Abstract
Escherichia coli ranks among the organisms most frequently isolated from cases of bacteremia. The relative contribution of the host and bacteria to E. coli bacteremia severity remains unknown. We conducted a prospective multicenter cohort study to identify host and bacterial factors associated with E. coli bacteremia severity. The primary endpoint was in-hospital death, up to 28 days after the first positive blood culture. Among 1,051 patients included, 136 (12.9%) died. Overall, 604 (57.5%) patients were female. The median age was 70 years, and 202 (19.2%) episodes were nosocomial. The most frequent comorbidities were immunocompromised status (37.9%), tobacco addiction (21.5%), and diabetes mellitus (20.1%). The most common portal of entry was the urinary tract (56.9%). Most E. coli isolates belonged to phylogenetic group B2 (52.0%). The multivariate analysis retained the following factors as predictive of death: older age (odds ratio [OR] = 1.25 [95% confidence interval {CI}, 1.09 to 1.43] for each 10-year increment), cirrhosis (OR = 4.85 [95% CI, 2.49 to 9.45]), hospitalization before bacteremia (OR = 4.13 [95% CI, 2.49 to 6.82]), being an immunocompromised patient not hospitalized before bacteremia (OR = 3.73 [95% CI, 2.25 to 6.18]), and a cutaneous portal of entry (OR = 6.45 [95% CI, 1.68 to 24.79]); a urinary tract portal of entry and the presence of the ireA virulence gene were negatively correlated with death (OR = 0.46 [95% CI, 0.30 to 0.70] and OR = 0.53 [95% CI, 0.30 to 0.91], respectively). In summary, host factors and the portal of entry outweigh bacterial determinants for predicting E. coli bacteremia severity.
INTRODUCTION
Recent reports indicate that the incidence of sepsis and the number of sepsis-related deaths are rising (29), placing it now among the 10 leading causes of death in the United States (15). Escherichia coli ranks among the organisms most frequently isolated from cases of sepsis, being the first most common cause of community-acquired and the fourth most common cause of nosocomial bacteremias (2, 11, 18, 26, 27, 49, 53). With a case-fatality rate of 5 to 30%, E. coli bacteremia represents an increasingly important endemic problem, accounting for hundreds of thousands of lives lost and billions of health care dollars spent each year (40). Worryingly, the spread, in recent years, of isolates producing extended-spectrum β-lactamases that are often resistant to most of the available antibiotic classes may further worsen the clinical and economic impact of E. coli bacteremia in the near future (43).
E. coli is found in its primary habitat, the digestive tract, as a commensal (46) but is also involved in various intestinal and extraintestinal diseases. The genetic structure of the species is roughly clonal, with the delineation of at least four major phylogenetic groups, groups A, B1, B2, and D (12). Pathogenic strains have been classified into various pathovars based on the conditions of their isolation and the presence of specific virulence genes (10). In the case of extraintestinal pathogenic E. coli (ExPEC) strains (41) isolated during septicemia, numerous epidemiologic and experimental data have pointed to the roles of the B2-phylogroup-belonging strains and of numerous virulence factors involved in adhesion, toxin production, iron capture, and cell protection in the pathogenicity of the strain (3, 21, 35).
Factors associated with E. coli bacteremia severity have not been clearly established. Relatively few studies have jointly examined the roles of host and bacterial factors in the severity and outcome of this infection (14, 17, 25, 30–32, 36, 50). The discrepancies among the conclusions of those studies might reflect the retrospective (14, 31, 36, 50) and/or monocenter (14, 30, 32, 36, 50) nature of most of them, the small number (30 to 185) of patients included (14, 17, 30–32, 36, 50), the small number of bacterial factors examined (14, 17, 25, 30–32, 36, 50), and the diversity of the disease's pathophysiological mechanisms.
To overcome those limitations, we conducted a large prospective multicenter study aimed at characterizing the risk factors for E. coli bacteremia severity. Herein, we thoroughly analyzed the host determinants and bacterial genetic and antibiotic resistance characteristics of more than 1,000 consecutive E. coli bacteremia episodes occurring in adults over a 1-year period.
(This work was presented in part at the 49th International Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 12 to 15 September 2009 [26a].)
MATERIALS AND METHODS
Study design and setting.
This prospective COLIBAFI study was conducted in 15 French hospitals (1 general and 14 university hospitals). Adults with E. coli bacteremia between January and December 2005 were enrolled in the study. Only patients receiving vasopressors before the onset of E. coli bacteremia or patients already included in the study for a previous episode were not considered for inclusion.
E. coli bacteremia was defined as the isolation of E. coli from ≥1 set of aseptically inoculated blood culture bottles. The primary endpoint was in-hospital death, up to 28 days after the first positive blood culture.
Clinical and bacteriological data were collected in each center at the time of bacteremia by a dedicated physician and microbiologist in tandem. All E. coli isolates were centralized in 1 research laboratory (INSERM, UMR722), which performed molecular epidemiology studies. The study was approved by the institutional Ethics Committee (Comité de Protection des Personnes, Hôpital Saint-Louis, Paris, France; approval number 2004-06).
Clinical characteristics.
Bacteremia episodes were defined as being nosocomial when the first positive blood culture was obtained ≥48 h following hospital admission. Otherwise, bacteremia was considered community acquired.
Immunocompromised patients were those presenting at least 1 of the following conditions: human immunodeficiency virus (HIV) infection with CD4 counts of <200 cells/mm3, underlying progressive solid cancer or malignant hemopathy, prior solid-organ or bone marrow transplantation, neutropenia of <500/mm3, congenital immunodeficiency, current immunosuppressive therapy (≥10 mg/day of a prednisone equivalent, immunomodulating treatment, or antineoplastic chemotherapy within the last month).
