Abstract
Children attending child care centers (CCCs) are at increased risk for infections, including those caused by methicillin-resistant Staphylococcus aureus (MRSA). Nasal colonization often precedes infection, and MRSA colonization has been associated with increased infection risk. Community-associated MRSA (CA-MRSA) has caused increased MRSA infections in the general population, including children. Little is known about the frequency of MRSA nasal colonization in young children, particularly in those attending CCCs where disease transmission is common. We sampled the nares of 1,163 children in 200 classrooms from 24 CCCs in North Carolina and Virginia to assess S. aureus colonization. MRSA strains were molecularly analyzed for staphylococcal cassette chromosome mec (SCCmec) type, Panton-Valentine leukocidin status, and multilocus sequence type. A case-control study was performed to identify risk factors for MRSA colonization. We found that 18.1% children were colonized with S. aureus and 1.3% with MRSA. Molecular analysis of the MRSA strains identified 47% as CA-MRSA and 53% as health care-associated MRSA (HA-MRSA). Although two centers had multiple children colonized with MRSA, genotyping indicated that no transmission had occurred within classrooms. The case-control study did not detect statistically significant risk factors for MRSA colonization. However, MRSA-colonized children were more likely to be nonwhite and to have increased exposure to antibiotics and skin infections in the home. Both CA-MRSA and HA-MRSA strains were found colonizing the nares of children attending CCCs. The low frequency of colonization observed highlights the need for a large multicenter study to determine risk factors for MRSA colonization and subsequent infection in this highly susceptible population.
INTRODUCTION
Staphylococcus aureus is a common cause of serious community- and health care-associated infections. The numbers of both community-associated and health care-associated staphylococcal infections have increased in recent decades (25). Methicillin was introduced into clinical use in 1960, and this introduction was closely followed by the first reports of methicillin-resistant S. aureus (MRSA), with their resistance arising through the production of a supplementary penicillin-binding protein (PBP) known as PBP2a or PBP2′ (40). The prevalence of health care-associated MRSA (HA-MRSA) infection has increased dramatically since the mid-1980s (2). In 1974, MRSA infections accounted for 2% of the total number of S. aureus infections; in 1995, it was 22%, and in 2004, it was 63% (4).
Beginning in the early 1990s in the United States, case reports and case series documented the increasing problem of community-associated MRSA (CA-MRSA). At-risk populations for CA-MRSA have included children, athletes, injection drug users, military personnel, persons living in correctional facilities or shelters, African-Americans, and veterinarians (2). Published reports using both population-based surveillance (29) and laboratory-based surveillance (12) have documented that CA-MRSA infections are more frequent in children, especially children less than 2 years of age.
Approximately one-third of healthy persons harbor S. aureus in their nose at any time (14, 21, 22). The nose appears to be the primary reservoir for replication and spread to other body areas (26). The fact that colonization precedes infection is supported by studies that have demonstrated that nasal S. aureus isolates are often identical to strains later causing clinical infection (38, 39) and that when colonization is eradicated the risk of clinical infection is reduced (21). The frequency of nasal colonization with MRSA has been less well described, but in general it has varied from approximately 1 to 12% (1, 14, 22). However, colonization with MRSA as opposed to methicillin-sensitive S. aureus (MSSA) has been associated with a 4-fold increase in the risk of infection (35).
In the United States in the summer of 2006, approximately 32.2% of the 19 million children younger than 5 years of age attended a child care center (CCC) or other organized out-of-home care on a regular basis (37). Of these, approximately 36% attend CCCs, defined as an arrangement where, at any one time, there are three or more unrelated preschool-age children receiving care for more than 4 h a day (37). Children attending child care centers have an increased risk for a variety of infections (6, 28) because they are immunologically naïve and vulnerable to infection. Complicating the issue is their tendency to contaminate the environment with respiratory tract secretions, urine, and feces, all of which can spread disease.
Given that young children are at higher risk for CA-MRSA and that attendance in a CCC is a well-described risk factor for respiratory, skin, and gastrointestinal infections, we undertook the following study of a large sample of children attending CCCs in North Carolina and Virginia. The goals of our study were to define the prevalence of MSSA and MRSA nasal colonization, microbiologically and molecularly characterize all isolated strains of MRSA, and perform a case-control study (in which the cases were children colonized with MRSA and the controls were noncolonized children and children colonized with MSSA) to assess risk factors for MRSA colonization.
MATERIALS AND METHODS
Study design.
This study was conducted between March 2007 and October 2009. In North Carolina, CCCs were eligible for participation if they were in New Hanover County and had access to a Child Care Health Consultant or public health nurse. The 17 centers recruited in North Carolina by the Child Care Health Consultants were based on their previous working relationship with the county health department. In Virginia, all Navy CCCs in the Hampton Roads area were eligible and participated. The inclusion criteria for subjects were as follows: child is enrolled in a designated CCC, child's age is between 0 and 5 years, family plans to remain in the CCC throughout the academic year, guardian signs informed consent and agrees to participate (if selected) in case-control questionnaire by telephone, and family is English speaking. Colonization was assessed by use of a nasal swab (see below) obtained by a study nurse or physician. Demographic information on North Carolina subjects was available from classroom lists provided by the CCC director but was not obtained in Virginia due to Navy-specific Institutional Review Board (IRB) requirements. Classroom- and CCC-level data were provided by the CCC director. Star-rated licenses ranging from one star (meets minimum qualification) to five stars (exceeds minimum qualifications) were available for North Carolina CCCs. Star ratings reflect indicators of a program's quality based on an objective evaluation of program standards and staff education (31). All military CCCs in Virginia are accredited by the National Association of Education of Young Children; this accreditation corresponds to the highest star rating. Additional demographic information and risk factors for staphylococcal colonization used in the case-control study were obtained via a telephone questionnaire of the child's primary caregiver.
