Abstract
The herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) is the only HSV-1 gene transcript abundantly expressed throughout latency. LAT null mutants have a significantly reduced reactivation phenotype. LAT's antiapoptosis activity is the major LAT factor involved in supporting the wild-type reactivation phenotype. During HSV-1 latency, some ganglionic neurons are surrounded by CD8 T cells, and it has been proposed that these CD8 T cells help maintain HSV-1 latency by suppressing viral reactivations. Surprisingly, despite injection of cytotoxic lytic granules by these CD8 T cells into latently infected neurons, neither apoptosis nor neuronal cell death appears to occur. We hypothesized that protection of latently infected neurons against cytotoxic CD8 T-cell killing is due to LAT's antiapoptosis activity. Since CD8 T-cell cytotoxic lytic granule-mediated apoptosis is critically dependent on granzyme B (GrB), we examined LAT's ability to block GrB-induced apoptosis. We report here that (i) LAT can interfere with GrB-induced apoptosis in cell cultures, (ii) LAT can block GrB-induced cleavage (activation) of caspase-3 both in cell culture and in a cell-free in vitro cell extract assay, and (iii) LAT can protect C1300 and Neuro2A cells from cytotoxic CD8 T-cell killing in vitro. These findings support the hypothesis that LAT's antiapoptosis activity can protect latently infected neurons from being killed by CD8 T-cell lytic granules in vivo.
Herpes simplex virus type 1 (HSV-1) is ubiquitous worldwide. Estimates of the percentage of adults that harbor latent HSV-1 range from just under 50% to over 90% (6, 7, 13, 19, 44, 47, 61, 69). HSV-1 can infect mucosal sites, in particular the eyes, mouth, and genitals. Following primary infection at the periphery, the virus travels up axons and becomes latent in sensory neurons of the host's peripheral nervous system (PNS). HSV-1 latency is lifelong. Sporadic spontaneous reactivations of the virus from the PNS can result in return of infectious virus to the original peripheral site of infection. This reactivated virus, in the absence of clinical disease, is shed from mucosal surfaces and can be transmitted to others. Less often, viral reactivations and shedding result in recurrent pathology at the site of the original infection, such as genital lesions, cold sores in and around the mouth, and corneal disease that can lead to loss of vision. In the United States, recurrent HSV-1-induced corneal disease is the leading cause of corneal blindness due to an infectious agent (48, 59). In addition, HSV-1 causes a severe form of focal necrotizing encephalitis that affects over 2,000 people in the United States each year (20, 40, 67, 68). Reactivations of HSV-1, rather than primary infections, are responsible for most incidences of HSV-1-induced disease and the majority of viral transmissions. Thus, understanding the molecular mechanisms of the HSV-1 latency-reactivation cycle is critical for developing methods of preventing both viral spread and HSV-1-induced disease.
During neuronal latency, high levels of HSV-1 latency-associated transcript (LAT) RNA can be readily and consistently detected (58, 62). Other viral RNAs are either not detectable or are present in comparatively small amounts, with the exception of AL and AL3, which overlap LAT in an antisense orientation (24, 56). HSV-1 LAT null mutants generally have a reduced reactivation phenotype (38, 55, 66), indicating that LAT plays an important role in the HSV-1 latency-reactivation cycle. LAT can block apoptosis (54), a finding that has been confirmed by a large body of evidence (2, 5, 9, 21, 23, 28, 30, 31, 45, 53, 57). Furthermore, wild-type levels of reactivation can be restored to a LAT null mutant virus by “rescuing” the virus with each of 3 different antiapoptosis genes (27, 31, 45, 57). Thus, LAT's antiapoptosis activity appears to be the dominant LAT function responsible for supporting the wild-type reactivation phenotype in small-animal models of HSV-1 latency.
LAT can block the two major mammalian apoptotic pathways, namely, the extrinsic apoptotic pathway (also called the caspase-8-dependent pathway or the tumor necrosis factor [TNF]/Fas ligand pathway) and the intrinsic pathway (also called the caspase-9-dependent pathway or the mitochondrial pathway) (22, 28, 53). However, the specific pathway details of the LAT-apoptotic factor interactions by which LAT influences apoptosis remain to be fully determined.
Granzyme B (GrB) is a serine protease present in cytotoxic granules of CD8 T cells and natural killer cells. Lytic granule-mediated apoptosis is critically dependent on GrB (51). GrB is important in eliminating virus-infected cells and cancer cells (11). It does this by inducing apoptosis, thus killing the cells. GrB is the most studied of the 5 known human and 11 known mouse granzymes. Similar to caspases, GrB cleaves after aspartic acid. GrB can directly cleave and activate caspase-3, as well as other proteins known to regulate apoptosis. Cleavage of caspase-3 appears to be the main mechanism by which GrB induces apoptosis (see references 11 and 51 for reviews of GrB).
Work by researchers in the Hendricks lab (37) and others (5) has shown that a subset of latently infected neurons in mouse trigeminal ganglia (TG) are surrounded by CD8 T cells. In human trigeminal ganglia that harbor latent HSV-1, there is also a chronic inflammatory response, which appears to be similar to what is seen in rodents latently infected with HSV-1 (63). In general, it is believed that the chronic inflammatory response, in particular the presence of CD8 T cells, promotes maintenance of latency. Support of this prediction comes from the finding that GrB can cleave (thus presumably inactivating) the essential HSV-1 immediately early (IE) protein ICP4 (37). Disruption of this essential HSV-1 gene product should terminate virus replication in neurons in which HSV-1 reactivation has begun. This would abort reactivation and prevent detection of infectious virus due to reactivation of virus from latently infected sensory neurons.
Surprisingly, in the study from the Hendricks lab, no activated caspases were detected in any of the 13 latently infected mouse sensory neurons into which lytic granules had been released by CD8 T cells and there was no evidence of neuronal cell death (37). We report here that LAT can interfere with GrB-induced apoptosis and that LAT can protect C1300 and Neuro2A cells from killing by CD8 T cells in vitro. These findings suggest that LAT's antiapoptosis activity protects latently infected neurons from being killed by apoptosis mediated by CD8 T-cell lytic granules in vivo.
MATERIALS AND METHODS
Cells.