The portal of entry was established according to compatible clinical and/or radiographic features and the isolation of E. coli from the presumed source of infection. When E. coli could not be isolated from the presumed portal of entry (i.e., previous antibiotic treatment leading to negative bacterial cultures or an undesirable invasive procedure needed to isolate E. coli from the portal of entry), the presumed portal of entry was assigned on the basis of a firm clinical suspicion, provided that all other possible sources of infection had been excluded. If the clinical data were ambiguous, the portal of entry was categorized as being “undetermined.” A secondary septic focus was defined as a metastatic focus of infection due to bacteremia that was anatomically distant from the portal of entry, if any.
The bacteremia was polymicrobial when at least 1 other microorganism was recovered from a set of blood culture bottles positive for E. coli. The antibiotic regimen was considered to be adequate when the E. coli isolate was susceptible in vitro to at least 1 of the antibiotics given.
Follow-up ended at hospital discharge or 28 days after the first E. coli-positive blood culture for patients still hospitalized 28 days after the bacteremia diagnosis.
Bacterial determinants.
Strains were assigned to 1 of the 4 main E. coli phylogenetic groups, i.e., groups A, B1, B2, and D, using a triplex PCR developed previously by Clermont et al. (6). The presence of 18 virulence factors representative of the main classes of identified E. coli extraintestinal virulence determinants (8, 20, 38, 39, 45), including adhesins (papC; papG, including papG alleles; sfa/foc; iha; hra; and ibeA), toxins (hlyC, cnf1, and sat), iron capture systems (fyuA, irp2, iroN, iucC, and ireA), protectins (neuC, chromosomal ompT, and traT), as well as a gene encoding a uropathogenic-specific protein, usp (24), were tested by PCR, as previously described (19). For each isolate, a virulence score, defined as the number of virulence factors present over the 18 tested, was calculated. As it is well known that numerous virulence genes are clustered on genomic islands called pathogenicity-associated islands (PAIs) (13), we deduced the presence of 6 PAIs from the presence of the individual virulence genes (4, 16, 24): PAIICFT073 (papGII, hly, and iucC positive), PAIIIJ96 (presence of at least 3 of the 4 following genes: papGIII, hly, cnf1, and hra), PAIIII536 (sfa/foc and iroN positive), PAIIV536, a high-pathogenicity island (HPI) (irp2 and fyuA positive), GimA (ibeA positive), and PAIUSP (usp positive). For each isolate, a PAI score, defined as the number of PAIs present over the 6 tested, was calculated.
Antimicrobial susceptibilities were determined for 18 antibiotics in each center with the disk diffusion method with Mueller-Hinton agar, as recommended by Comité de l'Antibiogramme de la Société Française de Microbiologie standards (http://www.sfm.asso.fr). A strain was considered to be resistant to expanded-spectrum cephalosporins if it was resistant to cefotaxime and/or ceftazidime according to MICs determined by the Etest diffusion method (AB Biodisk, Solna, Sweden) (9). A strain was considered to be multidrug resistant when it was resistant to at least amoxicillin, ofloxacin, and cotrimoxazole. For each strain, a resistance score was defined as the number of antibiotics to which it was resistant over the 5 following drugs: amoxicillin, cefotaxime, gentamicin, ofloxacin, and cotrimoxazole. The presence of integrons (classes I, II, and III), which are molecular markers of resistance, were detected by triplex real-time PCR (44).
Statistical methods.
Based on previous reports, the expected proportion of death was 15% (17, 25, 30, 53). We therefore planned to include 1,000 patients. With 150 deaths and based on the general rule of 10 events by covariate, that number was needed to test about 15 clinical characteristics and 15 bacterial determinants.
The risk factors associated with death were analyzed. First, univariate regression analyses were performed for clinical and bacteriological factors. The studied clinical factors were age; sex; place of birth; weight; body mass index; hospitalization before bacteremia; antibiotic therapy during the 2 weeks preceding bacteremia; comorbidities, including a history of bacteremia, pregnancy, chronic alcoholism, tobacco addiction, congestive heart failure, chronic respiratory insufficiency, chronic renal insufficiency, cystic fibrosis, diabetes mellitus, sickle-cell anemia, immunocompromise, cirrhosis, or hemochromatosis; nosocomial infection; a portal of entry including urinary tract, digestive tract, pulmonary, cutaneous, venous catheter, female genital tract, or surgical site; and prescription of an adequate antibiotic regimen within the first day. The bacteriological determinants were as follows: a phylogenetic group in 4 classes; group B2; the presence of each of the 18 virulence factors; virulence score; the presence of each of the 6 PAIs; PAI score; polymicrobial sample; resistance to each of the drugs amoxicillin, cefotaxime, gentamicin, ofloxacin, and cotrimoxazole; resistance to expanded-spectrum cephalosporins; multidrug resistance; resistance score; and the presence of an integron of classes I, II, and/or III. The clinical and bacteriological risk factors achieving a P value of <0.10 were then entered into the multivariate logistic regression model. A backward selection method was used to obtain a model in which all clinical risk factors had a P value of <0.05. After that selection step, all interactions between 2 variables were tested, and all significant ones were retained in the model. The predictability of the final model was assessed by using the C statistic.
The risk factors for death were then also analyzed for the 2 subgroups of patients with the most frequent portals of entry, i.e., urinary or digestive tract, using an approach similar to that described above. Comparisons of some factors between urinary or digestive tract portals of entry were performed by using a Wilcoxon test for continuous variables and the Fisher exact test for discrete variables.
All these analyses were done with SAS 9.1 software (SAS Institute Inc., Cary, NC).
