Abstract
Fusarium has recently emerged as an opportunistic pathogen of humans, but the histological differentiation of Fusarium from Aspergillus and Scedosporium is particularly difficult because these fungi may induce similar clinical features and exhibit filamentous development in host tissues. Thus, there is a need to establish rapid and reliable methods that are applicable to pathological diagnoses. The aim of this study was to evaluate and establish in situ hybridization (ISH) using peptide nucleic acid (PNA) probes targeting the 28S rRNA to identify Fusarium species in tissue sections. This technique was validated using both formalin-fixed and paraffin-embedded pulmonary tissues from mice infected with seven different species of fungi and cell blocks from fungal cultures of 30 strains. As a result, strong positive signals were observed within fungal organisms present in tissues of the lung from mice infected with Fusarium solani. Furthermore, this probe reacted strongly with both F. solani and Fusarium oxysporum in sections from cell blocks. Although some cross-reactivity occurred with the Pseudallescheria boydii in sections from cell blocks, the signal intensity was low and most hyphae were not reactive. In conclusion, it was confirmed that ISH with PNA probes is accurate and is a valuable tool for identifying Fusarium spp. among organisms that have identical morphological features in formalin-fixed and paraffin-embedded sections.
INTRODUCTION
Early diagnosis of invasive fungal infection is essential because the disease mostly occurs in patients with severely impaired defense mechanism. Fusarium species are widely distributed in soil, subterranean, aerial plant parts, plant debris, and other organic substrates, and mycotoxins produced by these organisms have often been associated with animal and human diseases (16). In humans, Fusarium spp. cause a broad spectrum of infections, including superficial (keratitis and onychomycosis), locally invasive, and disseminated infections in immunocompromised patients (18). In addition to that invasive and disseminated infections caused by Fusarium spp. are being diagnosed with increasing frequency in patients with hematological malignancies (3), it has been accepted that Fusarium spp. are resistant to most antifungal agents (5). Therefore, an early diagnosis of the infection is now required to improve the outcome of treatment for seriously debilitating conditions. Because of morphological similarities among molds in histopathological specimens, it has been difficult to differentiate histologically Fusarium spp. from other molds. Recently, sensitive and rapid molecular detection assays that use PCR-based methods have been introduced to detect Fusarium DNA in serum, total blood, and tissue samples (10). However, there have been a few attempts to use in situ hybridization (ISH) to identify Fusarium spp. in tissue sections for histological diagnosis (8, 14). We are describing the first report of ISH using peptide nucleic acid (PNA) as the probe targeting the 28S rRNA of Fusarium spp. to identify the fungus in formalin-fixed and paraffin-embedded tissue sections that are widely used as routine preparations for surgical and anatomical pathology in hospitals.
MATERIALS AND METHODS
Preparation of infected animals and tissue specimens.
To verify the specificity of probes, sections of formalin-fixed and paraffin-embedded tissues of lung were prepared from mice experimentally infected with seven different fungi. Lung has been understood as one of the commonest organ involved by invasive fungal infection. A part of this may be explained by the fact that the lung serves as a porta of infection. Therefore, in the present study, lungs from mice with intratracheal infection were used as a tissue specimen to evaluate the ISH procedure.
Six-week-old, male Institute of Cancer Research (ICR) mice (Sankyo Labo Service Corp., Inc., Tokyo, Japan) were used in the present study. Immune suppression was achieved by intraperitoneal injections of cyclophosphamide (Shionogi and Co., Ltd., Osaka, Japan) at a dose of 150 mg/kg (body weight) 3 days prior to infection. To prevent bacterial infection, the animals were also intraperitoneally administered with imipenem/cilastatin sodium (Banyu Pharmaceutical, Tokyo, Japan). Prior to inoculation, the animals were anesthetized intraperitoneally with 80 mg of ketamine (Daiichi Sankyo Co., Ltd., Tokyo, Japan) and 10 mg of xylazine (Bayer Health Care, Tokyo, Japan)/kg. The conidiae or yeast cells were injected intratracheally as previously described (19). A 25-μl aliquot of the conidiae or yeast cell suspension was injected into the trachea via a clinically used intravascular catheter (24G, Insyte-W; Becton Dickinson, Hollister, CA). The mice were infected with 3 × 105 conidiae or yeast cells of Aspergillus fumigatus (TIMM1776), Aspergillus terreus (TIMM2929), Aspergillus flavus (TIMM2935), Candida albicans, (TIMM1768), Rhizopus oryzae (TIMM1326), Fusarium solani (TIMM1303), and Pseudallescheria boydii (TIMM0952). The animals were sacrificed on the third day after infection, and the lungs were removed and fixed in 10% formalin, followed by dehydration with ethanol and embedding in paraffin. Tissue sections (3 μm) were mounted on aminoalkylsilane-coated slide glasses (Dako Japan, Tokyo, Japan). Pulmonary lesions induced by this procedure were confirmed by histological examination using these sections stained with hematoxylin and eosin (H&E) and Grocott's stains.
