Abstract
Early secretory antigen 6 (ESAT-6) and cell filtrate protein 10 (CFP-10) are two antigens secreted as a complex by the replicating Mycobacterium tuberculosis complex (MTC). Recently, an immunochromatographic assay (ICA) using a monoclonal antibody against the ESAT-6/CFP-10 complex was developed for the purpose of MTC detection. In this study, the efficacy of the assay was tested with 603 BACTEC cultures that were incubated for 3 additional days after positive signals appeared in the BACTEC MGIT 960 system. Bacterial isolates were recovered from these 603 BACTEC cultures, and 332 MTC isolates, 270 nontuberculosis mycobacterial isolates, and 1 Nocardia isolate were identified by using standard biochemical assays. The ESAT-6/CFP-10 assay detected 322 MTC cultures, resulting in a sensitivity of 97% and a specificity of 97.4%. To reduce the false-negative rate and improve the sensitivity, either serpentine cording in an acid-fast bacillus stain of the cultural smear, the ESAT-6/CFP-10 assay, or a combination of both was used for MTC detection. The sensitivity was then increased to 99.1%, and the negative predictive value increased to 98.9%, but the specificity decreased to 94.8% and the positive predictive value decreased to 95.9%. However, a combination of serpentine cording in cultural smears and the positivity of the ICA resulted in the specificity and positive predictive values of 100%. Therefore, BACTEC cultures with both serpentine cording and positivity of the ESAT-6/CFP-10 assay could be reported to contain MTC directly. The ESAT-6/CFP-10 assay may be an alternative of the Capilia assay (MPB64-ICA) as a convenient and cost-effective method for identification of MTC in culture.
INTRODUCTION
Tuberculosis (TB) is a major public health problem that leads to almost two million deaths annually. The disease is caused by Mycobacterium tuberculosis complex (MTC). Early diagnosis of patients infected with MTC and early treatment to prevent further spread are key issues in TB control (17).
The World Health Organization (WHO) recommends the use of liquid culture systems for mycobacterial species identification and drug susceptibility tests, regardless of whether they are carried out in developed, middle-income, or low-income countries (16). The BACTEC MGIT (mycobacteria growth indicator tube) 960 system (Becton Dickinson, Cockeysville, MD) is a fast, sensitive, and fully automated mycobacterial liquid culture system. Clinical specimens may be applied to culture tubes and cultivated in the system. After cultivation for 4 to 12 days, with a median of 5 days, tubes with mycobacterial growth are identified by the system (hereafter referred to as positive BACTEC cultures) based on oxygen usage in the medium and fluorescence emission. An acid-fast bacillus (AFB) smear is then performed on the positive BACTEC cultures to exclude the possibility of contamination by drug-resistant bacteria. With positive BACTEC cultures that are also AFB positive, colony isolation and identification are carried out using conventional biochemical methods for differentiation of MTC from nontuberculosis mycobacteria (NTM), which can take more than a month (25). The WHO has emphasized the importance of shortening the turnaround time of this process in mycobacterial laboratories (16). There are suitable molecular alternatives that are much faster than the conventional biochemical methods, such as AccuProbe (Gen-Probe, Inc., San Diego, CA), hsp65 PCR restriction enzyme analysis (hsp65 PRA), and 16S rRNA gene sequencing (8, 19, 20, 21). Because MTC grown in liquid medium often exhibits serpentine cording in the acid-fast stain, the presence of serpentine cords in cultural smears has been suggested for use as a rapid presumptive identification of MTC in positive BACTEC cultures (19).
Proteins that are secreted into the extracellular environment by MTC are known to elicit MTC-specific immune responses and are of diagnostic value. Such major proteins include MPT64, MPB70, MPT63, MPT80, MPT45, and ESAT-6/CFP-10 (15). The MPB64 antigen has been used in the immunochromatographic assay (ICA; the Capilia assay) for rapid differentiation of MTC in liquid cultures, including BACTEC cultures, with specificity and sensitivity values greater than 96% (9, 19, 23). False-negative findings, likely resulting from low bacterial amounts in the cultures or mutations in the mpb64 gene of the bacteria, have been reported (9).
Early secretory antigen 6 (ESAT-6, 6 kDa) and cell filtrate protein 10 (CFP-10, 10 kDa) are two specific MTC antigens. They are heterodimers in their natural state and are secreted in the early growth stage as MTC grows. These two antigens have been applied to the QuantiFERON TB-Gold and T-spot tests for rapid diagnosis of latent TB or active TB in blood samples (4, 5). Recently, an ICA using the ESAT-6/CFP-10-specific monoclonal antibody for detecting MTC in liquid cultures was developed (Formosa Biomedical Technology Corp., Taiwan). Like the Capilia assay and other ICAs, the ESAT-6/CFP-10 complex strip assay is rapid (15 min), is easy to use, and does not require expensive instruments. In the present study, the performance of the ESAT-6/CFP-10 strip assay and the serpentine cording of cultural smears for detection of MTC in positive BACTEC cultures were evaluated.