RESULTS
Among the 1,099 patients included in the study, 48 were excluded from the analysis because the E. coli isolates were not available (n = 18) or the patient's clinical research forms were not filled out (n = 30). Thus, 1,051 patients were retained for the analysis.
Clinical characteristics.
The characteristics of the patients with E. coli bacteremia are shown in Table 1. The patients were mostly >65 years old (638 patients [60.7%]) and predominantly female (57.5%). Overall, 19.2% of the bacteremias were nosocomial infections, and 9.1% occurred in patients living in nursing or retirement homes or a long-term care facility. At least one underlying immunocompromising comorbidity was reported for 37.9% of the patients. As expected, the most frequent portal of entry was the urinary tract (56.9% of the cases); a digestive tract portal of entry was identified for 13.1% of patients. Among 598 patients with a urinary tract source, 173 were male (including cases of presumed prostatitis [134 patients], pyelonephritis [32], and orchiepididymitis [6]) and 425 were female (all pyelonephritides). Among 138 patients with a digestive tract portal of entry, biliary tract infections predominated (angiocholitis [76 patients] and cholecystitis [33] but also pancreatitis [23], peritonitis [13], diverticulitis [10], appendicitis [4], and liver abscess [4]). Overall, 30 (2.9%) patients had at least 1 secondary septic focus of infection.
Table 1.
Demographic, epidemiological, and clinical characteristics of the 1,051 patients with E. coli bacteremia
| Characteristic | Valueb |
|---|---|
| Demographics | |
| Median age (yr) (range) | 70 (18–101) |
| No. (%) of males/no. (%) of females | 447 (42.5)/604 (57.5) |
| No. (%) of patients with a place of birth of: | |
| Europe | 918 (87.4) |
| Africa | 71 (6.8) |
| Asia | 8 (0.8) |
| America | 3 (0.3) |
| Unknown | 51 (4.9) |
| Origin of infection [no. (%) of patients] | |
| Patients with stay prior to bacteremia | |
| Home | 690 (65.9) |
| Institution | 95 (9.1) |
| Hospital | 262 (25.0) |
| Nosocomial infection | 202 (19.2) |
| Antibiotics within 2 wk preceding bacteremia | 176 (16.8) |
| Clinical [no. (%) of patients] | |
| Host predisposing conditionsa | |
| Solid cancer | 239 (23.5) |
| Malignant hemopathy | 106 (10.3) |
| Tobacco addiction | 216 (21.5) |
| Diabetes mellitus | 205 (20.1) |
| Chronic renal insufficiency | 150 (14.8) |
| Congestive heart failure | 133 (13.1) |
| Chronic alcoholism | 127 (12.6) |
| Prior bacteremia | 80 (7.9) |
| Cirrhosis | 52 (5.2) |
| HIV infection | 17 (1.7) |
| Immunocompromisea | 398 (37.9) |
| Progressive solid cancer/hemopathy | 321 (30.5) |
| Antiproliferative chemotherapy | 128 (12.2) |
| Past solid-organ or bone marrow transplant | 66 (6.6) |
| Current corticosteroid therapy | 78 (7.4) |
| Current immunomodulating treatment | 71 (7.0) |
| Neutropenia <500/mm3 | 62 (6.1) |
| HIV infection with <200 CD4 cells/mm3 | 5 (0.5) |
| Congenital immunodeficiency | 2 (0.2) |
| Portal of entry | |
| Urinary tract | 598 (56.9) |
| Digestive tract | 138 (13.1) |
| Respiratory tract | 19 (1.8) |
| Venous catheter | 11 (1.1) |
| Cutaneous | 10 (1.0) |
| Surgical site | 5 (0.5) |
| Female genital tract | 7 (0.7) |
| Two portals of entry | 19 (1.8) |
| Not determined | 282 (26.8) |
| Start of adequate antibiotic therapy | |
| ≤1 day after bacteremia | 758 (72.1) |
Some patients had >1 host predisposing condition and/or criterion for their immunocompromised status.
Because of missing values, percentages are calculated based on available data.
Bacterial determinants.
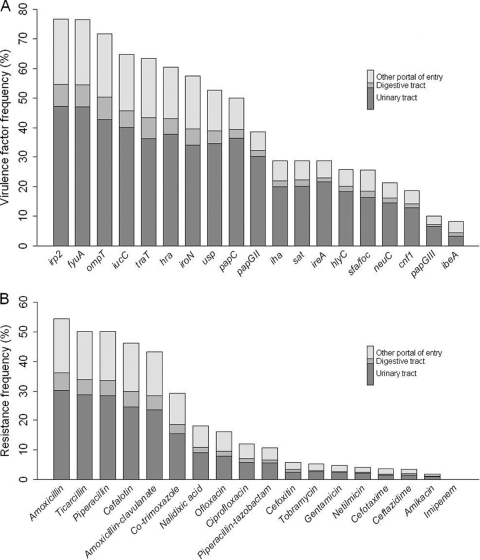
E. coli was isolated alone from the blood cultures of 988 patients, whereas for 63 (6.0%) patients bacteremias were polymicrobial. Five hundred forty-six strains (52.1%) and 219 strains (21.0%) belonged to the classical extraintestinal pathogenic E. coli phylogenetic groups (35), i.e., groups B2 and D, respectively; 236 (22.5%) belonged to phylogenetic group A, and only 48 (4.6%) belonged to group B1. The frequencies of the various virulence factors were highly variable, ranging from 8.3% for ibeA to 76.7% for irp2, a gene belonging to the high-pathogenicity island (42) (Fig. 1A). The median virulence score for isolates was 9 (range, 0 to 18). Likely, the frequencies of PAIs ranged from 8.3% for GimA to 76.6% for the HPI, with PAIICFT073, PAIIIJ96, PAIIII536, and PAIUSP at 13, 18.7, 25.3, and 57.2%, respectively. The median PAI score for isolates was 2 (range, 0 to 6).
