Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) G protein-coupled receptor (vGPCR) protein has been shown to induce several signaling pathways leading to the modulation of host gene expression. The hijacking of these pathways facilitates the viral life cycle and leads to tumorigenesis. In the present work, we show that transforming growth factor β (TGF-β)-activated kinase 1 (TAK1) is an important player in NF-κB activation induced by vGPCR. We observed that the expression of an inactive TAK1 kinase mutant (TAK1M) reduces vGPCR-induced NF-κB nuclear translocation and transcriptional activity. Consequently, the expression of several NF-κB target genes normally induced by vGPCR was blocked by TAK1M expression, including interleukin 8 (IL-8), Gro1, IκBα, COX-2, cIAP2, and Bcl2 genes. Similar results were obtained after downregulation of TAK1 by small interfering RNA (siRNA) technology. The expression of vGPCR recruited TAK1 to the plasma membrane, and vGPCR interacts with TAK1. vGPCR expression also induced TAK1 phosphorylation and lysine 63-linked polyubiquitination, the two markers of the kinase's activation. Finally, inhibition of TAK1 by celastrol inhibited vGPCR-induced NF-κB activation, indicating this natural compound could be used as a potential therapeutic drug against KSHV malignancies involving vGPCR.
Kaposi's sarcoma-associated herpesvirus (KSHV), or human herpesvirus 8 (HHV-8), is etiologically associated with the pathogenesis of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and some forms of multicentric Castleman's disease (MCD), a polyclonal B-cell angiolymphoproliferative disorder (11, 23). KS lesions are a multifocal angioproliferative disease composed of infiltrating inflammatory cells and spindle-shaped endothelial cells. KSHV replication can be divided into two phases, latent and lytic. During the latent phase, a limited number of latent genes and 11 microRNAs are transcribed (7, 23, 54, 66, 84), which mediates the inhibition of apoptosis, modulation of host anti-oncogenes, and maintenance of the latent KSHV genome. In the lytic phase, a cascade of immediate-early, early, and late viral genes is initiated, resulting in viral DNA replication, assembly, and release of progeny infectious virions. In addition, several KSHV lytic-cycle proteins are responsible for immune evasion, inhibition of apoptosis, host gene modulation, host protein expression shutoff, and modulation of signal transduction (23). In KS lesions, the majority of cells express the latent form of KSHV, while a low percentage of cells undergo a lytic cycle.
Viral GPCR (vGPCR) possesses limited homology to human chemokine receptors, such as the interleukin 8 (IL-8) receptor. Similar to the human chemokine receptors, vGPCR can be stimulated by Gro1 and IP10 or inhibited by SDF1. However, in contrast to human receptors, vGPCR is constitutively active (52). vGPCR has been shown to be involved in COS cell and endothelial cell proliferation (2, 28). Several laboratories have intensively documented the importance of vGPCR in cancer formation. vGPCR can transform NIH 3T3 fibroblasts in vitro, and vGPCR-expressing 3T3 cells form tumors in mice (3). In addition, it has been shown that vGPCR can immortalize human umbilical vein endothelial cells (HUVEC) and that it has antiapoptotic properties (4, 44). Mice expressing vGPCR in endothelial cells developed tumors resembling KS lesions. In addition, transgenic mice overexpressing vGPCR under a constitutive promoter or a T cell-specific promoter develop tumors resembling KS lesions (29, 81). The finding that T cell expression of vGPCR was able to promote KS-like lesions highlights the importance of paracrine signaling. Indeed, it has been shown by several laboratories that the expression of vGPCR leads to the secretion of several proliferative or angiogenic factors, including VEGF, VEGFR2 (KDR), Gro1, and IL-8 (4, 25, 29, 55, 63).
To understand how vGPCR could be involved in tumorigenesis, several laboratories characterized the different signaling pathways activated by its expression. It has been shown that vGPCR expression activates phosphatidylinositol 3-kinase (PI3-K) (4), mitogen-activated protein kinase (MAPK) cascades and p38 MAPK (63), and Akt/protein kinase B (8, 44), as well as some small GTPases, like RhoA and Rac1 (41, 45, 59). Consequently several transcription factors have been shown to be activated by vGPCR expression, including activating protein 1 (AP1), nuclear factor activator of T cells (NFAT), cyclic AMP response element binding protein (CREB), hypoxia-inducible factor 1 (HIF-1), and nuclear factor kappa B (NF-κB) (9, 10, 45, 63).
Members of the NF-κB family of proteins (p65/p50) are involved in the induction of a wide variety of genes involved in many processes (16, 24, 72), and constitutive activity of NF-κB has been documented in different kinds of malignancies (5, 51). A crucial step leading to NF-κB activation is phosphorylation of the inhibitor of the NF-κB family of proteins (IκB), which leads to its polyubiquitination and degradation by the proteasome. NF-κB, free from its inhibitor, translocates into the nucleus, binds to its target DNA sequences, and activates the transcription of its target genes. The kinases responsible for ΙκΒ-α phosphorylation belong to the IκB kinase (IKK) complex (33, 62, 72). The best-characterized components of this complex are the two kinases IKK1 and IKK2, as well as the regulatory scaffold protein NEMO (NF-κB essential modulator). Blocking IKK1 and IKK2 activity is currently being tested as an anticancer therapy (36).
Transforming growth factor β (TGF-β)-activated kinase 1 (TAK1) is a recently identified member of the MAPKKK family (also called Map3k7). After stimulation by a wide range of cytokines, such as TLR, IL-1, and tumor necrosis factor alpha (TNF-α), TAK1 is activated by a ubiquitin-dependent mechanism (without degradation) and phosphorylates the IKKs, which in turn activate NF-κB (1, 14, 47, 76). Expression of a dominant-negative kinase has demonstrated the role of TAK1 in the activation of both IKK and JNK induced by TNF-α (69). This result was confirmed when murine embryonic fibroblasts (MEF) deficient in TAK1 expression were shown to be defective in activating the IKK, JNK, and p38 kinases through TNF-α or IL-1 stimulation (61). During development, TAK1 modulates cell differentiation and apoptosis in Drosophila melanogaster and Xenopus laevis and regulates apoptosis in adult mammalian cells in vivo (60, 68, 79, 83).
In this study, we characterize the pathway resulting in NF-κB activation by vGPCR. We confirm that the IKK complex is involved in NF-κB activation induced by vGPCR, and we identify TAK1 as an important protein upstream of the IKK complex. We show that vGPCR colocalizes and interacts with TAK1, and consequently, that TAK1 is phosphorylated and polyubiquitinated, two steps necessary for its activation. Taken together, this study highlights the role of TAK1 in NF-κB activation induced by vGPCR, identifying a new therapeutic target for KSHV-associated Kaposi's sarcoma and other malignancies involving vGPCR.
MATERIALS AND METHODS
Cells.
Human embryonic kidney HEK293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 1 mM pyruvate (Invitrogen, Carlsbald, CA), 2 mM glutaMax (Invitrogen), 50 U/ml penicillin, 50 mg/ml streptomycin (Invitrogen), and 10% fetal calf serum (Fetalplex; Gemini, Logan, UT). SVEC-GFP and SVEC-vGPCR cells were a gift from S. Montaner, University of Maryland, Baltimore, MD (43). These cells were maintained as described above, with the exception of 10% fetal calf serum.
Reagents.
Celastrol and tubulin antibody were from Sigma-Aldrich, St. Louis, MO. Polyclonal rabbit anti-vGPCR peptide (amino acids 4 to 16) ([Y]EDFLTIFLDDDES[SC]) antibody was a gift from Gary Hayward, Johns Hopkins School of Medicine, Baltimore, MD (15). Ubiquitin (Ub) (P4D1), hemagglutinin (HA), Flag, TAK1, and green fluorescent protein (GFP) (B2) antibodies were from Santa Cruz (Santa Cruz, CA), and the p65 antibody was from Millipore, Billerica, MA.
Plasmids.
pSG5-vGPCR expression plasmids were from John Nicholas, Johns Hopkins School of Medicine, Baltimore, MD (38). PRK5-HA-ubiquitin wild type (WT) and mutants were from Ted Dawson (Addgene plasmids 17608, 17605, and 17606). IκBM, βTrCPM, IKK1M, IKK2M, and siTAK1 plasmids were from Inder Verma, Salk Institute for Biological Studies, La Jolla, CA. TAK1 WT and mutant expression plasmids were from Zhijian Chen, University of Texas Southwestern Medical Center, Dallas, TX (75).
Construction and production of lentiviral gene transfer vectors.
