Abstract
Poxviruses encode numerous proteins that inhibit apoptosis, a form of cell death critical to the elimination of virally infected cells. Sequencing of the deerpox virus genome revealed DPV022, a protein that lacks obvious homology to cellular members of the Bcl-2 family but shares limited regions of amino acid identity with two unique poxviral inhibitors of apoptosis, M11L and F1L. Given the limited homology, we sought to determine whether DPV022 could inhibit apoptosis. Here we show that DPV022 localized to the mitochondria, where it inhibited apoptosis. We used a Saccharomyces cerevisiae model system to demonstrate that in the absence of all other Bcl-2 family proteins, DPV022 interacted directly with Bak and Bax. We confirmed the ability of DPV022 to interact with Bak and Bax by immunoprecipitation and showed that DPV022 prevented apoptosis induced by Bak and Bax overexpression. Moreover, we showed that DPV022 blocked apoptosis even when all the endogenous mammalian antiapoptotic proteins were neutralized by a combination of selective BH3 ligands. During virus infection, DPV022 interacted with endogenous Bak and Bax and prevented the conformational activation of both of them. Thus, we have characterized a novel poxviral inhibitor of apoptosis with intriguing amino acid differences from the well-studied proteins M11L and F1L.
Viruses depend on living cells to reproduce and complete their life cycles. The initiation of apoptosis, a form of programmed cell death, therefore poses a significant barrier to viral infection. The systematic destruction of an infected cell by apoptosis robs the virus of the biochemical machinery upon which it relies. In order to keep cells alive, viruses have evolved numerous mechanisms that target specific points in the apoptotic cascade and prevent the premature death of an infected cell (25, 58).
Virus infection can trigger several cellular signaling pathways that all lead to apoptosis. Many of these pathways converge at the mitochondria and culminate in the release of cytochrome c, a proapoptotic molecule that activates caspases and leads to the systematic dismantling of the cell. The release of cytochrome c is ultimately controlled by the highly conserved Bcl-2 family of proteins, which consolidates multiple apoptotic signals, including those induced by viral infection, and regulates the integrity of the mitochondria (74). Bcl-2 proteins consist of both antiapoptotic and proapoptotic family members and are united by the presence of at least one of four conserved Bcl-2 homology (BH) domains. BH domains are thought to provide the structural basis by which Bcl-2 proteins interact to regulate cell death (50). The proapoptotic Bcl-2 proteins Bak and Bax possess BH domain 1 (BH1) to BH3 and are critical to the induction of apoptosis. Upon activation, both Bak and Bax undergo extensive conformational changes that result in the exposure of an N-terminal epitope and subsequent homo-oligomerization (3, 4, 30, 31, 47, 66). Bax and Bak homo-oligomers are thought to form pores that permeabilize the mitochondrial outer membrane and allow the release of cytochrome c (49). Because either Bak or Bax can function independently to facilitate the release of cytochrome c, both must be kept inactive to prevent apoptosis (44, 67, 77). In healthy cells, Bak and Bax are held in check by the antiapoptotic Bcl-2 family members, exemplified by Bcl-2, Bcl-xL, and Mcl-1, which possess all four BH domains (74). Members of a third Bcl-2 subfamily, the BH3-only proteins, possess only the BH3 domain and act as cytoplasmic sensors of various cell stresses (69). Once activated, BH3-only proteins induce apoptosis by activating Bak and Bax or by inhibiting the antiapoptotic Bcl-2 proteins (13). Together, it is the balance of interactions among the pro- and antiapoptotic Bcl-2 proteins that ultimately controls the fate of the cell.
Since Bcl-2 proteins play a central role in regulating the fate of an infected cell, many viruses have evolved proteins that inhibit their activity (16, 58). However, there is no single identical mechanism by which all viruses prevent cell death. Poxviruses, for example, encode multiple proteins that inhibit apoptosis but do so in slightly different ways (58). These include M11L, encoded by myxoma virus; F1L, encoded by vaccinia virus (VV); FPV039, encoded by fowlpox virus; and ORFV125, encoded by Orf virus (5, 6, 41, 54, 59, 61, 63, 68). Although each of these poxviral proteins inhibits apoptosis, they interact with a distinct profile of Bcl-2 proteins. Interestingly, only FPV039 exhibits obvious sequence identity with cellular Bcl-2 proteins, and none of these poxviral inhibitors of apoptosis bear sequence similarity with each other (5).
