Abstract
Mycobacterium tuberculosis that is resistant to both isoniazid (INH) and rifampin (RIF) is spreading. It has become a public health problem in part because the standard culture methods used to determine the appropriate treatment regimen for patients often take months following the presumptive diagnosis of tuberculosis. Furthermore, the misidentification of nontuberculosis mycobacteria (NTM) in patients presumably suffering from tuberculosis results in additional human and health care costs. The mechanisms of resistance for several drugs used to treat Mycobacterium tuberculosis are well understood and therefore should be amenable to determination by rapid molecular methods. We describe here the use of PCR followed by electrospray ionization mass spectrometry (PCR/ESI-MS) in an assay that simultaneously determines INH and RIF resistance in Mycobacterium tuberculosis and identifies and determines the species of NTMs. The assay panel included 16 primer pairs in eight multiplexed reactions and was validated using a collection of 1,340 DNA samples from cultured specimens collected in the New York City area, the Republic of Georgia, and South Africa. Compared with phenotypic data, the PCR/ESI-MS assay had 89.3% sensitivity and 95.8% specificity in the determination of INH resistance and 96.3% sensitivity and 98.6% specificity in the determination of RIF resistance. Based on a set of 264 previously characterized liquid culture specimens, the PCR/ESI-MS method had 97.0% sensitivity and 99.9% specificity for determination of NTM identity. The assay also provides information on ethambutol, fluoroquinolone, and diarylquinoline resistance and lineage-specific polymorphisms, to yield highly discriminative digital signatures potentially suitable for epidemiology tracking.
INTRODUCTION
The spread of multidrug-resistant (MDR) Mycobacterium tuberculosis resistant to both isoniazid (INH) and rifampin (RIF) is an increasingly worrisome health concern. Resistance testing requires 3 to 4 weeks in the best of laboratories using the state-of-the-art culture systems, due to the extremely slow growth rate of M. tuberculosis. At the same time, the spread of nontuberculosis mycobacteria (NTM) infections is also emerging as an issue that must be addressed (21, 39). The proper characterization of NTM is critical, as some species are known to be naturally resistant to one or more antitubercular agents and, under current testing paradigms, the presence of an NTM may be suspected only after the failure of a regular tuberculosis treatment (11). Molecular methods have been reported that directly identify and differentiate mycobacterial isolates from acid-fast bacillus (AFB) culture broth (23, 34, 38, 40, 41). Li et al. reported the use of broad-range PCR amplification followed by suspension array analysis to identify and differentiate 17 commonly encountered mycobacterial species in the clinical setting (25). However, the detection of resistance mutations and NTM characterization remain distinct tasks requiring multiple assays (24, 30).
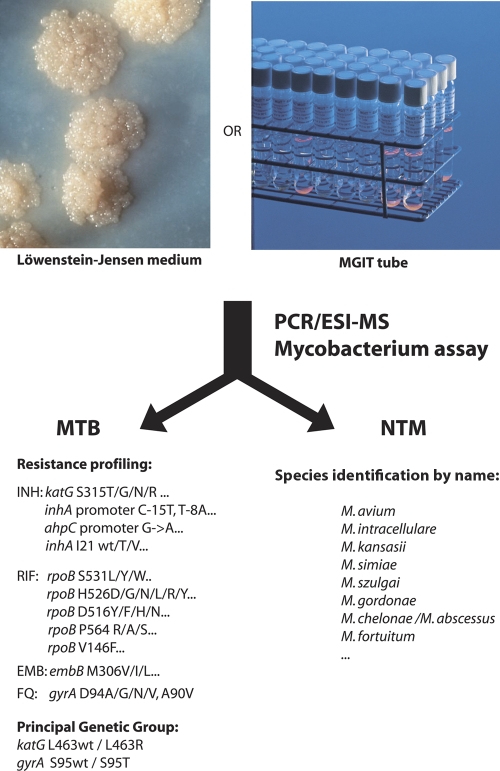
In the present study, we evaluated the use of an assay that employs PCR followed by amplicon characterization using electrospray ionization mass spectroscopy (PCR/ESI-MS) for identification of species and drug resistance characterization of mycobacteria, as illustrated in Fig. 1. PCR/ESI-MS is a rapid, high-throughput method for identification, characterization, and quantification of microorganisms (bacteria, fungi, and viruses) present in cultured specimens or patient samples (6, 7, 9). Several factors converge to make PCR/ESI-MS an ideal tool for M. tuberculosis genotyping, NTM characterization, and characterization of MDR. First, a PCR/ESI-MS assay provides extensive flexibility in the choice of custom-made primer pairs that specifically interrogate the desired markers (i.e., the determination of RIF resistance or the characterization of a specific lineage). Second, the process is automated and allows the batch analysis of up to 240 isolates per day, with the first isolate being analyzed in under 4 h (9). Third, the digital nature of PCR/ESI-MS results allows portability between different facilities. And finally, the type of information that needs to be queried for M. tuberculosis resistance profiling fits perfectly within the specifications of the methodology. In general, the main limitations of PCR/ESI-MS technology are found in the usable amplicon size (preferably less than 160 nucleotides long) and in the limited information content compared to traditional sequencing, as the linking order of the bases is not determined using PCR/ESI-MS. Neither limitation is significant in the case of M. tuberculosis profiling, as the targeted mutations are sparse and can be accurately identified using base composition alone (8). The genes carrying mutations that confer drug resistance in M. tuberculosis lack secondary (incidental) synonymous mutations, an observation that has been consistently verified by numerous genotyping studies (27). The virtual absence of background genomic variation ensures that any observed deviation from the expected mass represents an expressed mutation that is significant for the determination of drug resistance. These characteristics allowed us to assemble a PCR/ESI-MS assay using 16 primer pairs in eight multiplex reactions that target the main markers associated with RIF and INH resistance and allow identification of species of NTM. The assay was tested using a large collection of 1,340 isolates with various resistance profiles and a set of 264 previously characterized NTM. This study was performed using the Ibis T5000 biosensor, which allowed the simultaneous analysis of 12 samples on a 96-well microtiter plate. Sample extraction, PCR amplification, and T5000 analysis were typically performed in batches of up to 15 plates. Time to first answer was typically on the order of 6 to 8 h, with additional results becoming available by increments of 1 h per plate.
Fig. 1.
Basic flow chart of the PCR/ESI-MS assay.
MATERIALS AND METHODS
Isolates tested.