Figure 1 shows a schematic representation of the LAT regions inserted into each stable cell line. The C1300-derived mouse neuroblastoma cell lines DC-LAT6 and DC-ΔLAT311, which are stably transfected with LAT sequences, were described previously (9). Briefly, the DC-LAT6 cell line contains a NotI-NotI restriction fragment of HSV-1 that includes 361 nucleotides (nt) of the LAT promoter followed by the first 3,225 nt of the primary LAT sequence. DC-LAT6 cells express abundant levels of LAT and are resistant to cold shock-induced apoptosis (9). DC-ΔLAT311 cells (the LAT-nonexpressing [LAT−] control) contain the same NotI-NotI restriction fragment but lack the LAT TATA box and the start site for LAT transcription (nt −130 to +64, a PstI-PstI restriction fragment). These cells express low or undetectable levels of the 2-kb stable LAT intron and are not resistant to cold shock-induced apoptosis. JWLAT cells are Neuro2A cells that stably contain a HpaI-HpaI restriction fragment corresponding to LAT nt −1801 to +1499. These LAT+ cells express the first 1,499 nt of LAT driven by the LAT promoter and are resistant to cold shock-induced apoptosis (unpublished data). JW-ΔLAT cells are LAT− control cells containing the empty plasmid used for expression of LAT in the JWLAT cells. JWLAT and JW-ΔLAT cells were established by puromycin selection of Neuro2A cells that were transfected with the pPUR plasmid (Clontech) expressing the first 1,499 nt of LAT driven by the LAT promoter and with the empty pPUR vector alone, respectively. DC-LAT6 and DC-ΔLAT311 cells were grown in minimal essential medium (MEM) containing 10% (vol/vol) heat-inactivated fetal calf serum, penicillin (10 U/ml), streptomycin (100 μg/ml), 1 mM sodium pyruvate, 1× MEM nonessential amino acids, and 1 mg/ml G-418. The JWLAT and JW-ΔLAT cells were grown in the same medium but with 1 μg/ml puromycin in place of the G-418.
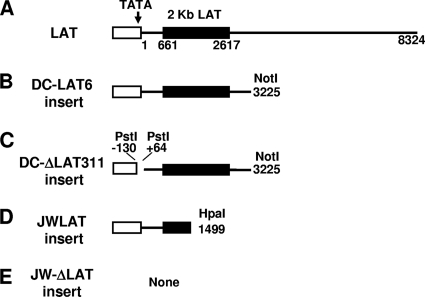
FIG. 1.
Schematic representation of the LAT regions expressed in C1300- and Neuro2A-derived stable cell lines. (A) The LAT promoter (hollow rectangle) containing the TATA box, the start of LAT transcription (nt 1), the beginning (nt 661) and end (nt 2617) of the stable 2-kb LAT intron (solid rectangle), and the end of the primary LAT sequence (nt 8324) are shown. (B) The DC-LAT6 insert contains the first 3,225 nt of the primary LAT driven by the LAT promoter, cloned into the NotI site of plasmid pSL301. (C) The DC-ΔLAT311 insert is identical to the DC-LAT6 insert except for a PstI-PstI deletion that deletes key LAT promoter elements, including the TATA box, resulting in only trace transcription of LAT (presumably from a cellular promoter). (D) The JWLAT insert contains the first 1,499 nt of LAT driven by the LAT promoter, cloned into the BamHI site of plasmid pPUR. (E) The JW-ΔLAT insert is the blank plasmid used for JWLAT cells. DC-LAT6 and DC-ΔLAT311 inserts are in C1300 cells and were described previously (9). JWLAT and JW-ΔLAT inserts are in Neuro2A cells and were constructed as described in Materials and Methods. The Neuro2A-based JWLAT and JW-ΔLAT cell lines and the C1300-based DC-LAT6 and DC-ΔLAT311 cell lines were generated at different times and by different people.
Delivery of granzyme B into cells.
Active recombinant mouse GrB (catalog no. G9278 [Sigma] and catalog no. 40-125 [ProSci]) was transduced into cells by using the BioPORTER QuikEase protein delivery kit (catalog no. BPQ24) according to the instructions of the manufacturer (Sigma) with some minor modifications. Briefly, 3 × 104 cells were seeded onto individual coverslips in wells of a 24-well culture plate and cultured overnight. GrB (1.25 μg) in a BioPORTER-protein mix was added to the medium in each well (yielding a final GrB concentration of 6 μg/ml). Cells were incubated at 37°C for 24 h, and then the numbers of surviving cells were determined by a trypan blue exclusion assay. For immunostaining to detect cleaved caspase-3, the cells were treated identically, except that incubation was for 4 h instead of 24 h. Preliminary experiments using GrB labeled with fluorescein isothiocyanate (FITC) indicated that 90% of cells took up significant amounts of the GrB.
Immunostaining.
Cells treated for 4 h with GrB were washed three times in phosphate-buffered saline (PBS) and then fixed in freshly prepared 4% paraformaldehyde-0.1% Triton X-100 in PBS for 30 min. The fixed cells were washed three times in PBS for 15 min each time and then permeabilized in 0.05% saponin in PBS for 30 min. Next, the cells were blocked for 60 min in blocking buffer (10% normal goat serum in PBS) and incubated for 4 h with rabbit polyclonal antibody specific for cleaved caspase-3 (diluted 1:1,000; catalog no. 9661 [Cell Signaling]). The cells were washed three times for 10 min each in 0.1% NP-40 in PBS and incubated for 1 h with fluorescein-conjugated goat anti-rabbit antibody (diluted 1:2,000; catalog no. A11070 [Invitrogen]). The cells were then washed three times for 10 min each in 0.1% NP-40 in PBS, subjected to staining with 1 μg/ml DAPI (4′,6-diamidino-2-phenylindole; Invitrogen), and examined under a Nikon Eclipse E600 fluorescence microscope.
Preparation of cell extracts.
Cells (106) were seeded into culture medium in a 100-mm dish and incubated for 24 h at 37°C in a 5% CO2 incubator. The cells were then harvested, collected by centrifugation (at 1,000 × g for 5 min at 4°C), washed once with ice-cold PBS, and resuspended in 5 volumes of ice-cold buffer A (20 mM HEPES-KOH [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM dithiothreitol [DTT], 0.1% Triton X-100, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) supplemented with protease inhibitors. After sitting on ice for 15 min, the cells were disrupted by 15 passages through a 22-gauge needle. The cell extracts were centrifuged at 1.8 × 104 × g for 30 min at 4°C in a tabletop centrifuge (Beckman). The supernatants were treated with a mixture of RNase A and RNase T1 (catalog no. AM2286; Ambion) at concentrations of 40 and 1,000 U/ml, respectively, for 30 min at 37°C. Active recombinant human GrB (final concentration, 0.05 μg/ml; catalog no. 1118-5 [BioVision]) was added to 60 μg of cell extract, the mixture was incubated at 37°C for 2 h, and caspase-3 cleavage was evaluated by Western blotting.
Western blot analysis.
Immunoblotting was performed with horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G (IgG) by using enhanced chemiluminescence (ECL) Western blotting detection reagents according to the instructions of the manufacturer (Pierce). Polyclonal antibody (catalog no. MAB374; Millipore) specific for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used to estimate relative GAPDH levels as a loading control.
CD8+ T-cell-mediated killing.