Strains tested.
For specificity testing of the probes, cells from the following molds and yeasts were tested: A. flavus var. flavus (NBRC 33021), Aspergillus niger (NBRC 33023), A. terreus (NBRC 33026), A. fumigatus (NBRC 6344), A. fumigatus var. fumigatus (NBRC 33022), F. solani (NBRC 5232), Fusarium oxysporum (NBRC 7152), P. boydii (NBRC 8078), R. oryzae (NBRC 5780), Cunninghamella elegans var. elegans (NBRC 4446), Rhizomucor pusillus (NBRC 9744), Mucor circinelloides f. sp. circinelloides (NBRC 4554), Penicillium commune (NBRC 5763), Pseudocochliobolus spicifer (NBRC 100222), C. albicans (ATCC 10231), Trichosporon asahii (CBS2479T), C. albicans var. stellatoidea (TIMM0310), Candida glabrata (TIMM1064), Candida guilliermondii (TIMM0260), Candida kefyr (TIMM0302), Candida krusei (TIMM0269), Candida lusitaniae (TIMM1668), Candida parapsilosis (TIMM0292), Candida tropicalis (TIMM0313), Cryptococcus neoformans (TIMM0354), Debaryomyces polymorphus (TIMM2937), Hansenula anomala (JCM3585), Pichia subpelliculosa (IFO0808), Saccharomyces cerevisiae (TIMM0925), and Schizosaccharomyces pombe (TIMM3376).
Preparation of cell blocks from cultured fungal cells.
Mold strains were grown for 48 to 72 h at 25°C in potato dextrose broth (Sigma Aldrich, St. Louis, MO). Yeast strains except Candida spp. were grown overnight at 25°C in YMPD broth (0.3% yeast extract, 0.3% malt extract, 0.5% peptone, 1.0% glucose) (Becton Dickinson), and Candida spp. were grown overnight at 37°C in Medium 199 (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) with 10% calf fetal serum (Sigma-Aldrich Co., St. Louis, MO). From cultures of the above-mentioned molds or yeasts, fungal cell suspensions were prepared in 10% formalin for fixation. Fixed molds were collected with centrifugation for 10 min at 2,000 rpm. The cluster of molds was carefully transferred onto filter paper (Advantec Toyo, Ltd., Tokyo, Japan) with pointed forceps. This was also wrapped with same filter paper and placed in an embedding cassette (Murazumi Industrial Co., Ltd., Hyogo, Japan) and then penetrated with paraffin by using an automated tissue processor (Tissue-Tek VIP Premier; Sakura Finetek Japan Co., Ltd., Tokyo, Japan) (12). The cluster of molds penetrated with paraffin was transferred to bottom of an embedding stainless dish, followed by filling solidifying of paraffin, and cut into 3-μm sections that were then mounted on aminoalkylsilane-coated slide glasses (Dako Japan, Tokyo, Japan).
To prevent the diffusion of yeast cells in suspension and to obtain a high density in paraffin blocks, we used agarose gel as an intermediate embedding medium. Yeast cells were collected with centrifugation for 10 min at 2,000 rpm and added with 50 μl of 2% liquid agarose at 65°C. This agarose gel was heaped onto the surface of cover glass (Matsunami Glass Ind., Ltd., Osaka, Japan), and solidified at room temperature. The solidified gel was penetrated with paraffin by using an automated tissue processor (Tissue-Tek VIP Premier), as well, and sections were prepared in same way (12).