All subjects from whom a culture was obtained were used in the analysis to determine the prevalence of colonization by MRSA and MSSA. An evaluation of risk factors for MRSA colonization was assessed by means of a case-control study. Cases consisted of all children colonized with MRSA. Two groups of controls were used: non-staphylococcally colonized children (control group 1) and children colonized with MSSA (control group 2). The ratio of cases to controls (cases/control group 1/control group 2) was 1:2:2. Controls were chosen using a random number generator from all eligible subjects in each control group.
Consent for CCC participation was obtained from the CCC director. Consent for subject participation was obtained from the child's guardian. This study was approved by the IRB of the University of North Carolina at Chapel Hill and the Naval Medical Center Portsmouth IRB.
Microbiology.
Nasal swabs were collected by swabbing both nares using a Copan (Brescia, Italy) liquid Stuart minitip transport swab. Swabs were shipped at ambient temperature via overnight Federal Express to the University of North Carolina at Chapel Hill for analysis. The swabs were plated to sheep blood agar and mannitol salt agar plates. Isolates were identified as S. aureus based on a positive tube coagulase test or a positive BactiStaph (Remel, Lenexa, KS) latex agglutination test. S. aureus isolates were identified as MRSA using oxacillin screening agar (Remel) and confirmed using cefoxitin disk diffusion (Becton Dickinson, Franklin Lakes, NJ). Susceptibilities of MRSA strains were tested using Kirby-Bauer disk diffusion for all drugs except daptomycin and vancomycin, which were assessed by Etests (AB bioMérieux, Sweden). All interpretations were based on CLSI guidelines (7). Antibiotics tested included clindamycin, daptomycin, doxycycline, erythromycin, gentamicin, levofloxacin, linezolid, trimethoprim-sulfamethoxazole, and vancomycin. The D-test was performed to detect inducible clindamycin resistance; D-test-positive organisms were reported as clindamycin resistant (19).
Molecular analyses.
DNA extraction was performed as previously described (34). The presence of Panton-Valentine leukocidin was assessed using real-time multiplex PCR assays as previously described (13, 30). Staphylococcal cassette chromosome mec (SCCmec) typing was performed by the method of Oliveira et al. (32). SCCmec typing classified as indeterminate by the Oliveira et al. method was performed as described by Boye et al. (3). Multilocus sequence typing (MLST) was performed and analyzed as outlined by Enright et al. (10, 11). MRSA strains were defined as health care associated or community associated based on MLST type cluster analysis along with SCCmec type.
Statistical analyses.
All data were entered into a Microsoft Access database. All analyses were performed using SAS 9.1 (SAS, Cary, NC). Descriptive measures were compared between the North Carolina and Virginia sites to evaluate differences between the CCCs in the two states. Logistic mixed models were fitted at the child level to test for relationships between positive swab status for MRSA and MSSA and six child-, classroom-, and center-level variables, using GLIMMIX. A total of 12 models were therefore fit. Odds ratios (OR), 95% confidence intervals, and P values were calculated. Multivariable models were not fit because of missing data which would have resulted in listwise deletion of all cases that contained any missing data. In the case-control study predictor, variables analyzed included hours per week in a CCC, playing sports, underlying diseases, chronic skin conditions, previous staphylococcal infections, pet(s) in the home, military persons or health care workers in the home, ethnicity, and household income (see Table 4 for complete list). Logistic regression models were fit for binary (i.e., yes/no) variables, and linear regression models were fit for scale or continuous variables. F-tests (two degrees of freedom [d.f.]) or χ2 tests were used to test for group differences.
Table 4.