C57BL/6 (H-2d) mice were immunized at the base of the tail with 107 Neuro2A cells in 100 μl of PBS. Neuro2A and C1300 cells, the parental cells for JWLAT/JW-ΔLAT and DC-LAT6/DC-ΔLAT311 cells, respectively, were derived independently from the same A/J (H-2a) mouse neuroblastoma and should induce similar immune responses. At 15 days after the initial injection, mice received a second immunization, and 3 days later they were euthanized. Draining lymph nodes were harvested, and CD8+ T cells were purified using a mouse CD8+ T-cell isolation kit (Miltenyi Biotec). CD8+ T cells were stimulated in vitro for 6 days with mitomycin C-treated Neuro2A cells in the presence of interleukin-2 (IL-2; 5 ng/ml) and IL-7 (10 ng/ml). The stimulated CD8+ T cells were then incubated with the LAT+ or LAT− stable cell lines that had been prelabeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) at effector/target (E/T) ratios of 0:1, 6:1, 12:1, and 24:1 for 2 to 3 h in fluorescence-activated cell sorter (FACS) tubes. The cells were then washed with FACS buffer and analyzed by flow cytometry using a FACScan cytometer (BD Biosciences). The acquired data were analyzed with CellQuest software (BD Biosciences).
RESULTS
C1300 cells stably expressing the first 3,225 nt of LAT are resistant to GrB-induced death.
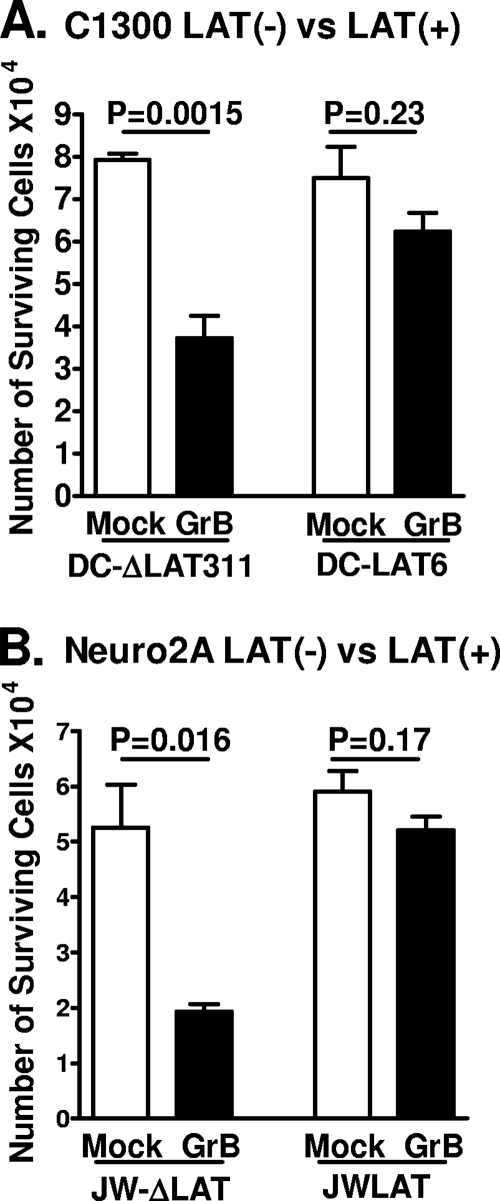
The mouse neuroblastoma cell line C1300 has been widely used as a neuronal tissue culture model to study various aspects of herpes simplex virus (9, 12, 17, 18, 33-35, 42, 43, 46, 50, 60, 70, 71). DC-LAT6 cells, which we described previously (9), are a clonal C1300-derived cell line expressing the first 3,225 nt of the primary 8.3-kb LAT (Fig. 1B). Cell cultures were treated with 6 μg/ml of GrB as described in Materials and Methods, and cell survival was evaluated by trypan blue exclusion 24 h later. DC-ΔLAT311 cells, which contain the same LAT sequences as DC-LAT6 cells but do not express LAT because a PstI-PstI restriction fragment containing the LAT TATA box is deleted (9) (Fig. 1C), were used as controls. GrB treatment of the LAT− cells significantly decreased cell survival compared to mock treatment (Fig. 2A, left) (P = 0.0015). In contrast, GrB treatment did not significantly decrease survival of the LAT+ DC-LAT6 cells (Fig. 2A, right) (P = 0.23). As expected, survival rates were similar for the two cells lines when cells did not receive GrB (P = 0.60). In addition, the survival rate was significantly higher for the GrB-treated LAT+ cells than for the GrB-treated LAT− cells (P = 0.02). Thus, LAT appeared to protect cells against GrB-induced death. Since GrB kills cells by inducing apoptosis (see references 11 and 51 for reviews), this finding supports the hypothesis that LAT's antiapoptosis activity can protect C1300 cells against GrB-induced apoptosis.
FIG. 2.
LAT expression in DC-LAT6 and JWLAT cells decreased GrB-induced cell death. (A) Subconfluent monolayers of LAT+ DC-LAT6 and LAT− DC-ΔLAT311 cells in 24-well plates were treated with 6 μg/ml of mouse GrB in serum-free medium for 24 h. The number of viable cells in each individual well was determined for at least 3 wells/group by trypan blue exclusion. The average number of viable cells per well ± the standard deviation (SD) for each group is shown. (B) Survival rates for LAT+ JWLAT cells and LAT− JW-ΔLAT cells were determined as described in the legend to panel A, except that GrB treatment in serum-free medium was done for 6 h rather than 24 h, following which the cells were fed with medium containing 10% serum and incubated overnight. Panel B shows results for 1 of 3 independently performed experiments, all with similar outcomes. Taken together, panels A and B therefore represent the results of 4 independent experiments, each done in triplicate.
Neuro2A cells stably expressing LAT nt 1 to 1499 are also resistant to GrB-induced death.
To confirm that the above-described result was not due to an artifact of the DC-LAT6 cell line, we constructed a second, completely independent cell line stably expressing a different portion of LAT, as described in Materials and Methods. This cell line was produced from Neuro2A cells, another commonly used mouse neuroblastoma-derived cell line (8, 29, 54). Neuro2A and C1300 cells are actually closely related since the two lines were isolated from different portions of the same mouse neuroblastoma. The second LAT+ cell line expresses LAT nt 1 to 1499 and is designated JWLAT (Fig. 1D). This LAT region is responsible for the majority of LAT's antiapoptosis activity (21, 23). The LAT− control cells (JW-ΔLAT) (Fig. 1E) for JWLAT cells are Neuro2A cells containing the empty pPUR plasmid used to construct the JWLAT cells. Cell monolayers were treated with GrB, and survival was assessed as described above. As with the C1300-based cell lines, (i)GrB treatment of the LAT− cells significantly decreased survival compared to mock treatment (Fig. 2B, left) (P = 0.016), (ii) GrB treatment did not significantly decrease survival of the LAT+ cells (Fig. 2B, right) (P = 0.17), (iii) survival rates were similar for the two cells lines when cells did not receive GrB (P = 0.48), and (iv) the survival rate was significantly higher for the GrB-treated LAT+ cells than for the GrB-treated LAT− cells (P = 0.0002). These results confirm the result obtained with the C1300 cell lines, that LAT can protect against GrB-induced cell death, and strongly suggest that the first 1.5 kb of LAT is sufficient for this protection.