The recent sequencing of the deerpox virus genome revealed the presence of yet another putative inhibitor of apoptosis, DPV022 (1). Surprisingly, although DPV022 lacks obvious sequence homology with cellular Bcl-2 proteins, DPV022 possesses limited regions of homology with both M11L and F1L (1). Based on this homology, we hypothesized that DPV022 would inhibit apoptosis, and we sought to characterize the mechanism of action. Here we show that like cellular Bcl-2 proteins and both M11L and F1L, DPV022 localized to the mitochondria, where it inhibited apoptosis induced by tumor necrosis factor alpha (TNF-α). DPV022 interacted directly with both exogenous Bak and Bax and inhibited apoptosis induced by Bak and Bax overexpression. In the context of virus infection, DPV022 interacted with endogenous Bak and Bax and inhibited their conformational activation. Together, our data indicate that DPV022 is an inhibitor of apoptosis that might be exploited to better understand the regulation of cell death.
MATERIALS AND METHODS
Cells and viruses.
HeLa, HEK 293T, HuTK−-143B, baby green monkey kidney (BGMK), and wild-type Jurkat cells were obtained from the ATCC and maintained as previously described (5, 11, 63, 71). Jurkat cells overexpressing Bcl-2 (Jurkat Bcl-2 cells) were generated and maintained as previously described (7). Bak- and Bax-deficient Jurkat cells (Jurkat Bak−/−/Bax−/− cells) were provided by H. Rabinowich (University of Pittsburgh School of Medicine, Pittsburgh, PA) (62). Mouse embryonic fibroblast (MEF) cells expressing the pMIH empty vector or pMIH-Flag-DPV022 were made by retroviral infection as described previously (11, 70). Hygromycin-resistant MEFs were selected, and protein expression was confirmed by intracellular flow cytometric analysis using an anti-FLAG antibody. Vaccinia virus (VV) strain Copenhagen expressing enhanced green fluorescent protein (GFP) (EGFP) was provided by G. McFadden (University of Florida, Gainesville, FL). VV strain Copenhagen lacking the F1L open reading frame VVΔF1L was generated as described previously (63, 65). Viruses were propagated in BGMK cells and isolated as previously described (53).
Plasmids.
Wild-type DPV022 from mule deer poxvirus strain W-848-83 (1) was codon optimized to remove rare codons, poly(A) sites, and RNA instability motifs. Codon optimization increased the GC content of DPV022 from 25% to 53% in order to prolong the mRNA half-life, and codon usage was adapted to the bias of Homo sapiens. Codon-optimized DPV022 was synthesized with 5′ EcoRI and 3′ BamHI restriction sites in backbone vector pMA (Geneart). Following restriction digestion with EcoRI and BamHI, DPV022 was cloned into the pGemT vector (Promega), and the construct was sequenced to ensure the absence of errors. DPV022 was subcloned into pEGFP-C2 (Clontech) to generate N-terminally EGFP-tagged DPV022. pEGFP-F1L, pEGFP-FPV039, and pEGFP-Bcl-xL were generated as described elsewhere previously (5, 6, 65). pcDNA3-HA-Bax, pcDNA3-HA-Bak, and pEGFP-M11L were provided by G. McFadden (University of Florida, Gainesville, FL), and pEGFP-Bcl-2 was provided by C. Bleackley (University of Alberta, Edmonton, Alberta, Canada). The Saccharomyces cerevisiae constructs were made by subcloning Bcl-xL, DPV022, Bak, and Bax into the pGALL(TRP) vector to place each gene under galactose-inducible control, as previously described (23). A retroviral construct expressing Bims2A was made by subcloning into the pMIG vector (murine stem cell virus-internal ribosome entry site [MSCV-IRES]-GFP, where the GFP sequence is that of EGFP), as previously described (11, 70). To generate the expression plasmid for DPV022, the GFP selection cassette was replaced with that of the hygromycin resistance gene to generate the pMIH-Flag-DPV022 construct (60). All of the genes used were of human origin except for the viral proteins.
Generation of VVΔF1L-Flag-DPV022.
