Abstract
Most retroviruses express all of their genes from a single primary transcript. In order to allow expression of more than one gene from this RNA, differential splicing is extensively used. Cellular quality control mechanisms retain and degrade unspliced or partially spliced RNAs in the nucleus. Two pathways have been described that explain how retroviruses circumvent this nuclear export inhibition. One involves a constitutive transport element in the viral RNA that interacts with the cellular mRNA transporter proteins NXF1 and NXT1 to facilitate nuclear export. The other pathway relies on the recognition of a viral RNA element by a virus-encoded protein that interacts with the karyopherin CRM1. In this report, we analyze the protein factors required for the nuclear export of unspliced foamy virus (FV) mRNA. We show that this export is CRM1 dependent. In contrast to other complex retroviruses, FVs do not encode an export-mediating protein. Cross-linking experiments indicated that the cellular protein HuR binds to the FV RNA. Inhibition studies showed that both ANP32A and ANP32B, which are known to bridge HuR and CRM1, are essential for FV RNA export. By using this export pathway, FVs solve a central problem of viral replication.
The nuclear export of RNA molecules in eukaryotic cells is a tightly regulated process (18, 59, 63, 70, 71). Nuclear exit is usually allowed only for fully spliced cellular mRNAs, while intron-containing mRNAs are retained in the nucleus and subsequently degraded (17, 18, 59, 63, 70). This defines a specific problem in the replication of retroviruses (RVs), since they must export not only fully spliced but also unspliced or partially spliced mRNAs into the cytoplasm. For the export of the two latter RNA species, retroviruses have found ways to escape both the splicing machinery and the degradation of incompletely spliced mRNAs by making use of either of two strategies for nuclear export of mRNAs with intact splice donor (SD) and acceptor (SA) pairs.
In complex retroviruses, such as lentiviruses, some betaretroviruses, and all deltaretroviruses, virus-encoded regulatory proteins (Rev, Rem, and Rex, respectively) bind to the unspliced or incompletely spliced viral mRNA on one hand and contact the karyopherin CRM1 on the other (1, 29, 33, 48, 49). Subsequently, this complex shuttles to the cytoplasm, where it delivers the RNA cargo in a regulated fashion that involves Ran in GTP-bound form. Normally CRM1 is used for nuclear export of ribosomal subunits, 5S rRNAs, cellular proteins containing a nuclear export signal (NES), and snRNAs (18, 27, 53, 63). This pathway can also be hitchhiked by endogenous human retroviruses (12, 47, 74). The presence of regulatory proteins acting at the posttranscriptional level enables complex retroviruses to use a biphasic mode of gene expression (“early” versus “late” phase), resulting in a gain of complexity better known from DNA viruses (16).
Alternatively, more simple retroviruses, such as the betaretrovirus Mason-Pfizer monkey virus (MPMV), can (42) contain a cis-acting constitutive RNA transport element (CTE) that, by directly contacting the NXF1 and NXT1 export factors, facilitates nuclear mRNA exit (28, 42, 72, 77). The NXF1/NXT1-mediated export pathway is normally used for the nuclear exit of fully spliced cellular mRNAs (17, 36-38). For the simple gammaretrovirus murine leukemia virus (MLV), a nuclear export pathway of unspliced transcripts that involves the packaging sequence (Ψ) at the 5′ end of the genomic RNA has been suggested, and very recently for the expression of unspliced mRNA of the simple Jaagsiekte retrovirus of sheep, a role for the env gene-encoded signal peptide and CRM1 has been described (3, 14). However, for most of the simple retroviruses, the way unspliced mRNA exits the nucleus has not been identified yet.
Foamy viruses (FVs) constitute the Spumaretrovirus subfamily of retroviruses (43, 61). They are complex retroviruses that encode accessory proteins (Fig. 1) in the 3′ region of the genome. Among these is a DNA-binding protein, the transcriptional transactivator Tas (7, 35, 39, 44, 62). However, despite intensive investigation, regulatory proteins acting at the RNA export level could not be identified (4, 76). The replication pathway of spumaretroviruses diverges in many ways from that of orthoretroviruses (43, 61). This aberrant replication strategy also involves the presence of two Tas-dependent promoters (8, 45, 46), differentially regulating the viral gene expression (35, 44, 61). The internal promoter (IP) is located in the env gene approximately 100 nucleotides upstream of the accessory genes (Fig. 1). This IP is driving the accessory gene expression in the early phase of viral transcription, while the U3 promoter in the long terminal repeat (LTR) overtakes it to direct the expression of structural genes in the second phase (Fig. 1). This mode of gene regulation allows a differential expression of FV genes; however, it does not circumvent the central problem of all retroviruses to export spliced as well as unspliced RNAs from the nucleus.
FIG. 1.
Genome organization and transcripts of PFV. Horizontal arrows indicate the U3 LTR and the internal promoter (IP). The vertical arrows indicate the position of frequently used 5′ and 3′ splice sites.
In orthoretroviruses, the genes are translated from three classes of mRNAs (58). The Gag and Gag-Pol precursor proteins are translated from the unspliced mRNA. This mRNA is also packaged into progeny virus and serves as the template for reverse transcription (RT) in the next round of infection. Single-spliced mRNAs specifying the env mRNA and completely spliced mRNAs encoding some accessory proteins of complex RVs are also generated and transferred from the nucleus to the cytoplasm (58). In FVs, the situation is even more complex, since they generate their Pol precursor protein independently of Gag from a spliced mRNA (9, 34, 75). Thus, FV gene expression involves extensive mRNA splicing (Fig. 1) that probably necessitates a kind of posttranscriptional regulation. Here we identify the potential cellular partners facilitating nuclear export of intron-containing FV mRNAs by using the prototype FV (PFV) isolate as a model.
MATERIALS AND METHODS
Cells and transfections with DNA.
HEK 293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM glutamine. The medium was exchanged prior to transfection. Cells were transfected with plasmid DNA by using Polyfect (Qiagen). The transfection mix contained 0.5 μg pEGFP, 1 μg pcHSRV2 (49) or derivatives, and the amounts of the other expression plasmids indicated in the figures. The total amount was adjusted to be 3.5 μg with pUC19 DNA. Transfection efficiencies were monitored by enhanced green fluorescent protein (EGFP) expression, when appropriate.
RNA preparation and analysis.