MATERIALS AND METHODS
Clinical specimens and smear morphology.
From 1 January to 31 December 2008, 3,010 sputum samples, transbronchial aspirates, or bronchoalveolar lavage specimens were collected from patients clinically suspected of having TB. The specimens were digested and decontaminated with N-acetyl-l-cysteine and 2% sodium hydroxide (NaLC-NaOH) (6). The processed specimens were concentrated using centrifugation, inoculated into MGIT culture tubes, and incubated in the BACTEC MGIT 960 system (Becton Dickinson, Cockeysville, MD). Once a positive signal was reported by the system, smears from the resultant deposits of the culture tubes were screened for AFB by using the Kinyoun method (6). Serpentine cording morphology was observed using microscopic examination (magnification, ×1,000) and recorded in a blinded fashion (i.e., by an observer who did not know the molecular or ICA results for that sample). Serpentine cord (or cord) morphology indicates rope-like aggregates in which the long axes of the bacteria parallels the cord. Spot morphology indicates short bacilli and cocci coexisting in small aggregates or in dispersed cells. Needle morphology indicates bacilli in palisade forms or in dispersed cells. Ladder morphology indicates bacilli in cross-barring (19).
Identification of mycobacterial species by using biochemical methods, hsp65 PCR-restriction enzyme analysis, and 16S rRNA gene sequencing.
Positive BACTEC cultures that were also acid-fast stain positive were subcultured on Lowenstein-Jensen (LJ) slants and incubated at 37°C in a 5% CO2 atmosphere. Colonies on the LJ slants were used for species identification using conventional culture and biochemical methods. These methods included growth rate, photoreactivity for pigment production, morphology in microcolonies on LJ slants, and biochemical tests, including the following: nitrate reduction, arylsulfatase (3 days), Tween 80 hydrolysis (3 days), urease, semiquantitative catalase, tolerance to 5% NaCl, and niacin production (6, 25).
In addition, positive BACTEC cultures also underwent species identification by hsp65 PCR-restriction enzyme analysis (hsp65 PRA) (20). DNA was extracted from either the positive BACTEC cultures using the DTB specimen processing kit (Becton Dickinson) (23) or from microcolonies on LJ slants using the 2% sodium dodecyl sulfate (SDS) and 10% Triton X-100 method (18). hsp65 PRA was performed according to the method of Telenti et al. (20) and the PRASITE database (http://app.chuv.ch/prasite/index.html).
If identification results using the biochemical methods were different from identification results by hsp65 PRA, 16S rRNA gene sequencing was performed. The mycobacterial DNA extracted from either the positive BACTEC cultures or from microcolonies on the LJ slants was used as a template, and the oligonucleotides 8FPL (5′-AGT TTG ATC CTG GCT CAG-3′) and 1492 (5′-GGT TAC CTT GTT ACG ACT T-3′) were used as primers for PCR and sequencing of the 16S rRNA gene (21). DNA sequences of the 16S rRNA gene were compared to the NCBI nucleotide database, and mycobacterial species were identified (21). In some cases, DNA extracted from MTC microcolonies on the LJ slants was further used for identification of M. bovis or M. tuberculosis using PCR, according to the method of Bakshi et al. (1).
Detection of MTC by the ESAT-6/CFP-10 ICA.
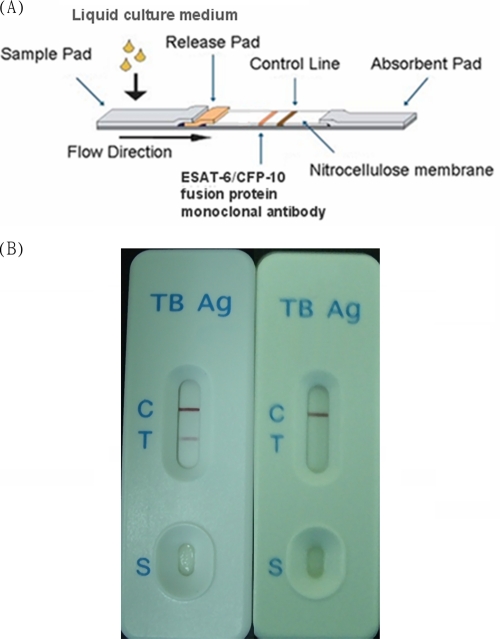
Positive BACTEC cultures that were also acid-fast stain positive were used for the ESAT-6/CFP-10 complex strip assay (Formosa Biomedical Technology Corp., Taiwan; Fig. 1A) according to the manufacturer's recommendations. To increase the detection sensitivity, the culture was left in the BACTEC MGIT 960 instrument for ESAT-6/CFP-10 ICA at day 3 after positive signals appeared.