Fig. 1.
Virulence factors and resistance to antibiotics of 1,051 E. coli isolates according to the portal of entry of the bacteremia. (A) Percentages of isolates harboring any of the 19 extraintestinal virulence factors tested (the papGII and papGIII alleles are individualized here). (B) Percentage of isolates resistant to the 18 antibiotics tested.
Rates of resistance to each antibiotic are shown in Fig. 1B; 108 strains (10.3%) were multidrug resistant. Strains had a median resistance score of 1, ranging from no resistance to resistance to all the antibiotics tested; 39 (3.7%) were resistant to expanded-spectrum cephalosporins, among which 76.9% were community acquired. Three hundred fifteen strains (30.0%) possessed at least 1 integron, predominantly class I integrons (294 strains); a few class II (24 strains) and no class III integrons were detected.
Risk factors for death.
Overall, 136 (12.9%) patients died. The median time to death was 6 days (range, 0 to 28). Clinical and bacterial factors associated with death were identified by univariate and multivariate analyses. The 13 clinical and 16 bacteriological risk factors significant in univariate analyses are reported in Table 2. A significant interaction between immunocompromise and hospitalization before bacteremia was also entered into the model and yielded a new variable with 3 classes: not immunocompromised and not hospitalized before bacteremia, immunocompromised and not hospitalized before bacteremia, and hospitalized before bacteremia with or without immunocompromise; their corresponding odds ratios (ORs) (and 95% confidence intervals [CIs]) are reported in Table 2.
Table 2.
Risk factors for death from E. coli bacteremia identified by univariate and multivariate analyses
| Risk factord | Value for group |
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| Nonsurvivors (n = 136) | Survivors (n = 915) | OR (95% CI) | P value | OR (95% CI) | P value | |
| Clinical | ||||||
| Median age (yr) (range) | 72 (28–99) | 70 (18–101) | 1.13 (1.01–1.26)a | 0.0267 | 1.25 (1.09–1.43)a | 0.0019 |
| No. (%) of patients | ||||||
| Male | 72 (52.94) | 375 (40.98) | 1.62 (1.13–2.33) | 0.0089 | ||
| Tobacco addiction | 37 (28.24) | 179 (20.46) | 1.53 (1.01–2.32) | 0.0441 | ||
| Chronic alcoholism | 27 (20.61) | 100 (11.35) | 2.03 (1.27–3.25) | 0.0033 | ||
| Cirrhosis | 18 (13.64) | 34 (3.88) | 3.91 (2.14–7.15) | <.0001 | 4.85 (2.49–9.45) | <.0001 |
| Nosocomial infection | 42 (30.88) | 160 (17.49) | 2.11 (1.41–3.15) | 0.0003 | ||
| Hospitalized before bacteremiab | 55 (40.74) | 207 (22.70) | 2.34 (1.61–3.41) | <.0001 | ||
| Immunocompromisedb | 79 (58.09) | 319 (34.86) | 2.59 (1.79–3.74) | <.0001 | ||
| Not I, not H, before Bc | 34 (25.19) | 507 (55.59) | 1 | – | 1 | – |
| H before B +/− Ic | 55 (40.74) | 207 (22.70) | 3.96 (2.51–6.26) | <.0001 | 4.13 (2.49–6.82) | <.0001 |
| I, not H, before Bc | 46 (34.07) | 198 (21.71) | 3.46 (2.16–5.56) | <.0001 | 3.73 (2.25–6.18) | <.0001 |
| Portal of entry | ||||||
| Urinary | 47 (34.56) | 551 (60.22) | 0.35 (0.24–0.51) | <.0001 | 0.46 (0.30–0.70) | 0.0002 |
| Venous catheter | 4 (2.94) | 7 (0.77) | 3.93 (1.14–13.61) | 0.0308 | ||
| Cutaneous | 4 (2.94) | 6 (0.66) | 4.59 (1.28–16.49) | 0.0194 | 6.45 (1.68–24.79) | 0.0066 |
| Bacteriological | ||||||
| No. (%) of patients with polymicrobial infection | 17 (12.50) | 46 (5.03) | 2.70 (1.50–4.86) | 0.0009 | ||
| No. (%) of patients with B2 phylogenetic group infection | 57 (41.91) | 489 (53.56) | 0.63 (0.44–0.90) | 0.0117 | ||
| Median virulence score (range) | 6 (0–15) | 9 (0–17) | 0.91 (0.88–0.95) | <.0001 | ||
| No. (%) of patients with virulence factor | ||||||
| papGII | 30 (22.06) | 376 (41.18) | 0.40 (0.26–0.62) | <.0001 | ||
| papC | 43 (31.62) | 482 (52.79) | 0.41 (0.28–0.61) | <.0001 | ||
| ireA | 21 (15.44) | 281 (30.78) | 0.41 (0.25–0.67) | 0.0003 | 0.53 (0.30–0.91) | 0.0205 |
| hra | 64 (47.06) | 571 (62.54) | 0.53 (0.37–0.77) | 0.0007 | ||
| irp2 | 90 (66.18) | 715 (78.31) | 0.54 (0.37–0.80) | 0.0020 | ||
| fyuA | 90 (66.18) | 713 (78.09) | 0.55 (0.37–0.81) | 0.0025 | ||
| neuC | 19 (13.97) | 204 (22.34) | 0.56 (0.34–0.94) | 0.0277 | ||
| traT | 74 (54.41) | 592 (64.84) | 0.65 (0.45–0.93) | 0.0190 | ||
| usp | 59 (43.38) | 494 (54.11) | 0.65 (0.45–0.94) | 0.0201 | ||
| iroN | 66 (48.53) | 538 (58.93) | 0.66 (0.46–0.94) | 0.0227 | ||
| Median PAI score (range) | 1 (0–5) | 2 (0–6) | 0.85 (0.75–0.95) | 0.0064 | ||
| No. (%) of isolates with expanded-spectrum cephalosporin resistance | 12 (8.82) | 27 (2.95) | 3.18 (1.57–6.45) | 0.0013 | ||
| No. (%) of isolates with multidrug resistance | 21 (15.44) | 87 (9.51) | 1.74 (1.04–2.91) | 0.0354 | ||
Age by 10 years.
