Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) encodes two RING finger E3 ubiquitin ligases (MIR1 and MIR2) that mediate ubiquitination and degradation of cellular proteins important for the establishment of an efficient antiviral immune response. MIR1 and MIR2 share 30% sequence identity; however, their substrate preferences are varied. MIR1 has been shown to primarily downregulate major histocompatibility complex class I (MHC-I), whereas MIR2 can downregulate a wide range of cell surface proteins. Many of the MIR substrates are thought to be present in lipid raft microdomains, a subregion of the plasma membrane known to be important for a wide range of signal transduction events. Palmitoylation is a posttranslational modification that increases recruitment of transmembrane proteins to lipid rafts. In this study, we investigated the importance of palmitoylation for MIR function. We present evidence that MIR2-mediated downregulation of MHC-I and platelet endothelial cell adhesion molecule 1 (PECAM-1) but not other substrates is inhibited in the presence of the drug 2-bromohexadecanoic acid (2-Br), a chemical inhibitor of palmitoylation. Biochemical analysis indicates that MIR2 is directly palmitoylated on cysteine 146. Mutation of this cysteine to a phenylalanine prevents MIR2 palmitoylation and blocks the ability of MIR2 to downregulate MHC-I and PECAM-I but not B7.2 and intercellular adhesion molecule 1 (ICAM-I), consistent with the phenotype observed after 2-Br treatment. Unpalmitoylated MIR2 does not interact with MHC-I and is thus unable to ubiquitinate and downregulate MHC-I from the cell surface. Furthermore, we observed that MIR2 is palmitoylated in vivo during lytic infection. Palmitoylation may act to regulate MIR2 function and localization during viral infection by allowing MIR2 to properly interact with and downregulate multiple substrates known to play an important role in the host immune response.
Kaposi's sarcoma-associated herpesvirus (KSHV) is a large, double-stranded DNA gamma-2 herpesvirus that is the causative agent of Kaposi's sarcoma as well as two lymphoproliferative disorders: primary effusion lymphoma and multicentric Castleman's disease (11, 34). KSHV is a persistent virus that devotes a large fraction of its genome to encoding proteins involved in immune evasion (4). Two such viral products are the Modulator of Immune Responses 1 and 2 (MIR1 and MIR2). These proteins are transmembrane RING finger E3 ligases that cause ubiquitination and downregulation of major histocompatibility complex class I (MHC-I) molecules and other plasma membrane proteins involved in immune responses (reviewed in reference 20). The MIR proteins are homologous to the membrane-associated RING-CH protein family (MARCH) of E3 ubiquitin ligases (20) found in a broad range of mammals.
Ubiquitination is a conserved and highly regulated process in all eukaryotic species (reviewed in references 14 and 27). Through an ATP hydrolysis reaction, the C-terminal glycine residue of ubiquitin forms a thioester bond with the catalytic cysteine of the E1 enzyme. Activated E1 can then transfer ubiquitin to the active cysteine residue of an E2 enzyme. RING finger E3 ligases, such as the MIR proteins, mediate the transfer of ubiquitin from an E2 directly to the substrate. Thus, by providing substrate specificity, E3 ligases act as key regulatory determinants for the ubiquitination reaction. MIR1 downregulates all human class I MHC, gamma interferon receptor (IFN-γR), and CD1d, whereas MIR2 downregulates a subset of MHC-I molecules (HLA-A and HLA-B), IFN-γR, CD1d, platelet endothelial cell adhesion molecule 1 (PECAM-l), intercellular adhesion molecule 1 (ICAM-l), B7.2, bone marrow stromal cell antigen 2 (BST-2), syntaxin 4, and activated leukocyte cell adhesion molecule (ALCAM; CD166) (2, 5, 6, 15, 16, 21, 23, 24, 30). Most of these substrates are known to reside in, or upon activation are recruited to, lipid rafts (8, 18, 32, 35), microdomains of the plasma membrane thought to be important for a wide range of signal transduction events, including T cell and B cell activation.
Posttranslational modifications can target proteins to specific regions of the plasma membrane. Palmitoylation is a type of fatty acid modification in which palmitic acid is attached to sulfur atoms of cysteine residues, making polypeptides more hydrophobic. This increase in affinity for more hydrophobic regions of the plasma membrane is believed to be important for targeting proteins to lipid rafts (reviewed in reference 22). Palmitoylation can occur either near a transmembrane region or on an intracytoplasmic cysteine, where it acts as an anchor to secure the polypeptide in the membrane.
We tested whether palmitoylation is important for MIR-mediated downregulation of their substrates. Interestingly, we found that while MIR1 does not require palmitoylation for proper function, palmitoylation is required for MIR2 to downregulate a subset of its substrates, such as MHC-I. We observed that MIR2 is directly palmitoylated both in vitro and in vivo and that this modification is required for MIR2 interaction with MHC-I. Palmitoylation may act to regulate MIR2 function and localization during viral infection, allowing MIR2 to properly interact with and downregulate multiple substrates known to play an important role in the host immune response.
MATERIALS AND METHODS
Cell culture and reagents.
The HEK293T cell line (no. CRL-11268; American Type Culture Collection), SLK endothelial cell line (provided by Don Ganem, University of California, San Francisco, CA), Phoenix cells (no. SD-3443; American Type Culture Collection), and HeLa S3 cells (no. CCL2.2; American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 100 μg/ml penicillin-streptomycin and transfected using Fugene HD reagent (Roche) according to the manufacturer's instructions. The TREx-BCBL1-Rta cell line (provided by Jae Jung, University of Southern California) was maintained in RPMI 1640 with 10% fetal bovine serum and 100 μg/ml penicillin-streptomycin. This cell line has been described in detail (26).