Fig. 1.
(A) ESAT-6/CFP-10 immunochromatographic assay. (B) A red-purple band appearing on the test band (T) indicates the presence of MTC in the cultures. No band indicates a lack of MTC in the cultures. The control band (C) containing anti-mouse immunoglobulin G antibody should be positive with each testing. The “S” indicates the area of sample loading.
A total of 100 μl of sample from positive BACTEC cultures was applied to the test well of the strip. A red-purple band appearing on the strip within 15 min indicated the presence of MTC in the cultures. No band appearing within 15 min indicated a lack of MTC in the cultures. The control band containing anti-mouse immunoglobulin G antibody should be positive in each test (Fig. 1B).
Agreement and discrepancy analysis.
Species identification using conventional biochemical methods, hsp65 PRA, and/or 16S rRNA gene sequencing was considered the gold standard. The proportion of agreement and the correlation coefficient between identification results from serpentine cording in cultural smears and the gold standard and those between identification results from the ESAT-6/CFP-10 strip assay and the gold standard were determined. They were represented as the sensitivity, specificity, positive predictive rate, negative predictive rate, and likelihood ratio.
If the results from identification using the ESAT-6/CFP-10 ICA were different from the gold standards, a repeated ESAT-6/CFP-10 ICA strip test was performed with the same culture that was left in the BACTEC MGIT 960 instrument for seven additional days after the initial strip test.
RESULTS
Mycobacterial species identification using conventional biochemical methods, hsp65 PRA, and 16S rRNA gene sequencing.
In all, 3,010 clinical specimens were collected and processed from 1 January to 31 December 2008, and 630 specimens were found to be positive for mycobacteria using the BACTEC MGIT 960 system. Of the 630 positive BACTEC cultures, 603 demonstrated a positive AFB smear in the Kinyoun acid-fast stain, and 23 of these were mixed cultures of AFB and other bacteria. The other 27 positive BACTEC cultures were negative for AFB smear. Thus, the contamination rate was 7.9% (50/630). The 603 mycobacterial strains were isolated and purified on LJ slants and identified by using the conventional biochemical methods. The results are shown in Table 1. Of the 603 positive MGIT samples, 332 cultures contained MTC, 1 culture contained Nocardia spp., and 270 cultures contained NTM, including M. intracellulare (n = 133), M. abscessus (n = 74), M. fortuitum (n = 14), M. chelonae (n = 1), M. kansasii (n = 17), M. gordonae (n = 14), M. scrofulaceum (n = 3), M. flavescens (n = 1), M. peregrinum (n = 2), M. simiae (n = 1), M. szulgai (n = 1), M. terrae (n = 1), and Mycobacterium spp. that could not be identified (referred to here as “unidentified”) using the biochemical methods (n = 8).
Table 1.
Identification of MTC in BACTEC cultures using the gold standard method (biochemical methods, hsp65 PRA, and/or 16S rRNA gene sequencing), serpentine cording in smears, and the ESAT-6/CFP-10 ICA
| Mycobacterial species identified using the gold standard method | No. of cultures (n = 603) | No. of positive cultures according to the following tests |
|
|---|---|---|---|
| Serpentine cording in smear (n = 314) | ESAT-6/CFP-10 ICA (n = 329) | ||
| MTC | 332 | 307 | 322 |
| NTM | 270 | 7 | 7 |
| M. intracellulare | 133 | 0 | 1 |
| M. abscessus | 74 | 6 | 2 |
| M. fortuitum | 14 | 0 | 1 |
| M. chelonae | 1 | 0 | 0 |
| M. kansasii | 17 | 0 | 1 |
| M. gordonae | 17 | 0 | 1 |
| M. flavescens | 1 | 0 | 0 |
| M. peregrinum | 2 | 0 | 0 |
| M. simiae | 1 | 0 | 0 |
| M. szulgai | 1 | 0 | 0 |
| M. terrae | 1 | 0 | 0 |
| Unidentified NTM | 8 | 1 | 1 |
| Nocardia spp. | 1 | 0 | 0 |
DNA from the 603 positive BACTEC cultures was extracted, and hsp65 PRA was performed for species identification directly from the cultures. As shown in Table 1, hsp65 PRA identified 591 cultures with the same results as the biochemical methods did, which included 332 MTC-containing cultures and 259 NTM-containing cultures. The remaining 12 cultures included 8 cultures and 1 culture that were identified using the biochemical methods to contain Mycobacterium spp. and Nocardia spp., respectively, but were unable to be identified by hsp65 PRA and 3 cultures that were identified using the biochemical methods to contain M. scrofulaceum but were found to contain M. gordonae (type 3) using hsp65 PRA. These 12 cultures were further identified using 16S rRNA gene sequencing, and the results were the same as those from identification using hsp65 PRA. We therefore concluded that the three cultures identified using the biochemical methods to contain M. scrofulaceum indeed contained M. gordonae (type 3).