A significant interaction between these two factors was found in the univariate analysis. Therefore, for the multivariate analysis, a new variable with 3 classes was created.
The 3 classes formed as a result of the significant interaction between the 2 risk factors hospitalization before bacteremia and immunocompromise.
H, hospitalized; B, bacteremia; I, immunocompromised; +/−, with or without.
After backward selection in the multivariate model, risk factors associated with death were older age, cirrhosis, hospitalization before bacteremia, immunocompromised patients not hospitalized before bacteremia, and a cutaneous portal of entry, whereas a urinary tract portal of entry and the presence of the bacteriological ireA virulence factor were negatively correlated with death (Table 2). The C statistic of the final model was 0.77 (95% CI, 0.73 to 0.81) and was similar for the model with the same clinical factors but without the virulence factor ireA. These results show the good predictability of the model and the limited added value of the virulence factor in addition to clinical risk factors.
Relationship among portal of entry, host characteristics, and bacterial determinants.
Because the urinary and the digestive tracts were the most frequently found portals of entry, and the pathophysiologies of E. coli bacteremias originating from the urinary or the digestive tract differ markedly, episodes were analyzed separately according to their urinary or digestive tract origin. Only isolates originating from patients with a single source (urinary or digestive tract) were considered for these subgroup analyses.
There were significantly more group B2 isolates among isolates of urinary tract origin (360/581 [62%]) than among those of digestive origin (43/129 [33.3%]) (P < 0.001). Virulence and PAI scores were significantly higher for isolates originating from the urinary tract than those from the digestive tract (medians, 10 [range, 0 to 17] and 2 [range, 0 to 6] versus 5 [range, 0 to 15] and 1 [range, 0 to 5], respectively; P < 0.001), whereas resistance scores did not differ (median, 1 [range, 0 to 5] for both portals of entry). All but 2 (traT and ibeA) of the 18 extraintestinal virulence factors and all but GimA of the PAIs analyzed were significantly more frequently found among isolates of urinary tract origin than among those of digestive tract origin (P < 0.05).
The rate of death was higher among patients whose sepsis was of digestive tract origin (14.7%) than among those whose sepsis was of urinary tract origin (7.6%) (P = 0.002). Table 3 reports the results of the multivariate analyses according to the urinary or digestive tract portal of entry. Clinical factors associated with death from sepsis of urinary tract origin were the same as those retained in the global model (Table 2), plus tobacco addiction. No bacteriological factor was retrieved in this subgroup. For the subgroup with a digestive tract portal of entry, only cirrhosis and polymicrobial bacteremia were associated with death.
Table 3.
Risk factors for death from E. coli bacteremia according to the portal of entry
| Portal of entry and variablec | Value for groupd |
Multivariate analysis OR (95% CI) | P value | |
|---|---|---|---|---|
| Nonsurvivors | Survivors | |||
| Urinary tract (n = 581) | (n = 44) | (n = 537) | ||
| Median age (yr) (range) | 76 (43–99) | 70 (18–101) | 1.48 (1.16–1.89)a | 0.0019 |
| No. (%) of patients | ||||
| Tobacco addiction | 14 (33.33) | 86 (16.48) | 2.4 (1.11–5.01) | 0.0251 |
| Cirrhosis | 7 (16.67) | 17 (3.26) | 7.0 (2.48–19.85) | 0.0002 |
| Not I, not H, before Bb | 14 (32.56) | 342 (64.04) | 1 | – |
| H before B +/− I | 16 (37.21) | 91 (17.04) | 4.34 (1.92–9.82) | 0.0004 |
| I, not H, before B | 13 (30.23) | 101 (18.91) | 3.87 (1.69–8.87) | 0.0014 |
| Digestive tract (n = 129) [no. (%) of patients] | (n = 19) | (n = 110) | ||
| Cirrhosis | 4 (22.22) | 9 (8.57) | 4.20 (1.07–16.41) | 0.0392 |
| Polymicrobial | 5 (26.32) | 11 (10.00) | 4.29 (1.22–15.15) | 0.0235 |
Age by 10 years.
The 3 classes formed as a result of the significant interaction between the 2 risk factors of hospitalization before bacteremia and immunocompromise.
H, hospitalized; B, bacteremia; I, immunocompromised; +/−, with or without.
For a urinary tract portal of entry, there were 44 nonsurvivors and 537 survivors. For a digestive tract portal of entry, there were 19 nonsurvivors and 110 survivors.
DISCUSSION
Through the prospective, multicenter, cohort COLIBAFI study, we thoroughly analyzed detailed host characteristics and numerous bacterial determinants that could potentially influence the E. coli bacteremia severity of >1,000 episodes. Our study was conducted during a recent 1-year period and thus provides original clinical and molecular epidemiological data on current aspects of E. coli bacteremia.