A Flag-tagged version of wild-type DPV022 from strain W-848-83 was generated from synthetic cDNA (Blue Heron Biotechnology) using the forward primer 5′-ATGGTGTCGACACCATGGACTACAAAGACGATGA-3′ containing a SalI restriction site and Flag tag and the reverse primer 5′-TCCCTGTTGCGGCCGCAACAGGGATTTCTTGTCTCC-3′ containing a NotI restriction site. Flag-DPV022 was subcloned into vector pSC66, placing Flag-DPV022 between two regions of homology to the VV thymidine kinase (TK) gene and under the control of a synthetic poxviral early/late promoter (19). To generate recombinant VV strain Copenhagen devoid of F1L but expressing Flag-DPV022, BGMK cells were infected at a multiplicity of infection (MOI) of 0.05 with VVΔF1L and transfected with 5 μg pSC66-DPV022 using Lipofectin (Invitrogen Life Technologies). Homologous recombination between the VV TK gene and the TK sequences flanking Flag-DPV022 resulted in the integration of Flag-DPV022 and the poxviral promoter and the interruption of the VV TK gene. Recombinant viruses were selected by growth on HuTK−-143B cells in the presence of 5′-bromodeoxyuridine (Sigma-Aldrich) and plaque purified by using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (Rose Scientific Ltd.) to visualize recombinant viruses (19). The presence of DPV022 was confirmed by PCR, and protein expression was confirmed by Western blotting using anti-Flag M2 antibody (Sigma-Aldrich).
Live-cell confocal microscopy.
HeLa cells (1 × 106 cells) were grown on glass-bottom no. 1.0 cell culture dishes (MatTek Corporation) and transiently transfected with 0.5 μg pEGFP or 2 μg of pEGFP-DPV022 using Lipofectamine 2000 according to the manufacturer's instructions (Gibco Invitrogen Corp.). Mitochondria were labeled with 15 ng/ml of MitoTracker Red CMXRos (Invitrogen Life Technologies). Live cells were examined by using an LSM510 laser scanning confocal microscope at 543 nm to detect MitoTracker fluorescence and 489 nm to detect EGFP fluorescence.
Measurement of mitochondrial membrane potential.
Changes in the mitochondrial membrane potential were quantified by staining with tetramethylrhodamine ethyl ester (TMRE) (Invitrogen Life Technologies) (20, 46). HeLa cells (1 × 106 cells) were transfected with pEGFP-C3, pEGFP-DPV022, pEGFP-FPV039, pEGFP-M11L, pEGFP-F1L, or pEGFP-Bcl-2 using Lipofectamine 2000, and apoptosis was induced by treating cells with 10 ng of tumor necrosis factor alpha (TNF-α) (Roche Diagnostics) and 5 μg of cycloheximide (ICN Biomedicals Inc.) for 6 h or by transfecting cells with 2 μg of pcDNA3-HA-Bak or 0.75 μg of pcDNA3-HA-Bax for 18 h. Following treatment, cells were stained with 0.2 μM TMRE (20, 46). Cells were analyzed by two-color flow cytometry (FACScan; Becton Dickinson) with TMRE fluorescence measured through the fluorescence parameter 2 (FL-2) channel equipped with a 585-nm filter (42-nm band pass) and EGFP fluorescence measured through the FL-1 channel equipped with a 489-nm filter (42-nm band pass). Data were acquired for 20,000 cells per sample with fluorescence signals at logarithmic gain, and analysis was performed by using CellQuest software. The overall percentage of cells that exhibited a loss in mitochondrial membrane potential was calculated, taking into account the cytotoxicity induced by transfection. The number of EGFP-positive, TMRE-negative cells was divided by the total number of EGFP-positive cells to determine the percentage of apoptotic cells in transfected samples treated with an apoptotic stimulus or left untreated. The value obtained for transfected but untreated cells was subtracted from the value obtained for transfected cells also treated with an apoptotic stimulus to give the overall percentage of apoptotic cells.
Bax cross-linking.
HEK 293T cells (1 × 106 cells) were transfected with pEGFP, pEGFP-Bcl-2, or pEGFP-DPV022 and pcDNA-HA-Bax. The peptide zVAD.fmk (50 μM; Kamiya Biomedical Company) was added following transfection to prevent the downstream activation of caspases. Cells were lysed in lysis buffer containing 2% CHAPS {(3-[3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} (Sigma-Aldrich), 150 mM NaCl, and 50 mM Tris (pH 8.0) and supplemented with EDTA-free proteinase inhibitor (Roche Diagnostics). Following lysis, nuclei and membranes were spun down, and the supernatant was incubated for 30 min at room temperature with 1 μM 1,6-bismaleimidohexane (BMH) (Thermo Scientific) dissolved in dimethyl sulfoxide (DMSO). Supernatants were acetone precipitated, and cross-linking was quenched by the addition of SDS loading buffer containing 100 mM 2-mercaptoethanol. Protein samples were separated by SDS-PAGE and analyzed by Western blotting with an anti-hemagglutinin (HA) antibody.