Case-control study
| Parameter | P | Result for group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (control) |
MSSA |
MRSA |
|||||||||||
| n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | ||
| Age (yr) | 0.37 | 30 | 4.5 | 1.3 | 30 | 5 | 1.6 | 15 | 4.5 | 1.4 | |||
| Males | 0.65 | 14 | 47 | 14 | 47 | 9 | 60 | ||||||
| Time (h/day) in child care centera | 0.68 | 16 | 34.4 | 11.2 | 10 | 34.7 | 11.8 | 9 | 30.2 | 15.5 | |||
| Sport participation (h/wk) | 0.66 | 30 | 1 | 1.6 | 30 | 1.2 | 1.8 | 15 | 0.7 | 1.7 | |||
| Underlying disease | 0.47 | 2 | 7 | 1 | 3 | 2 | 13 | ||||||
| Hospitalization | 1.00 | 4 | 13 | 4 | 13 | 2 | 13 | ||||||
| Antibiotics | 0.09 | 20 | 67 | 25 | 83 | 14 | 93 | ||||||
| Chronic skin condition | 0.10 | 2 | 7 | 7 | 23 | 3 | 20 | ||||||
| Skin infections | 0.37 | 10 | 33 | 13 | 43 | 8 | 53 | ||||||
| Eczema | 0.62 | 6 | 20 | 7 | 23 | 5 | 33 | ||||||
| History of staphylococci | 0.11 | 3 | 10 | 0 | 0 | 1 | 7 | ||||||
| Pet(s) (cat, dog, or rodent) | 0.23 | 18 | 60 | 23 | 77 | 11 | 73 | ||||||
| Single-family home | 0.84 | 26 | 87 | 25 | 83 | 12 | 80 | ||||||
| Home on military base or military person in home | 0.83 | 10 | 33 | 8 | 27 | 4 | 27 | ||||||
| Health care worker in home | 0.06 | 11 | 37 | 3 | 10 | 2 | 13 | ||||||
| Skin infections in home | 0.13 | 5 | 17 | 7 | 23 | 7 | 47 | ||||||
| No. of people in household | 0.71 | 30 | 4 | 1 | 30 | 3.8 | 1.1 | 15 | 4.1 | 1.0 | |||
| <12 yr old | 30 | 1.9 | 0.8 | 30 | 1.7 | 0.6 | 15 | 1.7 | 0.5 | ||||
| 12–18 yr old | 30 | 0.2 | 0.5 | 30 | 0.3 | 0.7 | 15 | 0.3 | 0.6 | ||||
| >18 yr old | 30 | 2 | 0.3 | 30 | 1.9 | 0.4 | 15 | 2.1 | 0.7 | ||||
| No. of rooms in home | 0.45 | 30 | 7.5 | 1.7 | 30 | 7.3 | 2.2 | 15 | 6.7 | 2.2 | |||
| Ethnicity | 0.07 | ||||||||||||
| White | 24 | 80 | 27 | 90 | 9 | 60 | |||||||
| Nonwhite | 6 | 20 | 3 | 10 | 6 | 40 | |||||||
| Household income | 0.61 | ||||||||||||
| <$50,000 | 5 | 17 | 7 | 23 | 5 | 33 | |||||||
| $50,001–$75,000 | 9 | 30 | 7 | 23 | 5 | 33 | |||||||
| $75,001–$100,000 | 11 | 37 | 7 | 23 | 1 | 7 | |||||||
| >$100,000 | 5 | 17 | 9 | 30 | 4 | 27 | |||||||
Number of hours at the time of case-control questionnaire.
RESULTS
Subject and child care center demographics.
The study population consisted of 1,163 subjects (811 in North Carolina and 352 in Virginia), from 200 classrooms (131 in North Carolina and 69 in Virginia) distributed among 24 centers (17 in North Carolina and 7 in Virginia), who had nasal cultures. Approximately 40% of children participated, with individual center participation ranging from 20% to 58%. Of note, some guardians did not consent for their child's participation because the child was already known to be positive for MRSA. The mean age of children in North Carolina was 3.3 years (standard deviation [SD], 1.4). Overall, gender distribution was similar for North Carolina (51% male) and Virginia (45% male).
Center-level and classroom-level data for the subjects are displayed in Table 1. Center-level data include number of classrooms per center, center type, percentage of children in center financially subsidized, and star rating for North Carolina CCCs. Since the Virginia CCCs were all Navy associated, they were all government-based centers, while North Carolina centers were a range of center types. The Virginia CCCs also had a higher percentage of subjects subsidized (mean, 29%; SD, 49%) than did North Carolina CCCs (mean, 18%; SD, 19%). Classroom-level data, including number of children in classroom by age group and child-to-teacher ratio by age group, were similar for North Carolina and Virginia classrooms.
Table 1.