Transfections of HEK 293T cells with plasmid DNA and the preparation of total or fractionated RNA were performed as described previously (13). The nuclear and cytoplasmic fractions were subjected to RNA extraction with the RNeasy kit (Qiagen) according to the manufacturer's protocol. The RNA detection was essentially performed as described before (6). Briefly, the RNA content was determined photometrically and by gel analysis. Five micrograms of RNA was loaded onto denaturing formaldehyde gels containing 1% agarose and transferred to Hybond-N+ membrane (Amersham) by capillary blotting. The blots were hybridized overnight at 60°C to specific probes (activity, >107 cpm), which had been labeled by random priming with the MegaPrime kit (Amersham). The blots were washed stringently, sealed, and exposed to X-ray films. Probes were derived from the PFV 5′ gag gene (nucleotides [nt] 531 to 930); the tas/bet genes (nt 1 to 905), which are also able to detect the env gene mRNA; the U3 of the LTR region (nt 10052 to 11017 after the start of transcription of the pcHSRV2 virus isolate); the human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene (nt 1011 to 1310); and the complete gene coding for EGFP.
Metabolic labeling.
A total of 4 × 106 HEK 293T cells were transfected with 20 μg pcHSRV2 by calcium phosphate coprecipitation (5). At 40 h posttransfection, the medium was changed and leptomycin B (LMB) was added to a final concentration of 20 nM. After 1 h, cells were labeled for 5 h with [35S]methionine (2 mCi/ml; Hartmann Analytic). Subsequent to labeling, the cells were washed with phosphate-buffered saline (PBS) and lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]). Cell lysates were cleared by centrifugation (13,000 × g for 15 min). The samples were immunoprecipitated with polyclonal rabbit anti-PFV Gag serum (4) and anti-PFV Tas serum able to detect the accessory Bet protein (4) coupled to protein A-Sepharose (Sigma). The immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis (PAGE). Gels were fixed in 30% methanol-10% acetic acid and dried. Immunprecipitates were visualized by autoradiography.
Immunoblotting and antibodies.
Immunoblotting was performed by standard procedures (5). The following primary antibodies were used. Mouse anti-PFV Gag (30, 32) or anti-PFV Pol (32) monoclonal antibodies (MAbs) were used at a dilution of 1:1,000 or 1:25, respectively. Mouse anti-GFP (Sigma), anti-GAPDH (Sigma), anti-ANP32A (Santa Cruz and Abnova), and anti-HuR (Invitrogen) MAbs and rabbit anti-ANP32B (26, Santa Cruz), anti-HuR (Sigma), and anti-Halo (Promega) antisera were used and diluted as recommended by the supplier. Immunocomplexes were stained with secondary anti-mouse or anti-rabbit serum coupled to horseradish peroxidase (Jackson).
Recombinant DNA.
Established procedures were employed to generate plasmid DNAs (2, 64). All recombinant molecular clones were verified by nucleic acid sequencing of the relevant parts.
Plasmid pcHSRV2 (50), the pol ATG-to-CTG mutant plasmid pcHSRV2-M54 (22), the gag ATG-to-TTG mutant plasmid pcHSRV2-M78 (30), pNLS-NES (73), pNLS-NES-M90 (73), p3CANc (57), the codon-optimized gag expression plasmid pcoPG4 (69), and pEGFP (Invitrogen) have been described previously.
The complete cDNAs of the ANP32A, ANP32B, and HuR genes were obtained from the German Resource Center for Genome Research (accession no. NM 006305, NM 006401, and NM 001419, respectively) and had already been inserted into pCMV-Sport6. The ANP32A/B and HuR genes were Halo tagged by cloning the respective open reading frames (ORFs) into the pHT-2 vector (Promega) by PCR using the primers Halo-pp32s (5′-CAGCTAGCATGGAGATGGGCAGACGGATTCATTTA), Halo-pp32as (5′-GGCGATATCATCATCTTCTCCCTCATCTTCAG), Halo-HuRs (5′-CAGCTAGCATGTCTAATGGTTATGAAGACCACATGGCC), Halo-HuRas (5′-GCGATATCTTTGTGGGACTTGTTGGTTTTGAAGGA), Halo-Aprils (5′CAGCTAGCATGGACATGAAGAGGAGGATCCACCTG), and Halo-Aprila (5′CGCCCGGGATCATCTTCTCCTTCATCATCTGTTTC). The ANP32A and -B expression plasmids insensitive to RNA interference (RNAi) treatment (Δ3′ untranslated region [UTR] plasmids) were generated by amplifying the respective ORFs lacking the targeted sequence in the 3′ UTR by using the oligonucleotides ANP32As (5′-CACCATGGAGATGGGCAGACGGATT), ANP32Aas (5′-TTAGTCATCATCTTCTCCCTCATCT), ANP32Bs (5′-CACCATGGACATGAAGAGGAGGATC), and ANP32Bas (5′-TTAATCATCTTCTCCTTCATCATCT). These amplimers were subsequently inserted into the pcDNA-3.1D-TOPO vector (Invitrogen).
Reversible cross-linking, immunoprecipitation, and RT-PCR.
The experiment followed a modified protocol described by Niranjanakumari et al. (52). In brief, HEK 293T cells were transfected with pcHSRV2 and Halo-tagged constructs by calcium phosphate coprecipitation. Two days after transfection, protein-RNA complexes were cross-linked with 1% formaldehyde for 10 min. The reaction was stopped with 0.25 M glycine. Cells were washed twice with PBS and lysed in RIPA buffer. Protein-RNA complexes were solubilized by Qiagen shredder and sonication. Immobilized complexes were bound to 20 μl HaloLink resin and washed with HaloLink buffer (Promega). Cross-linking was reversed at 65°C for 45 min, and RNAs were subsequently purified with Trizol (Invitrogen) reagent. The remaining cellular DNA was digested with 4 U RNase-free DNase I (Promega) at 37°C for 45 min. Approximately 500-nt PFV pol or GAPDH gene-specific fragments were amplified by reverse transcription-PCR (RT-PCR) applying 30 cycles of denaturation (94°C for 20 s), annealing (55°C for 40 s), and elongation (72°C for 30 s) with 5 pmol primers (GAPDHs, 5′-AGTGGATATTGTTGCCATCAATGAC; GAPDHas, 5′-GCCAGTAGAGGCAGGGATGATGTTC; PFVs, 5′-AAACAACACCTATAGCCCTGTATTA; and PFVas, 5′-GCACAACAAGTATAAAGCAGATATC). After a final elongation step, the amplimers were resolved by agarose gel electrophoresis.
siRNAs and transfections.
HEK 293T or HT1080 cells (as indicated in the figure legends) were transfected with small interfering RNAs (siRNAs), using HiPerFect (Qiagen) according to the manufacturer's protocols. siRNAs were obtained from Qiagen (ANP32A-1, 5′-CGGAGCTGGTTGAGCCTTCAA; ANP32A-2, 5′-AACGTTGCTGTGGGAACGAGA; ANP32B-3, 5′-CAGAAACAGAACTGTTCAGTA; ANP32B-4, 5′-ACCGTCTGTGGCTACCAGTTA; and HuR-5, 5′-AAGTAGCAGGACACAGCTTGG) and from Santa Cruz (order number for the unspecific control siRNA targeting glutathione S-transferase [GST], sc-37007). Transfection was repeated after 2 days, followed by transfection of pcHSRV2 with Fugene6 (Roche) as described before (15). Cells were harvested 2 days after plasmid transfection, and cellular lysates were analyzed for ANP32A, ANP32B, HuR, PFV Gag, EGFP, and GAPDH expression by immunoblotting or RNA analysis. For ANP32A and -B rescue experiments, siRNAs (ANP32A-1 and ANP32B-4) that targeted the 3′ UTR of the respective cDNAs and the plasmids pANP32A-Δ3′UTR and pANP32B-Δ3′UTR were used.