Colony formation assay.
A long-term survival assay was performed as described previously (11). Wild-type MEFs were stably transfected with either the empty vector pMIH or pMIH-Flag-DPV022, and the expression of Flag-DPV022 was verified by flow cytometry. The mock or DPV022-expressing MEFs were infected with a retrovirus expressing the empty pMIG vector or pMIG-BimS2A. Since cloning into the pMIG vector places the BH3-only sequence upstream of an IRES and the EGFP open reading frame, pMIG clones express the relevant BH3-only protein and EGFP simultaneously and to similar levels. EGFP-positive cells (and, therefore, BH3-only-positive cells) were sorted by fluorescence-activated cell sorter (FACS) analysis and seeded onto 6-well plates (150 cells/well). The MEFs infected with the retrovirus expressing BimS2A were cultured with or without 1 μM ABT-737. Cells were incubated for 5 days, and the number of EGFP-positive clones was scored by using a fluorescent microscope.
Yeast colony assays.
Saccharomyces cerevisiae W303α cells were cotransformed with the pGALL(TRP) vector only, pGALL(TRP)-Bcl-xL, or pGALL(TRP)-DPV022 and pGALL(TRP)-Bak or pGALL(TRP)-Bax. pGALL(TRP) places genes under the control of a galactose-inducible promoter (33). Cells were spotted as 5-fold serial dilutions onto medium containing 2% (wt/vol) galactose (inducing, or “on”), which induces protein expression, or 2% (wt/vol) glucose (repressing, or “off”), which prevents protein expression, as previously described (37). Plates were incubated for 48 h at 30°C and then photographed.
Conformational analysis of Bak and Bax by flow cytometry.
Jurkat cells (1 × 106 cells), Jurkat Bcl-2 cells, or Jurkat Bak−/−/Bax−/− cells were infected with VV-EGFP, VVΔF1L, or VVΔF1L-Flag-DPV022 for either 4 or 6 h at an MOI of 10. Following infection, cells were exposed to either 0.25 or 2 μM staurosporine (STS) (Sigma-Aldrich) for 2 h and then fixed in 0.25% paraformaldehyde. Cells were permeabilized with 500 μg/ml digitonin and stained with either 2 μg/ml anti-Bak Ab-1 (Oncogene Research Products) (30, 31), 2 μg/ml anti-Bax6A7 antibody (BD Biosciences) (35), or 2 μg/ml of an isotype control antibody specific for NK1.1 (PK136) (provided by K. Kane, University of Alberta, Edmonton, Alberta, Canada) (39) and counterstained with phycoerythrin-conjugated anti-mouse antibody (Jackson ImmunoResearch Laboratories Inc.). Antibody staining was analyzed by flow cytometry (FACScan; Becton Dickinson) with fluorescence measured through the FL-2 channel equipped with a 585-nm filter (42-nm band pass). Data were acquired for 20,000 cells per sample with fluorescence signals at logarithmic gain, and analysis was performed by using CellQuest software.
Immunoprecipitations and immunoblotting.
HEK 293T cells (1 × 106 cells) were transfected by using Lipofectamine 2000 and the following plasmids: pEGFP-C3, pEGFP-FPV039, pEGFP-M11L, pEGFP-F1L, pEGFP-DPV022, pEGFP-Bcl-xL, or pEGFP-Bcl-2 and either pcDNA3-HA-Bak or pcDNA3-HA-Bax. zVAD.fmk (50 μM; Kamiya Biomedical Company) was added following transfection to prevent the downstream activation of caspases. Cells were lysed in 2% CHAPS lysis buffer, followed by precipitation using goat anti-EGFP antibody (provided by Luc Berthiaume, University of Alberta, Edmonton, Alberta, Canada). Similar experiments were performed in the context of viral infection. HeLa cells (7 × 106 cells) were infected with VVΔF1L or VVΔF1L-Flag-DPV022 at an MOI of 2 for 10 h. Cells were lysed in 2% CHAPS (Sigma-Aldrich) and precleared for 30 min with protein A beads. Lysates were split equally into 2 volumes and immunoprecipitated with either mouse anti-Bax 2D2 (Trevigen) or rabbit anti-Bak NT (Upstate).
Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. The following antibodies were used for detection: mouse anti-EGFP antibody (1:5,000; Cedarlane Laboratories Ltd.), mouse anti-HA antibody (clone 12CA5) (1:4,000; Roche Diagnostics), mouse anti-Flag horseradish peroxidase (HRP) (1:2,000; Sigma-Aldrich), mouse anti-Bak (1:500; Pharmingen), and mouse anti-Bax2D2 (1:10,000; Trevigen). Proteins were visualized by using enhanced chemiluminescence according to the manufacturer's directions (GE Healthcare).
RESULTS
DPV022 shares sequence homology with F1L and M11L but not Bcl-2 family proteins.
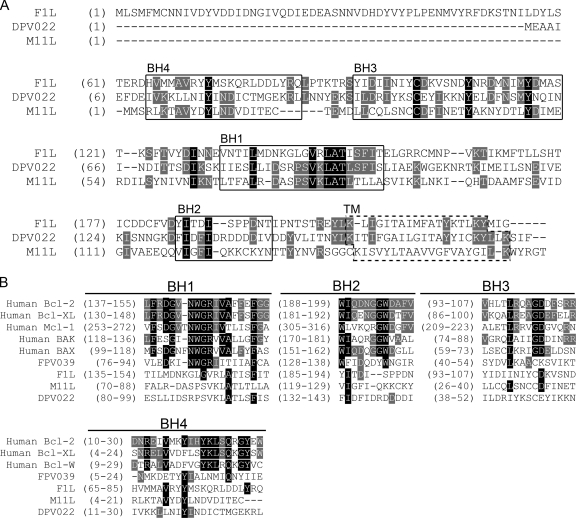
F1L, encoded by vaccinia virus, and M11L, encoded by myxoma virus, share no obvious sequence similarity with each other or other known modulators of apoptosis. It was therefore surprising that upon the sequencing of the deerpox virus genome, the open reading frame DPV022 was discovered to share 27% amino acid sequence identity with F1L (1). An alignment of the F1L and DPV022 amino acid sequences revealed identical amino acids distributed throughout the two proteins but not necessarily localized to any particular region, including the regions corresponding to the highly divergent F1L BH domains (Fig. 1A) (10). Interestingly, DPV022 also possesses 23% amino acid identity with M11L (Fig. 1A). An alignment of M11L and DPV022 amino acid sequences likewise showed identical amino acids distributed throughout the two proteins (Fig. 1A). Many of the amino acids shared by DPV022 and F1L differed from the amino acids shared by DPV022 and M11L, suggesting that DPV022 may represent an evolutionary intermediate between F1L and M11L. Moreover, although relatively few amino acids were identical among the three proteins, a number of identical amino acids were clustered within the regions corresponding to the highly divergent BH domains of F1L and M11L, particularly BH3 and BH1 (Fig. 1A) (10, 41, 42). These putative BH domains in DPV022, however, bear little sequence identity to the BH domains present in cellular Bcl-2 family proteins or the poxviral Bcl-2 homologue, FPV039 (Fig. 1B) (10). Like Bcl-2 family proteins and both F1L and M11L, DPV022 is predicted to possess a C-terminal hydrophobic domain responsible for targeting the protein to intracellular membranes, such as the mitochondria (Fig. 1A). Therefore, based on sequence identity and the predicted presence of a C-terminal transmembrane (TM) tail, we hypothesized that DPV022 would act at the mitochondria to inhibit apoptosis.
FIG. 1.
DPV022 shares regions of sequence homology with F1L and M11L but not cellular Bcl-2 proteins. (A) DPV022 shares regions of sequence homology with both F1L and M11L, particularly in the regions corresponding to F1L BH domains (boxed). The transmembrane (TM) domains for F1L and M11L and the putative TM domain for DPV022 are indicated (dashed box). (B) Alignment of the BH1, BH2, BH3, and BH4 domains from various cellular and viral Bcl-2 proteins with the corresponding regions of DPV022. Amino acids conserved among all three proteins are highlighted in black, and amino acids conserved among two of the three proteins are highlighted in gray (AlignX).
DPV022 localizes to the mitochondria and inhibits apoptosis.