Subject and child care center demographicsa
| Parameter | Result for location |
Result for both locations |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North Carolina |
Virginia |
||||||||||||||
| n | Mean | SD | Min | Max | n | Mean | SD | Min | Max | n | Mean | SD | Min | Max | |
| Age (yr) | 549 | 3.3 | 1.4 | <1 | 5 | 549 | 3.3 | 1.4 | <1 | 5 | |||||
| Gender | |||||||||||||||
| Male | 414 | 160 | 574 | ||||||||||||
| Female | 386 | 153 | 539 | ||||||||||||
| Unknown | 11 | 39 | 50 | ||||||||||||
| Avg no. of classrooms/center | 17 | 7.7 | 2.8 | 4 | 13 | 7 | 9.9 | 6.9 | 1 | 21 | 24 | 8.3 | 4.4 | 21 | |
| Center type | |||||||||||||||
| Church operated | 3 | 0 | 3 | ||||||||||||
| Government | 0 | 7 | 7 | ||||||||||||
| Other nonprofit | 2 | 0 | 2 | ||||||||||||
| Profit, franchise | 2 | 0 | 2 | ||||||||||||
| Profit, independent | 9 | 0 | 9 | ||||||||||||
| Work site, employer provided | 1 | 0 | 1 | ||||||||||||
| Star rating | 14 | 3.9 | 1.1 | 1 | 5 | 14 | 3.9 | 1.1 | 1 | 5 | |||||
| Subsidized children/center | 17 | 18% | 19% | 0% | 56% | 7 | 29% | 49% | 0% | 100% | 24 | 21% | 30% | 0% | 100% |
| No. children/classroom | |||||||||||||||
| Birth to 12 mo | 109 | 9.2 | 1.7 | 4 | 12 | 30 | 8.3 | 3.0 | 4 | 16 | 139 | 8.1 | 2.7 | 4 | 16 |
| 1 to 2 yr | 97 | 10.5 | 2.9 | 5 | 18 | 61 | 10.9 | 2.3 | 10 | 20 | 158 | 10.5 | 3.1 | 5 | 20 |
| 2 to 3 yr | 140 | 14.9 | 3.9 | 8 | 20 | 77 | 14.7 | 3.6 | 10 | 21 | 217 | 13.9 | 3.3 | 8 | 21 |
| 2 to 4 yr | 12 | 26.0 | 0.0 | 26 | 26 | 12 | 26.0 | 0.0 | 26 | 26 | |||||
| 3 to 4 yr | 137 | 16.7 | 4.5 | 10 | 25 | 37 | 21.0 | 2.0 | 18 | 24 | 174 | 16.6 | 4.7 | 10 | 25 |
| 3 to 5 yr | 98 | 22.4 | 2.6 | 16 | 24 | 98 | 20.9 | 4.2 | 12 | 24 | |||||
| 4 to 5 yr | 161 | 19.8 | 6.7 | 10 | 34 | 19 | 24.0 | 0.0 | 24 | 24 | 180 | 17.8 | 6.3 | 9 | 34 |
| 5 to 6 yr | 29 | 18.3 | 5.6 | 12 | 27 | 29 | 17.2 | 5.1 | 12 | 27 | |||||
| Child/teacher ratio | |||||||||||||||
| Birth to 12 mo | 109 | 4.5 | 0.7 | 3 | 5 | 30 | 4.0 | 0.0 | 4 | 4 | 139 | 4.4 | 0.7 | 3 | 5 |
| 1 to 2 yr | 97 | 5.5 | 1.2 | 4 | 9 | 37 | 5.0 | 0.0 | 5 | 5 | 134 | 5.3 | 1.0 | 4 | 9 |
| 2 to 3 yr | 76 | 7.8 | 1.3 | 6 | 9 | 77 | 6.6 | 0.8 | 5 | 7 | 153 | 7.2 | 1.2 | 5 | 9 |
| 2 to 4 yr | 12 | 9.0 | 0.0 | 9 | 9 | 12 | 9.0 | 0.0 | 9 | 9 | |||||
| 3 to 4 yr | 137 | 9.3 | 1.9 | 7 | 17 | 37 | 12.0 | 0.0 | 12 | 12 | 174 | 9.9 | 2.0 | 7 | 17 |
| 3 to 5 yr | 98 | 10.9 | 3.1 | 5 | 14 | 98 | 10.9 | 3.1 | 5 | 14 | |||||
| 4 to 5 yr | 172 | 11.1 | 2.9 | 6 | 17 | 19 | 12.0 | 0.0 | 12 | 12 | 191 | 11.2 | 2.7 | 6 | 17 |
| 5 to 6 yr | 29 | 11.5 | 3.3 | 8 | 15 | 29 | 11.5 | 3.3 | 8 | 15 | |||||
Min, minimum; Max, maximum.
MRSA prevalence.
Overall, 210 children (18.1%) were colonized with S. aureus. Of these, 195 (16.8% of total) were colonized with MSSA while 15 (1.3% of total) were colonized with MRSA (Table 2). The proportions were not different between North Carolina (MSSA, 16.8%; MRSA, 1.4%) and Virginia (MSSA, 16.8%; MRSA, 1.1%) subjects.
Table 2.