Vector plasmids were constructed for the production of lentiviral vectors that expressed the desired short hairpin RNA (shRNA). All vectors were designed to be self-inactivating (SIN) (42). The vectors expressed enhanced green fluorescent protein (EGFP) under a cytomegalovirus (CMV) promoter, and the shRNA cassette utilized the U6 polymerase III promoter. Lentiviral vectors were produced using a four-plasmid transfection system, as previously described (70). Briefly, HEK293T cells were transfected with vector and packaging plasmids, the supernatants were collected, and the lentiviral vectors were concentrated by centrifugation. The lentiviral vector titers were estimated by flow cytometry for GFP-positive cells.
Immunoblotting.
Cells were harvested in RIPA lysis buffer (125 mM NaCl, 0.01 M sodium phosphate, pH 7.2, 0.1% SDS, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, and 50 mM sodium fluoride) with protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma) or in lysis buffer (50 mM Tris-HCl, pH 8, 170 mM NaCl, 0.5% NP-40, 50 mM NaF) with protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma). Cellular debris was removed by centrifugation at 13,000 × g for 5 min at 4°C, and equal amounts of protein were resolved by SDS-10% PAGE, transferred to nitrocellulose, and subjected to Western blotting with the indicated antibodies. To confirm equal protein loading, blots were also probed with antibodies against human tubulin or actin. Secondary antibodies conjugated to horseradish peroxidase (Santa Cruz) were used for detection. Immunoreactive bands were developed by enhanced chemiluminescence (Lumi-Light Plus Western blotting substrate; Roche, Indianapolis, IN).
Calcium phosphate transfection.
HEK293T cells were transfected by the calcium phosphate method, as previously described (27). Briefly, for transfection, per well of a 6-well plate, 3 μg of DNA was mixed in 100 μl of 0.25 M CaCl2 and then added to an equal volume of BBS (50 mM BES [N,N-bis-(2-hydroxyethyl)-2-aminoethanesulfonic acid], 280 mM NaCl, 1.5 mM Na2HPO4, pH 6.95)-buffered solution. The DNA mixture was added to the HEK293T cells in 2 ml of complete medium and incubated overnight at 3% CO2. The medium was then changed, and the cells were allowed to recover for 24 h at 10% CO2.
Luciferase assays.
HEK293T cells were transiently transfected with the expression plasmid, a luciferase reporter construct, and a vector expressing the β-galactosidase (β-Gal) reporter gene controlled by a Rous sarcoma virus (RSV) promoter (used to normalize transfection efficiency) by the calcium phosphate method. At 36-h posttransfection, cells were harvested and lysed in lysis reporter buffer (Promega, Madison, WI). Soluble extracts were assayed for luciferase and β-Gal activities using Steady-Glo and Beta-Glo (Promega), respectively, following the manufacturer's instructions. The luciferase activity was normalized to β-galactosidase activity as a transfection control.
RNA extraction, reverse transcription, and real-time PCR.
Total RNA was extracted by using TRIzol reagent (Invitrogen) and quantified by densitometric analysis at 260 nm. Two micrograms of total RNA was treated with DNase for 1 h (DNA free; Ambion) and then reverse transcribed into cDNA by using random primers (hexamers) with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). For real-time (RT)-PCR, cDNA was used as a template with gene-specific primers that were designed with Primer Express 1.5a software or previously published. The primer sequences used are shown in Table 1. PCR was performed using an ABI Prism 7500 real-time PCR system utilizing SYBR green PCR master mix (Applied Biosystems).
TABLE 1.
Primer sequences used in the study
| Human protein | Primer |
|
|---|---|---|
| Forward | Reverse | |
| IL-8 | 5′-CTGGCCGTGGCTCTCTTG-3′ | 5′-CCTTGGCAAAACTGCACCTT-3′ |
| Gro1 | 5′-AACGTGAAGTCCCCCGGA-3′ | 5′-CGCCCATTCTTGAGTGTGG-3′ |
| lκB-α | 5′-GCTGAAGAAGGAGCGGCTACT-3′ | 5′-TCGTACTCCTCGTCTTTCATGGA-3′ |
| COX-2 | 5′-GAATCATTCACCAGGCAAATTG-3′ | 5′-TCTGTACTGCGGGTGGAACA-3′ |
| cIAP2 | 5′-GACAGGAGTTCATCCGTCAAGTTC-3′ | 5′-CCTGGGCTGTCTGATGTGG-3′ |
| Bcl2 | 5′-GAGGAGCTCTTCAGGGACGG-3′ | 5′-GGTGCCGGTTCAGGTACTCA-3′ |
| GAPDH | 5′-GAAGGTGAAGGTCGGAGTC-3′ | 5′-GAAGATGGTGATGGGATTTC-3′ |
| Tubulin | 5′-TCCAGATTGGCAATGCCTG-3′ | 5′-GGCCATCGGGCTGGAT-3′ |
Immunofluorescence assay.
For p65-GFP localization, cells were transfected using the calcium phosphate method as described above. At 36 h posttransfection, the cells were washed 3 times with PBS, fixed in 4% paraformaldehyde (PFA) for 15 min at room temperature, and washed again three times with PBS. Nuclei were visualized using DAPI (4′,6-diamidino-2-phenylindole) (Molecular Probes, Invitrogen, San Diego, CA), and the stained cells were viewed with the appropriate filters under a fluorescence microscope with a 20× objective lens and the Nikon MetaMorph Digital Imaging System.
Confocal microscopy.
For colocalization experiments, HEK293T cells were transfected as described previously. The cells were harvested 36 h posttransfection, washed 3 times in phosphate-buffered saline (PBS), fixed with a 1:1 acetone-to-methanol ratio for 10 min at room temperature, washed, and blocked with bovine serum albumin (BSA) for 30 min. The cells were stained with anti-rabbit vGPCR antibody and anti-mouse Flag antibody for 1 h at 37°C, followed by staining with Alexa Fluor 488-labeled goat anti-rabbit and Alexa Fluor 594-labeled goat anti-mouse secondary antibodies, respectively. An Olympus Fluoview 300 fluorescence confocal microscope was used for imaging, and analysis was performed using Fluoview software (Olympus, Melville, NY).
RESULTS
Mutants of proteins involved in classical pathway activation inhibit NF-κB activation by vGPCR in HEK293T cells.
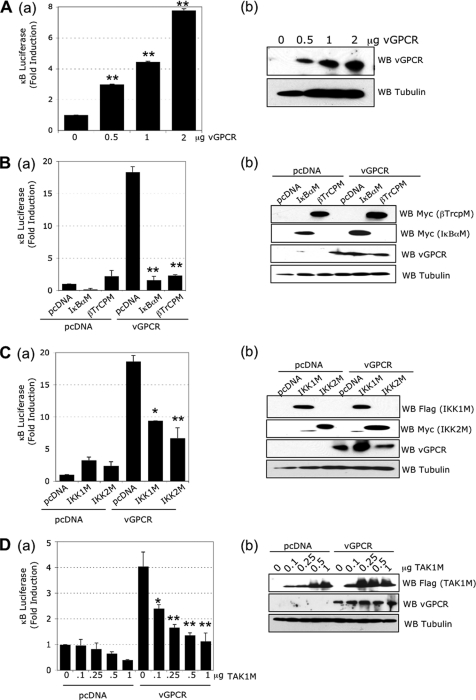
vGPCR has been shown to activate NF-κB signaling in different cell lines. To confirm this in HEK293T cells, we transfected the cells with increasing quantities of plasmid expressing vGPCR and measured the NF-κB activity by a luciferase assay in which luciferase is controlled by 6 consensus κB binding sites. We observed 3-, 4.5-, and 7.8-fold NF-κB activation with 0.5, 1, and 2 μg of vGPCR expression plasmid, respectively, compared to the transfection of an empty plasmid (Fig. 1 A, a). The transgene expression was confirmed by Western blot analysis (Fig. 1A, b).
FIG. 1.
The classical NF-κB pathway is induced by vGPCR. (A) (a) vGPCR induces NF-κB activation. HEK293T cells were transfected with pcDNA or increasing quantities of vGPCR expression plasmids, 1 μg of κB-luciferase construct, and 0.5 μg of β-Gal reporter construct. The luciferase and β-galactosidase activities were measured in triplicate using Steady-glo and Beta-glo Promega kits, respectively. (b) Western blots (WB) using lysates from transfected cells with the indicated antibodies. (B) (a) NF-κB activation induced by vGPCR is blocked by IκBα and βTrCP mutants. HEK293T cells were transfected with 1 μg of pcDNA or vGPCR expression plasmids in the presence or absence of 1 μg of IκBα mutant (IκBαM) or βTrCP mutant (βTrCPM) expression plasmid, along with κB-luciferase and β-Gal reporter constructs. (b) Western blots from lysates of transfected cells using the indicated antibodies. (C) (a) NF-κB activation induced by vGPCR is blocked by IKK1 and IKK2 mutants. HEK293T cells were transfected with 1 μg of pcDNA or vGPCR expression plasmids in the presence or absence of 1 μg of IKK1 or IKK2 mutant expression plasmids (IKK1M and IKK2M, respectively), along with κB-luciferase and β-Gal reporter constructs. (b) Western blots from transfected cell lysates using the indicated antibodies. (D) (a) NF-κB activation induced by vGPCR is blocked by a TAK1 mutant. HEK293T cells were transfected with 1 μg of pcDNA or vGPCR expression plasmids, an increasing quantity of TAK1M expression plasmid, along with κB-luciferase and β-Gal reporter constructs. Statistics compare vGPCR conditions with the corresponding pcDNA conditions at the same quantity of TAK1M plasmid. (b) Western blots from transfected cell lysates using the indicated antibodies. The bar graphs represent the mean and standard deviation (SD) of fold induction (n = 3). *, P < 0.05; **, P < 0.01 compared to the pcDNA control within the vGPCR group (unless stated otherwise).