Four sets of DNA samples isolated from clinical M. tuberculosis isolates were used in this study. First, 45 reference isolates were collected from the Public Health Research Institute (PHRI) for the initial primer pair testing and panel assembly. These isolates had been extensively characterized by IS6110 restriction fragment length polymorphism (RFLP) analysis, spoligotyping, mycobacterial interspersed repetitive unit (MIRU) typing, and principal genetic group analysis (27). Susceptibilities to INH, RIF, and ethambutol (EMB) were also previously determined using direct culture-based growth inhibition methods (29), and the presence of the corresponding mutations within the katG, inhA, rpoB, and gyrA genes was confirmed by direct sequencing.
Second, a total of 1,340 M. tuberculosis DNA samples was gathered to evaluate the drug resistance determination capabilities of the PCR/ESI-MS assay. This evaluation set was divided into three subsets. The first subset of 962 isolates originated primarily from the greater New York area, with 911 isolates provided by an ongoing collaboration between the PHRI TB Center, the New York City Bureau of Laboratories, and the New Jersey Department of Health and Senior Services (4). An additional 51 isolates were obtained from the Russian Federation and were characterized at the PHRI. The second subset of 188 M. tuberculosis isolates was collected in the Republic of Georgia (10), and the third subset included 190 isolates from South Africa. Statistical analyses of these three subsets were performed separately, since the drug susceptibility testing was performed at different locations.
Third, a panel of 47 mycobacterial reference strains was collected from the ATCC to provide reference NTM signatures. This set represented 33 Mycobacterium species: M. agri, M. asiaticum, M. aurum, M. avium, M. branderi, M. chelonae, M. engbaekii, M. farcinogenes, M. flavescens, M. fortuitum, M. gallinarum, M. gastri, M. gordonae, M. haemophilum, M. intracellulare, M. kansasii, M. lactis, M. lufu, M. malmoense, M. marinum, M. neoaurum, M. phlei, M. rhodesiae, M. scrofulaceum, M. senegalense, M. sherrisii, M. simiae, M. szulgai, M. terrae, M. tokaiense, M. ulcerans, M. vaccae, and M. xenopi.
Finally, to test the capabilities for identification of species by the assay, a total of 264 culture broth specimens that had tested positive for acid-fast bacilli using the BACTEC MGIT 960 system (BD Diagnostic Systems, Franklin Lakes, NJ) were collected at the Vanderbilt University Medical Center and the Veteran Affairs Tennessee Valley Health Care System (25).
Sample culture and drug susceptibility testing.
The strains were subcultured on Löwenstein-Jensen slants (29). Drug susceptibility testing (DST) was performed in the New York City Department of Health laboratory as described previously (29), with the indirect proportion method and Middlebrook 7H10 medium for the following anti-TB drugs (concentrations in parentheses): isoniazid (1.0 μg/ml), rifampin (1.0 μg/ml), streptomycin (10.0 μg/ml), and ethambutol (5.0 μg/ml).
Genome preparation and PCR.
Genomic material from cultured samples was prepared using the DNeasy tissue kit (Qiagen, Valencia, CA) according to the manufacturer's protocols. All PCR mixtures were assembled in 40-μl volumes in the 96-well microtiter plate format by using a Packard MPII liquid handling robotic platform and a Mastercycler Pro apparatus (Eppendorf, Hauppauge, NY). The primers used for PCR/ESI-MS analysis are shown in Table 1. The PCR mix consisted of 1 unit of Immolase (Bioline, Taunton, MA), 1× buffer II, 1.5 mM MgCl2, 0.4 M betaine, 800 μM deoxynucleoside triphosphate mix, and 250 nM each primer. The following PCR conditions were used to amplify the sequences used for PCR/ESI-MS analysis: 95°C for 10 min, followed by eight cycles of 95°C for 30 s, 48°C for 30 s, and 72°C for 30 s, with the 48°C annealing temperature increasing 0.9°C each cycle. The PCR was then continued for 37 additional cycles of 95°C for 15 s, 56°C for 20 s, and 72°C for 20 s.
Table 1.
Primer pair panels used in the present study
| Locusa | Target | Forward and reverse primer sequence | Full amplicon length (nt) | Sequence length (nt) between primers | Comments |
|---|---|---|---|---|---|
| BCT3633* | rpoB 515–516 | TCCAGCCAGCTGAGCCAATTC; TCCGACAGCGGGTTGTTCTG | 47 | 6 | RIF resistance; does not discriminate NTM from M. tuberculosis |
| BCT4235 | inhA 19–23 | TTGGTTAGCGGAATCATCACCGA; TTGGGCTACCCGTGCGATGT | 60 | 17 | INH resistance; NTM species identification |
| BCT3552* | inhA promoter | TGCTCGTGGACATACCGATTTCG; TCAGTGGCTGTGGCAGTCAC | 75 | 32 | INH resistance; does not prime NTM |
| BCT3908 | rpoB 526 | TCCGCTGTCGAGGTTGACC; TTCGGACAGTCGGCGCTT | 40 | 3 | RIF resistance; does not discriminate NTM from M. tuberculosis |
| BCT4234* | ahpC promoter | TGGGATGCCGATAAATATGGTGTGATA; TTCATCAAAGCGGACAATGCATTTG | 101 | 49 | INH resistance; does not prime NTM |
| BCT4364 | atpE 58–69 | TCACCGTTCTTCATCACCGTC; TGAAGACGAACAGCGCCATAAA | 79 | 36 | Diarylquinoline resistance; NTM species identification |
| BCT3551* | embB 306 | TGACGGCTACATCCTGGGC; TGCGTGGTCGGCGACTC | 43 | 7 | Ethambutol resistance; NTM species identification |
| BCT4236 | inhA 91–96 | TGGCAACAAGCTCGACGG; TCCGGTCTGCGGCATGA | 55 | 20 | INH resistance; NTM species identification |
| BCT3553* | katG 315 | TCGGTAAGGACGCGATCACC; TGTCCATACGACCTCGATGCC | 44 | 3 | INH resistance; does not discriminate NTM from M. tuberculosis |
| BCT4366 | rpoB 505–516 | TGCCGCGATCAAGGAGTTCT; TCCGACAGCGGGTTGTTCTG | 72 | 32 | RIF resistance; NTM species identification |
| BCT3554* | katG 463 | TGCCAGCCTTAAGAGCCAGATC; TGTGAGACAGTCAATCCCGATGC | 48 | 3 | PGG determination; does not discriminate NTM from M. tuberculosis |
| BCT3828 | rpoB 531–539 | TTGACCCACAAGCGCTGACTG; TAGCCCGGCACGCTCAC | 66 | 28 | RIF resistance; NTM species identification |
| BCT3555* | gyrA 90–95 | TCACCCGCACGGCGAC; TGGGCCATGCGCACCAG | 51 | 18 | PGG determination; NTM species identification |
| BCT4237 | rpoB 142–154 | TCACGTTCATCATCAACGGGAC; TTCAATGGTCTCGTCGAAGTACAC | 86 | 40 | RIF resistance; NTM species identification |
| BCT3556* | gyrA 95 | TCGACGCGTCGATCTACGAC; TGGGCCATGCGCACCAG | 40 | 3 | Fluoroquinolone resistance; does not discriminate NTM from M. tuberculosis |
| BCT3697 | rpoB 562–572 | TGCCGGATGTGCCCGATC; TGCGTACACCGACAGCGAG | 72 | 35 | RIF resistance; NTM species identification |
a *, the primer pair for which an internal positive control was designed and included in the assay.