LAT protects against caspase-3 cleavage in GrB-treated cells.
To confirm that the cell death seen following treatment of cells with GrB as described above was due to apoptosis, LAT+ (DC-LAT6 and JWLAT) cells and the respective LAT− (DC-ΔLAT311 and JW-ΔLAT) control cells were treated with GrB as described above and then stained with polyclonal antibody specific for cleaved (activated) caspase-3 followed by secondary FITC-labeled IgG antibody as described in Materials and Methods. Representative fluorescence photomicrographs of mock-treated and GrB-treated LAT− DC-ΔLAT311 cells are shown in Fig. 3A. Little or no cleaved-caspase-3 signal is seen in mock-treated [GrB (−)] cells, while significant signal is seen in the GrB-treated cells. The results for both LAT+ cell lines and the LAT− controls are quantitated in Fig. 3B and C. In DC-ΔLAT311 and DC-LAT6 cells not exposed to GrB, only low background levels of cleaved-caspase-3 staining were detected, and the percentages of positive cells were similar for the two cell lines (Fig. 3B, left) (P = 0.60). In contrast, when these cells were treated with GrB, a significantly higher percentage of LAT− DC-ΔLAT311 cells (∼60%) than of LAT+ DC-LAT6 cells (∼19%) were positive (Fig. 3B, right) (P < 0.0001). Similarly, only low levels of background staining were seen with untreated JWLAT and JW-ΔLAT cells (P = 1.0), while significantly more GrB-treated JW-ΔLAT cells (∼60%) than GrB-treated JWLAT cells (∼12%) had staining (Fig. 3C, right) (P < 0.0001). The results of these studies with two independent cell lines expressing LAT strongly suggest that, as expected, GrB treatment induces caspase-3 cleavage (a hallmark of apoptosis) in C1300 and Neuro2A cells and that this induction is blocked or significantly decreased by LAT. Thus, the cell death represented in Fig. 2 was consistent with GrB-induced apoptosis. Furthermore, these results indicate that C1300 and Neuro2A cells expressing LAT are protected against GrB-induced apoptosis and that LAT nt 1 to 1499 are sufficient for this protection.
FIG. 3.
LAT expression in DC-LAT6 and JWLAT cells decreased GrB-induced apoptosis as judged by intracellular staining for cleaved (activated) caspase-3. Subconfluent cell monolayers on coverslips were treated with GrB as described in the legend to Fig. 2 [GrB (+)] or mock treated [GrB (−)] and then stained for cleaved caspase-3 by using a rabbit antibody specific for activated, cleaved caspase-3. (A) Examples of activated-caspase-3 staining in LAT− LATΔ311 cells with and without GrB treatment are shown. Arrows indicate some cells with obvious apoptotic morphologies. (B) The percentages of DC-ΔLAT311 and DC-LAT6 apoptotic cells as judged by positivity for cleaved-caspase-3 staining are shown (on the y axis). The numbers above each bar are the number of cleaved-caspase-3-positive cells and the total number of cells counted. P values were determined by a two-sided chi-square test. (C) The percentages of cleaved-caspase-3-positive JWLAT and JW-ΔLAT cells are shown. The results shown for JWLAT and JW-ΔLAT cells are representative of results from 2 independent experiments.
LAT can inhibit GrB-induced cleavage of caspase-3 in an in vitro cell extract system.
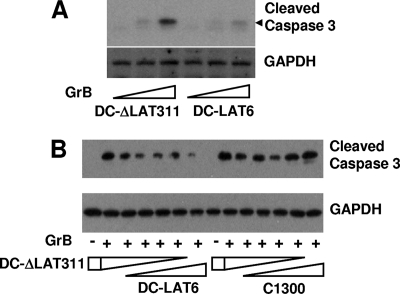
LAT+ DC-LAT6 cell extracts and LAT− DC-ΔLAT311 cell extracts were prepared as described in Materials and Methods. Increasing amounts of GrB were added, and the relative amounts of caspase-3 cleavage induced by GrB were visualized by Western blotting using polyclonal antibody specific for cleaved (activated) mouse caspase-3 (Fig. 4A). Less caspase-3 cleavage was seen in DC-LAT6 extracts than in DC-ΔLAT311 extracts, again strongly suggesting that LAT blocks GrB-induced cleavage of caspase-3. In a different study (Fig. 4B), DC-ΔLAT311 and DC-LAT6 extracts were mixed together at various ratios. The same amounts of GrB were added to the individual samples, and caspase-3 cleavage was examined by Western blotting as described above. As the amount of LAT+ DC-LAT6 extract relative to LAT− DC-ΔLAT311 extract increased, the amount of caspase-3 cleavage decreased. This again illustrated that LAT+ extracts were resistant to GrB-induced caspase-3 cleavage. The right side of Fig. 4B shows results for a control in which increasing amounts of parental C1300 cell extract were mixed with decreasing amounts of DC-ΔLAT311 cell extract. In other words, the LAT+ DC-LAT6 cell extract used for the left side of Fig. 4B was replaced by the same amounts of parental C1300 cell extract. As expected, no dramatic differences in levels of cleaved caspase-3 were detected.
FIG. 4.
GrB-induced caspase-3 cleavage in extracts of DC-LAT6 and DC-ΔLAT311 cells. (A) Cell extracts prepared as described in Materials and Methods were treated with increasing amounts of GrB in vitro as indicated (bottom) and analyzed by Western blotting using cleaved-caspase-3-specific rabbit antibody. Doses of GrB were 0, 0.025, and 0.05 μg/ml for 2 h. The predicted location of the cleaved-caspase-3 band is indicated by the black arrowhead. GAPDH is a loading control. The results shown are representative of results from 3 independent experiments. (B) Extracts from DC-ΔLAT311 and DC-LAT6 cells (left) or extracts from DC-ΔLAT311 and parental C1300 cells (right) were mixed together at various ratios (1:0, 4:1, 3:2, 2:3, 1:4, and 0:1) and treated with 0.2 μg/ml of GrB (+) or left untreated (−).
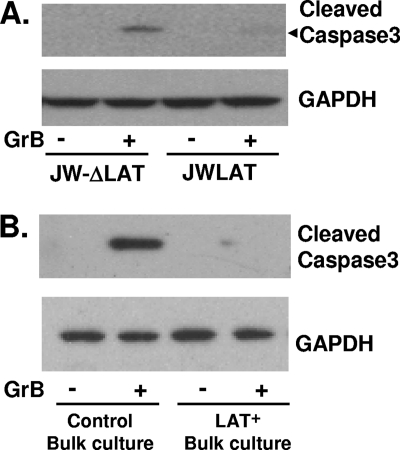
We similarly examined the effect of adding GrB to extracts of LAT+ JWLAT cells. In contrast to the LAT− JW-ΔLAT cell extracts, the LAT+ JWLAT cell extracts were resistant to GrB-induced cleavage of caspase-3 (Fig. 5). This result is similar to that seen for the LAT+ DC-LAT6 cells. Thus, in contrast to the respective control cell lines, both LAT-expressing cell lines were resistant to GrB-induced cleavage of caspase-3.