In the classical NF-κB pathway, some NF-κB family members, such as p65/p50 dimers, are inhibited by the IκBα protein. During classical pathway activation, in order for NF-κB to be active, IκBα has to be phosphorylated at serines 32 and 36, polyubiquitinated, and degraded by the proteasome. To confirm that vGPCR activates NF-κB through the classical pathway in HEK293T cells, we expressed a mutant IκBα (IκBαM) in which the critical serines are mutated to alanine, preventing its degradation and allowing it to act as a dominant-negative protein. Expression of IκBαM decreased basal NF-κB activity and totally abrogated the activation induced by vGPCR (Fig. 1B, a). Another way to block IκBα degradation is to express βTrCP ubiquitin ligase with a deleted F box domain (βTrCPM) (31). As shown in Fig. 1B, a, the expression of βTrCPM did not modify the basal NF-κB activity, and in contrast, we observed an important reduction in vGPCR-induced NF-κB activity. Transgene expression levels were confirmed by Western blot analysis (Fig. 1B, b).
Since the two main IκB kinases (IKK) responsible for IκBα phosphorylation are IKK1 and IKK2, we tested whether these kinases are crucial in vGPCR-induced NF-κB activation in our system. HEK293T cells were transfected with vGPCR in combination with inhibitory IKK1 and IKK2 ATP binding mutants (IKK1M and IKK2M). As shown in Fig. 1C, a, the expression of IKK1M, as well as IKK2M, significantly decreased vGPCR-induced NF-κB activation. Transgene expression levels were confirmed by Western blot analysis (Fig. 1C, b). Taken together, these studies confirmed that vGPCR-dependent NF-κB activation takes place through the classical activation of the IKK complex, followed by the phosphorylation and degradation of IκBα.
Mutant TAK1 inhibits NF-κB activation by vGPCR in HEK293T cells.
The TAK1 protein, a member of the MAPK kinase kinase family, has been shown to regulate IKK complex activity (47). Consequently, we decided to investigate the role of TAK1 in vGPCR-induced NF-κB activation. We expressed increasing quantities of a catalytically dead TAK1 mutant (TAK1M) in which the ATP binding lysine 63 is mutated to tryptophan. We observed that TAK1M could decrease basal NF-κB activity (Fig. 1D, a). In addition, we observed that vGPCR-induced NF-κB activity was also reduced in a dose-dependent manner (Fig. 1D, a). Transgene expression levels were confirmed by Western blot analysis (Fig. 1D, b). These results demonstrated for the first time a role for TAK1 in NF-κB activation induced by a G protein-coupled receptor.
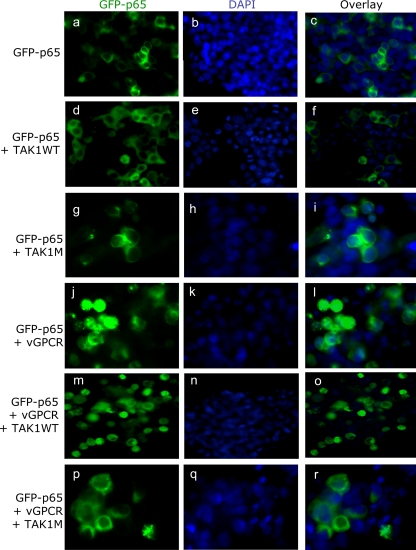
Expression of TAK1M blocks NF-κB nuclear translocation.
Once the ΙκΒα protein is degraded, NF-κB translocates into the nucleus. To confirm the role of TAK1 on NF-κB activation induced by vGPCR, we analyzed the localization of NF-κB (p65-GFP) using fluorescence microscopy. HEK293T cells were transfected with a GFP-p65 fusion construct alone or in combination with vGPCR and WT or mutant TAK1. As expected, under basal conditions, GFP-p65 was localized predominantly in the cytoplasm (Fig. 2 a to c). GFP-p65 remained in the cytoplasm when TAK1 WT and TAK1M were expressed alone (Fig. 2d to i), whereas nuclear translocation was observed with vGPCR expression (Fig. 2j to l). When WT TAK1 was expressed in association with vGPCR, the GFP-p65 fusion protein was predominantly localized in the nuclei of the cells (Fig. 2m to o). However, when TAK1M (functionally dead TAK1) was expressed in association with vGPCR, the GFP-p65 fusion protein was predominantly localized in the cytoplasm of the cells (Fig. 2p to r). This ability of TAK1M to abolish GFP-p65 nuclear localization further confirmed the role of TAK1 in vGPCR-induced NF-κB activation.
FIG. 2.
Expression of TAK1 mutant inhibits NF-κB nuclear translocation. HEK293T cells were transfected with 0.5 μg of GFP-p65 expression plasmid in combination with 1 μg of TAK1 WT or mutant and/or vGPCR expression plasmids. Thirty-six hours posttransfection, the cells were fixed in 4% PFA. Nuclei were visualized using DAPI. The cells were viewed with the appropriate filters under a fluorescence microscope with the Nikon MetaMorph Digital Imaging System. Magnification, ×20.
Expression of TAK1M blocks NF-κB-induced target gene expression.
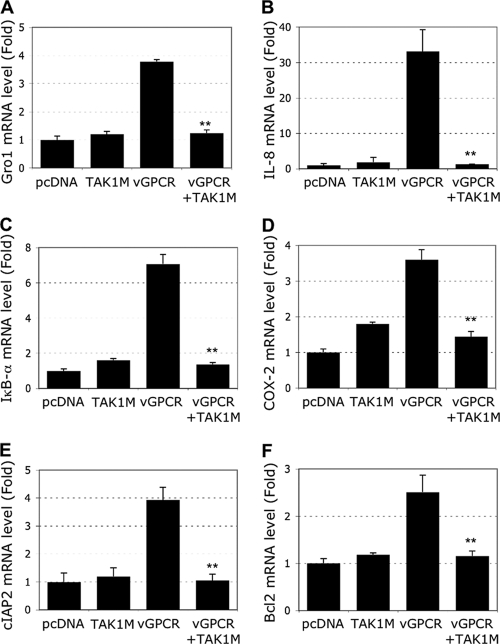
The expression of vGPCR has been shown to induce the transcription of multiple cellular genes, including NF-κB-dependent targets. In addition, it has been shown that NF-κB is necessary for vGPCR-induced tumorigenesis in endothelial cells (40). Hence, we next characterized the involvement of TAK1 in the expression of some of the NF-κB-dependent genes. HEK293T cells were transfected with the indicated expression plasmids, and then RNA was extracted and used in real-time RT-PCR analysis for several NF-κB target genes. These results were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Fig. 3) or tubulin (data not shown) housekeeping genes.
FIG. 3.
Expression of the TAK1 mutant reduces vGPCR-induced NF-κB target genes. HEK293T cells were transfected with 1 μg of vGPCR and/or 1 μg of TAK1M expression plasmids. Real-time PCR analysis measured the level of message for the indicated genes. (A) Gro1. (B) IL-8. (C) ΙκΒ-α. (D) COX-2. (E) cIAP2. (F) Bcl2. Each reaction was done in triplicate, and means and SD are shown. **, P < 0.01 comparing the vGPCR-plus-TAK1M condition with the vGPCR-alone condition.
It is well known that vGPCR induces the expression of IL-8 and the CXC chemokine Gro1 (25, 55), which in turn increases KSHV vGPCR activity above its constitutively active level (52). As shown in Fig. 3A and B, vGPCR increased the levels of Gro1 and IL-8 mRNAs by 4- and 30-fold, respectively. As expected, simultaneous expression of TAK1M totally abrogated Gro1 and IL-8 mRNA expression to basal levels. One of the first NF-κB target genes induced is often the NF-κB inhibitor IκBα itself. It has also been shown that measuring the level of IκBα gene expression provides a good indicator of NF-κB activation (6). We observed a 7-fold increase in IκBα mRNA levels when vGPCR was expressed that was totally blocked by the expression of TAK1M (Fig. 3C).