RESULTS
Nuclear export of PFV gag mRNA depends on CRM1.
To investigate whether nuclear export of unspliced PFV mRNA follows either of the two pathways previously characterized for orthoretroviruses (see above), we analyzed viral gene expression by blocking experiments. To abrogate the CRM1 pathway, we incubated HEK 293T cells transfected with the infectious molecular clone pcHSRV2 (50) 48 h previously in 20 nM specific CRM1 inhibitor (LMB) for 1 h. The pcHSRV2 plasmid expresses hybrid promoter-derived RNAs in a Tas-independent way (50). Subsequent to transfection, the cells were metabolically labeled with [35S]methionine/cysteine for 5 h and viral Gag and Bet proteins were immunoprecipitated with specific rabbit antisera (4). As depicted in Fig. 2 A, the expression of Bet, which is translated from a subgenomic fully spliced mRNA (4, 10, 51), was independent of LMB treatment of cells, while a strong reduction in Gag protein synthesis upon LMB incubation was observed.
FIG. 2.
Dependence of PFV Gag protein expression on CRM1. (A) Treatment of cells transfected with the replication-competent proviral plasmid pcHSRV2 with LMB and subsequent [35S]methionine labeling resulted in a strong reduction of Gag protein that could be immunoprecipitated from the cellular lysate, while the expression of Bet protein, translated from a completely spliced mRNA, remained unaffected. (B) Northern blot of total and cytoplasmic (Cyto.) PFV mRNAs upon treatment of transfected cells with LMB. Blocking CRM1-dependent nuclear export resulted in a reduction to hardly detectable levels of unspliced PFV gag mRNA in the cytoplasm. Furthermore, the singly spliced env mRNA was similarly affected, while the tas/bet message, derived from a multiply spliced mRNA, remained unchanged, as were the total mRNA content and the cytoplasmic appearance of the mRNAs for GAPDH (internal control) and for EGFP (derived from pEGFP). Total RNA of untransfected 293T cells was analyzed to prove the specificity of probes. The cytoplasmic RNA was analyzed in duplicate. Blots were hybridized to a PFV gag gene-derived probe and subsequently to the tas/bet probe (also able to detect env mRNA) and GAPDHand EGFP probes.
This finding was suggestive of a CRM1-dependent nuclear export pathway of PFV gag mRNA. To further elucidate this, we studied the amounts of total and cytoplasmic PFV RNA by Northern blotting. After transfection of HEK 293T cells with pcHSRV2 and an enhanced green fluorescent protein (EGFP)-encoding control plasmid (pEGFP), cells were incubated for 16 h with medium containing 10 nM LMB. RNA was extracted from the different cellular compartments and hybridized to a gag gene-specific probe. As shown in Fig. 2B, almost similar amounts of full-length transcripts were detected regardless of the presence of LMB in the fractions of total RNA. In the absence of LMB, PFV gag RNA could be easily detected in the cytoplasmic RNA fraction, while this transcript was significantly reduced in LMB-treated cells. Rehybridization of the membrane by using GAPDH- or EGFP-specific probes revealed the cytoplasmic accumulation of both mRNAs to be unaffected by LMB treatment (Fig. 2B). Subsequent rehybridization to a tas/bet-specific probe revealed the independence of nuclear export of these mRNAs from LMB treatment. Furthermore, since the probe also detects the env gene transcript, we can conclude that this singly spliced mRNA was as affected by LMB as the gag transcript (Fig. 2B). The expression levels of tas and bet genes were low in untreated and LMB-treated cells, probably due to the poor efficiency of the Tas-mediated transactivation of the IP in HEK 293T cells.
Nuclear export of unspliced PFV mRNA is independent of viral proteins.
Up to now, a CRM1-dependent nuclear export pathway of retroviral mRNAs had only been described in conjunction with a virus-encoded regulatory protein (18, 19). Previous searches for such a protein within the PFV genome were futile (4, 76). Expression of unspliced PFV mRNA is independent of Env and the accessory Tas and Bet proteins (4, 5, 25, 31, 65). However, Gag and/or Pol proteins could potentially, via an autoregulatory loop, positively regulate their own expression in the context of the wild-type genome. In PFV, both proteins have been reported to have a nuclear phase (32, 61, 66), which would be consistent with nuclear-cytoplasmic shuttle proteins. To address this question, we separately cotransfected cells with proviral constructs, in which either the start codon of the gag open reading frame (ORF) or the pol ORF ATG were mutated to TGG and CTG (22, 30), respectively, together with pUC or the pNLS-NES construct that blocks the CRM1-dependent pathway (73), and analyzed them for Gag and Pol proteins by immunoblotting.
As shown in Fig. 3 A and B, the prevented expression of either the gag or pol gene did not alter the dependence on the CRM1 pathway to express the other gene. Furthermore, cotransfection of cells with the pcHSRV2 mutants and the pNLS-NES-derived pM90 control plasmid (73) had no significant impact on the generation of the respective PFV proteins; however, cotransfection of cells with the p3CANc construct that blocks the CRM1-dependent pathway (11) severely impacted the detection of PFV Pol protein (Fig. 3B).
FIG. 3.
The nuclear export of unspliced PFV gag and single-spliced pol mRNA does not depend on PFV Gag and Pol proteins. HEK 293T cells were cotransfected with the two pcHSRV2 mutants M54 and M78, disabling the translation of Gag and Pol proteins, respectively, and the plasmids pNLS-NES and p3CANc, which block the CRM1 pathway, or the pNLS-NES M90 control plasmid, and cellular lysates were analyzed by immunoblotting for PFV Gag protein in case of the pol ATG mutant M54 (A) and for Pol protein in case of the gag ATG mutant M78 (B). WT, wild type. −, no CRM1 blocking expression plasmid was added to the transfection cocktail.
Since Renault et al. (60) recently proposed a model in which Gag supports the export of PFV genomic RNA, we sought to investigate the role of this protein in FV RNA export in particular.