Prevalence studya
| Parameter | Result for group |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (control) |
MSSA |
MRSA |
|||||||||||||||||
| n | Mean | SD | Min | Max | n | Mean | SD | Min | Max | OR (95% CI) | P | n | Mean | SD | Min | Max | OR (95% CI) | P | |
| Location of study | 1.03 (0.65, 1.62) | 0.9 | 0.74 (0.14, 3.86) | 0.73 | |||||||||||||||
| NC | 664 | 136 | 11 | ||||||||||||||||
| VA | 289 | 59 | 4 | ||||||||||||||||
| Total | 953 | 195 | 15 | ||||||||||||||||
| Age (yr) | 470 | 3.1 | 1.4 | <1 | 5.8 | 85 | 3.9 | 1.5 | <1 | 5.8 | 1.48 (1.22, 1.78) | <0.001 | 15 | 3.3 | 1.3 | 1 | 4.8 | 1.04 (0.72, 1.50) | 0.82 |
| Gender | 0.94 (0.68, 1.30) | 0.71 | 1.41 (0.50, 3.98) | 0.52 | |||||||||||||||
| Male | 472 | 93 | 9 | ||||||||||||||||
| Female | 443 | 90 | 6 | ||||||||||||||||
| Star rating | 570 | 3.9 | 1.1 | 1 | 5 | 101 | 4.1 | 1 | 1 | 5 | 1.17 (0.88, 1.54) | 0.3 | 10 | 3.4 | 1.1 | 1 | 5 | 0.62 (0.28, 1.40) | 0.27 |
| Subsidized children/center | 953 | 20% | 31% | 0% | 100% | 195 | 21% | 33% | 0% | 100% | 1.11 (0.59, 2.14) | 0.75 | 15 | 39% | 36% | 0% | 100% | 4.76 (0.61, 37.0) | 0.15 |
| No. children/classroom | 856 | 15.4 | 6.1 | 4 | 34 | 167 | 16.6 | 6.4 | 4 | 34 | 1.02 (0.99, 1.04) | 0.14 | 15 | 16.3 | 5.2 | 9 | 26 | 1.06 (0.97, 1.16) | 0.22 |
| Child/teacher ratio, by age | 0.93 (0.86, 1.01) | 0.08 | 1.10 (0.85, 1.42) | 0.48 | |||||||||||||||
| Birth to 12 months | 122 | 4.4 | 0.7 | 3 | 5 | 16 | 4.5 | 0.6 | 3 | 5 | 1 | 5.0 | 0.0 | 5 | 5 | ||||
| 1 to 2 yr | 118 | 5.4 | 1.1 | 4 | 9 | 13 | 5.2 | 0.4 | 5 | 6 | 3 | 5.7 | 0.6 | 5 | 6 | ||||
| 2 to 3 yr | 135 | 7.3 | 1.2 | 5 | 9 | 17 | 6.8 | 0.8 | 5 | 8 | 1 | 9.0 | 0.0 | 9 | 9 | ||||
| 2 to 4 yr | 10 | 9.0 | 0.0 | 9 | 9 | 2 | 9.0 | 0.0 | 9 | 9 | |||||||||
| 3 to 4 yr | 140 | 10.0 | 2.1 | 7 | 17 | 31 | 9.1 | 1.4 | 7 | 12 | 3 | 10.7 | 1.2 | 10 | 12 | ||||
| 3 to 5 yr | 78 | 10.9 | 3 | 5 | 14 | 19 | 11.2 | 3.1 | 5 | 14 | 1 | 5.0 | 0.0 | 5 | 5 | ||||
| 4 to 5 yr | 139 | 11.3 | 2.7 | 6 | 17 | 48 | 10.8 | 2.9 | 6 | 17 | 4 | 12.5 | 0.6 | 12 | 13 | ||||
| 5 to 6 yr | 17 | 11.7 | 3.3 | 8 | 15 | 12 | 11.2 | 3.4 | 8 | 15 | |||||||||
Min, minimum; Max, maximum; CI, confidence interval.
MRSA was isolated from children attending 10 different CCCs. Eight centers had one positive child each, one center (A) had four positive children, and one center (B) had three positive children. Center A children were found in two classrooms (two children each), and center B positivity involved three different classrooms (one child each). However, no transmission within classrooms was documented (see below).
Analysis of center-level and classroom-level variables based on subject colonization status (i.e., noncolonized, MSSA colonized, and MRSA colonized) is shown in Table 2. MSSA-colonized subjects were older (P < 0.001). However, there was no other statistically significant characteristic associated with MRSA or MSSA colonization. Although not reaching statistical significance, the child-to-teacher ratio of the MSSA-colonized classrooms was lower (P = 0.08), but the number of children in the classrooms of MSSA-colonized children (mean, 16.3; SD, 6.4) was higher (P = 0.14) than the number of children in classrooms of noncolonized children (mean, 15.4; SD, 6.1). The number of children in MRSA-colonized classrooms was higher as well (mean, 16.3; SD, 5.2). The MRSA-colonized children more often came from centers with a higher percentage of subsidized children (MRSA, 39%; MSSA, 21%; noncolonized, 20%), but this did not reach statistical significance (OR, 4.76, P = 0.15).
MRSA molecular analyses and antibiotic susceptibility patterns.
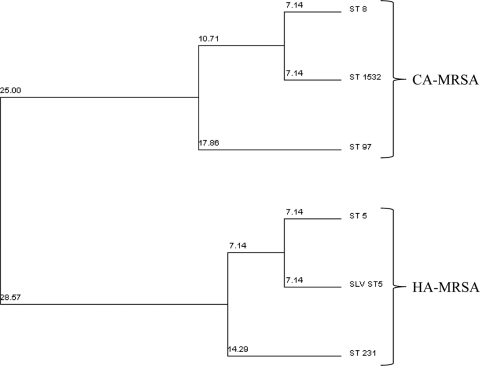
The 15 MRSA isolates were analyzed for SCCmec type, Panton-Valentine leukocidin (PVL) status, antibiotic susceptibilities, and multilocus sequence type (Table 3). The sequence type determined by MLST was used to define strains as either CA-MRSA or HA-MRSA based on their clonal complex (CC). Of the 15 MRSA strains, 7 (47%) were CA-MRSA (CC8, n = 6, and CC97, n = 1) and 8 (53%) were HA-MRSA (CC5). The five CC8 CA-MRSA isolates were PVL positive and SCCmec type IV, and the CC97 isolate was PVL negative and SCCmec type IV. The eight CC5 HA-MRSA isolates were PVL negative (n = 8) and SCCmec types II (n = 2) and IV (n = 6). A dendrogram showing the relatedness of the MRSA strains and their corresponding identification as CA-MRSA or HA-MRSA is shown in Fig. 1. MLST also revealed that none of the MRSA isolates obtained from children in the same classroom were identical.