PNA probes.
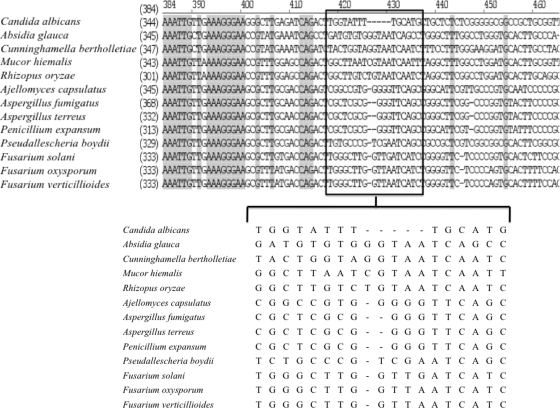
The design of the PNA probes for Fusarium spp. (N terminus-GAT GAT CAA CCA AGC CCA) and panfungal species (N terminus-TAC TTG TGC GCT ATC GGT) was derived from a comparison of 28S rRNA genes in the GenBank database. The alignment of the DNA sequence was performed by using Vector NTI Advance TM10 (Invitrogen, Carlsbad, CA). After alignment and visual assessment of the Fusarium and non-Fusarium sequences, the probe for Fusarium spp. was designed. As shown in Fig. 1, a Fusarium sp. antisense PNA probe targeting the 28S rRNA could be designed and evaluated on a genus-specific level. The sequence revealed at least 9 to 14 mismatches within the target region in sequences of nontarget organisms. Furthermore, to assess the retention of RNA in samples, we also designed a panfungal antisense PNA probe in the same way. Each selected sequence was checked for specificity against the GenBank database by using the Basic Local Alignment Search Tool (BLAST; http//www.ncbi.nlm.nih.gov/BLAST/). The selected sequence was then synthesized, and the N terminus of the oligomer was conjugated to fluorescein isothiocyanate (FITC) via a double aminoethoxyethoxyacetate (AEEA) linker (Fasmac Co., Ltd., Kanagawa, Japan).
Fig. 1.
Alignment of 28S rRNA sequences for Fusarium species, C. albicans, and other important hyalohyphomycetes for histological differentiation. The binding regions of the antisense probe used in the present study to detect Fusarium rRNA were boxed and magnified. Homologous regions were highlighted in gray. Species and GenBank accession numbers were as follows: C. albicans (AB436387), Absidia glauca (AF113447), Cunninghamella bertholletiae (AF113459), Mucor hiemalis (AF113468), Rhizopus oryzae (DQ466617), Ajellomyces capsulatus (AB176493), A. fumigatus (AB354577), A. terreus (AF454185), Penicillium expansum (AJ519347), P. boydii (EF151324), F. solani (AF178377), F. oxysporum (AF060383), and Fusarium verticillioides (U34526).
ISH.
The ISH procedure was performed as described previously (24). Briefly, sections were deparaffinized and rehydrated according to standard procedures. To expose target nucleic acids in the formalin-fixed tissue, the sections were treated with a 1 mM concentration of EDTA buffer (pH 8.0) in a water bath (Thermo Fisher Scientific K.K., Yokohama, Kanagawa) for 20 min at 98°C and digested with a 10-μg/ml concentration of proteinase K (Nippon Gene Co., Ltd., Tokyo, Japan) for 10 min at 37°C. Hybridization was performed at 56°C for 90 min with 1 μg of PNA probe/ml dissolved in hybridization medium (Dako Japan, Tokyo, Japan). After repeated washings with 2× standard saline citrate (SSC) at 56°C, the signals were detected by enzyme immunohistochemistry using an anti-FITC antibody (Roche Diagnostics K.K., Tokyo, Japan) and horseradish peroxidase-labeled polymer solution (Nichirei Biosciences, Inc., Tokyo, Japan). Finally, the sites of peroxidase were visualized by 3,3′-diaminobenzidine tetrahydrochloride (DAB; Dojindo Laboratories, Kumamoto, Japan) in the presence of H2O2 and nickel and cobalt ions (1). As negative controls, ISH procedures were performed with a C. albicans PNA probe (20).