The results of our multivariate analyses showed that death was strongly associated with certain patient characteristics: older age, cirrhosis, hospitalization before bacteremia, and immunocompromised status for patients not hospitalized before the episode. Previous studies identified advanced age, health care or hospital acquisition, and comorbidities as host determinants associated with a poor outcome (25, 30, 36). Surprisingly, a cutaneous portal of entry was also predictive of life-threatening disease. E. coli isolates from skin and soft tissue infections usually belong to phylogenetic group B2 and have a high virulence potential (34), but a link between that origin and E. coli bacteremia severity was never suggested previously. This result must be taken cautiously, as only 10 patients with a cutaneous portal of entry were included in the study. In contrast, a urinary tract portal of entry was associated with a less severe outcome, as previously reported (17, 25, 30, 36).
The only bacterial determinant that significantly influenced prognosis, although at a lower level than that of clinical determinants, was the presence of the ireA gene, which was found to negatively correlate with death (Table 2). The ireA (iron-responsive element) gene encodes a peptide (IreA) suggested to be involved in iron acquisition and to be important in urovirulence (39). IreA has been used with success as an immunizing agent in urinary tract infections (1) and bacteremia (52) in mice. Interestingly, this gene can be physically linked to the papC (pyelonephritis-associated pilus) gene and the papGII allele in the E. coli genome (22, 48), both also involved in urinary tract infection and subsequent bacteremia (23, 33). In our analysis, these two determinants have odds ratios similar to those of ireA in the univariate analysis (Table 2). Thus, this part of the E. coli genome plays a crucial role in the occurrence of kidney infection and subsequent bacteremia but not in the latter's severity. These bacterial determinants are markers of the urinary tract origin of the bacteremia, which is associated with a better prognosis. Our findings indicate that host factors and portal of entry outweigh bacterial determinants in predicting the severity of E. coli bacteremia. The good predictability of the model with 6 clinical risk factors encourages the building of a prognostic score of death following bacteremia, which should be prospectively evaluated.
We then postulated that risk factors predictive of death might differ according to the portal of entry. For a urinary tract origin, the host characteristics predictive of death were the same as those retained in the overall analysis, plus tobacco addiction. No bacterial determinant was identified. For the digestive tract origin, cirrhosis was associated with life-threatening bacteremia. Cirrhotic patients have impaired mechanisms of hepatosplenic clearance (7) and are at an increased risk of bacteremia caused by poorly virulent strains colonizing the digestive tract. It was previously reported that the bacteremia prognosis for cirrhotic patients is highly guarded (5, 47). We also found a link between the polymicrobial character of the bacteremia and death. It was previously shown that polymicrobial bacteremia was associated with higher mortality rates than unibacterial infection (37, 51), but no previous studies evidenced this parameter as a risk factor for death in a subgroup of patients with a digestive tract source of bacteremia.
Our study has several limitations. First, we did not analyze the impact of host genetic factors, which could have played a role in the severity of E. coli bacteremia. Second, we were unable to determine the portal of entry for 26.8% of the episodes. Third, although we looked at 18 virulence factors, either individually or grouped into PAIs, we are far from having tested all the genes of the variable E. coli gene pool (48) and their different combinations. It was shown from the complete sequence analysis of some natural isolates that multiple constellations of genes can lead to a virulent phenotype (48). High-throughput sequencing technologies (28) will allow the performance of comparative genomic analyses on numerous strains and the performance of phenotype-genotype association studies in the near future.
Despite these limitations, our analysis based on 15 centers and >1,000 patients, and its prospective design, enhanced our ability to draw conclusions. Death following E. coli bacteremia is associated mainly with host characteristics and the portal of entry. Thus, the early identification of clinical risk factors for severe progression is essential to optimize the timely management of patients with E. coli bacteremia.
ACKNOWLEDGMENTS
This study received grants from Réseau de Recherche Clinique (INSERM grant RBM-03-58) and the Projet Hospitalier de Recherche Clinique (Assistance Publique-Hôpitaux de Paris grant AOR 04 053). The sponsors (INSERM and Assistance Publique-Hôpitaux de Paris) had no role in designing and conducting the study; collection, management analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
We do not declare any conflicts of interest.
Members of the COLIBAFI Group in France include the following individuals. Clinical investigators are Michel Wolff, Loubna Alavoine, Xavier Duval, David Skurnik, Paul-Louis Woerther, and Antoine Andremont (CHU Bichat-Claude-Bernard, Paris); Etienne Carbonnelle, Olivier Lortholary, and Xavier Nassif (CHU Necker-Enfants Malades, Paris); Sophie Abgrall, Françoise Jaureguy, and Bertrand Picard (CHU Avicenne, Bobigny); Véronique Houdouin, Yannick Aujard, Stéphane Bonacorsi, and Edouard Bingen (CHU Robert-Debré, Paris); Agnès Meybeck, Guilène Barnaud, and Catherine Branger (CHU Louis-Mourier, Colombes); Agnès Lefort, Bruno Fantin, Claire Bellier, Frédéric Bert, and Marie-Hélène Nicolas-Chanoine (CHU Beaujon, Clichy); Bernard Page, Julie Cremniter, and Jean-Louis Gaillard (CHU Ambroise-Paré, Boulogne-Billancourt); Bernard Garo, Séverine Ansart, Geneviève Herry-Arnaud, and Didier Tandé (CHU Brest, Brest); Jean-Claude Renet, René Ze Bekolo, Renaud Verdon, and Roland Leclercq (CHU Caen, Caen); Claire de Gialluly, Jean-Marc Besnier, Laurent Mereghetti, and Roland Quentin (CHU Tours, Tours); Achille Kouatchet, Alain Mercat, and Marie Laure Joly-Guillou (CHU Angers, Angers); Catherine Dalebroux, Pascal Chavanet, and Catherine Neuwirth (CHU Dijon, Dijon); Camille Colliard, Martin Dary, Gilles Potel, and Jocelyne Caillon (CHU Nantes, Nantes); Françoise Leturdu, Jean-Pierre Sollet, and Gaëtan Plantefève (CH Argenteuil, Argenteuil); and Agnès de Patureaux, Pierre Tattevin, and Pierre-Yves Donnio (CHU Rennes, Rennes). Those responsible for bacterial genotyping are Erick Denamur, Olivier Clermont, Christine Amorin, and Jeremy Glodt (INSERM, UMR722, Université Paris-Diderot, Paris). Those responsible for methodology and biostatistics are Xavière Panhard, Ludovic Lassel, Quentin Dornic, and France Mentré (AP-HP, Hôpital Bichat, UF de Biostatistiques, Paris). Those responsible for methodology at the Clinical Research Unit are Estelle Marcault and Florence Tubach (CHU Bichat-Claude-Bernard, Paris).