Table 3.
Microbiological and molecular analyses of MRSA isolates
| Parameter | Result |
|---|---|
| No. of MRSA isolates | 15 |
| Percent susceptible | |
| Clindamycin | 67 |
| Erythromycin | 20 |
| Levofloxacin | 27 |
| No. with SCCmec type | |
| II | 2 |
| IV | 13 |
| No. with PVL result | |
| Positive | 6 |
| Negative | 9 |
| No. with sequence type (clonal complex) | |
| ST5 (CC5) | 6 |
| ST8 (CC8) | 5 |
| ST1532 (CC5) | 1 |
| ST231 (CC5) | 1 |
| ST97 (CC97) | 1 |
| SLVa ST5 (CC5) | 1 |
SLV, single-locus variant.
Fig. 1.
Cluster analysis of MRSA isolates. Shown is a dendrogram displaying the relatedness of MRSA strains isolated from children attending childcare centers. Two clusters are evident that separate CA-MRSA isolates from HA-MRSA isolates. ST, sequence type; SLV, single locus variant.
All 15 MRSA strains were uniformly susceptible to daptomycin, doxycycline, gentamicin, linezolid, trimethoprim-sulfamethoxazole, and vancomycin. The various susceptibilities to clindamycin, erythromycin, and levofloxacin are shown in Table 3.
Case-control study.
The case-control study did not reveal any statistically significant differences between the MRSA-colonized or MSSA-colonized children and the noncolonized children (Table 4). Interestingly, there was no increased risk for MRSA colonization associated with living on a military base or having a military person or health care worker in the home. However, children who were MRSA positive were 20 to 30% more likely to be nonwhite than those in the control groups (MRSA, 40%; MSSA, 10%; noncolonized, 20%), but this difference was not statistically significant (P = 0.07). MRSA-colonized children reported a history of antibiotic use 10 to 26% more frequently than those in the control groups (MRSA, 93%; MSSA, 83%; noncolonized, 67%; P = 0.09) and were 24 to 30% more likely to have skin infections in the home (MRSA, 47%; MSSA, 23%; noncolonized, 17%; P = 0.13). Of the eight MRSA-colonized subjects that had a history of skin infections, half were colonized with CA-MRSA and half with HA-MRSA.
DISCUSSION
S. aureus is a human pathogen which primarily colonizes the nose (25). In the past few decades, methicillin-resistant strains have come to predominate in both health care-associated and community-associated S. aureus infections (40). However, the epidemiology and microbiology of CA-MRSA and HA-MRSA strains have differed (8). CA-MRSA has involved younger patients and caused predominantly skin and soft tissue infections, while HA-MRSA has involved older patients who have typical health care-associated risk factors (e.g., indwelling catheter or percutaneous device). CA-MRSA strains have most commonly been SCCmec type IV and Panton-Valentine leukocidin positive, while HA-MRSA strains have most commonly been SCCmec types I, II, and III and Panton-Valentine leukocidin negative. However, recent studies describing CA-MRSA strains in the hospital setting (17, 27, 33) suggest that clinical presentation and epidemiologic risk factors are no longer sufficient to reliably define molecular strain types.
CA-MRSA infections have been demonstrated to be more common in children, especially children less than 2 years of age (12). Outbreaks of MRSA have been noted in CCCs (18), and CCC attendance has been identified as a risk factor for CA-MRSA infections in the United States (9). Only limited information is available regarding the prevalence of colonization of healthy children attending CCCs in the United States. Single child care center prevalence studies have reported frequencies of MRSA colonization in children of 1.2% (2 of 164) (36) and 6.7% (7 of 104) (15). These results are similar to the 2.5% (3 of 122) (16) and 1.7% (5 of 291) (5) frequencies of MRSA colonization found in healthy children attending outpatient pediatric clinics in Chicago and to the 0.9% prevalence in children in Boston communities (24).
Since all previous studies of MRSA colonization in children attending CCCs in the United States were single center based and small, we undertook our study to further define the prevalence of MRSA colonization, characterize the MRSA strains, and determine the risk factors for colonization. Our study of 1,163 subjects from 200 classrooms in 24 centers revealed that overall 18.1% were colonized with S. aureus and 1.3% were colonized with MRSA. The frequency of nasal colonization was lower than the 31.1% reported among 1,192 children under 5 years of age attending child care centers in Brazil but similar to the 1.2% frequency of MRSA colonization (23). The prevalence we determined may also be underestimated since some potential subjects had already been identified as MRSA positive and did not consent for participation in our study. Importantly, our study which used molecular typing did not reveal evidence of person-to-person transmission of MRSA strains within classrooms. This is in contrast to the transmission observed by Hewlett et al. in a single university-based facility (15).