RESULTS
Paraffin sections of both lungs of mice infected with different fungi and cell blocks mounted on the slide glasses were H&E and/or Grocott's stained, processed with ISH, and observed under light microscopy for evaluation of our ISH procedure.
Specificity of ISH for Fusarium spp. in infected animal models.
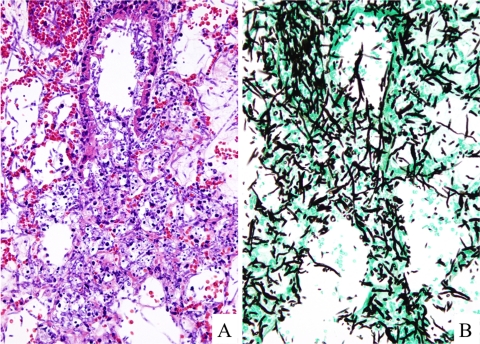
Histological examination revealed an extensive fungal growth both in alveoli and terminal bronchus with necrosis and minor polymorphonuclear leukocyte infiltrate (Fig. 2). The establishment of experimental pulmonary lesions was confirmed in mice infected with all seven of the different fungi that we examined.
Fig. 2.
Microphotographs of a pulmonary lesion in a mouse 3 days after intratracheal infection of F. solani (TIMM1303). (A) Histological examination revealed an extensive fungal growth both in alveoli and terminal bronchus with necrosis and minor polymorphonuclear leukocyte infiltrate with scattering nuclear debris (H&E stain; original magnification, ×100). (B) There is extensive hyphal growth of invading mold showing dichotomous branching (Grocott's stain; original magnification, ×100).
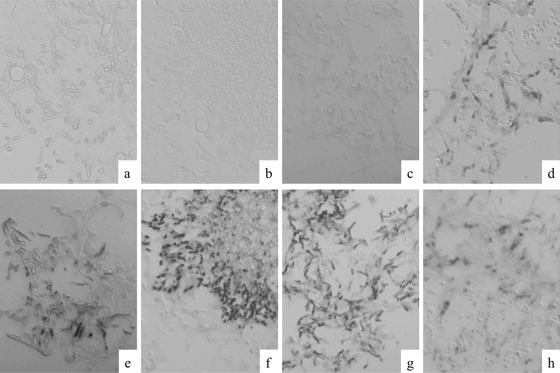
Formalin-fixed and paraffin-embedded pulmonary tissues from mice infected with seven different fungi were tested to assess whether the probe hybridized specifically with Fusarium spp. Strong positive signals against 28S rRNA of Fusarium spp. were observed within fungal organisms present in lung tissue from mice infected with F. solani (Fig. 3d). Positive organisms typically exhibited a signal visualized by a DAB reaction that was limited in a large part of cytoplasm and can be recognized as black fine dots. The signal intensity varied within and between fungal organisms in tissue sections. No substantial background signal was observed in any tissue. In addition, no hybridization was found in other fungi tested.
Fig. 3.
Specificity verification of the Fusarium sp. PNA probe and assessments of rRNA retention and its hybridizability in experimentally infected mice. The tissue sections were hybridized with Fusarium sp. PNA probe (a to d) or with panfungal PNA probe (e to h). Strong positive signals against 28S rRNA of Fusarium spp. were observed in lung tissues from mice infected with F. solani (d). The panfungal PNA probe reacted with all fungi tested (e to h). (a and e) A. fumigatus (TIMM1776); (b and f) A. terreus (TIMM2929); (c and g) P. boydii (TIMM0952); (d and h) F. solani (TIMM1303). Original magnification, ×400.
Specificity of ISH for Fusarium spp. in cell blocks of cultured fungi.