Another very important NF-κB target gene is the COX-2 gene, which is induced by KSHV infection and is a key factor in tumor-related inflammation, angiogenesis, cell survival, and invasion (57). We observed roughly 3.5-fold induction of COX-2 mRNA after vGPCR expression, and TAK1 appeared to be crucial in this induction, as TAK1M expression blocked COX-2 induction significantly (Fig. 3D). We also examined the role of vGPCR and the involvement of TAK1 in the expression of antiapoptotic molecules, such as cIAP2 and Bcl2, encoded by two well-characterized NF-κB target genes. vGPCR increased the expression levels of cIAP2 and Bcl2 by 4- and 2.5-fold, respectively, which was completely abolished by TAK1M expression (Fig. 3E and F). Taken together, these results suggested that vGPCR acts through TAK1 in the regulation of several NF-κB target genes, such as those encoding Gro1, IL-8, ΙκΒ-α, COX-2, cIAP2, and Bcl2, which are known to be involved in inflammatory and tumorigenic activities.
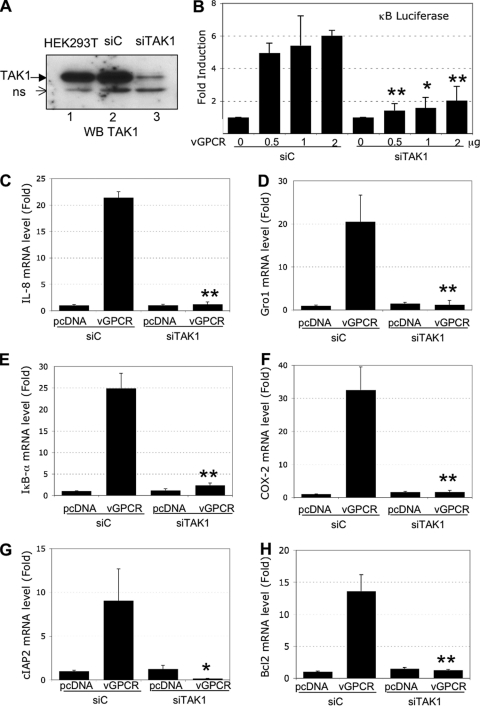
Expression of siRNA against TAK1 decreased vGPCR-induced NF-κB activation.
To confirm the role of TAK1 in NF-κB activation induced by vGPCR, we used small interfering RNA (siRNA) technology to knock down the level of vGPCR. HEK293T cells were transduced with a lentiviral vector expressing a control siRNA (siC) or an siRNA against TAK1 (siTAK1). The Western blot analysis shown in Fig. 4 A confirmed that TAK1 is efficiently reduced (75% decrease). The indicated nonspecific band was used as a loading control. The cells transduced with siRNA lentiviruses were transfected with increasing quantities of vGPCR expression plasmids, and NF-κB activity was measured by luciferase assay. We observed a dose-dependent increase of NF-κB activity in cells expressing siC (Fig. 4B). However, in the cells expressing siTAK1, NF-κB activity was inhibited significantly (Fig. 4B).
FIG. 4.
NF-κB activation induced by vGPCR is blocked by the expression of TAK1 siRNA. (A) TAK1 silencing. HEK293T cells were transduced with a lentivector expressing a control siRNA (siC) or an siRNA against TAK1 (siTAK1). One week later, the cells were lysed in RIPA lysis buffer, and 50 μg of protein was subjected to Western blot analysis using a TAK1 antibody. The detection of a nonspecific band (ns) was used as a loading control. (B) NF-κB luciferase activity induced by vGPCR is reduced when TAK1 is knocked down. HEK293T cells expressing siC or siTAK1 were transfected with pcDNA or increasing quantities of vGPCR expression plasmids, along with κB-luciferase and β-Gal reporter constructs (as described above). Thirty-six hours later, the luciferase and β-galactosidase activities were measured in triplicate using Steady-glo and β-glo Promega kits, respectively. The data represent the means and SD of fold induction (n = 3); *, P < 0.05; **, P < 0.01, comparing siTAK1 conditions with siC conditions with identical quantities of vGPCR-expressing plasmid. (C to H) HEK293T cells expressing siC or siTAK1 were transfected with or without 2 μg of vGPCR. Real-time PCR analysis measured the levels of message for the indicated genes. (C) IL-8. (D) Gro-1. (E) ΙκΒ-α. (F) COX-2. (G) cIAP2. (H) Bcl2. Each reaction was done in triplicate, and means and SD are shown. *, P < 0.05; **, P < 0.01, comparing vGPCR conditions in the siTAK1 cells with vGPCR conditions in the siC cells.
We next examined the effect of siTAK1 expression on the expression of NF-κB-dependent genes shown in Fig. 3. vGPCR induction of Gro1, IL-8, IκBα, COX-2, cIAP2, and Bcl2 mRNAs was observed in siC-transduced cells, which were totally blocked in siTAK1-transduced cells (Fig. 4C to H). This further confirmed that TAK1 plays a critical role in vGPCR induction of NF-κB.
TAK1 colocalizes with vGPCR.
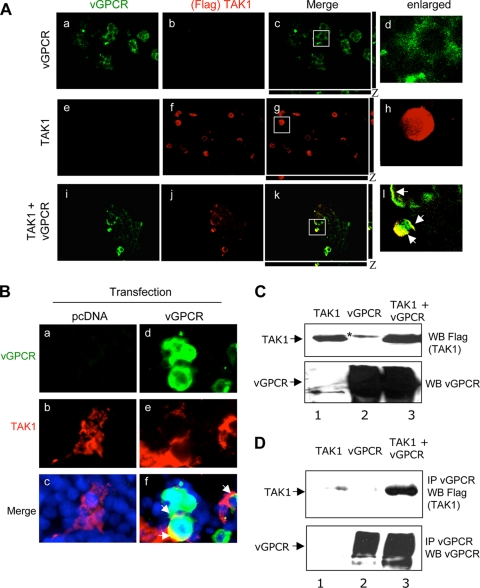
vGPCR is a seven-transmembrane receptor. Confocal analysis demonstrated the plasma membrane localization of vGPCR in HEK293T cells transfected with vGPCR plasmid (Fig. 5 A, a to d). When WT TAK1 was expressed alone, we detected the expected cytoplasmic localization (Fig. 5A, e to h). In contrast, when vGPCR was coexpressed with TAK1, colocalization of vGPCR and TAK1 in the plasma membrane was observed (Fig. 5A, i to l). This suggested that vGPCR is able to modify the localization of TAK1 to the plasma membrane. We also performed colocalization analysis between transfected vGPCR and endogenous TAK1 in HEK293T cells transfected with vGPCR. No colocalization was observed in control pcDNA-transfected cells (Fig. 5B, a, b, and c). In contrast, we observed a clear colocalization of transfected vGPCR with endogenous TAK1 (Fig. 5B, d, e, and f).
FIG. 5.
vGPCR and TAK1 colocalization and interaction. (A) HEK293T cells were transfected with 1 μg of vGPCR alone (a, b, c, and d), WT TAK1 alone (e, f, g, and h), or both expression plasmids together (i, j, k, and l). Thirty-six hours posttransfection, the cells were fixed, permeabilized, and stained with vGPCR (a, e, and i) or Flag (TAK1) (b, f, and j) antibodies and visualized by confocal microscopy. (c, g, and k) Merged images. (d, h, and l) Higher-magnification images of the indicated areas of images c, g, and k, respectively. The white arrows indicate the colocalization of vGPCR with TAK1. Magnification, ×40. (B) HEK293T cells were transfected with pcDNA (a, b, and c) or vGPCR (d, e, and f), fixed, permeabilized, stained with vGPCR (a and d) and TAK1 (b and e) antibodies, and visualized with a fluorescence microscope. (c and f) Merged images, with DAPI nuclear staining in blue. (C) HEK293T cells were transfected with 10 μg of vGPCR- and/or 10 μg of TAK1-expressing plasmids. At 36 h posttransfection, total protein was extracted and subjected to Western blot analysis using anti-Flag (TAK1) (top) or anti-vGPCR (bottom) antibodies. *, nonspecific bands. (D) The proteins from the same extraction were immunoprecipitated (IP) using a vGPCR antibody followed by Western blot analysis using anti-Flag (TAK1) (top) or anti-vGPCR (bottom) antibodies.
vGPCR interacts with TAK1.