PCR/ESI-MS gene targets and selection of primers.
General methods for PCR/ESI-MS have been described previously using the first PCR/ESI-MS instrument, the Ibis T5000 biosensor (6, 12), and they remain fully applicable with the currently available instrument, marketed as Plex-ID (9). The choice of the genes used for PCR/ESI-MS analysis was primarily dictated by the location of the main mutations associated with resistance to INH and RIF (e.g., katG codon 315) and to rifampin (e.g., rpoB codons 516, 526, and 531). Whenever possible, primer pairs were designed to exclusively amplify the codons of interest. Other primer pairs were designed to amplify a longer portion of a gene in regions where rarer mutations are known to occur at different locations, to allow query of multiple sites simultaneously. An example is primer pair BCT4366, designed to yield amplicons that encompass rpoB codons 505 to 516 of the rifampin resistance-determining region. This primer pair not only detects point mutations in individual codons (e.g., L511P or D516G) but also more complex insertion or deletion patterns of one or more codons (18). Such mutations are often not surveyed by alternate molecular typing methods that focus on point mutations. An internal positive control, containing a target for one of the two primer pairs per well, was designed, tested, and added at 150 molecules per well of the assay plates. This calibrant provided a distinct base composition signature as a positive control for the PCR.
Mass spectrometry and base composition analysis.
Following amplification, 15-μl aliquots of each PCR mixture were desalted and purified using a weak anion exchange protocol as described elsewhere (20). Accurate mass (±1 ppm), high-resolution mass spectra were acquired for each sample using the PCR/ESI-MS protocols described previously (19). For each sample, approximately 1.5 μl of analyte solution was consumed during the 74-s spectral acquisition. Raw mass spectra were postcalibrated with an internal mass standard and deconvolved to monoisotopic molecular masses. Unambiguous base compositions were derived from the exact mass measurements of the complementary single-stranded oligonucleotides. Quantitative results were obtained by comparing the peak heights with the internal PCR calibration standard (present in every PCR well at 150 molecules).
RESULTS
Assay panel.
We assembled a 16-primer pair, multiplexed assay panel; nucleic acid isolated from each specimen was amplified in eight reaction mixtures, each containing two primer pairs. This allowed the analysis of 12 samples on the same 96-well plate. We tested over 80 primer pairs in dilution-to-extinction experiments using purified DNA from the reference strain H37Rv and from strains selected to represent the variety of mutations expected within the surveyed loci. Information on the 16 primer pairs selected for final configuration is reported in Table 1. The assay primarily focuses on the determination of resistance to INH and RIF (five and six primer pairs, respectively). The panel was completed by the incorporation of primer pairs that target mutations associated with ethambutol, fluoroquinolone, and diarylquinoline resistance; all compounds are chiefly associated with mutations in single hot spots (27, 37). In addition, two primer pairs were included for the determination of the principal genetic group (PGG) classification (36) and provided a broad phylogenetic classification.
Linearity and dynamic range.
All the results described in this work are from examination of DNA extracted from isolated colonies or broths from MGIT culture. However, the assay has the potential for use when quantitative measurements are important. Therefore, we measured the linearity and dynamic range of the PCR/ESI-MS assay in serial limiting dilution experiments (Fig. 2). PCR/ESI-MS measurements are calibrated by spiking, during the manufacturing process, a precisely known amount of synthetic DNA into each PCR (19). This synthetic DNA has the same sequence as the target organism, with the exception of a small deletion in the amplicon region to distinguish it from amplicons generated from the target. In this assay the calibration standard was present at 150 molecules in each PCR. The target amplicon was quantitated by a comparison of the mass spectral peak heights from amplicons generated from the target organism and the calibration standard. As shown in Fig. 2, the assay was linear and had a dynamic quantitative response over a range of at least 300-fold.
Fig. 2.
Linearity and dynamic range of the PCR/ESI-MS assay. Synthetic controls, including the intended target sequence for each primer pair, were designed, and known quantities were analyzed by PCR/ESI-MS (x axis). The measured molecular count detected by ESI-MS is reported on the y axis for each of four primer pairs, with the same logarithmic scale as was used for the x axis. Each data point represents the average detection read for the same primer pair across five distinct plates, with the minimal and maximal values represented by vertical bars. Data are not represented for lower input values, as the amplification ceased to be reproducible below a theoretical input of eight molecules per well. The average response of the 16 primer pairs is indicated by the brown solid line.
Determination of reference base composition signatures for M. tuberculosis and NTM species.
We then established reference base composition signatures for a large panel of mycobacterial species. A collection of 92 isolates were tested in the PCR/ESI-MS assay: 23 isolates from diverse lineages of M. tuberculosis, 22 isolates representing other species within the M. tuberculosis complex (M. canettii, M. africanum, M. bovis, and M. microti), and 47 isolates representing 33 distinct NTM species. Typical signatures associated with a number of clinically relevant mycobacterial species are reported in Fig. 3.