Mycobacterial species identification using an acid-fast stain.
The Kinyoun acid-fast stain was performed on the 603 positive BACTEC cultures, and the smear morphology was recorded. Of the 603 AFB-positive cultures, 314 cultures showed serpentine cord morphology, including 307 MTC-containing cultures, 6 M. abscessus-containing cultures, and 1 culture containing unidentified mycobacterial species (Table 1). Therefore, the use of serpentine cord morphology in cultural smears for the diagnosis of MTC in positive BACTEC cultures had a sensitivity of 92.5% and a specificity of 97.4%.
Daily evaluation of the ESAT-6/CFP-10 ICA using 30 positive BACTEC cultures.
Totals of 20, 5, 2, and 3 positive BACTEC cultures containing MTC, M. kansasii, M. intracellulae, and M. abscessus, respectively, were chosen for daily evaluation of the ESAT-6/CFP-10 ICA. During a 2-week period, 100 μl of the cultures was taken daily from the MGIT tubes and assayed by the ESAT-6/CFP-10 ICA after positive signals appeared in the BACTEC MGIT 960 system. Nineteen and one MTC-containing cultures showed a visible positive pink signal on the ESAT-6/CFP-10 ICA from days 1 and 2, respectively, to day 14. Two, one, and one M. kansasii-containing cultures showed a positive pink signal from days 4, 10, and 12, respectively, to day 14 and one M. kansasii-containing culture only on day 14. The two M. intracellulae-containing cultures and three M. abscessus-containing cultures showed negative results from days 1 to day 14. For these selected 30 positive BACTEC cultures, detection of MTC by the ESAT-6/CFP-10 ICA on days 2 and 3 had the best sensitivity (100%) and specificity (100%).
Detection of MTC using ESAT-6/CFP-10 ICA.
The 603 positive BACTEC cultures were assayed by the ESAT-6/CFP-10 ICA on day 3 after positive signals appeared in the BACTEC MGIT 960 instrument. The results are shown in Table 1. Of these samples, 322 MTC-containing cultures and 7 NTM-containing cultures showed a visible positive pink signal in both the sample band and the control band within 15 min with the ESAT-6/CFP-10 ICA, whereas the remaining 10 MTC-containing cultures, 263 NTM-containing cultures, and 1 Nocardia-containing culture only exhibited a positive signal in the control band. The seven NTM-containing cultures that were positive in the ESAT-6/CFP-10 ICA (false positives; Table 1) included one M. kansasii-containing culture (type 6), one M. gordonae-containing culture (type 3), two M. abscessus-containing cultures, one M. fortuitum-containing culture, one M. intracellulare-containing culture, and one culture containing an unidentified mycobacterial strain. Therefore, detection of MTC on day 3 after positive signals appeared by ESAT-6/CFP-10 ICA had a sensitivity of 97% and a specificity of 97.4% (Table 2).
Table 2.
Comparison of results from the identification of MTC in BACTEC cultures based on serpentine cording in smear, the ESAT-6/CFP-10 ICA, and a combination of serpentine cording in smear with the ESAT-6/CFP-10 ICAa
| Parameter | Identification of MTC in BACTEC cultures based on: |
|||
|---|---|---|---|---|
| Serpentine cording in smear | Positivity in ESAT-6/CFP-10 ICA | Intersection of serpentine cording in smear and positivity in ESAT-6/CFP-10 ICAc | Union of serpentine cording in smear and positivity in ESAT-6/CFP-10 ICAd | |
| % Sensitivity | 92.5 | 97 | 90.4 | 99.1 |
| % Specificity | 97.4 | 97.4 | 100 | 94.8 |
| % PPV | 97.8 | 97.9 | 100 | 95.9 |
| % NPV | 91.4 | 96.4 | 89.4 | 98.9 |
| Likelihood ratiob | 35.6 | 37.3 | INe | 19.1 |
The data are from identifications of MTC in the 603 BACTEC cultures shown in Table 1.
Likelihood ratio = sensitivity/(1 − specificity).
This indicates that both serpentine cording in smear and positivity in the ESAT-6/CFP-10 ICA were required in order to identify the culture to contain MTC.
This means that either serpentine cording in smear, positivity in the ESAT-6/CFP-10 ICA, or a combination of both alone was sufficient in order to identify the culture to contain MTC.