293T cells were transfected with the gag ATG mutant (M78) mentioned above and M78, together with pcoPG4, a CMV promoter-driven codon-optimized gag expression plasmid. Cytoplasmic RNAs were prepared and hybrized to a U3-specific probe by Northern blotting and to Gag antibody by Western blotting. As shown in Fig. 4 A, we detected no difference concerning the nuclear export of full-length RNA between the samples with or without heterologous Gag protein. The specificity of both the Gag antiserum and U3 probe were analyzed in parallel (Fig. 4B).
FIG. 4.
Western and Northern blotting of HEK 293T cells transfected with the pcHSRV2-Gag-ATG mutant (M78) and pcoPG4, a codon-optimized Gag expression plasmid, in duplicates. The proteins (bottom panel) and RNA (upper panel) were extracted. Nuclear and cytoplasmic RNA fractions were prepared, and the cytoplasmic RNA was analyzed with a U3-derived LTR probe for PFV gag mRNA. Cellular RNAs of 293T cells and cells transfected with either pcoPG4 or HSRV were used to prove the specificity of the probe. Hybridization of a GAPDH-specific probe served as a loading control. Cellular lysates were analyzed for PFV Gag protein and for GAPDH. The coexpression of Gag protein did not lead to accumulation of unspliced PFV RNA. To prove the specificity of the Gag antiserum or the U3 probe, total lysates and RNA of 293T cells and cells transfected with either pcoPG4 or HRSV were analyzed by Western or Northern blotting.
From these experiments and previous results (4, 5, 25, 31, 65, 76), it can be concluded that no viral protein is involved in facilitating nuclear export of unspliced PFV mRNA, leaving the possibility of the involvement of a viral RNA element and so far unknown cellular factors different from those interacting with the MPMV CTE. As far as the viral element is concerned, an RNA element located in the 3′ pol genomic region and enabling the Rev/Rev-responsive element (RRE)-independent expression of human immunodeficiency virus (HIV) gag has been suggested for PFV (73). However, potential functional aspects of PFV nuclear mRNA export and the role of cellular proteins were not further investigated in that study.
Identification of cellular molecules mediating nuclear export of unspliced PFV mRNA.
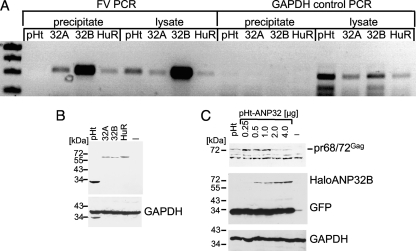
It has been reported recently that the cellular RNA-binding protein HuR and the adapter protein ANP32B were responsible for the CRM1-dependent nuclear export of CD83 mRNA (26). To investigate whether these proteins may also be involved in the nuclear export of unspliced PFV mRNA, we initially used an RNA-protein cross-linking and immunoprecipitation approach. The HuR and the CRM1 adapter proteins ANP32A and -B were Halo tagged by inserting the respective cDNAs into the pHt2 vector. HEK 293T cells were cotransfected with pcHSRV2 and pHt2, pHt2-ANP32A/B, or pHt2-HuR. Two days posttransfection, cells were harvested, cross-linked with formaldehyde, and lysed through sonication. Following precipitation with Halo resin, the cross-linked complexes were resolved and analyzed for the presence of PFV or GAPDH mRNAs as a control by reverse transcription (RT)-PCR with specific primers for GAPDH and PFV pol-derived fragments. For additional control reactions, RNA was isolated from 10% of the lysates and used as a template in control RT-PCRs. As shown in Fig. 5, we could amplify FV-specific RNAs after precipitation with Halo-ANP32A/B and -HuR, but not with the Halo protein alone. In addition, neither Halo alone nor any of the Halo-tagged proteins precipitated GAPDH RNA. The Halo-HuR and -ANP32A/B expression was monitored by Western blotting (Fig. 5B). The strong PCR products after ANP32B transfection prompted us to analyze whether higher ANP32B levels would influence Gag expression (Fig. 5C). This Western blotting analyses of increasing amounts of pHt-ANP32B showed that indeed Gag expression is ANP32B dependent (2 μg of the pHt vector was transfected as a control). Similar titrations of ANP32A and HuR did not result in significantly higher Gag levels (data not shown).
FIG. 5.
Detection of HuR and ANP32A and -B in a complex with PFV RNA was suggestive of a functional role of these proteins in nuclear export of viral mRNA. (A) RT-PCR results with PFV pol- or GAPDH gene-specific primers to amplify approximately 500 bp of PFV or GAPDH sequences from 10% of the input lysate or after precipitation of complexes with Halo-tagged HuR, ANP32A (32A), and ANP32B (32B) proteins or with Halo (pHt) alone. (B) The Halo-tagged HuR and ANP32A/B expression was monitored by Western blotting with a Halo-specific antibody. (C) Effects of ANP32B overexpression on Gag levels. 293T cells were cotransfected with pcHSRV2 and increasing amounts of pHt-ANP32B or with 2 μg of the pHt vector as a control.
This result suggested that PFV mRNA exists in a complex with HuR and both ANP32A and -B and that this complex may be responsible for PFV unspliced mRNA nuclear export. To substantiate this assumption, additional experiments employing siRNAs were performed.
Essential role of ANP32A/B and HuR for nuclear export of unspliced PFV mRNA.
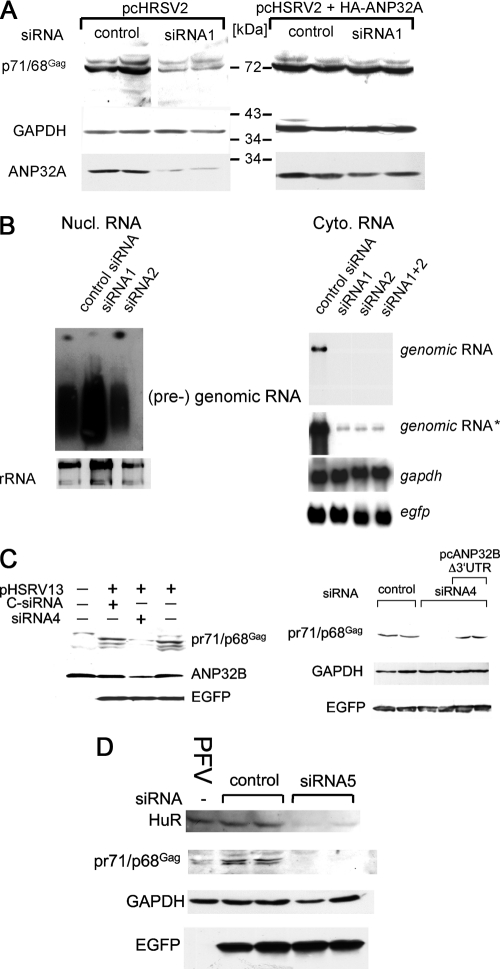
Two different siRNAs (siRNA1 and -2) to downregulate the expression of anp32A were designed. The transfection with specifically targeted siRNA1, but not with an unspecific control siRNA, led to a reduction in amounts of ANP32A, as detected by immunoblotting (Fig. 6 A). As shown in Fig. 6A, the generation of normal levels of PFV Gag protein depended on normal levels of ANP32A. To prove that the reduction in Gag expression was due to a specific siRNA-mediated effect, we generated an ANP32A clone (pcANP32A-Δ3′UTR), not targeted by the siRNA1. Reconstitution of ANP32A expression in siRNA-treated cells with this siRNA-insensitive clone restored the gag expression to wild-type levels (Fig. 6A).