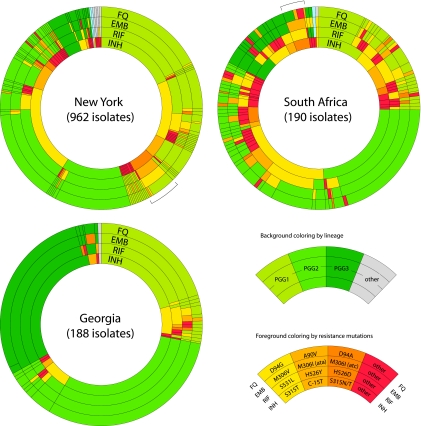
Fig. 3.
Characterization by PCR-ESI-MS of the 1,340 isolates used for the validation of resistance profiling. Isolates were first segregated into three distinct doughnut charts according to their origin. Within each chart, isolates were then sorted and color coded in accordance with their phylogenetic characterization (light, medium, and dark green slices for PGG1, -2, and -3, respectively; blue for M. africanum; gray for strain mixtures or undetermined lineages). Mutations for INH, RIF, EMB, and fluoroquinolone (FQ) resistance are indicated by the color of the corresponding sectors, with the three most common mutations indicated in yellow and light and dark orange (see color key). All other mutations or multiple detections are indicated with red sectors. Brackets indicate two distinct clusters of multiresistant strains sharing largely congruent PCR/ESI-MS signatures.
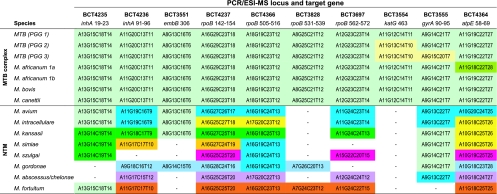
Base composition signatures for the pan-susceptible M. tuberculosis isolates revealed their PGG classification (Fig. 3, top three rows). Signatures for the other species within the M. tuberculosis complex were indistinguishable from the one for M. tuberculosis (PGG1), except for the four M. africanum strains, which had G-to-T mutations within the atpE locus (Fig. 3). This mutation (Rv1305_0207s) is one of the 11 synonymous (silent) single-nucleotide polymorphisms (sSNP) that characterize M. africanum type 1a, also known as M. tuberculosis lineage 6 (5, 17). In contrast, all NTM isolates outside the M. tuberculosis complex yielded distinct signatures, although only 9 out of the 16 primer pairs included in the assay actually contributed to identification of species; the two primer pairs that target the inhA and ahpC promoter sequences did not yield amplicons outside the M. tuberculosis complex, whereas the five primer pairs that result in amplification of only one or two codon regions yielded the same signature for NTM and M. tuberculosis. The nine primer pairs that target stretches of 7 to 40 nucleotides segregated mycobacterial species from each other (Fig. 3). Six of these loci provided the bulk of the resolving power: BCT4236 (inhA codons 91 to 96), BCT4237 (rpoB 142 to 154), BCT4366 (rpoB 505 to 516), BCT3697 (rpoB 562 to 572), BCT3555 (gyrA 90 to 96), and BCT4364 (atpE 58 to 69). The last three loci, BCT4235 (inhA 21), BCT3551 (embB 306), and BCT3828 (rpoB 531 to 538), mainly provide signatures specific for M. kansasii, M. simiae, M. szulgai, and M. gordonae. All species tested were clearly differentiated from M. tuberculosis in at least five loci. Furthermore, each species tested so far displayed unique base composition signatures in at least two distinct loci. Thus, the PCR/ESI-MS assay has potential for the characterization of NTM species.
Characterization of mycobacterial species from liquid culture broth.
Once base composition signatures for these reference samples were determined, we analyzed 264 DNA samples that had been characterized previously using the BACTEC MGIT 960 culture system (25). The correlation between the PCR/ESI-MS identification and the reference identification is shown in Table 2. Among the 50 M. tuberculosis-positive specimens, 49 showed typical 16-loci M. tuberculosis complex signatures when analyzed by PCR/ESI-MS and could be further segregated into PGG1, PGG2, and PGG3 (11, 31, and 7 isolates, respectively) based on our data. Only one resistance mutation was detected, an A-to-T change in BCT3908, indicating an rpoB H526L mutant (CAC→CTC). One sample yielded a highly unusual signature with only one locus compatible with M. tuberculosis; as the signature did not match those of any of the NTM isolates tested, the identity of this isolate remains unclear.
Table 2.
Correlation between PCR/ESI-MS and reference identifications for the collection of 264 NTM samples
| PCR/ESI-MS identification | Reference identification (no. of isolates)a |
All isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M. tuberculosis complex | MAI complex | M. abscessus | M. chelonae | M. fortuitum | M. gordonae | M. kansasii | M. simiae | M. szulgai | MAI and M. gordonae | ||
| MTB complex (three PGG variants) | 49 | 49 | |||||||||
| MAI complex | 74 | 74 | |||||||||
| M. avium (three variants) | |||||||||||
| M. intracellulare (six variants) | 76 | 1 | 1 | 78 | |||||||
| M. avium and M. intracellulare | 2 | 2 | |||||||||
| MAI complex sp. | 3 | 3 | |||||||||
| M. chelonae/M. abscessus complex | 14 | 1 | 15 | ||||||||
| M. fortuitum complex (three variants) | 7 | 7 | |||||||||
| M. gordonae (three variants) | 9 | 9 | |||||||||
| M. kansasii | 16 | 16 | |||||||||
| M. simiae | 1 | 1 | |||||||||
| M. szulgai (two variants) | 3 | 3 | |||||||||
| M. intracellulare and M. gordonae | 1 | 1 | |||||||||
| Unique signatures | 1 | 2 | 1 | 2 | 6 | ||||||
| All isolates | 50 | 155 | 16 | 1 | 7 | 10 | 19 | 1 | 3 | 2 | 264 |
Results shown in boldface indicate concordant results.
A total of 155 of the specimens were previously characterized as belonging to the M. avium/M. intracellulare (MAI) complex (25). Most of these were further characterized as either M. avium or M. intracellulare by PCR/ESI-MS (74 and 76 isolates, respectively). The species assignments were based on the retrieval of at least five out of the six MAI-specific base counts (Fig. 3). Seven of these isolates showed unexpected mutations in one locus, resulting in the identification of two new M. avium and five M. intracellulare signature variants in addition to the consensus signatures already established for these species. Further studies will be needed to determine if these mutations correlate with variations in drug susceptibility. In two of the 155 MAI isolates, both M. avium and M. intracellulare base counts were detected in the three loci where these species differ, revealing the simultaneous occurrence of both species within the same sample. Conversely, three isolates yielded partial matches to both species, allowing a partial identification to the MAI complex but not a definitive species assignment; these may represent evolutionarily intermediate strains, recombinants, or strains in which homoplasy has generated identical mutations in multiple lineages.