FIG. 5.
GrB-induced caspase-3 cleavage in extracts of JWLAT and JW-ΔLAT cells. (A) Extracts of JWLAT and JW-ΔLAT cells were prepared, treated with 0.05 μg/ml of GrB, and analyzed by Western blotting as described in the legend to Fig. 4A. The results shown are representative of results from 2 independent experiments. (B) Extracts of bulk Neuro2A cell cultures transfected with either the JWLAT or the JW-ΔLAT plasmid (see the text) were prepared and treated as described in the legend to panel A.
Although in the above-described experiments similar results were obtained with two completely independent clonal cell lines expressing LAT, it was useful to perform an additional study to ensure that the LAT+ clonal cell lines had not somehow acquired a mutation that rendered them insensitive to GrB-induced apoptosis. In an independent experiment, Neuro2A cells were therefore transfected with the same plasmids used to make the Neuro2A clonal cell lines (JWLAT and JW-ΔLAT), and antibiotic selection was initiated to eliminate cells that were not efficiently transfected. However, no cell cloning was performed. Instead, the total culture was then maintained with routine cell passage as needed. The resulting “bulk culture” thus comprised cells resistant to the antibiotic, the vast majority of which therefore contained the input plasmid. Cell extracts from the bulk cultures were then treated with GrB, and caspase-3 cleavage was examined (Fig. 5B) using the same methods used for the experiment represented in Fig. 5A. The results obtained with the bulk cultures were virtually identical to those obtained with the corresponding clonal cell lines (compare Fig. 5B with Fig. 5A). Little or no cleaved caspase-3 was seen in cells transfected with the JWLAT plasmid, in contrast to those transfected with the JW-ΔLAT plasmid. Thus, as with the LAT-expressing clonal cell lines, the uncloned bulk selected cells were resistant to GrB-induced cleavage of caspase-3. The results seen with the two independent LAT-expressing clonal cell lines were therefore due to the presence of LAT rather than unexpected cloning artifacts.
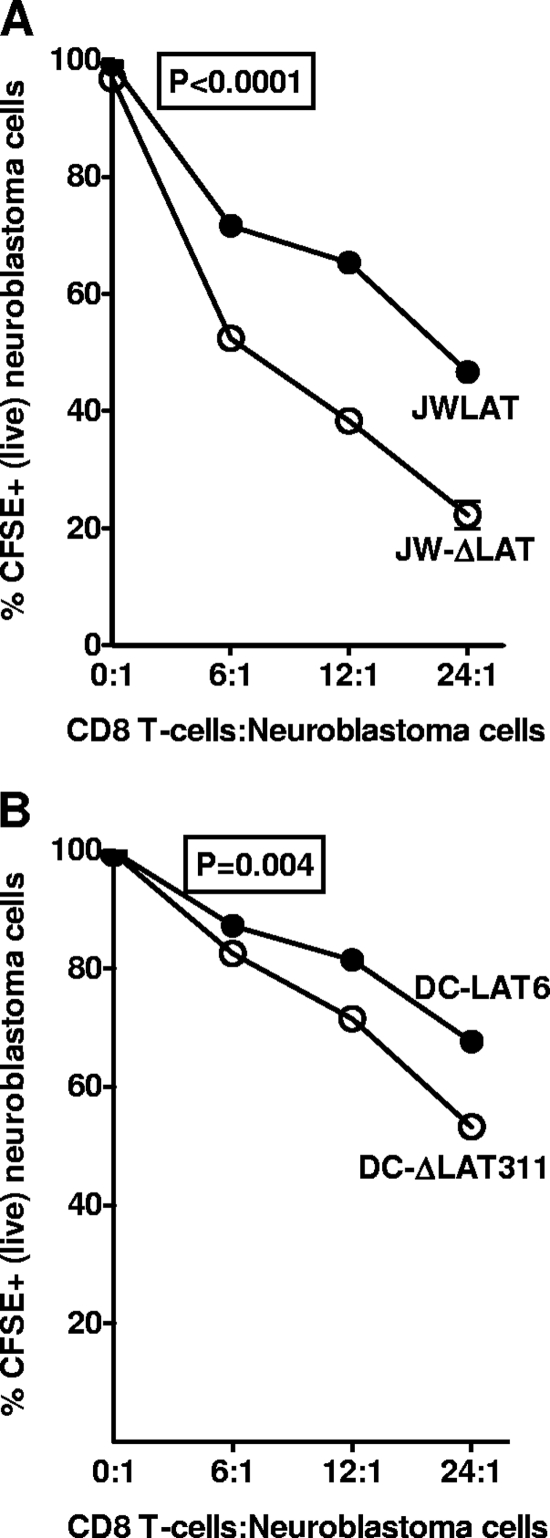
LAT expression protects JWLAT and DC-LAT6 cells against allogeneic CD8+ T-cell-mediated killing.
To determine if LAT can protect cells against cytotoxic CD8 T cells, we tested the ability of our LAT-expressing stable cell lines to resist killing by allogeneic CD8+ T cells. Allogeneic CD8+ T cells specific to Neuro2A cells were prepared as described in Materials and Methods. JWLAT and JW-ΔLAT cells were labeled with CFSE and then incubated with increasing concentrations of the allogeneic CD8 T cells. When cells die, either by apoptosis or necrosis, their membranes becomes leaky and CSFE is progressively lost, until the cells become CFSE negative. Thus, loss of CSFE indicates killing by the CD8 T cells. In contrast to LAT− JW-ΔLAT cells, the LAT+ JWLAT cells were resistant to CD8 T-cell-mediated killing (Fig. 6A) (P < 0.0001). The LAT+ DC-LAT6 cells were also more resistant to CD8 T-cell killing than the LAT− DC-ΔLAT311 control cells (Fig. 6B) (P = 0.004). Thus, cells expressing LAT were more resistant to CD8 T-cell-mediated killing.
FIG. 6.
LAT inhibits cell death caused by mouse CD8 T cells. CD8 T cells from mice immunized with Neuro2A cells were stimulated in vitro, and increasing amounts were incubated with LAT+ or LAT− cells that had been prelabeled with CFSE as described in Materials and Methods. P values were determined by a two-sided paired Student t test.
DISCUSSION
A typical host cell response to viral infection is apoptosis. This can be considered to be an innate immune response to viral infection. If apoptosis occurs rapidly, the host cell will die before viral replication is completed. This would decrease virus spread and overall host viral load. Thus, many viruses have evolved genes that block apoptosis (reviewed in reference 4). Induction of apoptosis by GrB is one mechanism by which cytotoxic CD8 T cells destroy targeted cells (see references 11 and 51 for reviews). Thus, LAT can be considered to be acting in an immune evasion capacity when it blocks GrB killing of latently infected neurons. To our knowledge, this is the first report to show that LAT can block a GrB function. Interestingly, we previously reported a different immune evasion tactic facilitated by LAT. LAT delays or alters production of interferon (IFN) in neuronal cells and TG of infected mice (52). Thus, LAT appears to have at least two distinct immune evasion mechanisms.