2-Bromohexadecanoic acid (2-bromopalmitic acid [2-Br]]) was purchased from Sigma Aldrich, and cells were treated with 100 μM 2-Br for 16 h. N-Ethylmaleimide crystalline was purchased from Sigma Aldrich. EZ-Link (1-biotinamido-4-(4′-[maleimidoethyl-cyclohexane]-carboxamido)butane) (biotin-BMCC), high-capacity NeutrAvidin agarose resin, and hydroxylamine hydrochloride were purchased from Thermo Fisher Scientific.
Plasmids.
pIRES2-EGFP is from BD Biosciences. pCR3.1 vectors expressing either MIR1 or MIR2 fused to enhanced green fluorescent protein (EGFP) have been previously described (29). The MIR2 cysteine mutants were created by site-directed mutagenesis and cloned into pIRES2 EGFP using the BglII and SalI restriction sites. FLAG-tagged MIR2 and MIR2-C146F were cloned into the BamHI and NotI sites of pMG hygro (provided by Michael Curran, University of California, Berkeley, CA). Cloning of pBMN B7.2 IN was previously described (7). PECAM-1 was amplified by PCR from HeLa cDNA using the forward (5′ ATGCAAGAAAACTCTTTCACAATCAACAGTGTTGAC 3′) and reverse (5′ CTAAGTTCCATCAAGGGAGCCTTCC 3′) primers. ICAM-1 was amplified by PCR from BJAB cDNA using the forward (5′ ATGCAGACATCTGTGTCCCCCTCA 3′) and reverse (5′ TCAGGGAGGCGTGGCTTGT 3′) primers. FLAG tags (3×) were added to the N termini of PECAM-1 and ICAM-1 by overlap PCR and were then cloned into pQCXIN using the NotI and PacI restriction sites. pBMN HLA-A2 IN was created by amplifying HLA-A2 from pCDNA3.1(+) HLA-A2 (17) using the forward (5′ CTAGGGATCCATGGCCGTCATGGCGCC 3′) and reverse (5′ CTAGGTCGACTCACACTTTACAAGCTGTGAGAGACACATC 3′) primers. The PCR product was digested with BamHI and SalI and ligated into pBMN IN. pBMN HLA-B7-IN construction was previously described (7).
Transfection and retroviral transduction.
HeLa, HEK293T, SLK, and Phoenix cells were transfected using the Fugene HD reagent (Roche) according to the manufacturer's instructions. Upon transfection with the retroviral vectors (pMG hygro, pBMN IN, or pQC IN), the Phoenix packaging cell line produces replication-defective viral particles that can be used to stably transduce mammalian cells. Phoenix cells were transfected, and the virus-containing supernatant was harvested 48 h after transfection, filtered through a 0.45-μm filter, and diluted with Polybrene (4 μg/ml, final dilution). HeLa cells were infected by spin infection (800 × g for 2 h at 20°C) using 2 ml of viral supernatant. Selection of transduced cells was started 36 h after infection by adding 600 μg/ml hygromycin B or 1,500 μg/ml G418 (neomycin).
Immunoprecipitation, Western blot analysis, and antibodies.
Dishes (10 cm) of HeLa cells stably expressing vector, MIR2, or MIR2-C146F were grown to confluence, the medium was removed, and the plates were washed with phosphate-buffered saline (PBS) and then lysed in RIPA buffer (50 mM Tris [pH 8], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and antiprotease). For coimmunoprecipitation, a special lysis buffer was used (0.5% NP-40, 150 mM NaCl, 20 mM Tris [pH 7.4], 10% glycerol, and protease inhibitor cocktail). The cells were scraped from the dishes and incubated at 4°C for 30 min. The lysates were cleared of debris by centrifugation in a microcentrifuge at 4°C for 20 min and incubated with 1 μg antibody (anti-MHC-I, anti-B7.2, or anti-MIR2). The antibody-bound proteins were then incubated and precipitated with protein A/G+ agarose beads. The bound proteins were eluted with 25 μl loading buffer (31 mM Tris [pH 6.8], 1% SDS, 0.002% bromophenol blue, 5% glycerol, and 5% β-mercaptoethanol). Samples were run on a 12% polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. Blots were probed with antiubiquitin, anti-FLAG, anti-MHC-1, or anti-MIR2 antibodies.
For flow cytometry analysis, all antibodies were used at a concentration of 1 μg per 106 cells. For Western blot analysis, all antibodies were used at a dilution of 1/1,000. Phycoerythrin (PE)-conjugated anti-MHC-I (w6/32) was purchased from Dako, anti-B7.2 (CD86) and anti-HLA-A2 were purchased from BD Pharmingen, anti-FLAG M2 antibody was purchased from Sigma-Aldrich, anti-HLA-B7 was purchased from AbD Serotec, goat F(ab′) anti-mouse IgG R-PE was purchased from Caltag Laboratories, anti-ubiquitin (P4D1) and protein A/G+ agarose were purchased from Santa Cruz Biotechnology, anti-MHC-I (HC10) was a gift from Hidde Ploegh, and anti-MIR2 was a gift from Klaus Früh.
Biotin switch assay.