For the remaining 59 samples, reference and PCR/ESI-MS characterizations were in good agreement overall. However, the species of six isolates (Table 2) could not be determined by PCR/ESI-MS, as the base composition signatures for these isolates contained multiple novel base counts and/or inconsistent or partial matches to base composition signatures of known species. The diagnostic performance of the PCR/ESI-MS assay for Mycobacterium species identification based on these 264 AFB-positive MGIT broth culture specimens is reported in Table 3.
Table 3.
Sensitivity, specificity, and predictive values of the PCR/ESI-MS Mycobacterium assay for identification and species identification of 264 mycobacterial isolates directly from AFB-positive MGIT broth culture mediaa
| Mycobacterial species, complex, or group, according to PCR/ESI-MS | No. of isolates with indicated PCR/ESI-MS result |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|
| Correct positive | Incorrect positive | Incorrect negative | Correct negative | |||||
| M. tuberculosis complex | 49 | 1 | 0 | 218 | 98.0 | 100.0 | 100.0 | 99.5 |
| M. avium/M. intracellulare | 157 | 0 | 1 | 106 | 100.0 | 99.1 | 99.4 | 100.0 |
| M. chelonae/M. abscessus group | 15 | 2 | 0 | 249 | 88.2 | 100.0 | 100.0 | 99.2 |
| M. fortuitum group | 7 | 0 | 0 | 259 | 100.0 | 100.0 | 100.0 | 100.0 |
| M. gordonae | 10 | 2 | 0 | 256 | 83.3 | 100.0 | 100.0 | 99.2 |
| M. kansasii | 16 | 3 | 0 | 249 | 84.2 | 100.0 | 100.0 | 98.8 |
| M. simiae | 1 | 0 | 0 | 267 | 100.0 | 100.0 | 100.0 | 100.0 |
| M. szulgai | 3 | 0 | 0 | 265 | 100.0 | 100.0 | 100.0 | 100.0 |
| Total | 258 | 8 | 1 | 1869 | 97.0 | 99.9 | 99.6 | 99.6 |
PPV, positive predictive value; NPV, negative predictive value.
Determination of drug resistance profiles.
A total of 1,340 DNA samples from M. tuberculosis isolates were tested to evaluate the utility of the PCR/ESI-MS assay for the determination of drug resistance. Phenotypic testing was performed previously for each of these isolates by the absolute concentration method on solid agar medium, allowing a direct evaluation of the reliability of the PCR/ESI-MS assay. Four isolates were characterized as M. avium by PCR/ESI-MS, whereas strain mixtures were identified in nine isolates through the recognition of multiple base composition signatures in at least two loci. The PCR/ESI-MS characterization of each subset of isolates is shown in Fig. 4.
Fig. 4.
Expected PCR/ESI-MS signatures for members of the M. tuberculosis complex and the most common NTM tested in this study. Only 10 loci (out of 16) are represented here; the 6 loci not shown did not provide lineage-specific signatures.
Mutations conferring resistance to INH were found in 745 of the 835 isolates previously shown to be INH resistant, for an overall sensitivity of 89.2% (Table 4). Interestingly, the INH mutations in regions targeted by our primer pairs were more often observed in the isolates with the MDR phenotype (339/356, or 95.2%) than in mono-INH-resistant isolates (406/479, or 84.8%). As expected, the most common mutations for INH resistance were found in the two main loci, katG S315T (ACC codon; 492 isolates, or 58.9%) and inhA promoter C-15T (151 isolates, or 18.1%). The signature katG S315N/T was also found in 89 isolates (10.7%); it corresponds to either the single mutant S315N (AAC) or the double mutant S315T (ACA) found in the W MDR strain. Both mutations have the same mass signature and are therefore indistinguishable by PCR/ESI-MS. Four instances of rarer katG mutations (S315T, S315R, or S315I) and 29 other mutations within the inhA operon were also detected. Mutations were simultaneously found in both katG 315 and the inhA promoter in 32 of the 835 INH-resistant isolates (3.8%). Three secondary loci were also evaluated for INH resistance in the PCR/ESI-MS assay. Within the ahpC promoter four distinct types of mutations were observed, but in only 16 of 37 instances were they seen in the absence of mutations within the inhA promoter or katG. Similarly, mutations within the inhA reading frame were observed alone in only 8 of 20 instances (five I21V, six I21T, and nine I94T). The inclusion of the ahpC promoter locus and the two inhA loci therefore allowed modest gains of 2% and 1%, respectively, in the overall level of INH susceptibility. Finally, mutations were found in 21 isolates with INH-susceptible phenotypes, including seven instances of katG S315T and nine instances of inhA C-15T. These findings highlight the still-incomplete understanding of the molecular mechanisms underlying INH resistance.
Table 4.
Determination of INH resistancea
| Source of isolates (n) | No. of isolates with indicated results (reference, PCR/ESI-MS) |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|
| R, R | R, S | S, R | S, S | |||||
| New York (962) | 598 | 67 | 16 | 281 | 89.9 | 94.6 | 97.4 | 80.7 |
| Georgia (188) | 30 | 9 | 5 | 144 | 76.9 | 96.6 | 85.7 | 94.1 |
| South Africa (190) | 117 | 14 | 0 | 59 | 89.3 | 100.0 | 100.0 | 80.8 |
| All isolates (1,340) | 745 | 90 | 21 | 484 | 89.2 | 95.8 | 97.3 | 84.3 |
R, resistant; S, susceptible; PPV, positive predictive value; NPV, negative predictive value.
For RIF, the correlation between phenotypes and PCR/ESI-MS profiles was higher than for INH, with only 14 missed detections among 393 RIF-resistant isolates (96.4% sensitivity) and 13 unexpected mutations among 933 RIF-susceptible isolates (98.5% specificity). The data are summarized in Table 5. Most mutations were found within the so-called rifampin resistance-determining region (RRDR), particularly in codons 531 (207 instances, mainly S5131L), 526 (115 instances), and 516 (58 instances). These finding are in agreement with the commonly held view that RIF resistance mutations are confined within the rpoB gene (3). Mutations conferring resistance to rifampin have also been characterized in secondary rpoB hot spots (13, 15, 26). We found 10 RIF-resistant isolates with the mutation V146F, and 5 isolates possessed the I572F mutation. These two mutations outside the RRDR accounted for 3.8% of the instances of RIF resistance. Neither mutation was present in the RIF-susceptible isolates. L511P, L533P, or D516Y mutations were found in seven RIF-susceptible isolates; these mutations are usually associated with low-level RIF resistance (13). The mutations H526Y, H526N, and S531L, commonly associated with RIF resistance, were observed in six isolates with a RIF-susceptible phenotype.