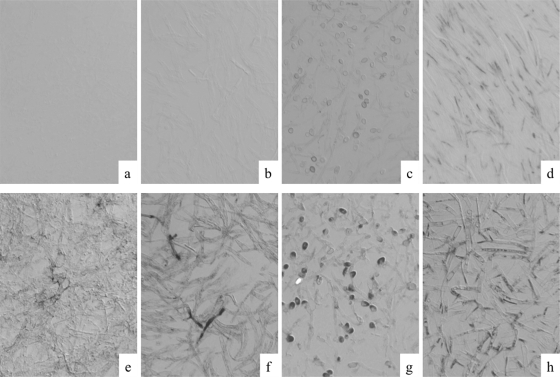
In total, 30 cell block sections from formalin-fixed and paraffin-embedded fungi of 30 strains were studied (see Fig. S1 in the supplemental material). Within the panel of 30 fungi, the Fusarium sp. PNA probe reacted strongly with both F. solani (Fig. 4d) and F. oxysporum in sections of cell blocks. The signal intensity and distribution in fungal organisms were similar to those observed in animal models. With the exception of the Fusarium spp., the P. boydii in sections of cell blocks (not of tissue sections) showed positive reactivity for the probe, but their signal intensity was low and most of the hyphae were negative (Fig. 4c). No hybridization was observed in other fungi tested.
Fig. 4.
Specificity verification of the Fusarium sp. PNA probe and assessments of rRNA retention and its hybridizability in strains of molds. Cell block sections from formalin-fixed and paraffin-embedded fungi were hybridized with Fusarium sp. PNA probe (a to d) or with panfungal PNA probe (e to h). Fusarium sp. PNA probe hybridized strongly with F. solani (d), whereas some cross-hybridization occurred with the P. boydii (c). The panfungal PNA probe reacted with all fungi tested (e to h). (a and e) A. fumigatus (NBRC 6344); (b and f) A. terreus (NBRC 33026); (c and g) P. boydii (NBRC 8078); (d and h) F. solani (NBRC 5232). Original magnification, ×400.
Control experiments.
To confirm the specificity of the 28S rRNA signals, adjacent sections were hybridized with a C. albicans PNA probe. A strong positive signal was detected in C. albicans in a tissue section, whereas no hybridization signal was found in the other fungi tested. The panfungal PNA probe reacted with all fungi tested (Fig. 3e to h, Fig. 4e to h). The intensity of ISH signals for the panfungal probe was similar to that with species-specific probes.
DISCUSSION
Recently, several genome databases have provided new information that can be used for field studies for the molecular identification and epidemiology of pathogenic fungi. Accordingly, sensitive and rapid molecular detection assays have been established by using PCR-based methods to detect fungal DNA (7, 11, 27). The application of these molecular techniques to formalin-fixed and paraffin-embedded tissue has also been reported (2, 13, 21). Although there have been a few attempts to use ISH to detect fungal agents in histopathological specimens (6, 8, 9, 14, 15), the use of ISH for the diagnosis of fungal infection in formalin-fixed and paraffin-embedded sections has not been systematically assessed. We have previously reported that a combination of high-temperature heating in solutions of high pH, followed by a 10-min proteinase K digestion step, gave better ISH results (24). The heating pretreatment used in the present study was adapted from antigen retrieval techniques used in conventional immunohistochemistry (23).
Our purpose was to evaluate and establish an ISH procedure for the detection of Fusarium spp. in formalin-fixed and paraffin-embedded sections. Diagnosis of fusariosis from cultures remains a difficult and time-consuming task, relying on morphological and physiological examinations and requiring some degree of expertise. Fusarium spp. are phylogenetically heterogeneous with variable antifungal susceptibilities (25). An approach based on PCR methods has been used to detect Fusarium DNA (10). Although there have been a few attempts to use ISH to identify Fusarium spp. in histopathological specimens (8, 14), to our knowledge there has been no report of ISH using a PNA probe. Our results obtained with mice experimentally infected with seven different fungi showed that F. solani can be specifically detected in infected tissues by ISH with a PNA probe targeting 28S rRNA of Fusarium spp. On the other hand, using cell block sections from formalin-fixed and paraffin-embedded fungi, this probe reacted strongly with both F. solani and F. oxysporum, but some cross-reactivity was observed in P. boydii hyphae. Part of this result may be explained by the fact that P. boydii has a sequence similar to the target of our probe.