HEK293T cells were transiently cotransfected with expression plasmids encoding Flag-tagged TAK1 and vGPCR. The transgene expression levels were determined by Western blot analysis of total lysates using antibodies against vGPCR (Fig. 5C, bottom) or Flag epitope (Fig. 5C, top). To determine if TAK1 and vGPCR interact, extracts of transfected cells were then immunoprecipitated with anti-vGPCR antibody and Western blotted with anti-Flag epitope antibody (Fig. 5D, top) or vGPCR antibody (Fig. 5D, bottom). vGPCR was efficiently immunoprecipitated when expressed alone or in combination with TAK1 (Fig. 5D, bottom, lanes 2 and 3). We detected the coimmunoprecipitation of TAK1 with vGPCR only when vGPCR and TAK1 were coexpressed (Fig. 5D, top, lane 3), which demonstrated the interaction of vGPCR with TAK1.
vGPCR induces TAK1 polyubiquitination.
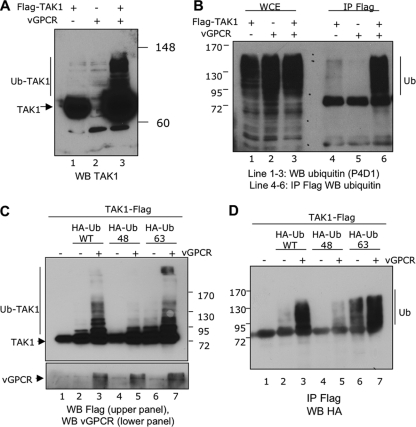
TAK1 kinase activity has recently been shown to be regulated by ubiquitination (22, 35, 65). When TAK1 was expressed alone, Western blot analysis showed a major band of about 70 kDa (Fig. 6 A, lane 1). However, when vGPCR was expressed in association with WT TAK1, TAK1 migrated as higher-molecular-weight bands in the Western blot analysis (Fig. 6A, lane 3). To determine whether the higher-molecular-weight bands represented polyubiquitinated forms of TAK1, we performed TAK1 immunoprecipitation using anti-Flag antibody (Flag-tagged WT TAK1), followed by Western blot analysis using an antibody against ubiquitin (Fig. 6B). When TAK1 was expressed in the presence of vGPCR, we observed an intense ubiquitin signal (Fig. 6B, compare lane 6 to lane 4), which suggested polyubiquitination of TAK1 kinase.
FIG. 6.
vGPCR expression induces lysine 63 TAK1 polyubiquitination. (A and B) vGPCR expression induces TAK1 polyubiquitination. HEK293T cells were transfected with 10 μg of Flag-TAK1 and/or 10 μg of vGPCR expression plasmids. (Lanes 4 to 6) Thirty-six hours posttransfection, Western blot analysis was done using TAK1 antibody (A) or the cells were subjected to TAK1 immunoprecipitation using Flag antibody followed by Western blot analysis using a ubiquitin antibody (P4D1 Ab) (B). (Lanes 1 to 3) Whole-cell extracts (WCE) reacted with P4D1 Ab. (C and D) TAK1 polyubiquitination on lysine 68. HEK293T cells were transfected with 10 μg of TAK1-Flag, along with 5 μg of the indicated ubiquitin expression plasmids with or without 10 μg of vGPCR expression plasmid. At 36 h posttransfection, total protein was extracted in lysis buffer and subjected to a Western blot experiment using Flag antibody (C, top) or vGPCR antibody (C, bottom) or subjected to Flag immunoprecipitation (TAK1 IP) followed by Western blot analysis using an HA antibody (ubiquitin Western blotting) (D).
The two main polyubiquitination reactions involve lysine 48 or lysine 63 of the ubiquitin molecule. We next determined if vGPCR expression induced Lys 48- or Lys 63-linked polyubiquitination. We transfected HEK293T cells with Flag-tagged WT TAK1 with or without vGPCR expression plasmids in combination with HA-tagged ubiquitin WT or mutant expression plasmids. HA-Ub48 contains all the mutated lysines except lysine 48, whereas HA-Ub63 contains all the mutated lysines except lysine 63. When TAK1 was expressed in association with vGPCR plus HA-Ub WT or HA-Ub63, anti-TAK1 antibody recognized the high-molecular-weight bands in Western blots (Fig. 6C, top, lanes 3 and 7). These bands were not observed when HA-Ub48 was expressed (Fig. 6C, top, lane 5). We confirmed the expression of vGPCR in these transfections (Fig. 6C, bottom). To confirm that these high-molecular-weight bands were due to polyubiquitination, TAK1 was immunoprecipitated with anti-Flag antibody, followed by Western blot analysis using an antibody against the HA epitope. As shown in Fig. 6D, we detected polyubiquitinated forms of TAK1 when vGPCR was expressed and in the presence of WT HA-Ub (Fig. 6D, lanes 2 and 3) or HA-Ub63 (Fig. 6D, lanes 6 and 7). Taken together, these results indicated that the expression of vGPCR can induce Lys 63-linked TAK1 polyubiquitination.
Celastrol blocks vGPCR-induced NF-κB activation in HEK293T cells.
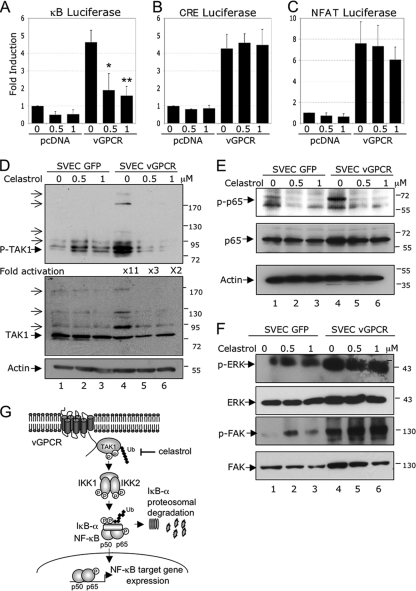
Celastrol, a compound used in Chinese medicine, is extracted from the “thunder of God vine” and is well known for its antioxidative and anti-inflammatory effects. Celastrol has also been shown to be a potent NF-κB inhibitor (37, 46). We next tested the effect of increasing concentrations of celastrol on vGPCR-induced NF-κB activation in a luciferase assay. At the tested concentrations, the compound was not cytotoxic for HEK293T cells (data not shown). HEK293T cells were transfected with κB-luciferase reporter plasmid in the presence or absence of vGPCR expression plasmid. At 36 h posttransfection, the cells were treated overnight with the indicated concentrations of celastrol. As shown in Fig. 7 A, increasing concentrations of celastrol decreased basal NF-κB activity. In addition, celastrol significantly reduced the NF-κB activity induced by vGPCR expression. As a control, we evaluated whether celastrol affects other pathways activated by vGPCR. Celastrol did not affect vGPCR-induced cAMP response element (CRE) luciferase activity (Fig. 7B) or NFAT luciferase activity (Fig. 7C). This indicated that celastrol specifically inhibited vGPCR-induced NF-κB activation.
FIG. 7.
Celastrol inhibits NF-κB activation induced by vGPCR in SVECs. (A) Celastrol inhibits NF-κB luciferase activity induced by vGPCR. HEK293T cells were transfected with 2 μg of pcDNA or vGPCR expression plasmids, along with κB-luciferase and β-Gal reporter constructs (as described in Materials and Methods). Thirty-six hours posttransfection, the cells were treated overnight (16 h) with increasing quantities of celastrol. The luciferase and β-galactosidase activities were measured in triplicate using Steady-glo and Beta-glo Promega kits, respectively. The data represent the mean and SD of fold induction of 4 independent experiments; *, P < 0.05; **, P < 0.01. (B and C) Celastrol has no effect on CRE and NFAT luciferase activities induced by vGPCR. HEK293T cells were transfected with 2 μg of pcDNA or vGPCR expression plasmids, along with 1 μg of CRE-luciferase construct (B) or NFAT-luciferase construct (C) and 0.5 μg of β-Gal reporter construct and analyzed as for κB-luciferase. (D) Celastrol inhibits vGPCR-induced TAK1 activation in SVECs. SVECs expressing GFP (SVEC-GFP) or vGPCR (SVEC-vGPCR) were treated overnight (16 h) under low-serum conditions (0.5% serum) with increasing quantities of celastrol. After treatment, the cells were lysed in RIPA buffer, and 50 μg of protein was subjected to Western blot analysis using a phospho-TAK1 antibody, a TAK1 antibody, or a β-actin antibody (loading control). (E) SVEC-GFP and SVEC-vGPCR were treated and analyzed as indicated in panel D. Western blotting was performed using a phospho-p65 antibody, a p65 antibody, or a β-actin antibody (loading control). (F) Celastrol has no effect on vGPCR-induced FAK and ERK activation in SVECs. SVEC-GFP and SVEC-vGPCR were treated as indicated in panel D. Western blot analysis was performed using phospho-FAK, total FAK, phospho-ERK, and total ERK antibodies. (G) Model of vGPCR-induced activation of NF-κB through TAK1. Expression of vGPCR recruits TAK1 near the plasma membrane, and TAK-1 interaction with vGPCR results in the activation of TAK1 by phosphorylation and Lys 63-linked polyubiquitination. The downstream substrates IKK1 and IKK2 are then activated, which induces IκBα phosphorylation, Lys 48-linked polyubiquitination, and degradation by the proteosome, thus allowing NF-κB to translocate to the nucleus, where it induces target genes, including those encoding IL-8, Gro1, IκBα, COX-2, cIAP2, and Bcl2. Celastrol inhibits TAK1 activation, which can serve as a potential anticancer drug against KSHV malignancies involving vGPCR.