IN, infinite.
Detection of MTC by combining the ESAT-6/CFP-10 ICA with serpentine cording in cultural smears.
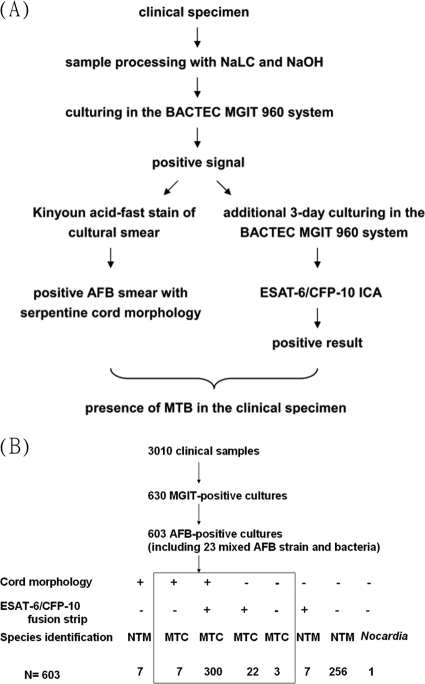
The ESAT-6/CFP-10 ICA misidentified 10 MTC-containing cultures (false negatives; Table 1). To reduce the false-negative rate and improve the sensitivity, serpentine cord formation, positivity in ESAT-6/CFP-10 ICA, or a combination of both methods was used to detect MTC and the sensitivity and the negative predictive value (NPV) were increased to 99.1 and 98.9%, respectively, but the specificity and the positive predictive value (PPV) were decreased to 94.8 and 95.9%, respectively (Table 2). If MTC was identified only when both the serpentine cord morphology and the ESAT-6/CFP-10 ICA were positive, the sensitivity and NPV were decreased to 90.4 and 89.4%, respectively, but the specificity and PPV both improved to 100% (Table 2 and Fig. 2).
Fig. 2.
(A) Flow chart showing the identification of MTC from clinical specimens using serpentine cord morphology and the ESAT-6/CFP-10 complex strip assay. (B) Summary of identification results.
Because the combination of the serpentine cord morphology and the ESAT-6/CFP-10 strip assay showed specificity and PPV of 100%, positive BACTEC cultures that were also positive using the ESAT-6/CFP-10 ICA and showed cord morphology could be reported to contain MTC directly (Table 2 and Fig. 2). For positive BACTEC cultures that were either negative with both assays or positive with one assay and negative with the other assay, the cultures could contain MTC or NTM. Positive BACTEC cultures that were negative with both assays could also contain Nocardia spp. (Fig. 2).
Analysis of the false-negative and false-positive cultures using a repeated ESAT-6/CFP-10 ICA.
A total of 46 positive BACTEC cultures, including 32 MTC-containing cultures and 14 NTM-containing cultures, showed discordant results from identification using the gold standard method (biochemical methods, hsp65 PRA, and 16S rRNA gene sequencing), serpentine cording in cultural smears, and the ESAT-6/CFP-10 ICA. Of the 32 MTC-containing cultures with discordant results, 7 cultures showed cord morphology but were ESAT-6/CFP-10 ICA-negative, 22 cultures showed non-cord morphology (11 cultures, spot morphology; 11 cultures, needle morphology) but were ESAT-6/CFP-10 ICA positive, and 3 cultures showed non-cord morphology (needle morphology) and were ESAT-6/CFP-10 ICA negative. Of the 14 NTM-containing cultures with discordant results, 7 showed cord morphology but were ESAT-6/CFP-10 ICA negative (6 cultures contained M. abscessus and 1 culture contained an unidentified NTM), and 7 cultures showed non-cord morphology but were ESAT-6/CFP-10 ICA positive (2 cultures contained M. abscessus; M. fortuitum, M. intracellulare, M. kansasii [type 6], M. gordonae [type 3], and an unidentified NTM were found in 1 culture each and showed spot, needle, needle, ladder, spot, and spot morphologies, respectively).
Regarding the ESAT-6/CFP-10 ICA, all of the 10 false-negative and 7 false-positive cultures were assayed again 1 week after the initial assay. In addition, since M. kansasii, M. szulgai, and M. marinum were reported to secrete ESAT-6 and CFP-10, cultures of these NTM species might show cross-reactivity with the ESAT-6/CFP-10 ICA (22). Thus, the other 16 M. kansasii-containing cultures (9 type 1 and 7 type 2) and the 1 M. szulgai-containing culture that were ESAT-6/CFP-10 ICA negative also underwent another strip assay 1 week after the initial assay. During this week, these cultures remained in the BACTEC MGIT 960 system for incubation. The results showed that, of the 10 false-negative MTC cultures, 9 were positive but 1 was still negative with the repeat strip assay. The method of Bakshi et al. (1) was then used to further identify whether these 10 MTC cultures contained M. bovis, M. tuberculosis, or other MTC members. The results indicated that the culture that was negative in both initial and repeat assays contained M. bovis, while the other nine cultures negative with the initial but positive with the repeat assay contained M. tuberculosis.