PNA molecules are DNA mimics in which the negatively charged sugar-phosphate backbone is replaced by a neutral polyamide backbone, formed by repetitive units of N-glycine. This structure enables PNA probes to hybridize to complementary nucleic acid targets with high specificity and rapid binding kinetics (4, 17). Due to the novel properties of its hybridization, PNA is beginning to be applied in ISH to detect fungal nucleic acids (20, 22, 26, 28); however, there has been no report of the application of such a probe to formalin-fixed and paraffin-embedded tissues. Better outcomes are obtained with PNA probes compared to conventional DNA probes (26). In our first approach, we confirmed that PNA probes required shorter hybridization times than double-stranded DNA probes. From the standpoint of decreased assay turnaround time, the application of PNA probes is especially attractive.
Recently, Montone reported that the use of dual fluorogenic-labeled locked nucleic acids (LNA) probes of ISH were able to differentiate Fusarium from Aspergillus organisms (14) and that the LNA probe produced a stronger signal compared to a DNA probe with the same sequence (15). Our probe could differentiate Fusarium from 23 fungal species other than Aspergillus. These novel findings demonstrate the feasibility of the approach and strongly suggest that LNA and PNA can be widely used as probes of ISH in the near future.
The 28S rRNA sequence was selected as a detection target because its large size may reveal adequate differences in distinguishing closely related organisms. In addition, it has been accepted that multiple copies of ribosomal genes are present in fungi, which can be transcribed into rRNA. It is essential that assessment of retention of rRNA and its hybridizability should be performed, because loss of rRNA or failure of the accessibility of probes in processed tissue sections can lead to misleading results. In the present study, we designed a panfungal PNA probe and confirmed that the intensity of ISH signals of this probe was similar to those of species-specific probes. These findings suggest that ISH with the panfungal probe may be useful for the estimation of hybridizable rRNA for the specific detection of human pathogenic fungi.
In conclusion, we have shown the superiority and the usefulness of ISH with PNA probes for identifying Fusarium spp. in formalin-fixed and paraffin-embedded sections. Further studies are needed to establish ISH with PNA probes as an accurate and rapid diagnostic procedure for tissue sections from patients with suspected fusariosis.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Health Science Research grants for Research on Emerging and Re-Emerging Infectious Diseases (H16-Shinko-6, H19-Shinko-8, and H22-Shinko-8) and Measures for Intractable Diseases (H20 Nannchi Ippann 35) from the Ministry of Health, Labor, and Welfare of Japan and by the Grant of the Strategic Basis on Research Grounds for Non-Governmental Schools at Heisei 20th from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to K.S.
We are grateful to K Makimura, K Uchida, and H Yamaguchi for kindly providing important advice.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 24 November 2010.
REFERENCES
- 1. Adams J. C. 1981. Heavy metal intensification of DAB-based HRP reaction product. J. Histochem Cytochem. 29:775. [DOI] [PubMed] [Google Scholar]
- 2. Bialek R., et al. 2005. PCR based identification and discrimination of agents of mucormycosis and aspergillosis in paraffin wax embedded tissue. J. Clin. Pathol. 58:1180–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boutati E. I., Anaissie E. J. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999–1008 [PubMed] [Google Scholar]
- 4. Demidov V. V., Yavnilovich M. V., Belotserkovskii B. P., Frank-Kamenetskii M. D., Nielsen P. E. 1995. Kinetics and mechanism of polyamide (“peptide”) nucleic acid binding to duplex DNA. Proc. Natl. Acad. Sci. U. S. A. 92:2637–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guinea J., Peláez T., Recio S., Torres-Narbona M., Bouza E. 2008. In vitro antifungal activity of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycetes, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob. Agents Chemother. 52:1396–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanazawa R., Murayama S. Y., Yamaguchi H. 2000. In-situ detection of Aspergillus fumigatus. J. Med. Microbiol. 49:285–290 [DOI] [PubMed] [Google Scholar]