Table 5.
Determination of RIF resistancea
| Source of isolates (n) | No. of isolates with indicated results (reference, PCR/ESI-MS) |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|
| R, R | R, S | S, R | S, S | |||||
| New York (962) | 238 | 9 | 8 | 707 | 96.4 | 98.9 | 96.7 | 98.7 |
| Georgia (188) | 16 | 0 | 4 | 168 | 100 | 97.7 | 80.0 | 100 |
| South Africa (190) | 125 | 5 | 1 | 59 | 96.2 | 98.3 | 99.2 | 92.2 |
| All isolates (1,340) | 379 | 14 | 13 | 934 | 96.4 | 98.6 | 96.7 | 98.5 |
R, resistant; S, susceptible; PPV, positive predictive value; NPV, negative predictive value.
The PCR/ESI-MS assay also includes three markers for ethambutol, fluoroquinolone, and diarylquinoline resistance (2, 27, 31, 37). Phenotypes for EMB resistance were available for 755 isolates (Table 6). Mutations at embB codon 306 were observed in 92 of the 125 EMB-resistant isolates (73.6% sensitivity) and in 17 of the 630 EMB-susceptible isolates (97.3% specificity). The mutations identified were M306V (69 isolates), M306L (4 isolates), and the three M306I variants (21, 13, and 2 isolates with ATA, ATC, and ATT codons, respectively). The M306I mutants were noticeably overrepresented in the isolates with the EMB-susceptible phenotype (9/17, 53%) compared to the ones with the EMB-resistant phenotype (27/92, 29%). No phenotypes were available for fluoroquinolone resistance analysis, but at least eight distinct mutations were detected within the quinolone resistance-determining region of 49 isolates, including 5 isolates that were both INH and RIF susceptible. Finally, analysis of amplicons from the primer pair targeting the atpE locus indicated diarylquinolone resistance in two isolates and allowed the identification of the M. africanum lineage in seven isolates.
Table 6.
Determination of EMB resistance (755 isolates)
| Source of isolates (n) | No. of isolates with indicated resultsa (reference, PCR/ESI-MS) |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|
| R, R | R, S | S, R | S, S | |||||
| New York (567) | 87 | 32 | 9 | 439 | 73.1 | 98.0 | 90.6 | 93.2 |
| Georgia (188) | 5 | 1 | 8 | 174 | 83.3 | 95.6 | 38.5 | 99.4 |
| All isolates (755) | 92 | 33 | 17 | 613 | 73.6 | 97.3 | 84.4 | 94.9 |
R, resistant; S, susceptible; PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
In the present study we investigate the use of the PCR/ESI-MS technology for the molecular characterization of M. tuberculosis and other mycobacteria species. The PCR/ESI-MS mycobacterium assay provides, on the time frame of a few hours and with a throughput of 300 specimens per day, rapid species identification of mycobacteria present in a sample (M. tuberculosis versus NTM, the latter being identified at the species level), the characterization of drug resistance to the primary front-line drugs (INH and RIF) if M. tuberculosis is present, the characterization of ethambutol and fluoroquinolone resistance, and the partial characterization of lineages within the M. tuberculosis complex itself. A unique feature of this assay is the simultaneous targeting of specific mutations conferring drug resistance and lineage markers, based on use of complementary priming strategies. In addition to the traditional MDR markers that were captured individually, primer pairs targeting the atpE, the rpoB, and the inhA loci were designed to capture rare resistance mutations that may occur in MDR M. tuberculosis. In addition, these primer pairs targeting housekeeping genes cover stretches of DNA long enough to capture species-specific variations, thus rendering the characterization of NTM feasible with the same assay used for M. tuberculosis drug resistance testing. A prime example is the atpE primer pair, which amplifies a region associated with mutations that provide resistance to diarylquinoline (2, 31) but is also useful for NTM characterization.
Although there was excellent correlation between PCR/ESI-MS data and previously determined rifampin resistance of the analyzed isolates (96.4% sensitivity and 98.6% specificity overall), the PCR/ESI-MS assay did not perform as well for the determination of INH resistance (89.2% sensitivity and 95.8% specificity). The sensitivity and specificity of the PCR/ESI-MS assay, however, compare favorably with other molecular methods for resistance determination (28). In our analysis of the PCR/ESI-MS assay, we characterized 1,340 isolates collected on three continents to maximize strain diversity. We also included an unusually large contingent of isolates with INH-only resistance phenotypes (479 INH-only versus 356 MDR). The PCR/ESI-MS assay interrogates five regions commonly associated with INH resistance. These mutations were less often present in the isolates resistant only to INH (406/479, 84.8%) than in the MDR isolates (339/356, 95.2%). Demonstrating the diversity due to geography, in the Georgian isolates, these common INH resistance mutations were found in 18 of 26 (69%) INH-only isolates versus 12 of 13 (92%) MDR isolates, hence, the significantly lower sensitivity achieved for INH testing with the Georgian isolates (Table 4). The isolates where INH mutations were expected but not observed were evenly distributed between the three principal genetic groups with no apparent sampling bias, and further investigation will be required to identify the molecular basis for INH resistance in these isolates. Other discrepancies, however, may find an explanation in the questionable portability of phenotyping methods (22). For example, we found embB M306I mutants primarily in samples with an EMB-susceptible phenotype. Previous studies showed that the marginal increase in EMB MIC levels resulting from the incorporation of a M306I only brings the EMB MIC close to the critical concentration and therefore an isolate could be inadvertently defined as EMB susceptible (33, 37).