Celastrol inhibits TAK1 and NF-κB activation in endothelial cells.
Since KSHV infection targets endothelial cells, which leads to the development of KS, we next decided to confirm the vGPCR-TAK1-NF-κB link in a physiologically relevant endothelial cell model. Simian virus 40 (SV40) large T-antigen-immortalized murine endothelial cells (SVECs) are a well-established model used to ascertain the role of vGPCR in KS progression (43). SVECs expressing GFP or vGPCR were treated with increasing concentrations of celastrol, and extracted proteins were subjected to Western blot analysis. We observed an 11-fold increase in TAK1 phosphorylation on the threonines in the activation loop of the protein (threonines 184 and 187) (Fig. 7D, top, compare lane 4 to lane 1). Autophosphorylation of these residues has been shown to be necessary for TAK1 activation (53). In the presence of vGPCR expression, in addition to the main 70-kDa TAK1 band, we also detected additional high-molecular-weight bands that could represent polyubiquitinated forms of the protein (Fig. 7D, top and middle, compare lane 4 to lane 1). Interestingly, when the cells were treated with celastrol, detection of both phosphorylated and potentially polyubiquinated TAK1 was reduced. These results indicated that vGPCR induced TAK1 phosphorylation and polyubiquitination in endothelial cells and that celastrol inhibits these hallmarks of TAK1 activation.
Since the optimal activation of NF-κB requires phosphorylation of its transactivation domain, we next investigated the effect of vGPCR expression and celastrol treatment on p65 phosphorylation at serine 536. We detected a significant increase in p65 phosphorylation when vGPCR was expressed (Fig. 7E, top, compare lane 4 to lane 1). Celastrol, by blocking TAK1 activation, was not only able to block basal p65 phosphorylation (Fig. 7E, top, compare lanes 2 and 3 to lane 1), but also totally abolished p65 phosphorylation induced by vGPCR (Fig. 7E, top, compare lanes 5 and 6 to lane 4). As a control, we evaluated the effect of celastrol on the activation of the tyrosine kinase FAK and the serine kinase ERK (Fig. 7F). vGPCR was able to induce the phosphorylation of both kinases (compare lane 4 to lane 1), and celastrol did not decrease vGPCR-induced FAK or ERK phosphorylation (compare lanes 5 and 6 to lane 4). Taken together, these results indicated that vGPCR induced TAK1 phosphorylation and ubiquitination, two modifications necessary for TAK1 activation. In addition, the inhibition of TAK1 activation by celastrol was able to block NF-κB activation.
DISCUSSION
The comprehensive study presented here investigated the signaling pathway induced by KSHV vGPCR that leads to NF-κB activation. We confirmed that the IKK complex is involved in NF-κB activation induced by vGPCR, and we identified TAK1 as an important protein upstream of the IKK complex. We showed for the first time that vGPCR colocalizes and interacts with TAK1, and consequently, TAK1 is phosphorylated and polyubiquitinated, two steps necessary for its activation.
Our observation that expression of mutants of IκBα, βTrCP, IKK1, or IKK2 blocks vGPCR-induced NF-κB activity supports several previous studies and suggests the importance of this pathway in vGPCR-induced tumorigenesis. Expression of dominant-negative IκBα or treatment with the NF-κB inhibitor BAY-11-7082 has been shown to inhibit the formation of vGPCR-induced cell colonies in soft-agar assays (18). Pati et al. were able to inhibit NF-κB activation induced by vGPCR with the expression of dominant-negative IKK1 or IKK2 (49). A full-genome microarray showed that expression of NF-κB-regulated genes is a predominant feature of vGPCR-expressing cells, as well as of cells exposed to vGPCR conditional media (40). In addition, whereas p65-expressing cells were unable to induce tumors in vivo, inhibition of the NF-κB pathway by the expression of mutant IκBα totally blocked vGPCR-induced tumorigenesis in vivo. Taken together, these results suggest that NF-κB is necessary but not sufficient for vGPCR-induced tumorigenesis (40).
We demonstrated for the first time that vGPCR activates NF-κB through the activation of TAK1. The expression of a TAK1 inactive mutant blocked both the nuclear translocation of NF-κB and its activity in a luciferase assay, which was also confirmed using an siRNA-mediated TAK1 knockdown approach. In addition, this is the first report to demonstrate that a KSHV protein activates TAK1. Other viruses have been shown to activate TAK1 to induce downstream signaling pathways. For example, hepatitis B virus protein X (HBx) induces the activation of NF-κB through a TRAF2/TAK1 signaling pathway (85). Although the human T lymphotropic virus (HTLV) oncoprotein Tax constitutively activates TAK1, the role of TAK1 in NF-κB activation induced by Tax is not clear. Suzuki et al. have shown that TAK1 activation is necessary for JNK and p38 MAPK activation induced by Tax but is not involved in NF-κB activation (67). In addition, knockdown of TAK1 does not decrease NF-κB activation induced by Tax in HEK293T cells (26). However, several other groups have identified TAK1 as an important molecule involved in Tax activation of NF-κB. Wu and Sun have shown that Tax physically interacts with TAK1 and mediates the recruitment of the IKK complex to TAK1 (78). Using TAK1-deleted mouse embryonic fibroblasts, it has been shown that TAK1 is essential for Tax-induced IKK activation. TAK1-associated binding protein 2 (TAB2) has been shown to be an adaptor between Tax and TAK1 in which TAB2 binding to Tax is required for Tax-induced TAK1 activation and to increase Tax-induced NF-κB activity (82).
The gammaherpesvirus Epstein-Barr virus (EBV) latent membrane protein 1 (LMP-1) has also been shown to activate TAK1. LMP-1 mimics members of the TNF receptor family and constitutively activates JNK and p38 MAPK, as well as the NF-κB pathway (21, 64). In addition, the activation of the JNK and NF-κB pathways by LMP-1 contributes to the oncogenic effect of LMP-1. Using a catalytically dead mutant of TAK1 or knockdown by siRNA technology, Wan et al. have shown that TAK1 is involved in LMP-1-mediated JNK and p38 MAPK activation (74). The role of TAK1 in JNK activation by LMP-1 has been more recently confirmed (71). The role of TAK1 in NF-κB induction, however, is controversial in EBV biology. Whereas Uemura et al. showed that TAK1 seems to be dispensable for NF-κB activation induced by LMP-1 (71), Wu et al. demonstrated that the CTAR2 domain of LMP-1 recruits TRAF6 to activate TAK1, which consequently activates NF-κB signaling (77).
Our studies characterized the mechanism by which vGPCR activates TAK1 and NF-κB. We observed colocalization and interaction between vGPCR and TAK1, and vGPCR induced TAK1 Lys 63-linked polyubiquitination. TAK1 activation has been shown to be dependent on a ubiquitination process (62). TAK1 is found in a complex with three other structural proteins, TAB1, TAB2, and TAB3 (13). TAB2 and TAB3 contain a ubiquitin-binding zinc finger domain that binds to the Lys 63-linked polyubiquitin chain on TRAF6. This Ub-TRAF6/TAB interaction was proposed to be responsible for TAK1 activation induced by IL-1 signaling (19, 47). However, a direct polyubiquitination of TAK1 has been identified only recently. In response to TGF-β receptor activation, TAK1 is a direct substrate for TRAF6 and mediates Lys 63-linked polyubiquitination of TAK1 on lysine 34 (65). This modification was necessary for TAK1 activation and subsequent p38 activation. TNF-α and IL-1β have also been shown to induce Lys 63-linked TAK1 polyubiquitination (22). In this study, lysine 158 located in the kinase domain was polyubiquitinated by TRAF2 and TRAF6. This modification was associated with IKK complex activation and subsequent NF-κB activation, but also with JNK/AP1 activation. TAK1 Lys 63-linked polyubiquitination is required for NF-κB activation induced by the Helicobacter pylori virulence factor CagA (35). The authors showed an interaction between CagA and TAK1 and demonstrated the role of TRAF6 in CagA-induced TAK1 polyubiquitination using the expression of a dominant-negative protein, as well as siRNA. Here, we observed a Lys 63-linked polyubiquitination of TAK1 after vGPCR expression. However, preliminary data suggest that this ubiquitination is independent of TRAF6 and TRAF2 (data not shown). Future experiments are essential to determine the mechanism of TAK1 polyubiquitination induced by vGPCR. In addition, it would be interesting to determine whether TAK1 polyubiquitination could also be induced by cellular G protein-coupled receptors.
