2008. Epidemiological features of Clostridium difficile-associated disease among inpatients at children's hospitals in the United States, 2001–2006. Pediatrics 122:1266–1270 [DOI] [PubMed] [Google Scholar]
- 21. Knudsen J. D., Tvede M. 1993. Demonstration of toxin A and B by polymerase chain reaction and McCoy cell assay in clinical isolates of Clostridium difficile from Denmark. APMIS 101:18–22 [DOI] [PubMed] [Google Scholar]
- 22. Kyne L., Hamel M. B., Polavaram R., Kelly C. P. 2002. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin. Infect. Dis. 34:346–353 [DOI] [PubMed] [Google Scholar]
- 23. Lemee L., et al. 2004. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (toxin A), and tcdB (toxin B) genes for toxigenic culture of Clostridium difficile. J. Clin. Microbiol. 42:5710–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loo V. G., et al. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442–2449 [DOI] [PubMed] [Google Scholar]
- 25. Luna R. A., et al. 2007. DNA pyrosequencing-based bacterial pathogen identification in a pediatric hospital setting. J. Clin. Microbiol. 45:2985–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonald L. C., et al. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433–2441 [DOI] [PubMed] [Google Scholar]
- 27. McDonald L. C., Owings M., Jernigan D. B. 2006. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg. Infect. Dis. 12:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mohan S. S., McDermott B. P., Parchuri S., Cunha B. A. 2006. Lack of value of repeat stool testing for Clostridium difficile toxin. Am. J. Med. 119:356 e7-8 [DOI] [PubMed] [Google Scholar]
- 29. O'Brien J. A., Lahue B. J., Caro J. J., Davidson D. M. 2007. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect. Control Hosp. Epidemiol. 28:1219–1227 [DOI] [PubMed] [Google Scholar]
- 30. Peterson L. R., Kelly P. J. 1993. The role of the clinical microbiology laboratory in the management of Clostridium difficile-associated diarrhea. Infect. Dis. Clin. North Am. 7:277–293 [PubMed] [Google Scholar]
- 31. Peterson L. R., et al. 2007. Detection of toxigenic Clostridium difficile in stool samples by real-time polymerase chain reaction for the diagnosis of C. difficile-associated diarrhea. Clin. Infect. Dis. 45:1152–1160 [DOI] [PubMed] [Google Scholar]
- 32. Redelings M. D., Sorvillo F., Mascola L. 2007. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg. Infect. Dis. 13:1417–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reller M. E., et al. 2007. Yield of stool culture with isolate toxin testing versus a two-step algorithm including stool toxin testing for detection of toxigenic Clostridium difficile. J. Clin. Microbiol. 45:3601–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rexach C. E., Tang-Feldman Y. J., Cantrell M. C., Cohen S. H. 2006. Epidemiologic surveillance of Clostridium difficile diarrhea in a freestanding pediatric hospital and a pediatric hospital at a university medical center. Diagn. Microbiol. Infect. Dis. 56:109–114 [DOI] [PubMed] [Google Scholar]
- 35. Shanholtzer C. J., et al. 1992. Comparison of the VIDAS Clostridium difficile toxin A immunoassay with C. difficile culture and cytotoxin and latex tests. J. Clin. Microbiol. 30:1837–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sloan L. M., Duresko B. J., Gustafson D. R., Rosenblatt J. E. 2008. Comparison of real-time PCR for detection of the tcdC gene with four toxin immunoassays and culture in diagnosis of Clostridium difficile infection. J. Clin. Microbiol. 46:1996–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stabler R. A., et al. 2006. Comparative phylogenomics of Clostridium difficile reveals clade specificity and microevolution of hypervirulent strains. J. Bacteriol. 188:7297–7305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stamper P. D., et al. 2009. Comparison of a commercial real-time PCR assay for tcdB detection to a cell culture cytotoxicity assay and toxigenic culture for direct detection of toxin-producing Clostridium difficile in clinical samples. J. Clin. Microbiol. 47:373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Staneck J. L., et al. 1996. Multicenter evaluation of four methods for Clostridium difficile detection: ImmunoCard C. difficile, cytotoxin assay, culture, and latex agglutination. J. Clin. Microbiol. 34:2718–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ticehurst J. R., et al. 2006. Effective detection of toxigenic Clostridium difficile by a two-step algorithm including tests for antigen and cytotoxin. J. Clin. Microbiol. 44:1145–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van den Berg R. J., et al. 2005. Prospective multicenter evaluation of a new immunoassay and real-time PCR for rapid diagnosis of Clostridium difficile-associated diarrhea in hospitalized patients. J. Clin. Microbiol. 43:5338–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van den Berg R. J., Kuijper E. J., van Coppenraet L. E., Claas E. C. 2006. Rapid diagnosis of toxinogenic Clostridium difficile in faecal samples with internally controlled real-time PCR. Clin. Microbiol. Infect. 12:184–186 [DOI] [PubMed] [Google Scholar]
- 43. van den Berg R. J., et al. 2007. Evaluation of real-time PCR and conventional diagnostic methods for the detection of Clostridium difficile-associated diarrhoea in a prospective multicentre study. J. Med. Microbiol. 56:36–42 [DOI] [PubMed] [Google Scholar]
- 44. Voth D. E., Ballard J. D. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zilberberg M. D., Tillotson G. S., McDonald C. 2010. Clostridium difficile infections among hospitalized children, United States, 1997–2006. Emerg. Infect. Dis. 16:604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.