GrB is a cell death-inducing protein present in cytotoxic granules of cytotoxic T lymphocytes and natural killer cells (see reference 11 for a review). It is a serine protease that cleaves after aspartic acid residues (reviewed in references 41 and 65), at the same sites cleaved by caspases. Thus, GrB can induce apoptosis by cleaving (activating) various caspases. This includes caspase-3, a key executioner or effector caspase. Direct cleavage of caspase-3 is thought to be the main method by which mouse GrB kills targeted cells (1, 15). Human GrB also kills targeted cells by cleaving caspase-3. In addition, human GrB can induce apoptosis by directly cleaving Bid or ICAD (10, 14, 64). Thus, if caspase inhibitors are present, human GrB can kill target cells by inducing apoptosis via mitochondrial damage or DNA damage. There is some species specificity to the mouse and human forms of GrB. Mouse GrB cleaves mouse caspase-3 more efficiently than human GrB, while human GrB cleaves human caspase-3 more efficiently than mouse GrB.
We have shown previously that LAT's antiapoptosis activity is crucial with respect to how LAT enhances the reactivation phenotype, since the wild-type reactivation phenotype can be restored to a LAT− mutant by replacing the LAT gene with several different antiapoptotic genes (27, 31, 45, 57). In this study, we showed that two different neuron-derived cell lines that stably express LAT are resistant to apoptosis induced by GrB. Since GrB can directly cleave caspase-3, this finding suggests that LAT, a LAT product, or a LAT-induced cell product can either (i) bind to caspase-3 and prevent its cleavage, (ii) bind to GrB and prevent it from cleaving caspase-3, or (iii) bind to a caspase-3-GrB complex, thus preventing caspase-3 cleavage. The analyses presented in Fig. 2, 3, and 4 of this report were performed using mouse cells (C1300 and Neuro2A cells), and mouse GrB was therefore used for examining the effects of LAT on cell survival (Fig. 2) and the cleavage of caspase-3 (Fig. 2 and 3). Since humans are the natural host for HSV-1 and since mouse and human forms of GrB may differ, it was of interest to confirm that LAT can also block caspase-3 cleavage induced by human GrB. Thus, human GrB was used in the cell extract experiments (Fig. 4 and 5). Similar results were obtained using mouse GrB in cell extract experiments (data not shown). Our results strongly suggest that mouse neuron-derived cells expressing LAT are resistant to activation of caspase-3 by both human and mouse forms of GrB.
Previous studies by researchers in the Hendricks lab (16, 36, 37, 39) have shown that a small number of sensory neurons in the TG of mice latently infected with wild-type HSV-1 are surrounded by CD8 T cells. The authors proposed that these CD8 T cells maintain latency by preventing reactivation. They then showed that the CD8 T cells inject lytic granules into these neurons and that the GrB in these lytic granules can specifically cleave and inactivate the critical HSV-1 immediate early protein ICP4 without killing the neuron (37). This would be expected to inhibit virus replication, thus preventing reactivated virus from producing infectious virus. This outcome would give the appearance that the CD8 T cells prevented reactivation, although we would prefer the interpretation that the CD8 T cells abort or significantly decrease the production of infectious virus once reactivation has begun or that CD8 T cells promote maintenance of latency by reducing the frequency of reactivation events that go to completion. Without LAT, the presence of GrB would be expected to lead to a reduced number of latently infected neurons over time. This pattern has been observed in mice latently infected with LAT mutants (32).
CD8 T-cell lytic granules typically kill the target cell by inducing apoptosis. However, the CD8 T-cell studies found no evidence of apoptosis in the neurons and no evidence that neurons were being killed (37). This raises the question of why the latently infected neurons that were injected with lytic granules did not undergo apoptosis and die. The key proapoptotic factor in lytic granules is GrB. We showed previously that LAT can block apoptosis induced by a variety of factors (23, 53, 54). In this study, we have shown for the first time that LAT can block GrB-induced apoptosis and, specifically, GrB cleavage of caspase-3. Furthermore, we showed that LAT can protect cells against CD8 T-cell mediated killing in vitro. This finding suggests that in vivo LAT can protect latently infected neurons (expressing LAT) from fatal attacks by cytotoxic CD8 T cells.
Thus, it appears that LAT is critical for survival of latently infected sensory neurons that are targeted by cytotoxic CD8 T cells. Although HSV-1 has several other antiapoptotic genes, including the US3, US5, ICP22, and ICP27 genes (3, 25, 26, 49), only the LAT gene is expressed at high levels during latency. Therefore, the LAT gene is the only HSV-1 antiapoptosis gene able to exert a significant antiapoptotic effect during latency and/or in the very early stages of reactivation. Thus, in the absence of continuous LAT expression during latency, the CD8 T cells would likely kill many of the neurons that initiate reactivation. If this killing occurred with a large number of neurons during the lifetime of a latently infected individual, the outcome would be loss of sensory neurons and a significantly reduced pool of latently infected neurons (32). This would result in decreasing HSV-1 reactivation rates with time and reduced viral transmission, situations that do not appear to occur.
It should also be noted here that we and others have shown that LAT can block the Fas-mediated apoptosis pathway (2, 21, 53). The GrB- and Fas-mediated apoptosis pathways are the two main pathways by which activated CD8 T cells induce apoptosis of targeted cells. Thus, LAT is likely to be able to block apoptosis induced by activated CD8 T cells in general, rather than blocking only GrB cleavage of caspase-3. Consistent with this possibility, we showed here for the first time that LAT can block CD8 T-cell-mediated killing in an in vitro cytotoxicity assay. This finding strongly suggests that cells expressing LAT in vivo, for instance, neurons latently infected with HSV-1, are resistant to being killed by cytotoxic CD8 T cells. Thus, the ability of LAT to block both GrB- and Fas-induced apoptosis is consistent with the expression of LAT's rendering cells resistant to CD8 T-cell killing.
In summary, we report here that mouse neuron-derived cells constitutively expressing LAT are refractory to apoptosis induced by GrB. This appears to be a result of LAT's directly or indirectly preventing cleavage of caspase-3 by GrB. Since human GrB can also induce apoptosis via additional apoptotic pathways, it will be of interest to determine if LAT can block human GrB-induced apoptosis in human neuron-derived cells expressing LAT. Importantly, we also showed here that cells expressing LAT are resistant to CD8 T-cell killing in an in vitro cytotoxic killing assay.