It has been proposed that vGPCR-expressing cells are tumorigenic by promoting the recruitment, proliferation, and transformation of adjacent cells through the secretion of cytokines and growth factors, such as IL-1β, IL-2, IL-4, IL-6, IL-8, Gro1, TNF-α, bFGF, MCP-1, MIP1 and -2, SDF1b, VEGF-A, VEGF-C, and angiopoietin 2 (3, 17, 25, 29, 43, 45, 49, 50, 55, 63, 73). We observed that the inhibition of TAK1 by the expression of a mutant or its knockdown by siRNA inhibited the induction of several NF-κB target genes, including those encoding IL-8 and Gro-1. In addition, we observed that vGPCR induced COX-2 in a TAK1-dependent manner. vGPCR has also been shown to be important in the activation of COX-2 in vascular endothelial cells (58). A recent study highlighted the role of COX-2 in KSHV-infected endothelial cells (57).
We also observed that vGPCR induces the antiapoptotic genes encoding cIAP2 and Bcl-2 via TAK1. The antiapoptotic role of NF-κB has been intensively documented (20). It has been shown that the expression of a kinase-inactive TAK1 mutant was able to block the NF-κB, JNK, and p38 MAPK pathways (69). Consequently, the cells were sensitized to TNF-α-induced apoptosis (69). TAK1 mutants suppressed the basal, as well as TNF-α-inducible, expression of several inflammatory genes (69). Recently, TAK1 activation of NF-κB has been shown to be required for the survival of cells treated with TNF-related apoptosis-inducing ligand (TRAIL) (39). Taken together, our studies demonstrate that TAK1 is involved in NF-κB activation and in the transcription of several genes involved in vGPCR-mediated tumorigenesis.
In an endothelial cell model, we showed that the natural anti-inflammatory molecule celastrol is able to inhibit TAK1 and NF-κB activation induced by vGPCR (Fig. 7G). Celastrol has been shown to inhibit the production of inflammatory mediators and has been proposed as a potential target for the treatment of inflammatory diseases (34, 37). In addition, the antitumor activity of celastrol has been investigated in vivo. More specifically, the compound has been shown to suppress human breast tumor and prostate cancer growth in athymic nude mice (37, 48, 80). When administered at a minimally toxic dose, celastrol has been shown to enhance the cytotoxicity of other anticancer drugs for oral squamous cell carcinoma and melanoma cells (12, 30). Moreover, celastrol has been shown to suppress endothelial cell angiogenesis both in vitro and in vivo (48). The mechanism by which celastrol inhibits inflammation and cancer has not been fully investigated. However, Sethi et al. have shown that TAK1 activation of NF-κB is inhibited by celastrol (56), and more recently, it has been demonstrated that TAK1 phosphorylation induced by TNF-α and IL-1β is reduced by celastrol (32). Our results confirm that celastrol is an NF-κB inhibitor acting at the level, or upstream, of TAK1. Future studies are essential to shed light on the effect of celastrol on vGPCR-induced tumorigenesis.
In conclusion, the work presented here has described for the first time the role of TAK1 in vGPCR-induced NF-κB activation (Fig. 7G) and demonstrated that vGPCR interacts with TAK1 to induce its phosphorylation and polyubiquitination. Celastrol, as a TAK1/NF-κB inhibitor, may serve as a potential therapeutic molecule to ameliorate vGPCR/KSHV-induced tumors.
Acknowledgments
This study was supported in part by Public Health Service grants CA 075911 and CA099925 and the RFUMS-H.M. Bligh Cancer Research Fund to B.C.
We are grateful to the laboratories of John Nicholas (pSG5-vGPCR expression plasmids), Sylvia Montaner (SVECs), Inder Verma (IκBα, IKK1, IKK2, βTrCP, and siTAK1 expression plasmids), Zhijian Chen (TAK1 WT and mutant expression plasmids), and Ted Dawson (PRK5-HA-ubiquitin WT and mutants plasmids) for providing the plasmids used in this study. We thank the Microscopy and Imaging Facility, Rosalind Franklin University of Medicine and Science, North Chicago, IL. We thank Keith Philibert for critically reading the manuscript.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1.Adhikari, A., M. Xu, and Z. J. Chen. 2007. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 26:3214-3226. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 3.Bais, C., et al. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 4.Bais, C., et al. 2003. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/KDR. Cancer Cell 3:131-143. [DOI] [PubMed] [Google Scholar]
- 5.Bassères, D. S., and A. S. Baldwin. 2006. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 25:6817-6830. [DOI] [PubMed] [Google Scholar]
- 6.Bottero, V., V. Imbert, C. Frelin, J. L. Formento, and J. F. Peyron. 2003. Monitoring NF-kappa B transactivation potential via real-time PCR quantification of I kappa B-alpha gene expression. Mol. Diagn. 7:187-194. [DOI] [PubMed] [Google Scholar]
- 7.Cai, X., et al. 2005. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl. Acad. Sci. U. S. A. 102:5570-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon, M. 2007. The KSHV and other human herpesviral G protein-coupled receptors. Curr. Top. Microbiol. Immunol. 312:137-156. [DOI] [PubMed] [Google Scholar]
- 9.Cannon, M., N. J. Philpott, and E. Cesarman. 2003. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells. J. Virol. 77:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon, M. L., and E. Cesarman. 2004. The KSHV G protein-coupled receptor signals via multiple pathways to induce transcription factor activation in primary effusion lymphoma cells. Oncogene 23:514-523. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 12.Chen, M., A. E. Rose, N. Doudican, I. Osman, and S. J. Orlow. 2009. Celastrol synergistically enhances temozolomide cytotoxicity in melanoma cells. Mol. Cancer Res. 7:1946-1953. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Z. J. 2005. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 7:758-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, Z. J., V. Bhoj, and R. B. Seth. 2006. Ubiquitin, TAK1 and IKK: is there a connection? Cell Death Differ. 13:687-692. [DOI] [PubMed] [Google Scholar]
- 15.Chiou, C. J., et al. 2002. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3421-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courtois, G., and T. D. Gilmore. 2006. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 25:6831-6843. [DOI] [PubMed] [Google Scholar]
- 17.Couty, J. P., E. Geras-Raaka, B. B. Weksler, and M. C. Gershengorn. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J. Biol. Chem. 276:33805-33811. [DOI] [PubMed] [Google Scholar]
- 18.Dadke, D., B. H. Fryer, E. A. Golemis, and J. Field. 2003. Activation of p21-activated kinase 1-nuclear factor kappaB signaling by Kaposi's sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res. 63:8837-8847. [PubMed] [Google Scholar]
- 19.Deng, L., et al. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351-361. [DOI] [PubMed] [Google Scholar]
- 20.Dutta, J., Y. Fan, N. Gupta, G. Fan, and C. Gelinas. 2006. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 25:6800-6816. [DOI] [PubMed] [Google Scholar]
- 21.Eliopoulos, A. G., and L. S. Young. 2001. LMP1 structure and signal transduction. Semin. Cancer Biol. 11:435-444. [DOI] [PubMed] [Google Scholar]
- 22.Fan, Y., et al. 2010. Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J. Biol. Chem. 285:5347-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganem, D. 2007. Kaposi's sarcoma-associated herpesvirus, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA.