FIG. 6.
Dependence of PFV gag mRNA nuclear export on ANP32A (A and B), ANP32B (C), and HuR (D), as revealed by siRNA inhibition experiments. The experiments in panels A, C, and D are shown in duplicates. (A) siRNA1 abrogates ANP32A and PFV Gag protein expression, in contrast to unspecific control siRNA and rescue of Gag detection by reconstitution of ANP32A expression in siRNA1-treated cells with an siRNA-insensitive clone (Δ3′UTR). Analysis of GAPDH expression served as a loading control. (Both panels were derived from the same gel.) (B) ANP32A siRNA1 and -2 led to nuclear retention of unspliced PFV RNA. HEK 293T cells were transfected with pcHSRV2 and the indicated siRNAs, and nuclear (Nucl.) and cytoplasmic (Cyto.) fractions were prepared and analyzed by Northern blotting for PFV RNA with a gag gene-derived probe. An rRNA loading control was used for the nuclear fraction, and GAPDH or EGFP was used for hybridization for loading and transfection efficiency control of the cytoplasmic (cyto.) fraction. Two different exposures of the cytoplasmic gag Northern blot are shown; the shorter exposure corresponds to the upper panel. (C) Detection of PFV Gag requires ANP32B. HEK 293T cells were transfected with siRNA4 targeting ANP32B or an irrelevant control siRNA, pcHSRV2, and pEGFP. Cellular lysates were analyzed for ANP32B, PFV Gag, and EGFP by immunoblotting. The additional ANP32B-specific siRNA4 also downregulated PFV Gag protein detection, while this was restored with an untargeted ANP32B clone (Δ3′UTR) and siRNA4. Analysis of GAPDH and EGFP expression served as a loading, specificity, and transfection control. (D) Role of the cellular RNA-binding protein HuR for the expression of structural PFV proteins. HT1080 cells were cotransfected with siRNA5 targeting HuR mRNA or an irrelevant siRNA (control), pcHSRV2, and pEGFP. Cellular lysates were prepared and analyzed for HuR, PFV Gag, GAPDH, and EGFP by immunoblotting. The PFV lane contains lysate from a culture infected with PFV.
To analyze whether this effect was due to a nuclear cytoplasmic transport defect of the unspliced PFV transcript, the relative amounts of unspliced PFV transcripts in nuclear and cytoplasmic fractions were analyzed by Northern blotting with a gag gene-specific probe after cotransfection of cells with either ANP32A-specific siRNAs or control siRNA and pcHSRV2. The result presented in Fig. 6B revealed that while large amounts of PFV RNA were detected in nuclear fractions, the use of the ANP32A siRNAs led to a significant reduction in its cytoplasmic accumulation, while the unrelated siRNA was not able to prevent the detection of full-length PFV mRNA in the cytoplasm. Furthermore, the cytoplasmic levels of the cellular GAPDH mRNA remained unchanged by using the specific siRNAs, indicating an ANP32A-independent nuclear export of this mRNA (Fig. 6B).
During this study, we discovered that ANP32A also interacts with the viral transactivator Tas. The ANP32A siRNA treatment led to a decrease in transactivation of the PFV IP by Tas (our unpublished observation). Therefore, and because the bet mRNA levels are generally low in HEK 293T cells (see above), this mRNA was hardly detectable in both the total and cytoplasmic fractions (data not shown). However, to exclude influences on the CMV promoter driving gag gene expression of pcHSRV2, we cotransfected the cells with pEGFP. Levels of EGFP mRNA were not altered by siRNA treatment (Fig. 6B), demonstrating that the CMV promoter activity and the nuclear export of mRNA coding for EGFP are not ANP32A dependent.
To analyze this involvement, ANP32B-specific siRNAs were designed that efficiently inhibited ANP32B mRNA translation (Fig. 6C). The siRNAs were used for cotransfection of HEK 293T cells together with pcHSRV2 and pEGFP. Lysates were prepared and analyzed for the presence of PFV Gag and EGFP. Figure 6C illustrates that the detection of PFV Gag protein was ANP32B dependent, while amounts of EGFP were unaffected, likely because the EGFP mRNA is using a different nuclear export pathway. To exclude unspecific side effects of the siRNA treatment, we used an ANP32-specific siRNA and constructed an siRNA-insensitive ANP32B mutant clone (pcANP32B-Δ3′UTR), lacking the siRNA-targeted 3′ UTR of ANP32B. While again Gag protein in ANP32B siRNA-treated cells was significantly reduced, the reconstitution of ANP32B by cotransfection of siRNA-treated cells with pcANP32B-Δ3′UTR and pcHRSV2 did indeed restore PFV gag gene expression to wild-type levels (Fig. 6C). GAPDH expression was analyzed by immunoblotting as a loading control and remained unchanged irrespective of the siRNA treatment. This result was highly suggestive of both proteins ANP32A and -B essentially contributing to the cytoplasmic export out of the nucleus of unspliced PFV mRNA.
ANP32A and -B do not posses RNA-binding motifs, but both are known adapter proteins with functional NESs. They interact with CRM1 on the one side and the RNA-binding shuttle protein HuR on the other (23, 24, 57). The latter is involved in protecting mRNAs with AU-rich elements (AREs) in their 3′ UTR from rapid cytoplasmic degradation by counteracting the degradation-enhancing activity of other ARE-binding proteins (24). Thus, HuR has a strong protective effect on certain cellular mRNAs (24). To substantiate the finding that HuR is indeed involved in nuclear-cytoplasmic shuttling of unspliced PFV mRNA, we cotransfected cells with pcHSRV2 and an HuR siRNA or an unrelated siRNA as a control. As illustrated in Fig. 6D, only the specific siRNA led to downregulation of HuR and PFV Gag protein, whereas the detection of GAPDH protein remained unaffected, as was the detection of the EGFP mRNA derived from the pEGFP plasmid, which was used for cotransfection of cells.