This technique has been previously described (10, 33). After transfection of HEK293T cells, total cell lysates were treated with 25 mM N-ethylmaleimide to block free sulfhydral groups on unmodified cysteine residues for 30 min on ice. Lysates were cleared by microcentrifugation at maximum speed for 20 min at 4°C. The lysate was treated (+) with 600 mM hydroxylamine (pH 7.4) to remove palmitate) or left untreated (−). Free cysteines were then biotinylated with 300 μM biotin-BMCC for 2 h at 4°C. Biotinylated proteins were then precipitated with 100 μl NeutrAvidin beads (Pierce) for 1 h at 4°C. Samples were run on 12% polyacrylamide gels, transferred to PVDF, and blotted with anti-FLAG or anti-MIR2. For in vivo studies, TREx BCBL1-Rta cells were activated 24 h prior to cell lysis with 20 μg/ml phorbol 12-myristate 13-acetate (PMA), 1 μg/ml doxycycline, and 0.5 μg/ml ionomycin (all purchased from Sigma Aldrich). The same numbers of latent and activated cells (107 cells) were used in the assay.
RESULTS
Palmitoylation is required for MIR2 function.
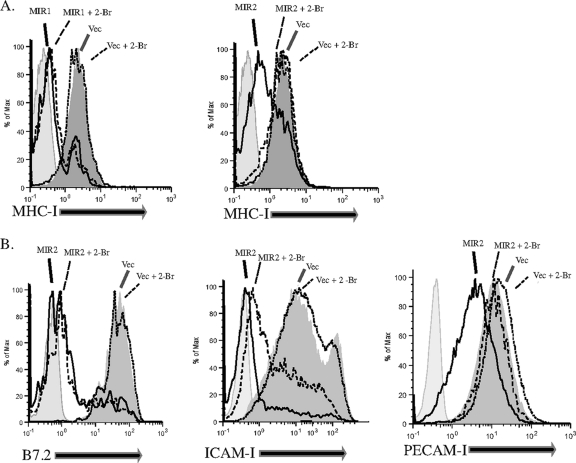
In order to investigate the role of palmitoylation in MIR-mediated downregulation of their substrates, HeLa cells stably expressing MIR1 or MIR2 were treated with the drug 2-bromohexadecanoic acid (2-Br), a nonmetabolizable analog of palmitate, known to block palmitoylation. MIR-mediated downregulation of MHC-I was examined by staining for surface MHC-I 24 h after the addition of the drug. Remarkably, while MIR1 activity was unaffected by the presence of 2-Br, MIR2 downregulation of MHC-I was greatly inhibited (Fig. 1A). Although MIR1 and MIR2 are thought to function similarly, our results suggest that palmitoylation is required for the function of MIR2 but not MIR1, highlighting a substantial difference between these two highly conserved E3 ligases.
FIG. 1.
Palmitoylation is required for MIR2 function. (A) HeLa cells were transfected with either MIR1 (left panel) or MIR2 (right panel) fused to enhanced green fluorescent protein (EGFP) or with EGFP alone. Cells were either treated with 100 μM 2-bromohexadecanoic acid (2-Br) or with vehicle alone, stained with phycoerythrin (PE)-conjugated antibody against MHC-I (w6/32), and analyzed by flow cytometry. (B) HeLa cells stably transduced with B7.2 (left panel), FLAG-tagged ICAM-1 (center panel), or FLAG-tagged PECAM-1 (right panel) were transfected with MIR2-EGFP or EGFP alone, treated with 100 μM 2-Br or vehicle alone, stained with mouse-raised primary antibody against B7.2 or against FLAG, stained with PE-conjugated secondary antibody against mouse IgG, and analyzed by flow cytometry. Histograms represent GFP+ cells only.
We next examined the effect of 2-Br on several other MIR2 substrates. HeLa cells stably expressing FLAG-tagged PECAM-1, FLAG-tagged ICAM-I, or B7.2 were transfected with MIR2 fused to EGFP (MIR2-EGFP) or EGFP alone. Cells were then treated with 2-Br (or ethanol as a control) for 24 h, and surface levels of PECAM-I, ICAM-I, and B7.2 in GFP-positive cells were examined by flow cytometry. We observed that in the presence of 2-Br, MIR2's ability to downregulate its substrates varies: while B7.2 and ICAM-I downregulation was not (or was modestly) affected, PECAM-I downregulation was completely abrogated (Fig. 1B). These results indicate that MIR2 requires a functioning palmitoylation system to downregulate a subset of its substrates (MHC-I and PECAM-I).
Substrate palmitoylation is not required for MIR2-mediated downregulation of MHC-I.