When the 17 M. kansasii-containing cultures (9 type 1, 7 type 2, and 1 type 6) and the 1 M. szulgai-containing culture were tested, all were positive in the repeat assay despite only 1 M. kansasii-containing culture (type 6) being positive with the initial assay. It was concluded that M. kansasii strains secreted ESAT-6 and CFP-10 antigens no matter whether they belonged to M. kansasii type 1, 2, or 6.
The remaining six false-positive cultures included one, one, one, two, and one cultures that contained M. gordonae (type 3), M. intracellulare, M. fortuitum, M. abscessus, and an unidentified NTM, respectively. All of them showed non-cord morphology and positivity with the initial strip assay. With the repeat assay, the one M. gordonae-containing culture (type 3) and the one culture that contained an unidentified NTM were still positive, but the other four cultures were negative. Then, the other 16 M. gordonae-containing cultures (6 type 1, 6 type 2, and 4 type 3) and 7 unidentified NTM-containing cultures that were ESAT-6/CFP-10 ICA negative in the initial strip assay also underwent the repeat strip assay. One M. gordonae-containing culture (type 3) turned out to be positive, while the other 22 cultures remained negative with the repeat assay. It appeared that some M. gordonae type 3 strains were able to secrete ESAT-6 and CFP-10 antigens.
Quantitative analysis of the detection sensitivity of ESAT-6/CFP-10 ICA.
In order to analyze the detection sensitivity of ESAT-6/CFP-10 ICA, one MTC-containing BACTEC culture was randomly chosen and adjusted to a turbidity of McFarland standard 1.0 with MGIT medium, which corresponds to a mycobacterial concentration of 3 × 108 CFU/ml. The culture was serially 10-fold diluted with MGIT medium, and 100-μl portions of the dilutions were assayed using the ESAT-6/CFP-10 ICA. The minimal concentration of MTC in the BACTEC culture for identification by ESAT-6/CFP-10 ICA was found to be between 3 × 104 and 3 × 105 CFU/ml.
DISCUSSION
Early diagnosis and early treatment are the most important policies for TB control. However, treatment delay or management delay has frequently been mentioned. In most cases, the prolonged time for identification of MTC in the suspected specimens is the bottleneck for such delays. Even though MTC may already be highly suspected in some cases, doctors must still wait for final culture and identification results to decide whether to proceed with antibiotic treatment (12). It is vitally important to reduce the turnaround time of culturing and identification based on the WHO's recommendation.
ESAT-6 and CFP-10 are secreted in the early growth stage of MTC. A combination of these two molecules provides specific and sensitive targets for the detection of MTC infection. ESAT-6 and CFP-10 naturally form a tight 1:1 complex, and both proteins adapt to form a stable, fully folded structure as a heterodimer (18). ESAT-6/CFP-10 has been used for vaccines, skin tests, and the gamma interferon release assay (IGRA) (2, 13, 24). In our study, we used a monoclonal antibody of ESAT-6/CFP-10 on a strip to detect the early secretory antigens ESAT-6 or CFP-10 in the culture of specimens. The MGIT culture tube inoculated with mycobacterium-containing specimens would be reported positive rapidly (5 to 14 days) in the BACTEC MGIT 960 system. After the positive signals appeared, 3 days of further incubation in the system provided more secretory MPB64 or ESAT-6/CFP-10 (7). Our results indicated that this additional 3-day incubation allowed clear positive bands in the ESAT-6/CFP-10 ICA with the MTC-containing cultures, and false-negatives were therefore reduced (Table 2). Although longer incubations further reduced the number of false-negatives compared to the initial strip test, the number of false-positives increased, mostly from cultures containing M. kansasii.
There are 23 ESAT-6 family member genes, including esat-6, in M. tuberculosis. Like esat-6, the 21 other family member genes form a gene pair with the CFP-10 family member genes and the proteins are elaborated as CFP-10-like protein/ESAT-6-like protein (3, 10, 11). CFP-10 and the CFP-10-like proteins show more than 90% amino acid sequence identity, similar to ESAT-6 and ESAT-6-like proteins. In addition to ESAT-6 and CFP-10, at least three ESAT-6-like proteins and five CFP-10-like proteins have been found in culture supernatants of M. tuberculosis (3, 11), which might contribute to the sensitivity of the ESAT-6/CFP-10 ICA.