DISCUSSION
In this report, we describe the identification and partial characterization of a novel retroviral RNA export pathway. So far, two independent mechanisms for nuclear RNA export of incompletely spliced retroviral RNAs have been described in detail. One is specified as CTE and NXF1/NXT1 dependent, while the other is virus-encoded regulatory protein and response element and CRM1 dependent. FVs appear to make use of a third distinctive pathway that has acquired features of both previously characterized nuclear exit strategies. The PFV pathway is CRM1 dependent without involving a virus-encoded regulatory protein. Instead, incompletely spliced PFV mRNA appears to be transported into the cytoplasm by contacting the cellular RNA-binding shuttle protein HuR and further adapter molecules. Although formal proof is still lacking, our results suggest a direct contact between HuR and supposedly PFV-specific HuR-interacting RNA elements. HuR has been shown previously to bind to a region of 150 nt adopting a stable RNA stem-loop in the CD83 mRNA (57). This interaction results in CRM1-dependent nuclear export (57).
However, the secondary structure involved in the CD83 mRNA-HuR interaction (57) is not present in the PFV genome, suggesting a different mode of binding. Furthermore, contrary to a previous study on the nuclear export of CD83 mRNA that indicated the requirement of only ANP32B (26), the results presented here strongly suggest the essential involvement of both adapter proteins ANP32A (pp32) and ANP32B (April), in facilitating nuclear export of unspliced PFV mRNA. Especially overexpression of ANP32B resulted in higher cellular Gag levels (Fig. 5C), indicating that ANP32B might be a limiting factor for Gag expression.
For the MLV-related intracisternal A-type particle (IAP), an RNA element (IAPE) has been described that maps to the 3′ pol gene region (73). IAPE was found to be required for IAP gag gene expression and also allowed the expression of HIV-1 gag independently of the Rev/RRE pathway (73). Consequently, IAPE-mediated nuclear export of viral RNA depended on NXF1/NXT1 and was independent of CRM1 (73). A structural and functional homology between a PFV pol gene fragment and IAPE was noted, the PFV fragment was shown to facilitate HIV-1 gag gene expression, and a CTE for PFV was suggested (73). However, our results strongly indicate that the PFV-specific nuclear export pathway is CRM1 dependent, suggesting that more than one RNA element in the PFV genome may exist to direct the nuclear export of unspliced mRNA by using alternative pathways or that the proposed CTE acts differently, as suggested in reference 73.
This study shows that the unspliced gag mRNA of PFV makes use of the newly identified nuclear export pathway. Although we did not investigate this point in detail, it is likely that this also applies to the singly spliced pol and env mRNAs, because additional and, during viral replication, often used SD-SA pairs are present within and downstream of these genes (Fig. 1). In addition, the export of the PFV env mRNA is LMB sensitive (Fig. 2), supporting a CRM1-dependent export pathway and suggesting an (additional) HuR binding motif contained in the env mRNA. This point should be resolved by future experiments.
FVs make use of a replication strategy that functionally bridges that of orthoretroviruses and hepadnaviruses (41, 43, 61), with respect to the way nuclear RNA export hepadnaviruses make use of various pathways (68). While posttranscriptional regulatory elements (PREs) have been identified in both the human and woodchuck hepatitis B viruses (HPRE and WPRE, respectively), their modes of action appear to be different, and the cellular proteins interacting with the elements are elusive (68). The nuclear export pathway specified by HPRE is CRM1 independent, and the pathway specified by WPRE depends on CRM1 (54, 56). However, both elements adopt an elaborated secondary structure (21, 55, 67, 73), and it remains to be seen where a probably structured element interacting with nuclear export factors is located within the FV (pre-) genome.
In complex orthoretroviruses, the use of the CRM1-dependent and virus-encoded posttranscriptional regulatory protein plus regulatory RNA element-dependent pathway serves at least the following four functions. (i) It enables the virus to differentially regulate its gene expression (16). As described above, FVs accomplish this by using two viral promoters (44). (ii) It facilitates the nuclear export of unspliced and singly spliced mRNAs, which would otherwise be retained and degraded (18). FVs appear to make use of the newly discovered mRNA export pathway to do this. (iii) It specifically speeds up the nuclear export of certain viral mRNAs by using a different NXF1/NXT1-independent pathway that might not be saturated by cellular mRNAs (38). FVs might also benefit from using an alternative CRM1-dependent pathway. Finally, (iv) evidence has been presented that at least in the Rev-RRE interaction of HIV-1 mRNAs encoding the structural proteins, there exists an enhancement effect on translational, in addition to the nuclear export component (20, 40). By exploitation of the HuR-mediated nuclear export of mRNA, FVs appear to have found an elegant way to translate their structural mRNAs preferentially over cellular mRNAs.
HuR had been described initially as a protein protecting certain cellular mRNAs from degradation and enhancing their cytoplasmic translation (24). An additional function in mediating the nuclear export of rare cellular mRNAs emerged only recently (57). Although far from being completely analyzed, the finding that structural gene expression of a retroviral subfamily member completely relies on HuR now points to the importance of what has so far been regarded as the minor function of this cellular protein.
Acknowledgments
We thank J. Hauber and J. Chemnitz for the precious ANP32B antiserum, J. Bohne and H.-G. Kräusslich for plasmids, and Melanie Wunram for technical assistance.
This work was supported in part by DFG grants (SFB479, RE627/7, RE627/8, and BO3006/1-2).
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1.Ahmed, Y. F., S. M. Hanly, M. H. Malim, B. R. Cullen, and W. C. Greene. 1990. Structure-function analyses of the HTLV-I Rex and HIV-1 Rev RNA response elements: insights into the mechanism of Rex and Rev action. Genes Dev. 4:1014-1022. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., et al. 1987. Current protocols in molecular biology. John Wiley, New York, NY.