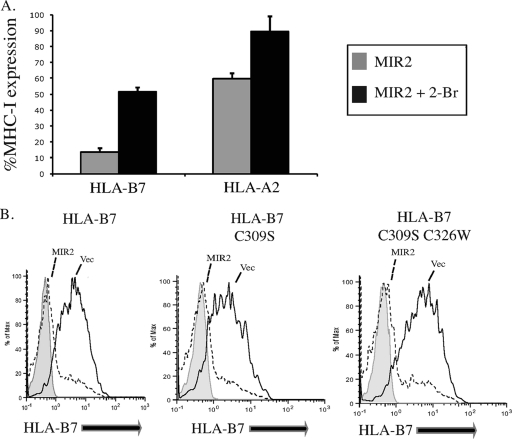
Next we addressed whether palmitoylation of the substrate itself is required for MIR2-mediated downregulation. MIR2 is known to downregulate only some MHC-I molecules: HLA-A and HLA-B but not HLA-C. Furthermore, MIR2 downregulates HLA-B7 from the cell surface to a greater extent than HLA-A2 for reasons that still remain unclear (Fig. 2A and data not shown). Studies of MHC-I palmitoylation have shown that HLA-A molecules do not have a cysteine residue in an optimal position for palmitoylation and therefore are unpalmitoylated (12). In contrast, HLA-B proteins contain either one or two candidate cysteines, both of which are palmitoylated (12). We tested whether downregulation of HLA-A and HLA-B by MIR2 was affected differentially upon treatment with 2-Br. Figure 2A shows that in the presence of 2-Br, surface expression of the two molecules was rescued to similar extents (30% for HLA-A2 and 38% for HLA-B7). This suggests that substrate palmitoylation is not responsible for the effect of 2-Br on MIR2. Additionally, we mutated the two cysteine residues in HLA-B7 that are known to be palmitoylated (cysteines 309 and 326 on the intracytoplasmic tail) (12) and examined their contribution to MIR2 downregulation. HeLa cells stably expressing HLA-B7, HLA-B7-C309W, or HLA-B7-C309W C326S were transfected with MIR2-EGFP, and HLA-B7 surface expression was analyzed by flow cytometry. All three HLA-B7 molecules were expressed to similar levels and were downregulated from the cell surface by MIR2 to the same extent (Fig. 2B). Our results indicate that MIR2 is capable of downregulating HLA-B7 in the absence of palmitoylation, providing additional evidence that substrate palmitoylation is unnecessary for MIR2-mediated downregulation.
FIG. 2.
Substrate palmitoylation is not required for MIR2-mediated downregulation of MHC-I. (A) HeLa cells stably transduced with HLA-A2 or HLA-B7 were transfected with MIR2-EGFP or EGFP alone, treated with 100 μM 2-Br or vehicle alone, stained with mouse-raised primary antibody against HLA-A2 or HLA-B7, stained with PE-conjugated secondary antibody against mouse IgG, and analyzed by flow cytometry. The level of MHC-I expression in MIR2-expressing cells was calculated by determining the ratio of MHC-I expression (mean PE value in GFP+ cells) in MIR2-EGFP-expressing cells to that in EGFP alone. Bar graphs represent the results of three consecutive experiments. (B) HeLa cells stably transduced with wild-type HLA-B7, HLA-B7-C309S, or HLA-B7-C309S C326W (unpalmitoylated) were transfected with MIR2-EGFP or EGFP alone. Samples were stained with a mouse-raised primary antibody against HLA-B7, stained with PE-conjugated secondary antibody against mouse IgG, and analyzed by flow cytometry.
MIR2 is palmitoylated on cysteine 146.
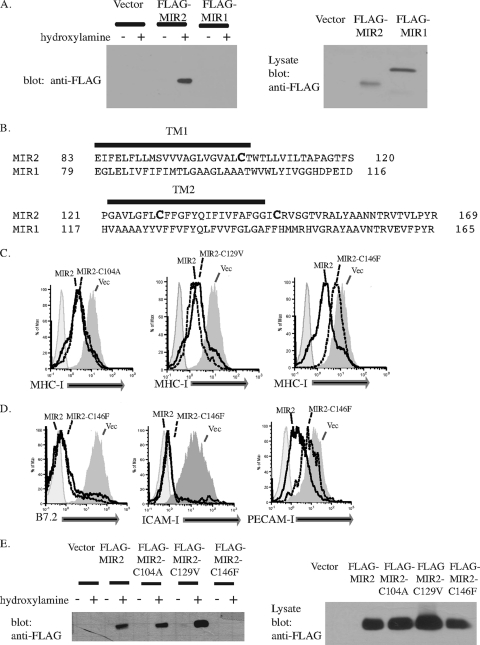
We next examined whether MIR2 is directly palmitoylated using the biotin-switch assay (10, 33). This assay specifically biotinylates proteins that are palmitoylated in a cellular lysate. Biotinylated proteins are immunoprecipitated, and the presence of the protein of interest in the immunoprecipitate is determined by Western blotting. 293T cells were transfected with FLAG-tagged MIR1 or FLAG-tagged MIR2, lysates were subjected to the biotin-switch assay, and the presence of MIR1 or MIR2 was determined using an anti-FLAG antibody. Our results indicate that while both MIR1 and MIR2 are expressed in cellular lysates (Fig. 3A, right panel), only MIR2 is palmitoylated (Fig. 3A, left panel).
FIG. 3.