Footnotes
Published ahead of print on 22 December 2010.
REFERENCES
- 1. Alteri C. J., Hagan E. C., Sivick K. E., Smith S. N., Mobley H. L. 2009. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 5:e1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biedenbach D. J., Moet G. J., Jones R. N. 2004. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997-2002). Diagn. Microbiol. Infect. Dis. 50:59–69 [DOI] [PubMed] [Google Scholar]
- 3. Bingen E., et al. 1998. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J. Infect. Dis. 177:642–650 [DOI] [PubMed] [Google Scholar]
- 4. Bingen-Bidois M., et al. 2002. Phylogenetic analysis and prevalence of urosepsis strains of Escherichia coli bearing pathogenicity island-like domains. Infect. Immun. 70:3216–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campillo B., Richardet J. P., Kheo T., Dupeyron C. 2002. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin. Infect. Dis. 35:1–10 [DOI] [PubMed] [Google Scholar]
- 6. Clermont O., Bonacorsi S., Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conn H. O. 1964. Spontaneous peritonitis and bacteremia in Laennec's cirrhosis caused by enteric organisms. A relatively common but rarely recognized syndrome. Ann. Intern. Med. 60:568–580 [DOI] [PubMed] [Google Scholar]
- 8. Cortes M. A., et al. 2008. Inactivation of ibeA and ibeT results in decreased expression of type 1 fimbriae in extraintestinal pathogenic Escherichia coli strain BEN2908. Infect. Immun. 76:4129–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Courpon-Claudinon A., et al. 23 July 2010. Bacteremia due to third-generation cephalosporin-resistant Escherichia coli in France: prevalence, molecular epidemiology and clinical features. Clin. Microbiol. Infect. [Epub ahead of print.] doi:10.1111/j.1469-0691.2010.03298.x [DOI] [PubMed] [Google Scholar]
- 10. Croxen M. A., Finlay B. B. 2010. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8:26–38 [DOI] [PubMed] [Google Scholar]
- 11. Diekema D. J., et al. 2003. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J. Clin. Microbiol. 41:3655–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon D. M., Clermont O., Tolley H., Denamur E. 2008. Assigning Escherichia coli strains to phylogenetic groups: multi-locus sequence typing versus the PCR triplex method. Environ. Microbiol. 10:2484–2496 [DOI] [PubMed] [Google Scholar]
- 13. Hacker J., Blum-Oehler G., Muhldorfer I., Tschape H. 1997. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 23:1089–1097 [DOI] [PubMed] [Google Scholar]
- 14. Hekker T. A., et al. 2000. Role of bacterial virulence factors and host factors in the outcome of Escherichia coli bacteraemia. Eur. J. Clin. Microbiol. Infect. Dis. 19:312–316 [DOI] [PubMed] [Google Scholar]
- 15. Heron M. 2007. Deaths: leading causes for 2004. Natl. Vital Stat. Rep. 56:1–95 [PubMed] [Google Scholar]
- 16. Huang S. H., et al. 2001. A novel genetic island of meningitic Escherichia coli K1 containing the ibeA invasion gene (GimA): functional annotation and carbon-source-regulated invasion of human brain microvascular endothelial cells. Funct. Integr. Genomics 1:312–322 [DOI] [PubMed] [Google Scholar]
- 17. Jaureguy F., et al. 2007. Host and bacterial determinants of initial severity and outcome of Escherichia coli sepsis. Clin. Microbiol. Infect. 13:854–862 [DOI] [PubMed] [Google Scholar]
- 18. Javaloyas M., Garcia-Somoza D., Gudiol F. 2002. Epidemiology and prognosis of bacteremia: a 10-y study in a community hospital. Scand. J. Infect. Dis. 34:436–441 [DOI] [PubMed] [Google Scholar]
- 19. Johnson J. R., et al. 2006. Experimental mouse lethality of Escherichia coli isolates, in relation to accessory traits, phylogenetic group, and ecological source. J. Infect. Dis. 194:1141–1150 [DOI] [PubMed] [Google Scholar]
- 20. Johnson J. R., Delavari P., Kuskowski M., Stell A. L. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 183:78–88 [DOI] [PubMed] [Google Scholar]
- 21. Johnson J. R., Russo T. A. 2005. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int. J. Med. Microbiol. 295:383–404 [DOI] [PubMed] [Google Scholar]
- 22. Kariyawasam S., Johnson T. J., Nolan L. K. 2006. The pap operon of avian pathogenic Escherichia coli strain O1:K1 is located on a novel pathogenicity island. Infect. Immun. 74:744–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korhonen T. K., Virkola R., Holthofer H. 1986. Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect. Immun. 54:328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurazono H., et al. 2000. Characterization of a putative virulence island in the chromosome of uropathogenic Escherichia coli possessing a gene encoding a uropathogenic-specific protein. Microb. Pathog. 28:183–189 [DOI] [PubMed] [Google Scholar]
- 25. Laupland K. B., Gregson D. B., Church D. L., Ross T., Pitout J. D. 2008. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin. Microbiol. Infect. 14:1041–1047 [DOI] [PubMed] [Google Scholar]
- 26. Laupland K. B., et al. 2007. Burden of community-onset bloodstream infection: a population-based assessment. Epidemiol. Infect. 135:1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a. Lefort A., et al. 2009. Predictive factors of the severity of Escherichia coli bacteremia: the Colibafi Study, abstr. K-260. Abstr. 49th Int. Conf. Antimicrob. Agents Chemother., San Francisco, CA [Google Scholar]