- 3.Basyuk, E., S. Boulon, F. Skou Pedersen, E. Bertrand, and S. Vestergaard Rasmussen. 2005. The packaging signal of MLV is an integrated module that mediates intracellular transport of genomic RNAs. J. Mol. Biol. 354:330-339. [DOI] [PubMed] [Google Scholar]
- 4.Baunach, G., B. Maurer, H. Hahn, M. Kranz, and A. Rethwilm. 1993. Functional analysis of human foamy virus accessory reading frames. J. Virol. 67:5411-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieniasz, P. D., O. Erlwein, A. Aguzzi, A. Rethwilm, and M. O. McClure. 1997. Gene transfer using replication-defective human foamy virus vectors. Virology 235:65-72. [DOI] [PubMed] [Google Scholar]
- 6.Bodem, J., et al. 2000. TIF-IA, the factor mediating growth-dependent control of ribosomal RNA synthesis, is the mammalian homolog of yeast Rrn3p. EMBO Rep. 1:171-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodem, J., Y. Kang, and R. M. Flügel. 2004. Comparative functional characterization of the feline foamy virus transactivator reveals its species specificity. Virology 318:32-36. [DOI] [PubMed] [Google Scholar]
- 8.Bodem, J., M. Löchelt, H. Delius, and R. M. Flügel. 1998. Detection of subgenomic cDNAs and mapping of feline foamy virus mRNAs reveals complex patterns of transcription. Virology 244:417-426. [DOI] [PubMed] [Google Scholar]
- 9.Bodem, J., et al. 1996. Characterization of the spliced pol transcript of feline foamy virus: the splice acceptor site of the pol transcript is located in gag of foamy viruses. J. Virol. 70:9024-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodem, J., M. Zemba, and R. M. Flügel. 1998. Nuclear localization of the functional Bel 1 transactivator but not of the gag proteins of the feline foamy virus. Virology 251:22-27. [DOI] [PubMed] [Google Scholar]
- 11.Bogerd, H. P., A. Echarri, T. M. Ross, and B. R. Cullen. 1998. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J. Virol. 72:8627-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogerd, H. P., H. L. Wiegand, J. Yang, and B. R. Cullen. 2000. Mutational definition of functional domains within the Rev homolog encoded by human endogenous retrovirus K. J. Virol. 74:9353-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohne, J., and H. Kräusslich. 2004. Mutation of the major 5′ splice site renders a CMV-driven HIV-1 proviral clone Tat-dependent: connections between transcription and splicing. FEBS Lett. 563:113-118. [DOI] [PubMed] [Google Scholar]
- 14.Caporale, M., et al. 2009. The signal peptide of a simple retrovirus envelope functions as a posttranscriptional regulator of viral gene expression. J. Virol. 83:4591-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cigler, P., et al. 2005. From nonpeptide toward noncarbon protease inhibitors: metallacarboranes as specific and potent inhibitors of HIV protease. Proc. Natl. Acad. Sci. U. S. A. 102:15394-15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullen, B. R. 1991. Human immunodeficiency virus as a prototypic complex retrovirus. J. Virol. 65:1053-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullen, B. R. 2003. Nuclear mRNA export: insights from virology. Trends Biochem. Sci. 28:419-424. [DOI] [PubMed] [Google Scholar]
- 18.Cullen, B. R. 2003. Nuclear RNA export. J. Cell Sci. 116:587-597. [DOI] [PubMed] [Google Scholar]
- 19.Cullen, B. R. 2002. Using retroviruses to study the nuclear export of mRNA. Results Probl. Cell Differ. 35:151-168. [DOI] [PubMed] [Google Scholar]
- 20.D'Agostino, D. M., B. K. Felber, J. E. Harrison, and G. N. Pavlakis. 1992. The Rev protein of human immunodeficiency virus type 1 promotes polysomal association and translation of gag/pol and vpu/env mRNAs. Mol. Cell. Biol. 12:1375-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donello, J. E., J. E. Loeb, and T. J. Hope. 1998. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J. Virol. 72:5085-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enssle, J., I. Jordan, B. Mauer, and A. Rethwilm. 1996. Foamy virus reverse transcriptase is expressed independently from the Gag protein. Proc. Natl. Acad. Sci. U. S. A. 93:4137-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan, X. C., and J. A. Steitz. 1998. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl. Acad. Sci. U. S. A. 95:15293-15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan, X. C., and J. A. Steitz. 1998. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 17:3448-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer, N., et al. 1998. Foamy virus particle formation. J. Virol. 72:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fries, B., et al. 2007. Analysis of nucleocytoplasmic trafficking of the HuR ligand APRIL and its influence on CD83 expression. J. Biol. Chem. 282:4504-4515. [DOI] [PubMed] [Google Scholar]
- 27.Gorlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607-660. [DOI] [PubMed] [Google Scholar]
- 28.Gruter, P., et al. 1998. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell 1:649-659. [DOI] [PubMed] [Google Scholar]
- 29.Hanly, S. M., et al. 1989. Comparative analysis of the HTLV-I Rex and HIV-1 Rev trans-regulatory proteins and their RNA response elements. Genes Dev. 3:1534-1544. [DOI] [PubMed] [Google Scholar]
- 30.Heinkelein, M., et al. 2002. Improved primate foamy virus vectors and packaging constructs. J. Virol. 76:3774-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinkelein, M., et al. 1998. Characterization of a cis-acting sequence in the Pol region required to transfer human foamy virus vectors. J. Virol. 72:6307-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imrich, H., M. Heinkelein, O. Herchenröder, and A. Rethwilm. 2000. Primate foamy virus Pol proteins are imported into the nucleus. J. Gen. Virol. 81:2941-2947. [DOI] [PubMed] [Google Scholar]
- 33.Indik, S., W. H. Gunzburg, B. Salmons, and F. Rouault. 2005. A novel, mouse mammary tumor virus encoded protein with Rev-like properties. Virology 337:1-6. [DOI] [PubMed] [Google Scholar]
- 34.Jordan, I., J. Enssle, E. Guttler, B. Mauer, and A. Rethwilm. 1996. Expression of human foamy virus reverse transcriptase involves a spliced pol mRNA. Virology 224:314-319. [DOI] [PubMed] [Google Scholar]
- 35.Kang, Y., W. S. Blair, and B. R. Cullen. 1998. Identification and functional characterization of a high-affinity Bel-1 DNA binding site located in the human foamy virus internal promoter. J. Virol. 72:504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang, Y., H. P. Bogerd, and B. R. Cullen. 2000. Analysis of cellular factors that mediate nuclear export of RNAs bearing the Mason-Pfizer monkey virus constitutive transport element. J. Virol. 74:5863-5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang, Y., H. P. Bogerd, J. Yang, and B. R. Cullen. 1999. Analysis of the RNA binding specificity of the human tap protein, a constitutive transport element-specific nuclear RNA export factor. Virology 262:200-209. [DOI] [PubMed] [Google Scholar]
- 38.Kang, Y., and B. R. Cullen. 1999. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 13:1126-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller, A., et al. 1991. Characterization of the transcriptional trans activator of human foamy retrovirus. J. Virol. 65:2589-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence, J. B., A. W. Cochrane, C. V. Johnson, A. Perkins, and C. A. Rosen. 1991. The HIV-1 Rev protein: a model system for coupled RNA transport and translation. New Biol. 3:1220-1232. [PubMed] [Google Scholar]
- 41.Lecellier, C. H., and A. Saib. 2000. Foamy viruses: between retroviruses and pararetroviruses. Virology 271:1-8. [DOI] [PubMed] [Google Scholar]
- 42.Li, Y., et al. 2006. An intron with a constitutive transport element is retained in a Tap messenger RNA. Nature 443:234-237. [DOI] [PubMed] [Google Scholar]
- 43.Linial, M. L. 2007. Foamy viruses. Lippincott Williams & Wilkins, Philadelphia, PA.