The molecular analysis of the colonizing MRSA strains revealed that six isolates were both PVL positive and SCCmec type IV, which is the classic molecular definition of CA-MRSA. Of those that were PVL negative (n = 9), only two were SCCmec type II—the molecular classification for HA-MRSA. When multilocus sequence typing with subsequent cluster analysis was applied (Fig. 1), the identification of one CA-MRSA strain was PVL negative and six HA-MRSA strains were SCCmec type IV. The latter isolates are likely related to the USA 800 pediatric clone. Interestingly, there was no difference in proportions of children with a history of hospitalization (Table 4) among those who were molecularly classified as CA-MRSA versus HA-MRSA. Our study suggests that the terms “community associated” and “health care associated” no longer apply in the CCC setting based on their traditional epidemiologic definitions (20). Similarly, molecular typing beyond SCCmec and PVL determination is necessary to accurately categorize MRSA strains and therefore the types of infections they are likely to cause (e.g., CA-MRSA and skin and soft tissue infections).
Multiple factors have been associated with CA-MRSA infection, including participation in contact sports, pet ownership, concurrent skin and soft tissue infections, and close contact (in the same household) with a person colonized or infected with MRSA. Our case-control study was not able to demonstrate any risk factors specific for MRSA colonization in our study population. However, this was likely due to our small number of colonized subjects. If the data from our study and that conducted in Brazil are generalizable to the United States, then determining risk factors for MRSA colonization would require an enormously larger study. Based on our MRSA prevalence of 1.3%, we would need 3,909 subjects to detect an odds ratio of 2.0 and over 12,000 subjects to detect an odds ratio of 1.5.
Additional potential limitations of the current study should be acknowledged. First, the participation rate among eligible children was 40%, which may make it difficult to generalize our results to a larger population of healthy children. However, the cross-section analysis of children enrolled in each center was demographically representative of the center in general based on center-level demographic data obtained (data not shown). Another limitation was the difference in demographic information obtained in children enrolled in North Carolina versus those enrolled in Virginia due to IRB-specific guidelines. This resulted in the lack of demographic information available for Virginia children making it impossible to compare the population of children in North Carolina and Virginia and to analyze the relationship between age and colonization status in Virginia, unless the subject was in the case-control study. Due to missing data for some of the variables, only bivariate associations could be analyzed in both the prevalence data and the case-control study (Tables 2 and 4).
In summary, we have demonstrated that approximately 1% of young children attending child care centers are colonized with MRSA. We did not document any person-to-person transmission of MRSA strains within classrooms. We were unable to statistically document risk factors associated with MRSA colonization but were limited by the small number of colonized children. It will be important for much larger multicenter studies to be performed to identify risk factors associated with MRSA colonization and subsequent infection in this highly susceptible population.
ACKNOWLEDGMENTS
The views expressed in this article are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the United States Government.
We gratefully acknowledge Cubist Pharmaceuticals for its financial support of this study.
Footnotes
Published ahead of print on 29 December 2010.
REFERENCES
- 1. Acton D. S., Plat-Sinnige M. J., van Wamel W., de Groot N., van Belkum A. 2009. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur. J. Clin. Microbiol. Infect. Dis. 28:115–127 [DOI] [PubMed] [Google Scholar]
- 2. Boucher H. W., Corey G. R. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl. 5):S344–S349 [DOI] [PubMed] [Google Scholar]
- 3. Boye K., Bartels M. D., Andersen I. S., Moller J. A., Westh H. 2007. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I-V. Clin. Microbiol. Infect. 13:725–727 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention 3 March 2010, posting date Healthcare-associated methicillin resistant Staphylococcus aureus (HA-MRSA). http://www.cdc.gov/ncidod/dhqp/ar_mrsa.html
- 5. Cheng Immergluck L., et al. 2004. Prevalence of Streptococcus pneumoniae and Staphylococcus aureus nasopharyngeal colonization in healthy children in the United States. Epidemiol. Infect. 132:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Churchill R. B., Pickering L. K. 1996. Infections in child care centers. Curr. Opin. Infect. Dis. 9:176–180 [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial susceptibility testing. Nineteenth informational supplement. M100-S19, p. 52-59 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Diederen B. M., Kluytmans J. A. 2006. The emergence of infections with community-associated methicillin resistant Staphylococcus aureus. J. Infect. 52:157–168 [DOI] [PubMed] [Google Scholar]
- 9. Dietrich D. W., Auld D. B., Mermel L. A. 2004. Community-acquired methicillin-resistant Staphylococcus aureus in southern New England children. Pediatrics 113:e347–e352 [DOI] [PubMed] [Google Scholar]