MIR2 is palmitoylated on cysteine 146. (A) Palmitoylation of MIR1 or MIR2 was detected by a biotin switch assay where protein palmitoylation is replaced with biotinylation. After transfection of HEK293T cells, total cell lysates were treated with N-ethylmaleimide to block free sulfhydral groups on unmodified cysteine residues. The lysate was treated (+) with hydroxylamine to remove palmitate or untreated (−). Free cysteines were then biotinylated with biotin-BMCC. Biotinylated proteins were then precipitated with NeutrAvidin beads (Pierce) and blotted for FLAG (left panel). Lysate samples from MIR-expressing cells were also probed with FLAG to verify MIR expression (right panel). Equal amounts of protein were loaded in each lane. (B) An alignment of MIR1 and MIR2 transmembrane domains. (C) MIR2 or MIR2 mutants fused to EGFP were individually transfected into HeLa cells. Twenty-four hours posttransfection, cells were stained with PE-conjugated antibody against MHC-I and analyzed by flow cytometry. Histograms were gated on GFP+ cells. (D) MIR2 or MIR2-C146F fused to EGFP was transiently transfected into HeLa cells stably transduced with B7.2 (left panel), FLAG-tagged ICAM-I (center panel), or FLAG-tagged PECAM-I (right panel). Cells were stained with mouse-raised primary antibody against B7.2 or against FLAG, stained with PE-conjugated secondary antibody against mouse IgG, and analyzed by flow cytometry. (E) The palmitoylation status of MIR2 mutants was determined using the biotin switch assay where protein palmitoylation is replaced with biotinylation. HEK 293T cells were transfected with individual MIR2 mutants, total cell lysates were subjected to the treatment described above for panel A, and the presence of biotinylated MIR2 was detected by probing Western blots for FLAG (left panel). Lysate samples from MIR2 or MIR2 mutant-expressing cells were also probed with FLAG to verify expression (right panel). Equal amounts of protein were loaded in each lane.
Upon examination of the MIR2 sequence, we identified three cysteines (Cys 104, Cys 129, and Cys 146) that were located within or close to the transmembrane regions and thus were likely candidates for palmitoylation. These residues were notably absent from MIR1 (Fig. 3B). We individually mutated these three cysteines to the cognate amino acid found in the transmembrane regions of MIR1 in an attempt to minimize transmembrane perturbation. HeLa cells were transfected with wild-type MIR2, MIR2-C104A, MIR2-C129V, or MIR2-C146F fused to EGFP, and cell surface expression of MHC-I was examined by flow cytometry. While MIR2-C104A and MIR2-C129V were able to downregulate MHC-I to the same extent as wild-type MIR2, MIR2-C146F was substantially inhibited (Fig. 3C).
We further examined the ability of MIR2-C146F to downregulate additional MIR2 substrates. HeLa cells stably expressing FLAG-tagged PECAM-1, FLAG-tagged ICAM-I, or B7.2 were transfected with MIR2-EGFP, MIR2-C146F-EGFP, or EGFP alone. Cell surface levels of MIR2 substrates were examined by flow cytometry 24 h after transfection. In addition to its inability to downregulate MHC-I, MIR2-C146F was unable to downregulate PECAM-I, while B7.2 and ICAM-I downregulation was unaffected (Fig. 3D). Together these results support the data obtained with 2-Br treatment (Fig. 1) and further suggest that MIR2 is palmitoylated on Cys 146.
Next we examined which MIR2 cysteine mutants were palmitoylated using the biotin-switch assay. 293T cells transfected with FLAG-tagged MIR2, MIR2-C104A, MIR2-C129V, or MIR2-C146F were lysed and subjected to the biotin-switch assay. Palmitoylated proteins were precipitated using NeutrAvidin beads, and the presence of MIR2 or the MIR2 mutants in the precipitate was determined by Western blotting using an anti-FLAG antibody (Fig. 3E, left panel). As a control, FLAG expression in lysate samples was also examined by Western blotting to verify that MIR2 and the MIR2 mutants were expressed to the same extents (Fig. 3E, right panel). Our results indicate that while MIR2, MIR2-C104A, and MIR2-C129V are palmitoylated, MIR2-C146F is not. These results indicate that MIR2 is palmitoylated on Cys 146 and that palmitoylation is important for MIR2-mediated degradation of a subset of its targets.
Palmitoylation of MIR2 is required for its ability to ubiquitinate MHC-I.
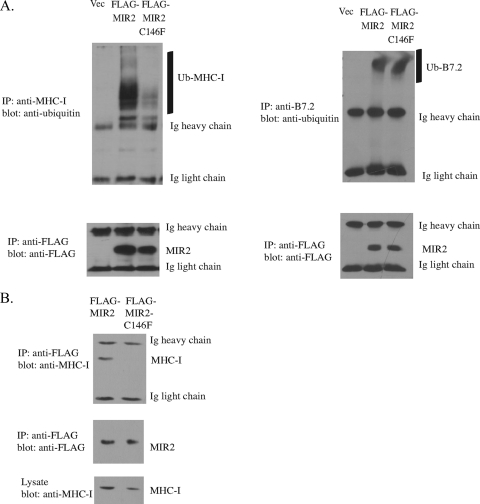
Our previous data indicate that MIR2-C146F is defective in its ability to downregulate MHC-I from the cell surface. We next determined whether this defect was due to an inability of MIR2 to ubiquitinate MHC-I or whether the defect was downstream of ubiquitination, such as MHC-I endocytosis and degradation. Lysates from HeLa cells expressing MIR2 or MIR2-C146F were immunoprecipitated with an anti-MHC-I antibody. The MHC-I ubiquitination status was determined by Western blotting using an anti-ubiquitin antibody (Fig. 4A, top left panel). In addition, HeLa cells stably expressing B7.2 were transfected with MIR2 or MIR2-C146F and immunoprecipitated with an anti-B7.2 antibody to examine the ubiquitination status of B7.2 (Fig. 4A, top right panel). FLAG-MIR2 and FLAG-MIR2-C146F were also immunoprecipitated from the same cellular lysates with an anti-FLAG antibody, and FLAG expression was examined by Western blotting to verify equal expression levels (Fig. 4A, bottom panels). As shown in Fig. 4A, we observed that MHC-I is poorly ubiquitinated in cells expressing MIR2-C146F, in contrast to MHC-I immunoprecipitated from cells expressing MIR2. However, B7.2 is ubiquitinated by both MIR2 and MIR2-C146F to similar extents. These data indicate that downregulation of MIR2 substrates from the cell surface by MIR2-C146F is associated with its ability to ubiquitinate its substrate. This suggests that MIR2 palmitoylation directly correlates with MIR2's ability to ubiquitinate its substrate and therefore to carry out its function as an E3 ligase.