The sensitivity and specificity of the ESAT-6/CFP-10 ICA for identification of MTC in the positive BACTEC cultures were 97 and 97.4%, respectively, similar to those of the Capilia assay (96.9 and 98.6%, respectively [19]). When the ESAT-6/CFP-10 ICA was combined with cord morphology, the specificity and PPV increased to 100% (Table 2). In a previous study, we combined cord morphology with the Capilia assay for identification of MTC in the MGIT tube and also obtained 100% specificity and PPV (19). Thus, BACTEC cultures that showed cord morphology in cultural smears and were ESAT-6/CFP-10 ICA positive can be reported to contain MTC directly. In BACTEC cultures that were either negative with both assays or positive with one assay and negative with the other assay, the cultures could contain MTC, NTM, or Nocardia spp. Further evaluations, such as traditional biochemical methods, hsp65 PRA, 16S rRNA gene sequencing, or the Capilia assay, were required to determine whether or not the culture contained MTC (Fig. 2).
The cost of identification with the ESAT-6/CFP-10 ICA (US $4) is less than that with the Capilia assay ($10), AccuProbe ($19), hsp65 or 16S rRNA gene sequencing ($33), or a conventional biochemical test panel ($99). Identification using a conventional biochemical test panel usually takes 26 days on average, whereas identification by the other four methods only takes 3 days or less. The ESAT-6/CFP-10 ICA and Capilia assay are technically the least challenging to perform (14). The ESAT-6/CFP-10 ICA is slower than the other three molecular methods by 2 to 3 days. The extra 3-day incubation in the BACTEC MGIT 960 system is a limitation of the ESAT-6/CFP-10 ICA.
Another limitation of the ESAT-6/CFP-10 ICA is its cross-reactivity with some NTM-containing cultures. Genes coding for ESAT-6-like proteins and CFP-10-like proteins were demonstrated in M. kansasii, M. marinum, M. szulgai, M. flavescens, M. gastri, and M. smegmatis (22). According to phylogenetic trees based on sequence alignment of the esat-6 and cfp-10 homologs, M. kansasii subtypes had distinct sequences compared to the esat-6 and cfp-10 sequences of M. tuberculosis, but M. marinum and M. szulgai had more related sequences. Among the 17 cultures containing M. kansasii, only 1 culture was positive in the initial strip test, and it was identified to contain M. kansasii type 6. Nine and seven cultures containing M. kansasii type 1 and M. kansasii type 2, respectively, were initially ICA negative but were positive in the repeat assay. This was also true of the one culture containing M. szulgai. This result means that although these M. kansasii type 1 and type 2 and M. szulgai strains might secrete ESAT-6-like proteins and CFP-10-like proteins, they did not perturb the initial ICA results but did perturb the repeat ICA results. Homologs of esat-6 and cfp-10 have not previously been reported in M. gordonae, but in the present study two cultures containing M. gordonae type 3 were positive in the ESAT-6/CFP-10 ICA. One culture was positive in the initial and the repeat tests, and the other was only positive in the repeat test. Two, one, and one culture that contained M. intracellulare, M. fortuitum, and M. abscessus, respectively, were positive in the initial ICA but negative in the repeat ICA. This might be due to the initial interpretation of a suspected weak positive.
In conclusion, the ESAT-6/CFP-10 ICA can be used for the identification of MTC in BACTEC cultures. The ICA is rapid, easy to perform, and cost-effective. This test could shorten the turnaround time of MTC identification and avoid the complexities of nucleic acid amplification-based identification. However, the ICA is slower than other molecular methods by 2 to 3 days, which is the major limitation of the method. Combination with cord morphology in the cultural smear could increase specificity and avoid false-positives.