Since the PCR/ESI-MS technology uses aggregate base composition signatures and not sequencing, potentially ambiguous results might occur if two mutations that result in no mass change (e.g., G→C and C→G) are simultaneously present within the same amplicon (8). This concern guided the primer pair design and panel assembly for the M. tuberculosis assay, and a subsequent survey of the literature revealed that such a situation may have been encountered in at least one instance in past studies: gyrA D94H + T95S (GAC→CAC + ACC→AGC). This ambiguity was alleviated by the inclusion of a primer pair that unambiguously resolved codon gyrA 95 alone (BCT3556). In this study, four isolates harbored both mutations, and each occurrence was identified. Mutations at this position characterize the sublineage that includes strain H37Rv and are the only known example of lineage-specific mutations appearing within a resistance-determining region (14).
The other lineage-specific mutation used for PGG typing, katG L463R, was recently confirmed by multilocus analysis to be congruent with the whole Euro-American lineage (5). Although the PGG classification does not distinguish older and less frequent lineages, it does provide valuable insights into the global phylogeny of sample collections and helps delineate clonal outbreaks. The characterization of these lineage-specific mutations within the katG and gyrA loci provides a basic framework upon which mutation profiles can be mapped and interpreted (Fig. 4). For example, a cluster of 36 PGG1 isolates from New York shared the same string of resistance mutations [katG S315T(ACA), rpoB H526Y, and embB M306V]. This combination was seen only in the New York PGG1 isolates and, based upon IS6110 and spoligotyping analysis, indicated the W strain. Similarly, another cluster of six PGG3 South African isolates was defined by simultaneous mutations of katG S315N(AAC), rpoB H526Y, and embB M306V. Three distinct gyrA mutations were also retrieved within this cluster, hinting that extensively drug-resistant strains may be common within this particular lineage. The inclusion of the atpE marker in the PCR/ESI-MS assay allowed characterization of one of the two M. tuberculosis lineages commonly known as M. africanum (17). The future inclusion of additional markers would similarly provide unambiguous characterization of the remaining M. tuberculosis lineages, providing valuable information for epidemiological tracking. To our knowledge, there is only one study that has previously attempted to identify species of mycobacteria and assess drug resistance with the same assay (35). This reverse line blot hybridization assay, however, uses a panel of 40 specific probes to target the main mutations associated with RIF, INH, and streptomycin resistance and a parallel set of 16 probes for NTM characterization. This assay achieved sensitivity levels comparable with our own; the set of isolates evaluated appears to have been less diverse than those evaluated here, as there was an unusually high prevalence of rpoB S531L and katG S315T mutations detected in the Shenai et al. study.
Most of the initial testing and validation work performed in this study involved isolates cultured on Löwenstein-Jensen medium obtained through existing collaborations. Current laboratory techniques for identification of mycobacteria to the species level involve growth in broth and then nucleic acid probing or subculture to solid media for phenotypic tests, which can take weeks. Use of DNA probes (AccuProbe; GenProbe, San Diego, CA), which are specific for the M. tuberculosis complex, MAI complex, M. kansasii, and M. gordonae, on samples taken directly from BACTEC TB broth culture has dramatically reduced the time to identification and differentiation of mycobacterial infections (1, 32). Recent work (16) has demonstrated that the characterization of resistance mutations directly from sputum samples is achievable, and validation of the PCR/ESI-MS assay using direct sputum specimens is planned.
The present study was primarily performed using the Ibis T5000 biosensor, a prototype of the next generation of PCR/ESI-MS instrument commercialized by Abbott under the Plex-ID brand (9). Since the completion of this study, the M. tuberculosis assay has been further validated for use with the Plex-ID instrument. While the core of the technology and the assays remain the same, the new instrument platform allows significant cost reductions and a faster turnover, with an average analysis time of 30 s per well, half of what was needed with the T5000.
Finally, it should be noted that, in order to be used as a clinical diagnostic tool, the technology must be FDA approved in the United States and CE marked in Europe. That process has been initiated, but approval is still pending. Consequently, the assay is for now available for research use only.
ACKNOWLEDGMENTS
We thank Dorothy Fallows and Gilla Kaplan from the PHRI Center for providing the South African isolates and Kristin Sannes-Lowery, Jenna Cromwell, and Maria Tobar-Mosquera for their help during sample analysis. We also thank Jacqueline Wyatt and David Metzgar for editorial assistance.
This study was supported in part by grants R44 AI078694-03 from the Centers for Disease Control and Prevention and GEB2-2605-TB-04 from the U.S. Civilian Research and Development Foundation.
Footnotes
Published ahead of print on 29 December 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Alcaide F., Benitez M. A., Escriba J. M., Martin R. 2000. Evaluation of the BACTEC MGIT 960 and the MB/BacT systems for recovery of mycobacteria from clinical specimens and for species identification by DNA AccuProbe. J. Clin. Microbiol. 38:398–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andries K., et al. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227 [DOI] [PubMed] [Google Scholar]
- 3. Chakravorty S., et al. 2008. Rifampin resistance, Beijing-W clade-single nucleotide polymorphism cluster group 2 phylogeny, and the Rv2629 191-C allele in Mycobacterium tuberculosis strains. J. Clin. Microbiol. 46:2555–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clark C. M., et al. 2006. Universal genotyping in tuberculosis control program, New York City, 2001–2003. Emerg. Infect. Dis. 12:719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Comas I., Homolka S., Niemann S., Gagneux S. 2009. Genotyping of genetically monomorphic bacteria: DNA sequencing in Mycobacterium tuberculosis highlights the limitations of current methodologies. PLoS One. 4:e7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ecker J. A., et al. 2006. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J. Clin. Microbiol. 44:2921–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ecker D. J., et al. 2008. Ibis T5000: a universal biosensor approach for microbiology. Nat. Rev. Microbiol. 6:553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ecker D. J., et al. 2009. Molecular genotyping of microbes by multilocus PCR and mass spectrometry: a new tool for hospital infection control and public health surveillance. Methods Mol. Biol. 551:71–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ecker D. J., et al. 2010. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev. Mol. Diagn. 10:399–415 [DOI] [PubMed] [Google Scholar]