- 10. Enright M. C., Day N. P., Davies C. E., Peacock S. J., Spratt B. G. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Enright M. C., Day N. P., Davies C. E., Peacock S. J., Spratt B. G. 11 May 2010, posting date The seven loci and the primers and conditions used for PCR. http://saureus.mlst.net/misc/info.asp
- 12. Fridkin S. K., et al. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 13. Goodrich J. S., et al. 2009. Prevalence of community-associated methicillin-resistant Staphylococcus aureus in patients with cystic fibrosis. J. Clin. Microbiol. 47:1231–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graham P. L., III, Lin S. X., Larson E. L. 2006. A U.S. population-based survey of Staphylococcus aureus colonization. Ann. Intern. Med. 144:318–325 [DOI] [PubMed] [Google Scholar]
- 15. Hewlett A. L., Falk P. S., Hughes K. S., Mayhall C. G. 2009. Epidemiology of methicillin-resistant Staphylococcus aureus in a university medical center day care facility. Infect. Control Hosp. Epidemiol. 30:985–992 [DOI] [PubMed] [Google Scholar]
- 16. Hussain F. M., Boyle-Vavra S., Daum R. S. 2001. Community-acquired methicillin-resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr. Infect. Dis. J. 20:763–767 [DOI] [PubMed] [Google Scholar]
- 17. Jenkins T. C., et al. 2009. Epidemiology of healthcare-associated bloodstream infection caused by USA300 strains of methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Infect. Control Hosp. Epidemiol. 30:233–241 [DOI] [PubMed] [Google Scholar]
- 18. Jensen J. U., et al. 2006. Control of a methicillin-resistant Staphylococcus aureus (MRSA) outbreak in a day-care institution. J. Hosp. Infect. 63:84–92 [DOI] [PubMed] [Google Scholar]
- 19. Jorgensen J. H., Crawford S. A., McElmeel M. L., Fiebelkorn K. R. 2004. Detection of inducible clindamycin resistance of staphylococci in conjunction with performance of automated broth susceptibility testing. J. Clin. Microbiol. 42:1800–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klevens R. M., et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 21. Kluytmans J., van Belkum A., Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuehnert M. J., et al. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J. Infect. Dis. 193:172–179 [DOI] [PubMed] [Google Scholar]
- 23. Lamaro-Cardoso J., et al. 2009. Molecular epidemiology and risk factors for nasal carriage of Staphylococcus aureus and methicillin-resistant S. aureus in infants attending day care centers in Brazil. J. Clin. Microbiol. 47:3991–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee G. M., et al. 2009. Epidemiology and risk factors for Staphylococcus aureus colonization in children in the post-PCV7 era. BMC Infect. Dis. 9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lowy F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 26. Miller L. G., Diep B. A. 2008. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 46:752–760 [DOI] [PubMed] [Google Scholar]
- 27. Milstone A. M. 2010. Community-associated methicillin-resistant Staphylococcus aureus strains in pediatric intensive care unit. Emerg. Infect. Dis. 16:647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mink C. M., Yeh S. 2009. Infections in child-care facilities and schools. Pediatr. Rev. 30:259–269 [DOI] [PubMed] [Google Scholar]
- 29. Naimi T. S., et al. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976–2984 [DOI] [PubMed] [Google Scholar]
- 30. Nakagawa S., et al. 2005. Gene sequences and specific detection for Panton-Valentine leukocidin. Biochem. Biophys. Res. Commun. 328:995–1002 [DOI] [PubMed] [Google Scholar]
- 31. North Carolina Department of Health and Human Services, North Carolina Division of Child Development 28 January 2011, accession date Star rated license overview. http://ncchildcare.dhhs.state.nc.us/providers/pv_sn2_ov_sr.asp
- 32. Oliveira D. C., de Lencastre H. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orendi J. M., et al. 2010. Community and nosocomial transmission of Panton-Valentine leucocidin-positive community-associated methicillin-resistant Staphylococcus aureus: implications for healthcare. J. Hosp. Infect. 75:258–264 [DOI] [PubMed] [Google Scholar]
- 34. Reischl U., Linde H. J., Metz M., Leppmeier B., Lehn N. 2000. Rapid identification of methicillin-resistant Staphylococcus aureus and simultaneous species confirmation using real-time fluorescence PCR. J. Clin. Microbiol. 38:2429–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Safdar N., Bradley E. A. 2008. The risk of infection after nasal colonization with Staphylococcus aureus. Am. J. Med. 121:310–315 [DOI] [PubMed] [Google Scholar]
- 36. Shahin R., et al. 1999. Methicillin-resistant Staphylococcus aureus carriage in a child care center following a case of disease. Toronto Child Care Center Study Group. Arch. Pediatr. Adolesc. Med. 153:864–868 [DOI] [PubMed] [Google Scholar]
- 37. U.S. Census Bureau 28 January 2011, accession date Who's minding the kids? Child care arrangements: summer 2006 detailed tables. http://www.census.gov/population/www/socdemo/child/tables-2006.html
- 38. von Eiff C., Becker K., Machka K., Stammer H., Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11–16 [DOI] [PubMed] [Google Scholar]
- 39. Wertheim H. F., et al. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705 [DOI] [PubMed] [Google Scholar]
- 40. Woodford N., Livermore D. M. 2009. Infections caused by Gram-positive bacteria: a review of the global challenge. J. Infect. 59(Suppl. 1):S4–S16 [DOI] [PubMed] [Google Scholar]