Acknowledgments
This work was supported by NIH grants EY013191, EY018171, EY014900, EY019896, NS33768, and 1P20RR15635, USDA grants 08-00891 and 09-01653, the Discovery Eye Foundation, the Henry L. Guenther Foundation, and a Research to Prevent Blindness (RPB) Challenge Grant.
S.L.W. is an RPB Senior Scientific Investigator.
Footnotes
Published ahead of print on 22 December 2010.
REFERENCES
- 1.Adrain, C., B. M. Murphy, and S. J. Martin. 2005. Molecular ordering of the caspase activation cascade initiated by the cytotoxic T lymphocyte/natural killer (CTL/NK) protease granzyme B. J. Biol. Chem. 280:4663-4673. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, M., M. Lock, C. G. Miller, and N. W. Fraser. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 76:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, M., and J. A. Blaho. 2001. Modulation of apoptosis during herpes simplex virus infection in human cells. Microbes Infect. 3:859-866. [DOI] [PubMed] [Google Scholar]
- 4.Best, S. M. 2008. Viral subversion of apoptotic enzymes: escape from death row. Annu. Rev. Microbiol. 62:171-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branco, F. J., and N. W. Fraser. 2005. Herpes simplex virus type 1 latency-associated transcript expression protects trigeminal ganglion neurons from apoptosis. J. Virol. 79:9019-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brugha, R., K. Keersmaekers, A. Renton, and A. Meheus. 1997. Genital herpes infection: a review. Int. J. Epidemiol. 26:698-709. [DOI] [PubMed] [Google Scholar]
- 7.Bunzli, D., V. Wietlisbach, F. Barazzoni, R. Sahli, and P. R. Meylan. 2004. Seroepidemiology of Herpes Simplex virus type 1 and 2 in Western and Southern Switzerland in adults aged 25-74 in 1992-93: a population-based study. BMC Infect. Dis. 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter, D., et al. 2008. Introducing point mutations into the ATGs of the putative open reading frames of the HSV-1 gene encoding the latency associated transcript (LAT) reduces its anti-apoptosis activity. Microb. Pathog. 44:98-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter, D., et al. 2007. Stable cell lines expressing high levels of the herpes simplex virus type 1 LAT are refractory to caspase 3 activation and DNA laddering following cold shock induced apoptosis. Virology 369:12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casciola-Rosen, L., et al. 2007. Mouse and human granzyme B have distinct tetrapeptide specificities and abilities to recruit the Bid pathway. J. Biol. Chem. 282:4545-4552. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury, D., and J. Lieberman. 2008. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu. Rev. Immunol. 26:389-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook, M. L., R. L. Thompson, and J. G. Stevens. 1986. A herpes simplex virus mutant is temperature sensitive for reactivation from the latent state: evidence for selective restriction in neuronal cells. Virology 155:293-296. [DOI] [PubMed] [Google Scholar]
- 13.Corey, L., and P. G. Spear. 1986. Infections with herpes simplex viruses (1). N. Engl. J. Med. 314:686-691. [DOI] [PubMed] [Google Scholar]
- 14.Cullen, S. P., C. Adrain, A. U. Luthi, P. J. Duriez, and S. J. Martin. 2007. Human and murine granzyme B exhibit divergent substrate preferences. J. Cell Biol. 176:435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darmon, A. J., D. W. Nicholson, and R. C. Bleackley. 1995. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature 377:446-448. [DOI] [PubMed] [Google Scholar]
- 16.Decman, V., P. R. Kinchington, S. A. Harvey, and R. L. Hendricks. 2005. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 79:10339-10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de De La Pena, N. C., E. Bal, L. Puricelli, A. Diaz, and E. S. De Lustig. 1978. Interferon in the replication of herpes simplex virus in normal and pathological nerve cells. IARC Sci. Publ. 24:1055-1066. [PubMed] [Google Scholar]
- 18.Estridge, J. K., L. M. Kemp, and D. S. Latchman. 1990. The herpes simplex virus protein Vmw65 can trans-activate both viral and cellular promoters in neuronal cells. Biochem. J. 271:273-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatahzadeh, M., and R. A. Schwartz. 2007. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 57:737-766. [DOI] [PubMed] [Google Scholar]
- 20.Gesser, R. M., and S. C. Koo. 1997. Latent herpes simplex virus type 1 gene expression in ganglia innervating the human gastrointestinal tract. J. Virol. 71:4103-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, G., et al. 2002. Regulation of caspase 8- and caspase 9-induced apoptosis by the herpes simplex virus type 1 latency-associated transcript. J. Neurovirol. 8:103-111. [DOI] [PubMed] [Google Scholar]
- 22.Henderson, G., G. C. Perng, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2004. The latency-related gene encoded by bovine herpesvirus 1 can suppress caspase 3 and caspase 9 cleavage during productive infection. J. Neurovirol. 10:64-70. [DOI] [PubMed] [Google Scholar]
- 23.Inman, M., et al. 2001. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J. Virol. 75:3636-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaber, T., et al. 2009. Identification of a novel herpes simplex virus type 1 transcript and protein (AL3) expressed during latency. J. Gen. Virol. 90:2342-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jerome, K. R., et al. 2001. HSV and glycoprotein J. inhibit caspase activation and apoptosis induced by granzyme B or Fas. J. Immunol. 167:3928-3935. [DOI] [PubMed] [Google Scholar]
- 26.Jerome, K. R., et al. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin, L., et al. 2008. Cellular FLIP can substitute for the herpes simplex virus type 1 LAT gene to support a wild type reactivation phenotype in mice. J. Neurovirol. 14:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin, L., et al. 2003. Identification of herpes simplex virus type 1 latency-associated transcript sequences that both inhibit apoptosis and enhance the spontaneous reactivation phenotype. J. Virol. 77:6556-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin, L., et al. 2004. Methods for detecting the HSV-1 LAT anti-apoptosis activity in virus infected tissue culture cells. J. Virol. Methods 118:9-13. [DOI] [PubMed] [Google Scholar]
- 30.Jin, L., et al. 2007. Reactivation phenotype in rabbits of a herpes simplex virus type 1 mutant containing an unrelated antiapoptosis gene in place of latency-associated transcript. J. Neurovirol. 13:78-84. [DOI] [PubMed] [Google Scholar]
- 31.Jin, L., et al. 2005. A herpes simplex virus type 1 mutant expressing a baculovirus inhibitor of apoptosis gene in place of latency-associated transcript has a wild-type reactivation phenotype in the mouse. J. Virol. 79:12286-12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang, W., R. Mukerjee, and N. W. Fraser. 2003. Establishment and maintenance of HSV latent infection is mediated through correct splicing of the LAT primary transcript. Virology 312:233-244. [DOI] [PubMed] [Google Scholar]