ACKNOWLEDGMENTS
This study was supported by grants from the National Science Foundation (NSC-94-2311-B005-012), the Centers for Disease Control (DOH-95-DC-1106), and the Taichung Veterans General Hospital (TCVGH-973202A) of Taiwan, Republic of China.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1. Bakshi C. S., Shah D. H., Verma R., Singh R. K., Malik M. 2005. Rapid differentiation of Mycobacterium bovis and Mycobacterium tuberculosis based on a 12.7-kb fragment by a single tube multiplex-PCR. Vet. Microbiol. 109:211–216 [DOI] [PubMed] [Google Scholar]
- 2. Bua A., et al. 2007. QuantiFERON TB Gold: a new method for latent tuberculosis infection. New Microbiol. 30:477–480 [PubMed] [Google Scholar]
- 3. Champion P. A., Stanley S. A., Champion M. M., Brown E. J., Cox J. S. 2006. C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 313:1632–1636 [DOI] [PubMed] [Google Scholar]
- 4. Chen D. Y., Shen G. H., Hsieh T. Y., Hsieh C. W., Lan J. L. 2008. Effectiveness of the combination of a whole-blood interferon-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum. 59:800–806 [DOI] [PubMed] [Google Scholar]
- 5. Dheda K., Smit R. Z., Badri M., Pai M. 2009. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden versus low-burden settings. Curr. Opin. Pulm. Med. 15:188–200 [DOI] [PubMed] [Google Scholar]
- 6. Hanna A. B. 1996. Diagnosis of tuberculosis by microbiologic techniques, p. 149–159 In Rom W. N., Garay S. M., Bloom B. R. (ed.), Tuberculosis. Little, Brown, and Company, Boston, MA [Google Scholar]
- 7. Hasegawa N., et al. 2002. New simple and rapid test for culture confirmation of Mycobacterium tuberculosis complex: a multicenter study. J. Clin. Microbiol. 40:908–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan I. U., Yadav J. S. 2004. Development of a single-tube, cell lysis-based, genus-specific PCR method for rapid identification of mycobacteria: optimization of cell lysis, PCR primers and conditions, and restriction pattern analysis. J. Clin. Microbiol. 42:453–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirano K., Aono A., Takahashi M., Abe C. 2004. Mutations including IS6110 insertion in the gene encoding the MPB64 protein of Capilia TB-negative Mycobacterium tuberculosis isolates. J. Clin. Microbiol. 42:390–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lightbody K. L., et al. 2008. Molecular features governing the stability and specificity of functional complex formation by Mycobacterium tuberculosis CFP-10/ESAT-6 family proteins. J. Biol. Chem. 283:17681–17690 [DOI] [PubMed] [Google Scholar]
- 11. Lightbody K. L., et al. 2004. Characterisation of complex formation between members of the Mycobacterium tuberculosis complex CFP-10/ESAT-6 protein family: toward an understanding of the rules governing complex formation and thereby functional flexibility. FEMS Microbiol. Lett. 238:255–262 [DOI] [PubMed] [Google Scholar]
- 12. Lin H. P., Deng C. Y., Chou P. 2009. Diagnosis and treatment delay among pulmonary tuberculosis patients identified using the Taiwan reporting enquiry system, 2002–2006. BMC Public Health 9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maue A. C., et al. 2007. An ESAT-6:CFP-10 DNA vaccine administered in conjunction with Mycobacterium bovis BCG confers protection to cattle challenged with virulent M. bovis. Vaccine 25:4735–4746 [DOI] [PubMed] [Google Scholar]
- 14. McNabb A., Adie K., Rodrigues M., Black W. A., Isaac-Renton J. 2006. Direct identification of mycobacteria in primary liquid detection media by partial sequencing of the 65-kilodalton heat shock protein gene. J. Clin. Microbiol. 44:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagai S., Wiker H. G., Harboe M., Kinomoto M. 1991. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect. Immun. 59:372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nyendak M. R., Lewinsohn D. A., Lewinsohn D. M. 2009. New diagnostic methods for tuberculosis. Curr. Opin. Infect. Dis. 22:174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ongut G., et al. 2006. Evaluation of the ICT Tuberculosis test for the routine diagnosis of tuberculosis. BMC Infect. Dis. 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Renshaw P. S., et al. 2002. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex: implications for pathogenesis and virulence. J. Biol. Chem. 277:21598–21603 [DOI] [PubMed] [Google Scholar]
- 19. Shen G. H., et al. 2009. Combining the Capilia TB assay with smear morphology for the identification of Mycobacterium tuberculosis complex. Int. J. Tuberc. Lung Dis. 13:371–376 [PubMed] [Google Scholar]
- 20. Telenti A., et al. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turenne C. Y., Tschetter L., Wolfe J., Kabani A. 2001. Necessity of quality-controlled 16S rRNA gene sequence databases: identifying nontuberculous Mycobacterium species. J. Clin. Microbiol. 39:3637–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Ingen J., de Zwaan R., Dekhuijzen R., Boeree M., van Soolingen D. 2009. Region of difference 1 in nontuberculous Mycobacterium species adds a phylogenetic and taxonomical character. J. Bacteriol. 191:5865–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J. Y., et al. 2007. Performance assessment of the Capilia TB assay and the BD ProbeTec ET system for rapid culture confirmation of Mycobacterium tuberculosis. Diagn. Microbiol. Infect. Dis. 59:395–399 [DOI] [PubMed] [Google Scholar]
- 24. Weldingh K., Andersen P. 2008. ESAT-6/CFP10 skin test predicts disease in Mycobacterium tuberculosis-infected guinea pigs. PLoS One 3:e1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Witebsky F. G., Kruczak-Filipov P. 1996. Identification of mycobacteria by conventional methods. Clin. Lab. Med. 16:569–601 [PubMed] [Google Scholar]