- 10. Gegia M., et al. 2008. Prevalence of and molecular basis for tuberculosis drug resistance in the Republic of Georgia: validation of a QIAplex system for detection of drug resistance-related mutations. Antimicrob. Agents Chemother. 52:725–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grubek-Jaworska H., et al. 2009. Nontuberculous mycobacterial infections among patients suspected of pulmonary tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 28:739–744 [DOI] [PubMed] [Google Scholar]
- 12. Hannis J. C., et al. 2008. High-resolution genotyping of Campylobacter species by use of PCR and high-throughput mass spectrometry. J. Clin. Microbiol. 46:1220–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hauck Y., Fabre M., Vergnaud G., Soler C., Pourcel C. 2009. Comparison of two commercial assays for the characterization of rpoB mutations in Mycobacterium tuberculosis and description of new mutations conferring weak resistance to rifampicin. J. Antimicrob. Chemother. 64:259–262 [DOI] [PubMed] [Google Scholar]
- 14. Hazbon M. H., et al. 2008. Convergent evolutionary analysis identifies significant mutations in drug resistance targets of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 52:3369–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heep M., Rieger U., Beck D., Lehn N. 2000. Mutations in the beginning of the rpoB gene can induce resistance to rifamycins in both Helicobacter pylori and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:1075–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Helb D., et al. 2010. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J. Clin. Microbiol. 48:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hershberg R., et al. 2008. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 6:e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirano K., Abe C., Takahashi M. 1999. Mutations in the rpoB gene of rifampin-resistant Mycobacterium tuberculosis strains isolated mostly in Asian countries and their rapid detection by line probe assay. J. Clin. Microbiol. 37:2663–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hofstadler S. A., et al. 2005. TIGER: the universal biosensor. Int. J. Mass Spectrom. 242:23–41 [Google Scholar]
- 20. Jiang Y., Hofstadler S. A. 2003. A highly efficient and automated method of purifying and desalting PCR products for analysis by electrospray ionization mass spectrometry. Anal. Biochem. 316:50–57 [DOI] [PubMed] [Google Scholar]
- 21. Katoch V. M. 2004. Infections due to non-tuberculous mycobacteria (NTM). Indian J. Med. Res. 120:290–304 [PubMed] [Google Scholar]
- 22. Kim S. J. 2005. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur. Respir. J. 25:564–569 [DOI] [PubMed] [Google Scholar]
- 23. Kontos F., et al. 2003. Combined use of the fully automated Bactec MGIT 960 system and a PCR-restriction fragment length polymorphism analysis for routine detection and identification of mycobacteria from clinical samples. J. Microbiol. Methods 52:137–140 [DOI] [PubMed] [Google Scholar]
- 24. Leung K. L., et al. 2009. Development of a simple and low-cost real-time PCR method for the identification of commonly encountered mycobacteria in a high throughput laboratory. Appl. Microbiol. 107:1433–1439 [DOI] [PubMed] [Google Scholar]
- 25. Li H., et al. 2009. Identification and differentiation of clinically relevant mycobacterium species directly from acid-fast bacillus-positive culture broth. J. Clin. Microbiol. 47:3814–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCammon M. T., et al. 2005. Detection of rpoB mutations associated with rifampin resistance in Mycobacterium tuberculosis using denaturing gradient gel electrophoresis. Antimicrob. Agents Chemother. 49:2200–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mathema B., Kurepina N. E., Bifani P. J., Kreiswirth B. N. 2006. Molecular epidemiology of tuberculosis: current insights. Clin. Microbiol. Rev. 19:658–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miotto P., Piana F., Cirillo D. M. 2008. Genotype MTBDRplus: a further step toward rapid identification of drug-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 46:393–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Munsiff S. S., et al. 2006. Trends in drug-resistant Mycobacterium tuberculosis in New York City, 1991–2003. Clin. Infect. Dis. 42:1702–1710 [DOI] [PubMed] [Google Scholar]
- 30. Pai M., Minion J., Steingart K., Ramsay A. 2010. New and improved tuberculosis diagnostics: evidence, policy, practice and impact. Curr. Opin. Pulm. Med. 16:271–284 [DOI] [PubMed] [Google Scholar]
- 31. Petrella S., et al. 2006. Genetic basis for natural and acquired resistance to the diarylquinoline R207910 in mycobacteria. Antimicrob. Agents Chemother. 50:2853–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reisner B. S., Gatson A. M., Woods G. L. 1994. Use of Gen-Probe AccuProbes to identify Mycobacterium avium complex, Mycobacterium tuberculosis complex, Mycobacterium kansasii, and Mycobacterium gordonae directly from BACTEC TB broth cultures. J. Clin. Microbiol. 32:2995–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Safi H., Sayers B., Hazbon M. H., Alland D. 2008. Transfer of embB 306 mutations into clinical Mycobacterium tuberculosis alters susceptibility to ethambutol, isoniazid, and rifampin. Antimicrob. Agents Chemother. 52:2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seagar A. L., et al. 2008. Evaluation of the GenoType Mycobacteria Direct assay for the simultaneous detection of the Mycobacterium tuberculosis complex and four atypical mycobacterial species in smear-positive respiratory specimens. J. Med. Microbiol. 57:605–611 [DOI] [PubMed] [Google Scholar]
- 35. Shenai S., Rodrigues C., Mehta A. 2009. Rapid speciation of 15 clinically relevant Mycobacteria with simultaneous detection of resistance to rifampin, isoniazid, and streptomycin in Mycobacterium tuberculosis complex. Int. J. Infect. Dis. 13:46–58 [DOI] [PubMed] [Google Scholar]
- 36. Sreevatsan S., et al. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. U. S. A. 94:9869–9874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Starks A. M., Gumusboga A., Plikaytis B. B., Shinnick T. M., Posey J. E. 2009. Mutations at embB codon 306 are an important molecular indicator of ethambutol resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 53:1061–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun R., Lee S. Y., Perng S. L., Lu J. J. 2009. Detecting Mycobacterium tuberculosis in Bactec MGIT 960 cultures by inhouse IS6110-based PCR assay in routine clinical practice. J. Formos. Med. Assoc. 108:119–125 [DOI] [PubMed] [Google Scholar]
- 39. Tabarsi P., et al. 2009. Nontuberculous mycobacteria among patients who are suspected for multidrug-resistant tuberculosis: need for earlier identification of nontuberculosis mycobacteria. Am. J. Med. Sci. 337:182–184 [DOI] [PubMed] [Google Scholar]
- 40. Tuohy M. J., Hall G. S., Sholtis M., Procop G. W. 2005. Pyrosequencing as a tool for the identification of common isolates of Mycobacterium sp. Diagn. Microbiol. Infect. Dis. 51:245–250 [DOI] [PubMed] [Google Scholar]
- 41. Wang H., Yue J., Han M., Yang J., Zhao Y. 2010. Rapid method for identification of six common species of mycobacteria based on multiplex SNP analysis. J. Clin. Microbiol. 48:247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]