- 33.Kemp, L. M., C. L. Dent, and D. S. Latchman. 1990. Octamer motif mediates transcriptional repression of HSV immediate-early genes and octamer-containing cellular promoters in neuronal cells. Neuron 4:215-222. [DOI] [PubMed] [Google Scholar]
- 34.Kemp, L. M., and D. S. Latchman. 1989. Regulated transcription of herpes simplex virus immediate-early genes in neuroblastoma cells. Virology 171:607-610. [DOI] [PubMed] [Google Scholar]
- 35.Kenny, J. J., et al. 1994. Identification of a second ATF/CREB-like element in the herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) promoter. Virology 200:220-235. [DOI] [PubMed] [Google Scholar]
- 36.Khanna, K. M., A. J. Lepisto, V. Decman, and R. L. Hendricks. 2004. Immune control of herpes simplex virus during latency. Curr. Opin. Immunol. 16:463-469. [DOI] [PubMed] [Google Scholar]
- 37.Knickelbein, J. E., et al. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leib, D. A., et al. 1989. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J. Virol. 63:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, T., K. M. Khanna, X. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lohr, J. M., J. A. Nelson, and M. B. Oldstone. 1990. Is herpes simplex virus associated with peptic ulcer disease? J. Virol. 64:2168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lord, S. J., R. V. Rajotte, G. S. Korbutt, and R. C. Bleackley. 2003. Granzyme B: a natural born killer. Immunol. Rev. 193:31-38. [DOI] [PubMed] [Google Scholar]
- 42.Lundstrom, M., S. Jeansson, and S. Olofsson. 1987. Host cell-induced differences in the O-glycosylation of herpes simplex virus gC-1. II. Demonstration of cell-specific galactosyltransferase essential for formation of O-linked oligosaccharides. Virology 161:395-402. [DOI] [PubMed] [Google Scholar]
- 43.Mador, N., A. Panet, D. Latchman, and I. Steiner. 1995. Expression and splicing of the latency-associated transcripts of herpes simplex virus type 1 in neuronal and non-neuronal cell lines. J. Biochem. 117:1288-1297. [DOI] [PubMed] [Google Scholar]
- 44.Miller, M. F., and I. I. Ship. 1977. A retrospective study of the prevalence and incidence of recurrent aphthous ulcers in a professional population, 1958-1971. Oral Surg. Oral Med. Oral Pathol. 43:532-537. [DOI] [PubMed] [Google Scholar]
- 45.Mott, K. R., et al. 2003. The bovine herpesvirus-1 LR ORF2 is critical for this gene's ability to restore the high wild-type reactivation phenotype to a herpes simplex virus-1 LAT null mutant. J. Gen. Virol. 84:2975-2985. [DOI] [PubMed] [Google Scholar]
- 46.Muggeridge, M. I., and N. W. Fraser. 1986. Chromosomal organization of the herpes simplex virus genome during acute infection of the mouse central nervous system. J. Virol. 59:764-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nahmias, A. J., F. K. Lee, and S. Beckman-Nahmias. 1990. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. Suppl. 69:19-36. [PubMed] [Google Scholar]
- 48.Nesburn, A. B. (ed.). 1983. Report of the corneal disease panel: vision research: a national plan 1983-1987, vol. II, part III. C. V. Mosby Co., St. Louis, MO.
- 49.Nguyen, M. L., and J. A. Blaho. 2007. Apoptosis during herpes simplex virus infection. Adv. Virus Res. 69:67-97. [DOI] [PubMed] [Google Scholar]
- 50.Olofsson, S., I. Sjoblom, and S. Jeansson. 1990. Activity of herpes simplex virus type 1-specified glycoprotein C antigenic site II epitopes reversibly modulated by peripheral fucose or galactose units of glycoprotein oligosaccharides. J. Gen. Virol. 71:889-895. [DOI] [PubMed] [Google Scholar]
- 51.Pardo, J., et al. 2009. The biology of cytotoxic cell granule exocytosis pathway: granzymes have evolved to induce cell death and inflammation. Microbes Infect. 11:452-459. [DOI] [PubMed] [Google Scholar]
- 52.Peng, W., et al. 2005. The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal ganglia of acutely infected mice. J. Virol. 79:6162-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng, W., et al. 2004. Mapping herpes simplex virus type 1 latency-associated transcript sequences that protect from apoptosis mediated by a plasmid expressing caspase-8. J. Neurovirol. 10:260-265. [DOI] [PubMed] [Google Scholar]
- 54.Perng, G., et al. 2000. Virus induced neuronal apoptosis blocked by the herpes simplex virus latency associated transcript (LAT). Science 287:1500-1503. [DOI] [PubMed] [Google Scholar]
- 55.Perng, G. C., et al. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045-8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perng, G. C., et al. 2002. A novel herpes simplex virus type 1 transcript (AL-RNA) antisense to the 5′ end of the latency-associated transcript produces a protein in infected rabbits. J. Virol. 76:8003-8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perng, G. C., et al. 2002. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J. Virol. 76:1224-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rock, D. L., et al. 1987. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol. 61:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith, R. E., H. R. McDonald, A. B. Nesburn, and D. S. Minckler. 1980. Penetrating keratoplasty: changing indications, 1947 to 1978. Arch. Ophthalmol. 98:1226-1229. [DOI] [PubMed] [Google Scholar]
- 60.Spivack, J. G., D. R. O'Boyle II, and N. W. Fraser. 1987. Novobiocin and coumermycin A1 inhibit viral replication and the reactivation of herpes simplex virus type 1 from the trigeminal ganglia of latently infected mice. J. Virol. 61:3288-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spruance, S. L. 1992. The natural history of recurrent oral-facial herpes simplex virus infection. Semin. Dermatol. 11:200-206. [PubMed] [Google Scholar]
- 62.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056-1059. [DOI] [PubMed] [Google Scholar]
- 63.Theil, D., et al. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 163:2179-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas, D. A., C. Du, M. Xu, X. Wang, and T. J. Ley. 2000. DFF45/ICAD can be directly processed by granzyme B during the induction of apoptosis. Immunity 12:621-632. [DOI] [PubMed] [Google Scholar]
- 65.Trapani, J. A., and V. R. Sutton. 2003. Granzyme B: pro-apoptotic, antiviral and antitumor functions. Curr. Opin. Immunol. 15:533-543. [DOI] [PubMed] [Google Scholar]
- 66.Trousdale, M. D., et al. 1991. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J. Virol. 65:6989-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitley, R. J. 1997. Herpes simplex virus. Lippincott-Raven Publishers, Philadelphia, PA.
- 68.Whitley, R. J. 1991. Herpes simplex virus infections of the central nervous system. Encephalitis and neonatal herpes. Drugs 42:406-427. [DOI] [PubMed] [Google Scholar]
- 69.Xu, F., et al. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964-973. [DOI] [PubMed] [Google Scholar]
- 70.Zwaagstra, J. C., H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1991. Identification of a major regulatory sequence in the latency associated transcript (LAT) promoter of herpes simplex virus type 1 (HSV-1). Virology 182:287-297. [DOI] [PubMed] [Google Scholar]
- 71.Zwaagstra, J. C., et al. 1990. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J. Virol. 64:5019-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]