FIG. 4.
Palmitoylation of MIR2 is necessary for MIR2-mediated ubiquitination of MHC-I. (A) HeLa cells or HeLa cells stably transduced with B7.2 were transfected with FLAG-tagged MIR2, FLAG-tagged MIR2-C146F, or vector. MHC-I or B7.2 was immunoprecipitated (IP) from cellular lysates using an anti-MHC-I or anti-B7.2 antibody, and the ubiquitination status of MHC-I (top left panel) or B7.2 (top right panel) was analyzed by Western blot analysis using an antiubiquitin antibody. FLAG-MIR2 and FLAG-MIR2-C146F were also immunoprecipitated from cellular lysates using an anti-FLAG antibody (M2 Sigma) and analyzed by Western blot analysis to verify equal expression levels (bottom panels). (B) HeLa cells stably transduced with FLAG-tagged MIR2 or FLAG-tagged MIR-C146F were lysed in co-IP lysis buffer. FLAG-MIR2 and FLAG-MIR2-C146F were immunoprecipitated from cellular lysates using an anti-FLAG antibody, and the levels of coimmunoprecipitating MHC-I were determined using the HC10 antibody (top panel). FLAG-MIR2 or FLAG-MIR2-C146F levels were analyzed by Western blot analysis using an anti-FLAG antibody (middle panel). As a control, levels of MHC-I in lysate samples were determined using the HC10 antibody (bottom panel).
Palmitoylation is required for MIR2 to interact with MHC-I.
The inability of MIR2-C146F to ubiquitinate MHC-I could result from an inability of MIR2 to directly interact with its substrate or an inability of MIR2 to interact with proteins important for substrate ubiquitination: for instance, an E2-conjugating enzyme. In order to determine if MIR2-C146F is still able to interact with MHC-I, HeLa cells stably expressing FLAG-tagged MIR2 or FLAG-tagged MIR2-C146F were lysed and subjected to immunoprecipitation with an anti-FLAG antibody, and the presence of coimmunoprecipitating MHC-I was examined using an anti-MHC-I antibody (Fig. 4B, top panel). Blots were stripped and reprobed using an anti-FLAG antibody to verify equal expression levels of MIR2 and MIR2-C146F (Fig. 4B, middle panel). To ensure that equal quantities of proteins were immunoprecipitated, lysate samples were run on a Western blot and probed for MHC-I (Fig. 4B, bottom panel). Figure 4B clearly shows that while MHC-I can coimmunoprecipitate with wild-type MIR2, it does not interact with MIR2-C146F. These results suggest that MIR2 palmitoylation is required for proper interaction of MIR2 with its substrate.
MIR2 is palmitoylated in vivo.
We next determined whether MIR2 was palmitoylated in vivo. TREx-BCBL1-Rta cells are derived from a human B-cell lymphoma that harbors latent KSHV (26). Addition of phorbol esters, doxycycline, and ionomycin to these cells allows the virus to lytically reactivate. MIR2 is expressed only during lytic replication. To determine if MIR2 is palmitoylated in the context of an infection, latently infected or lytically reactivated TREx-BCBL1-Rta cells were lysed, and lysates were subjected to the biotin switch assay. Palmitoylated proteins were precipitated using NeutrAvidin beads, and the presence of MIR2 in the precipitate was determined by Western blotting using an anti-MIR2 antibody. Figure 5 shows that MIR2 is palmitoylated in vivo following lytic reactivation. This result validates the data we obtained by transfection of MIR2, suggesting that MIR2 palmitoylation plays a key role in MIR2 function in vivo.
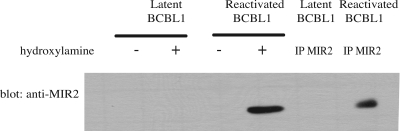
FIG. 5.
MIR2 is palmitoylated in vivo. Cells infected with KSHV were used to determine the palmitoylation status of MIR2. Cellular lysates from latently infected TREx-BCBL1-Rta cells or TREx-BCBL1-Rta cells that were lytically reactivated were subjected to the biotin switch assay where protein palmitoylation is replaced with biotinylation as described for Fig. 3A. The presence of biotinylated MIR2 was detected by probing Western blots for MIR2. MIR2 was also immunoprecipitated from cellular lysates using an anti-MIR2 antibody to confirm the presence of MIR2 in reactivated cellular lysates.
DISCUSSION
Our results from 2-Br treatment in conjunction with the results of the biotin-switch assay indicate that MIR2 is palmitoylated on Cys 146 and that palmitoylation is required for MHC-I and PECAM-I downregulation. Palmitoylation of MIR2 is required for MIR2 to interact with and subsequently ubiquitinate MHC-I. Therefore, palmitoylation is likely an important prerequisite for MIR2-mediated immune evasion during viral infection.