- 7. Hata D. J., Buckwalter S. P., Pritt B. S., Roberts G. D., Wengenack N. L. 2008. Real-time PCR method for detection of zygomycetes. J. Clin. Microbiol. 46:2353–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayden R. T., et al. 2003. In situ hybridization for the differentiation of Aspergillus, Fusarium, and Pseudallescheria species in tissue section. Diagn. Mol. Pathol. 12:21–26 [DOI] [PubMed] [Google Scholar]
- 9. Hayden R. T., Qian X., Procop G. W., Roberts G. D., Lloyd R. V. 2002. In situ hybridization for the identification of filamentous fungi in tissue section. Diagn. Mol. Pathol. 11:119–126 [DOI] [PubMed] [Google Scholar]
- 10. Hue F. X., Huerre M., Rouffault M. A., de Bievre C. 1999. Specific detection of Fusarium species in blood and tissues by a PCR technique. J. Clin. Microbiol. 37:2434–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hummel M., et al. 2006. Detection of Aspergillus DNA in cerebrospinal fluid from patients with cerebral aspergillosis by a nested PCR assay. J. Clin. Microbiol. 44:3989–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kerstens H. M., et al. 2000. AgarCyto: a novel cell-processing method for multiple molecular diagnostic analyses of the uterine cervix. J. Histochem. Cytochem. 48:709–718 [DOI] [PubMed] [Google Scholar]
- 13. Lau A., et al. 2007. Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J. Clin. Microbiol. 45:380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montone K. T. 2009. Differentiation of Fusarium from Aspergillus species by colorimetric in situ hybridization in formalin-fixed, paraffin-embedded tissue sections using dual fluorogenic-labeled LNA probes. Am. J. Clin. Pathol. 132:866–870 [DOI] [PubMed] [Google Scholar]
- 15. Montone K. T., Feldman M. D. 2009. In situ detection of Aspergillus 18S rRNA Sequences using a terminally biotinylated locked nucleic acid (LNA) probe. Diagn. Mol. Pathol. 18:239–242 [DOI] [PubMed] [Google Scholar]
- 16. Nelson P. E., Dignani M. C., Anaissie E. J. 1994. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7:479–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nielsen P. E., Egholm M., Berg R. H., Buchardt O. 1991. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 254:1497–1500 [DOI] [PubMed] [Google Scholar]
- 18. Nucci M., Anaissie E. 2002. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin. Infect. Dis. 35:909–920 [DOI] [PubMed] [Google Scholar]
- 19. Ochiai E., et al. 2008. Inhalation of Stachybotrys chartarum causes pulmonary arterial hypertension in mice. Int. J. Exp. Pathol. 89:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliveira K., Haase G., Kurtzman C., Hyldig-Nielsen J. J., Stender H. 2001. Differentiation of Candida albicans and Candida dubliniensis by fluorescent in situ hybridization with peptide nucleic acid probes. J. Clin. Microbiol. 39:4138–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paterson P. J., Seaton S., McLaughlin J., Kibbler C. C. 2003. Development of molecular methods for the identification of aspergillus and emerging moulds in paraffin wax embedded tissue sections. Mol. Pathol. 56:368–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rigby S., et al. 2002. Fluorescence in situ hybridization with peptide nucleic acid probes for rapid identification of Candida albicans directly from blood culture bottles. J. Clin. Microbiol. 40:2182–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi S. R., Key M. E., Kalra K. L. 1991. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 39:741–748 [DOI] [PubMed] [Google Scholar]
- 24. Shinozaki M., et al. 2009. Application of in situ hybridization to tissue sections for identification of molds causing invasive fungal infection. Nippon Ishinkin Gakkai Zasshi 50:75–83 [DOI] [PubMed] [Google Scholar]
- 25. Stanzani M., Tumietto F., Vianelli N., Baccarani M. 2007. Update on the treatment of disseminated fusariosis: focus on voriconazole. Ther. Clin. Risk Manag. 3:1165–1173 [PMC free article] [PubMed] [Google Scholar]
- 26. Teertstra W. R., Lugones L. G., Wösten H. A. 2004. In situ hybridisation in filamentous fungi using peptide nucleic acid probes. Fungal Genet. Biol. 41:1099–1103 [DOI] [PubMed] [Google Scholar]
- 27. Vollmer T., Störmer M., Kleesiek K., Dreier J. 2008. Evaluation of novel broad-range real-time PCR assay for rapid detection of human pathogenic fungi in various clinical specimens. J. Clin. Microbiol. 46:1919–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson D. A., et al. 2005. Multicenter evaluation of a Candida albicans peptide nucleic acid fluorescent in situ hybridization probe for characterization of yeast isolates from blood cultures. J. Clin. Microbiol. 43:2909–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.