- 24.Gerondakis, S., et al. 2006. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene 25:6781-6799. [DOI] [PubMed] [Google Scholar]
- 25.Glaunsinger, B., and D. Ganem. 2004. Highly selective escape from KSHV-mediated host mRNA shutoff and its implications for viral pathogenesis. J. Exp. Med. 200:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gohda, J., et al. 2007. HTLV-1 Tax-induced NFkappaB activation is independent of Lys-63-linked-type polyubiquitination. Biochem. Biophys. Res. Commun. 357:225-230. [DOI] [PubMed] [Google Scholar]
- 27.Graham, F. L., and A. J. van der Eb. 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456-467. [DOI] [PubMed] [Google Scholar]
- 28.Grisotto, M. G., et al. 2006. The human herpesvirus 8 chemokine receptor vGPCR triggers autonomous proliferation of endothelial cells. J. Clin. Invest. 116:1264-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo, H. G., et al. 2003. Kaposi's sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J. Virol. 77:2631-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He, D., et al. 2009. The NF-kappa B inhibitor, celastrol, could enhance the anti-cancer effect of gambogic acid on oral squamous cell carcinoma. BMC Cancer 9:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, T. T., S. L. Feinberg, S. Suryanarayanan, and S. Miyamoto. 2002. The zinc finger domain of NEMO is selectively required for NF-kappa B activation by UV radiation and topoisomerase inhibitors. Mol. Cell. Biol. 22:5813-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Idris, A. I., et al. 2009. Pharmacologic inhibitors of IkappaB kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasis in vivo. Mol. Cancer Ther. 8:2339-2347. [DOI] [PubMed] [Google Scholar]
- 33.Israel, A. 2010. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harbor Perspect. Biol. 2:a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, D. H., et al. 2009. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur. J. Clin. Invest. 39:819-827. [DOI] [PubMed] [Google Scholar]
- 35.Lamb, A., et al. 2009. Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 10:1242-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, D. F., and M. C. Hung. 2008. Advances in targeting IKK and IKK-related kinases for cancer therapy. Clin. Cancer Res. 14:5656-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, J. H., et al. 2006. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem. Pharmacol. 72:1311-1321. [DOI] [PubMed] [Google Scholar]
- 38.Liu, C., G. Sandford, G. Fei, and J. Nicholas. 2004. Galpha protein selectivity determinant specified by a viral chemokine receptor-conserved region in the C tail of the human herpesvirus 8 g protein-coupled receptor. J. Virol. 78:2460-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lluis, J. M., et al. 2010. TAK1 is required for survival of mouse fibroblasts treated with TRAIL, and does so by NF-kappaB dependent induction of cFLIPL. PLoS One 5:e8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin, D., R. Galisteo, Y. Ji, S. Montaner, and J. S. Gutkind. 2008. An NF-kappaB gene expression signature contributes to Kaposi's sarcoma virus vGPCR-induced direct and paracrine neoplasia. Oncogene 27:1844-1852. [DOI] [PubMed] [Google Scholar]
- 41.Martín, M. J., et al. 2007. The Galpha12/13 family of heterotrimeric G proteins and the small GTPase RhoA link the Kaposi sarcoma-associated herpes virus G protein-coupled receptor to heme oxygenase-1 expression and tumorigenesis. J. Biol. Chem. 282:34510-34524. [DOI] [PubMed] [Google Scholar]
- 42.Miyoshi, H., U. Blomer, M. Takahashi, F. H. Gage, and I. M. Verma. 1998. Development of a self-inactivating lentivirus vector. J. Virol. 72:8150-8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montaner, S., et al. 2003. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3:23-36. [DOI] [PubMed] [Google Scholar]
- 44.Montaner, S., A. Sodhi, S. Pece, E. A. Mesri, and J. S. Gutkind. 2001. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 61:2641-2648. [PubMed] [Google Scholar]
- 45.Montaner, S., et al. 2004. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood 104:2903-2911. [DOI] [PubMed] [Google Scholar]
- 46.Nam, N. H. 2006. Naturally occurring NF-kappaB inhibitors. Mini Rev. Med. Chem. 6:945-951. [DOI] [PubMed] [Google Scholar]
- 47.Ninomiya-Tsuji, J., et al. 1999. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398:252-256. [DOI] [PubMed] [Google Scholar]
- 48.Pang, X., et al. 2010. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res. 70:1951-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pati, S., et al. 2001. Activation of NF-kappaB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polson, A. G., D. Wang, J. DeRisi, and D. Ganem. 2002. Modulation of host gene expression by the constitutively active G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus. Cancer Res. 62:4525-4530. [PubMed] [Google Scholar]
- 51.Rayet, B., and C. Gelinas. 1999. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18:6938-6947. [DOI] [PubMed] [Google Scholar]
- 52.Rosenkilde, M. M., T. N. Kledal, H. Brauner-Osborne, and T. W. Schwartz. 1999. Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J. Biol. Chem. 274:956-961. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai, H., H. Miyoshi, J. Mizukami, and T. Sugita. 2000. Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett. 474:141-145. [DOI] [PubMed] [Google Scholar]
- 54.Samols, M. A., J. Hu, R. L. Skalsky, and R. Renne. 2005. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:9301-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarz, M., and P. M. Murphy. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J. Immunol. 167:505-513. [DOI] [PubMed] [Google Scholar]
- 56.Sethi, G., K. S. Ahn, M. K. Pandey, and B. B. Aggarwal. 2007. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood 109:2727-2735. [DOI] [PubMed] [Google Scholar]
- 57.Sharma-Walia, N., et al. 2010. Kaposi's sarcoma associated herpes virus (KSHV) induced COX-2: a key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 6:e1000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shelby, B. D., et al. 2007. Kaposi's sarcoma associated herpesvirus G-protein coupled receptor activation of cyclooxygenase-2 in vascular endothelial cells. Virol. J. 4:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shepard, L. W., et al. 2001. Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus involve G alpha(13) and RhoA. J. Biol. Chem. 276:45979-45987. [DOI] [PubMed] [Google Scholar]
- 60.Shibuya, H., et al. 1998. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J. 17:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shim, J. H., et al. 2005. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19:2668-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skaug, B., X. Jiang, and Z. J. Chen. 2009. The role of ubiquitin in NF-kappaB regulatory pathways. Annu. Rev. Biochem. 78:769-796. [DOI] [PubMed] [Google Scholar]
- 63.Sodhi, A., et al. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 64.Soni, V., E. Cahir-McFarland, and E. Kieff. 2007. LMP1 TRAFficking activates growth and survival pathways. Adv. Exp. Med. Biol. 597:173-187. [DOI] [PubMed] [Google Scholar]
- 65.Sorrentino, A., et al. 2008. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat. Cell Biol. 10:1199-1207. [DOI] [PubMed] [Google Scholar]
- 66.Staskus, K. A., et al. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki, S., et al. 2007. Constitutive activation of TAK1 by HTLV-1 tax-dependent overexpression of TAB2 induces activation of JNK-ATF2 but not IKK-NF-kappaB. J. Biol. Chem. 282:25177-25181. [DOI] [PubMed] [Google Scholar]
- 68.Takatsu, Y., et al. 2000. TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Mol. Cell. Biol. 20:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thiefes, A., et al. 2005. Simultaneous blockade of NFkappaB, JNK, and p38 MAPK by a kinase-inactive mutant of the protein kinase TAK1 sensitizes cells to apoptosis and affects a distinct spectrum of tumor necrosis factor target genes. J. Biol. Chem. 280:27728-27741. [DOI] [PubMed] [Google Scholar]
- 70.Tiscornia, G., O. Singer, and I. M. Verma. 2006. Production and purification of lentiviral vectors. Nat. Protoc. 1:241-245. [DOI] [PubMed] [Google Scholar]
- 71.Uemura, N., et al. 2006. TAK1 is a component of the Epstein-Barr virus LMP1 complex and is essential for activation of JNK but not of NF-kappaB. J. Biol. Chem. 281:7863-7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vallabhapurapu, S., and M. Karin. 2009. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27:693-733. [DOI] [PubMed] [Google Scholar]
- 73.Vart, R. J., et al. 2007. Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6 and G-protein-coupled receptor regulate angiopoietin-2 expression in lymphatic endothelial cells. Cancer Res. 67:4042-4051. [DOI] [PubMed] [Google Scholar]
- 74.Wan, J., et al. 2004. Elucidation of the c-Jun N-terminal kinase pathway mediated by Epstein-Barr virus-encoded latent membrane protein 1. Mol. Cell. Biol. 24:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang, C., et al. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346-351. [DOI] [PubMed] [Google Scholar]
- 76.Wertz, I. E., and V. M. Dixit. 2010. Signaling to NF-kappaB: regulation by ubiquitination. Cold Spring Harbor Perspect. Biol. 2:a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu, L., H. Nakano, and Z. Wu. 2006. The C-terminal activating region 2 of the Epstein-Barr virus-encoded latent membrane protein 1 activates NF-kappaB through TRAF6 and TAK1. J. Biol. Chem. 281:2162-2169. [DOI] [PubMed] [Google Scholar]
- 78.Wu, X., and S. C. Sun. 2007. Retroviral oncoprotein Tax deregulates NF-kappaB by activating Tak1 and mediating the physical association of Tak1-IKK. EMBO Rep. 8:510-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamaguchi, K., et al. 1995. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 270:2008-2011. [DOI] [PubMed] [Google Scholar]
- 80.Yang, H., D. Chen, Q. C. Cui, X. Yuan, and Q. P. Dou. 2006. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 66:4758-4765. [DOI] [PubMed] [Google Scholar]
- 81.Yang, T. Y., et al. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu, Q., et al. 2008. HTLV-1 Tax-mediated TAK1 activation involves TAB2 adapter protein. Biochem. Biophys. Res. Commun. 365:189-194. [DOI] [PubMed] [Google Scholar]
- 83.Zhang, D., et al. 2000. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat. Med. 6:556-563. [DOI] [PubMed] [Google Scholar]
- 84.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. U. S. A. 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou, Y., et al. 2010. HBx-induced expression of the CXC chemokine IP-10 is mediated through activation of NF-kappaB and increases migration of leukocytes. J. Biol. Chem. 285:12159-12168. [DOI] [PMC free article] [PubMed] [Google Scholar]