While other lipid modifications (myristoylation, O-palmitoleoylation, and prenylation) can be postranslationally added to proteins, they are unlikely to account for the observed phenotype (reviewed in reference 13). For instance, myristoylation and O-palmitoleoylation occur on noncysteine residues and thus cannot account for the modification observed on Cys 146. Although prenylation occurs on cysteine residues, it forms a thioether bond, which should be insensitive to hydroxylamine hydrochloride cleavage (and thus would not be detectable in our biotin switch assay). In addition, prenylation does not occur near transmembrane domains (such as Cys 146), but it occurs at the C termini of proteins, generally on a cysteine residue present in a CAAX consensus sequence which is absent from MIR2. For these reasons, we believe our experiments examine the direct effect of palmitoylation on a virally encoded E3 ligase.
To our knowledge, only two other E3 ligases have been shown to require palmitoylation for proper function, RNF11 and Sakura. In both cases, palmitoylation is thought to be required for proper membrane localization (1, 31). Similarly, it is possible that palmitoylation of MIR2 may favor MIR2 trafficking to some subcellular compartments, such as the plasma membrane (lipid raft) or other post-Golgi endosomal compartment where MIR2 is thought to act on MHC-I. Unfortunately, the vast majority of MIR2 expression is localized to the endoplasmic reticulum (ER), and therefore results of our attempts to address this possibility using microscopy and fractionation have been extremely difficult to interpret (data not shown). Further studies are required to definitively determine whether MIR2 is found in a subcellular compartment that MIR2-C146F cannot access. Intriguingly, some of the viral and mammalian homologues of the MIR proteins display localizations and substrate specificities comparable to those of MIR1 and MIR2. Similar to MIR2, MARCH proteins contain cysteine residues proximal to the transmembrane regions (data not shown), suggesting that the function of these proteins may also be regulated by palmitoylation.
It is unknown whether MIR1 and MIR2 target MHC-I from a similar or distinct subcellular compartment. Unlike MIR2, MIR1 does not require palmitoylation to downregulate MHC-I, suggesting that both of these highly similar E3 ligases may act in different compartments. Palmitoylation may also be required for MIR2 to overcome difficult interactions with some of its substrates. For example, palmitoylation of MIR2 may juxtapose regions in the tertiary structure, potentially increasing MIR2's affinity for some of its substrates. MIR1 and MIR2 have slightly different transmembrane regions, and it is possible that MIR1 transmembrane domains have a greater affinity for MHC-I.
It is interesting that PECAM-I downregulation by MIR2 is dependent on palmitoylation even though PECAM-1 has been shown to be downregulated from both the ER and the plasma membrane (23). Palmitoylation is known to occur in the ER, along the secretory pathway, and at the plasma membrane (reviewed in reference 9), and therefore MIR2's palmitoylation status while in the ER is unknown. If nascent MIR2 is unpalmitoylated in the ER, it is possible that palmitoylated MIR2 traffics back to the ER in order to downregulate PECAM-1. Alternatively, unpalmitoylated MIR2 may be able to downregulate PECAM-1 from the ER, while only palmitoylated MIR2 can downregulate PECAM-1 from the cell surface. Further studies are required to formally test these hypotheses.
There are several examples of viral proteins requiring palmitoylation for proper localization. For instance, Epstein-Barr virus (EBV)-encoded LMP1 and LMP2A are both palmitoylated, associate with lipid rafts, and are thought to play a substantial role in manipulating host signal transduction events to promote viral survival (25). It is quite likely that other KSHV-encoded proteins are also palmitoylated, and this palmitoylation may be integral to their function. For example, K15, a homologue of LMP2A, is thought to target the major B cell Src kinase Lyn to lipid rafts (3, 28). There are many cysteine residues present in K15, and at least one is predicted to be cytosolic. It would be quite interesting to determine if K15 is palmitoylated and if this palmitoylation is required for K15-mediated activation of Lyn, which is known to modulate transcription of NFAT and AP1 (3). Another attractive candidate is K1, a transmembrane glycoprotein with structural similarities to the B cell receptor. K1 has several cysteine residues located close to or within the intracytoplasmic tail that could potentially be palmitoylated. K1 has been shown to be constitutively active, possibly due to oligomerization via conserved extracellular cysteine residues (19). It is possible that K1 is palmitoylated, and this palmitoylation could potentially prolong its presence in lipid rafts, allowing for increased interaction with cellular partners.
In conclusion, our work has shown that palmitoylation of MIR2 is required for its ability to downregulate MHC-I and PECAM-1. This host-acquired posttranslational modification allows MIR2 greater access to its substrates, thereby providing KSHV with an additional mechanism to control cellular signaling pathways.
Acknowledgments
We gratefully acknowledge Lesley Pasman and Elena Bekerman for their contributions to this work. We are also grateful to Klaus Früh for generously providing us with the antibody against MIR2, Hidde Ploegh for providing us with the HC10 antibody, and Jae Jung for use of the TREx BCBL1-Rta cell line.