- 27. Lyytikainen O., et al. 2002. Nosocomial bloodstream infections in Finnish hospitals during 1999-2000. Clin. Infect. Dis. 35:e14–e19 [DOI] [PubMed] [Google Scholar]
- 28. MacLean D., Jones J. D., Studholme D. J. 2009. Application of ‘next-generation’ sequencing technologies to microbial genetics. Nat. Rev. Microbiol. 7:287–296 [DOI] [PubMed] [Google Scholar]
- 29. Martin G. S., Mannino D. M., Eaton S., Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 30. Martinez J. A., et al. 2006. Relationship of phylogenetic background, biofilm production, and time to detection of growth in blood culture vials with clinical variables and prognosis associated with Escherichia coli bacteremia. J. Clin. Microbiol. 44:1468–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maslow J. N., Mulligan M. E., Adams K. S., Justis J. C., Arbeit R. D. 1993. Bacterial adhesins and host factors: role in the development and outcome of Escherichia coli bacteremia. Clin. Infect. Dis. 17:89–97 [DOI] [PubMed] [Google Scholar]
- 32. McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. 1978. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J. Infect. Dis. 138:33–41 [DOI] [PubMed] [Google Scholar]
- 33. O'Hanley P., Lark D., Falkow S., Schoolnik G. 1985. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J. Clin. Invest. 75:347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petkovsek Z., Elersic K., Gubina M., Zgur-Bertok D., Erjavec M. S. 2009. Virulence potential of Escherichia coli isolates from skin and soft tissue infections. J. Clin. Microbiol. 47:1811–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Picard B., et al. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Picard B., Goullet P. 1988. Correlation between electrophoretic types B1 and B2 of carboxylesterase B and host-dependent factors in Escherichia coli septicaemia. Epidemiol. Infect. 100:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pittet D., Li N., Woolson R. F., Wenzel R. P. 1997. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin. Infect. Dis. 24:1068–1078 [DOI] [PubMed] [Google Scholar]
- 38. Restieri C., Garriss G., Locas M. C., Dozois C. M. 2007. Autotransporter-encoding sequences are phylogenetically distributed among Escherichia coli clinical isolates and reference strains. Appl. Environ. Microbiol. 73:1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russo T. A., Carlino U. B., Johnson J. R. 2001. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 69:6209–6216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Russo T. A., Johnson J. R. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 5:449–456 [DOI] [PubMed] [Google Scholar]
- 41. Russo T. A., Johnson J. R. 2000. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 181:1753–1754 [DOI] [PubMed] [Google Scholar]
- 42. Schubert S., Rakin A., Karch H., Carniel E., Heesemann J. 1998. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect. Immun. 66:480–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwaber M. J., et al. 2006. Clinical and economic impact of bacteremia with extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 50:1257–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Skurnik D., et al. 2005. Integron-associated antibiotic resistance and phylogenetic grouping of Escherichia coli isolates from healthy subjects free of recent antibiotic exposure. Antimicrob. Agents Chemother. 49:3062–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Srinivasan U., Foxman B., Marrs C. F. 2003. Identification of a gene encoding heat-resistant agglutinin in Escherichia coli as a putative virulence factor in urinary tract infection. J. Clin. Microbiol. 41:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tenaillon O., Skurnik D., Picard B., Denamur E. 2010. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 8:207–217 [DOI] [PubMed] [Google Scholar]
- 47. Thulstrup A. M., Sorensen H. T., Schonheyder H. C., Moller J. K., Tage-Jensen U. 2000. Population-based study of the risk and short-term prognosis for bacteremia in patients with liver cirrhosis. Clin. Infect. Dis. 31:1357–1361 [DOI] [PubMed] [Google Scholar]
- 48. Touchon M., et al. 2009. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 5:e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Uslan D. Z., et al. 2007. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch. Intern. Med. 167:834–839 [DOI] [PubMed] [Google Scholar]
- 50. Vazquez F., Mendoza M. C., Viejo G., Mendez F. J. 1992. Survey of Escherichia coli septicemia over a six-year period. Eur. J. Clin. Microbiol. Infect. Dis. 11:110–117 [DOI] [PubMed] [Google Scholar]
- 51. Weinstein M. P., Reller L. B., Murphy J. R. 1986. Clinical importance of polymicrobial bacteremia. Diagn. Microbiol. Infect. Dis. 5:185–196 [DOI] [PubMed] [Google Scholar]
- 52. Wieser A., et al. 2010. A multiepitope subunit vaccine conveys protection against extraintestinal pathogenic Escherichia coli in mice. Infect. Immun. 78:3432–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wisplinghoff H., et al. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]