- 44.Löchelt, M. 2003. Foamy virus transactivation and gene expression. Curr. Top. Microbiol. Immunol. 277:27-61. [DOI] [PubMed] [Google Scholar]
- 45.Löchelt, M., M. Aboud, and R. M. Flügel. 1993. Increase in the basal transcriptional activity of the human foamy virus internal promoter by the homologous long terminal repeat promoter in cis. Nucleic Acids Res. 21:4226-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Löchelt, M., W. Muranyi, and R. M. Flügel. 1993. Human foamy virus genome possesses an internal, Bel-1-dependent and functional promoter. Proc. Natl. Acad. Sci. U. S. A. 90:7317-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Löwer, R., R. R. Tönjes, C. Korbmacher, R. Kurth, and J. Löwer. 1995. Identification of a Rev-related protein by analysis of spliced transcripts of the human endogenous retroviruses HTDV/HERV-K. J. Virol. 69:141-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malim, M. H., J. Hauber, R. Fenrick, and B. R. Cullen. 1988. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature 335:181-183. [DOI] [PubMed] [Google Scholar]
- 49.Mertz, J. A., M. S. Simper, M. M. Lozano, S. M. Payne, and J. P. Dudley. 2005. Mouse mammary tumor virus encodes a self-regulatory RNA export protein and is a complex retrovirus. J. Virol. 79:14737-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moebes, A., et al. 1997. Human foamy virus reverse transcription that occurs late in the viral replication cycle. J. Virol. 71:7305-7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muranyi, W., and R. M. Flügel. 1991. Analysis of splicing patterns of human spumaretrovirus by polymerase chain reaction reveals complex RNA structures. J. Virol. 65:727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niranjanakumari, S., E. Lasda, R. Brazas, and M. Garcia-Blanco. 2002. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods 26:182-190. [DOI] [PubMed] [Google Scholar]
- 53.Ohno, M., M. Fornerod, and I. W. Mattaj. 1998. Nucleocytoplasmic transport: the last 200 nanometers. Cell 92:327-336. [DOI] [PubMed] [Google Scholar]
- 54.Otero, G. C., M. E. Harris, J. E. Donello, and T. J. Hope. 1998. Leptomycin B inhibits equine infectious anemia virus Rev and feline immunodeficiency virus rev function but not the function of the hepatitis B virus posttranscriptional regulatory element. J. Virol. 72:7593-7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patzel, V., and G. Sczakiel. 1997. The hepatitis B virus posttranscriptional regulatory element contains a highly stable RNA secondary structure. Biochem. Biophys. Res. Commun. 231:864-867. [DOI] [PubMed] [Google Scholar]
- 56.Popa, I., M. E. Harris, J. E. Donello, and T. J. Hope. 2002. CRM1-dependent function of a cis-acting RNA export element. Mol. Cell. Biol. 22:2057-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prechtel, A. T., et al. 2006. Expression of CD83 is regulated by HuR via a novel cis-active coding region RNA element. J. Biol. Chem. 281:10912-10925. [DOI] [PubMed] [Google Scholar]
- 58.Rabson, A. B., and B. J. Graves. 1997. Synthesis and processing of viral RNA, p. 205-261. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Plainview, NY. [PubMed]
- 59.Reed, R. 2003. Coupling transcription, splicing and mRNA export. Curr. Opin. Cell Biol. 15:326-331. [DOI] [PubMed] [Google Scholar]
- 60.Renault, N., A. Coiffic, and A. Saib. 2008. L'export nucléaire des ARN messagers rétroviraux. Virologie 12:429-437. [DOI] [PubMed] [Google Scholar]
- 61.Rethwilm, A. 2005. Foamy viruses, p. 1304-1321. In B. W. J. Mahy and V. ter Meulen (ed.), Topley & Wilson's microbiology and microbial infections—virology, 10th ed., vol. 2. Hodder Arnold, London, United Kingdom.
- 62.Rethwilm, A., O. Erlwein, G. Baunach, B. Maurer, and V. ter Meulen. 1991. The transcriptional transactivator of human foamy virus maps to the bel 1 genomic region. Proc. Natl. Acad. Sci. U. S. A. 88:941-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez, M. S., C. Dargemont, and F. Stutz. 2004. Nuclear export of RNA. Biol. Cell 96:639-655. [DOI] [PubMed] [Google Scholar]
- 64.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 65.Schenk, T., J. Enssle, N. Fischer, and A. Rethwilm. 1999. Replication of a foamy virus mutant with a constitutively active U3 promoter and deleted accessory genes. J. Gen. Virol. 80:1591-1598. [DOI] [PubMed] [Google Scholar]
- 66.Schliephake, A. W., and A. Rethwilm. 1994. Nuclear localization of foamy virus Gag precursor protein. J. Virol. 68:4946-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith, G. J., III, J. E. Donello, R. Luck, G. Steger, and T. J. Hope. 1998. The hepatitis B virus post-transcriptional regulatory element contains two conserved RNA stem-loops which are required for function. Nucleic Acids Res. 26:4818-4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sommer, G., and T. Heise. 2008. Posttranscriptional control of HBV gene expression. Front. Biosci. 13:5533-5547. [DOI] [PubMed] [Google Scholar]
- 69.Stirnnagel, K., et al. 2010. Analysis of prototype foamy virus particle-host cell interaction with autofluorescent retroviral particles. Retrovirology 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vinciguerra, P., and F. Stutz. 2004. mRNA export: an assembly line from genes to nuclear pores. Curr. Opin. Cell Biol. 16:285-292. [DOI] [PubMed] [Google Scholar]
- 71.Weis, K. 2003. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 112:441-451. [DOI] [PubMed] [Google Scholar]
- 72.Wiegand, H. L., et al. 2002. Formation of Tap/NXT1 heterodimers activates Tap-dependent nuclear mRNA export by enhancing recruitment to nuclear pore complexes. Mol. Cell. Biol. 22:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wodrich, H., J. Bohne, E. Gumz, R. Welker, and H. G. Kräusslich. 2001. A new RNA element located in the coding region of a murine endogenous retrovirus can functionally replace the Rev/Rev-responsive element system in human immunodeficiency virus type 1 Gag expression. J. Virol. 75:10670-10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang, J., et al. 1999. An ancient family of human endogenous retroviruses encodes a functional homolog of the HIV-1 Rev protein. Proc. Natl. Acad. Sci. U. S. A. 96:13404-13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu, S. F., et al. 1996. The carboxyl terminus of the human foamy virus Gag protein contains separable nucleic acid binding and nuclear transport domains. J. Virol. 70:8255-8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu, S. F., and M. L. Linial. 1993. Analysis of the role of the bel and bet open reading frames of human foamy virus by using a new quantitative assay. J. Virol. 67:6618-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zolotukhin, A. S., D. Michalowski, S. Smulevitch, and B. K. Felber. 2001. Retroviral constitutive transport element evolved from cellular TAP(NXF1)-binding sequences. J. Virol. 75:5567-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]