This work was supported by grants from the NIH.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1.Araki, K., et al. 2003. A palmitoylated RING finger ubiquitin ligase and its homologue in the brain membranes. J. Neurochem. 86:749-762. [DOI] [PubMed] [Google Scholar]
- 2.Bartee, E., A. McCormack, and K. Fruh. 2006. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho, N. H., Y. K. Choi, and J. K. Choi. 2008. Multi-transmembrane protein K15 of Kaposi's sarcoma-associated herpesvirus targets Lyn kinase in the membrane raft and induces NFAT/AP1 activities. Exp. Mol. Med. 40:565-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coscoy, L. 2007. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat. Rev. Immunol. 7:391-401. [DOI] [PubMed] [Google Scholar]
- 5.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. U. S. A. 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Invest. 107:1599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coscoy, L., D. J. Sanchez, and D. Ganem. 2001. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol. 155:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damjanovich, S., et al. 1995. Structural hierarchy in the clustering of HLA class I molecules in the plasma membrane of human lymphoblastoid cells. Proc. Natl. Acad. Sci. U. S. A. 92:1122-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drisdel, R. C., J. K. Alexander, A. Sayeed, and W. N. Green. 2006. Assays of protein palmitoylation. Methods 40:127-134. [DOI] [PubMed] [Google Scholar]
- 10.Drisdel, R. C., and W. N. Green. 2004. Labeling and quantifying sites of protein palmitoylation. Biotechniques 36:276-285. [DOI] [PubMed] [Google Scholar]
- 11.Ganem, D. 1997. KSHV and Kaposi's sarcoma: the end of the beginning? Cell 91:157-160. [DOI] [PubMed] [Google Scholar]
- 12.Gruda, R., et al. 2007. Intracellular cysteine residues in the tail of MHC class I proteins are crucial for extracellular recognition by leukocyte Ig-like receptor 1. J. Immunol. 179:3655-3661. [DOI] [PubMed] [Google Scholar]
- 13.Hannoush, R. N., and J. Sun. 2010. The chemical toolbox for monitoring protein fatty acylation and prenylation. Nat. Chem. Biol. 6:498-506. [DOI] [PubMed] [Google Scholar]
- 14.Hicke, L., and R. Dunn. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141-172. [DOI] [PubMed] [Google Scholar]
- 15.Ishido, S., et al. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13:365-374. [DOI] [PubMed] [Google Scholar]
- 16.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikkert, M., et al. 2001. Ubiquitination is essential for human cytomegalovirus US11-mediated dislocation of MHC class I molecules from the endoplasmic reticulum to the cytosol. Biochem. J. 358:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacs, B., et al. 2005. Ligation of CD28 by its natural ligand CD86 in the absence of TCR stimulation induces lipid raft polarization in human CD4 T cells. J. Immunol. 175:7848-7854. [DOI] [PubMed] [Google Scholar]
- 19.Lagunoff, M., R. Majeti, A. Weiss, and D. Ganem. 1999. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. U. S. A. 96:5704-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehner, P. J., S. Hoer, R. Dodd, and L. M. Duncan. 2005. Downregulation of cell surface receptors by the K3 family of viral and cellular ubiquitin E3 ligases. Immunol. Rev. 207:112-125. [DOI] [PubMed] [Google Scholar]
- 21.Li, Q., R. Means, S. Lang, and J. U. Jung. 2007. Downregulation of gamma interferon receptor 1 by Kaposi's sarcoma-associated herpesvirus K3 and K5. J. Virol. 81:2117-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linder, M. E., and R. J. Deschenes. 2007. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8:74-84. [DOI] [PubMed] [Google Scholar]
- 23.Mansouri, M., et al. 2006. Kaposi sarcoma herpesvirus K5 removes CD31/PECAM from endothelial cells. Blood 108:1932-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansouri, M., et al. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:9672-9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matskova, L., I. Ernberg, T. Pawson, and G. Winberg. 2001. C-terminal domain of the Epstein-Barr virus LMP2A membrane protein contains a clustering signal. J. Virol. 75:10941-10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura, H., et al. 2003. Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J. Virol. 77:4205-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickart, C. M. 2001. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70:503-533. [DOI] [PubMed] [Google Scholar]
- 28.Pietrek, M., et al. 2010. Role of the Kaposi's sarcoma-associated herpesvirus K15 SH3 binding site in inflammatory signaling and B-cell activation. J. Virol. 84:8231-8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez, D. J., L. Coscoy, and D. Ganem. 2002. Functional organization of MIR2, a novel viral regulator of selective endocytosis. J. Biol. Chem. 277:6124-6130. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez, D. J., J. E. Gumperz, and D. Ganem. 2005. Regulation of CD1d expression and function by a herpesvirus infection. J. Clin. Invest. 115:1369-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santonico, E., et al. 2010. Multiple modification and protein interaction signals drive the Ring finger protein 11 (RNF11) E3 ligase to the endosomal compartment. Oncogene 29:5604-5618. [DOI] [PubMed] [Google Scholar]
- 32.Sardjono, C. T., et al. 2006. Palmitoylation at Cys595 is essential for PECAM-1 localisation into membrane microdomains and for efficient PECAM-1-mediated cytoprotection. Thromb. Haemost. 96:756-766. [PubMed] [Google Scholar]
- 33.Wan, J., A. F. Roth, A. O. Bailey, and N. G. Davis. 2007. Palmitoylated proteins: purification and identification. Nat. Protoc. 2:1573-1584. [DOI] [PubMed] [Google Scholar]
- 34.Whitby, D., and C. Boshoff. 1998. Kaposi's sarcoma herpesvirus as a new paradigm for virus-induced oncogenesis. Curr. Opin. Oncol. 10:405-412. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, S., et al. 2009. Lipid rafts uncouple surface expression of transmembrane TNF-alpha from its cytotoxicity associated with ICAM-1 clustering in Raji cells. Mol. Immunol. 46:1551-1560. [DOI] [PubMed] [Google Scholar]