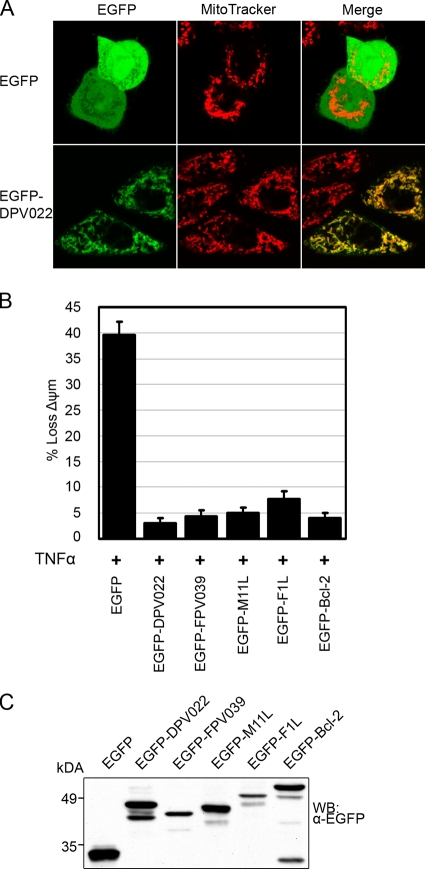
Most cellular Bcl-2 proteins and viral inhibitors of apoptosis possess hydrophobic C-terminal transmembrane tails that posttranslationally anchor the proteins to an intracellular membrane (8). Subcellular localization to the mitochondria is typical for cellular Bcl-2 proteins and, in many cases, a prerequisite for their antiapoptotic function (52). Sequence analysis of DPV022 revealed a C-terminal hydrophobic region that may act as a tail anchor (Fig. 1A). To determine whether DPV022 localized to the mitochondria, HeLa cells were transfected with pEGFP or pEGFP-DPV022 and analyzed by live-cell confocal microscopy (Fig. 2A). Cells transfected with pEGFP displayed a diffuse, cytoplasmic fluorescence pattern that did not colocalize with MitoTracker, a dye that specifically labels mitochondria. Conversely, cells transfected with pEGFP-DPV022 displayed a punctate fluorescence pattern that colocalized with MitoTracker, indicating that, like F1L and M11L, DPV022 is a mitochondrion-localized protein.
FIG. 2.
DPV022 localizes to the mitochondria and inhibits TNF-α-induced apoptosis. (A) HeLa cells were transfected with pEGFP or pEGFP-DPV022, and mitochondria were stained with MitoTracker dye. Colocalization was visualized by confocal microscopy. (B) HeLa cells were transfected with pEGFP, pEGFP-DPV022, pEGFP-FPV039, pEGFP-M11L, pEGFP-F1L, or pEGFP-Bcl-2 and treated with 10 ng/ml TNF-α and 5 mg/ml cycloheximide for 6 h. Apoptosis was assessed by quantifying TMRE fluorescence via flow cytometry, and the percentage of cells that demonstrated a loss of mitochondrial membrane potential (ΔΨm) is given on the y axis. Standard deviations were calculated from three independent experiments. (C) The expression level of each EGFP-tagged construct was assessed by Western blotting (WB).
Given that DPV022 bears limited sequence homology to F1L and M11L, two well-characterized poxviral inhibitors of apoptosis, we next sought to determine whether DPV022 could inhibit apoptosis. HeLa cells were transfected with pEGFP, pEGFP-DPV022, pEGFP-FPV039, pEGFP-M11L, pEGFP-F1L, or pEGFP-Bcl-2 and treated with TNF-α to induce apoptosis. Cells were stained with TMRE, a fluorescent dye incorporated by healthy, respiring mitochondria, and apoptosis was quantified via flow cytometric analysis of EGFP-expressing cells (Fig. 2B) (20, 46). Nearly 40% of cells expressing EGFP exhibited a decrease in TMRE staining upon TNF-α treatment, indicating a loss of mitochondrial membrane potential and the induction of apoptosis (Fig. 2B). Cells expressing EGFP-FPV039, the antiapoptotic Bcl-2 homologue encoded by fowlpox virus, EGFP-M11L, or EGFP-F1L were protected from the loss of mitochondrial membrane potential, with apoptosis being prevented in at least 92% of transfected cells. EGFP-Bcl-2, the prototypical antiapoptotic member of the cellular Bcl-2 family, prevented TNF-α-induced apoptosis in 96% of transfected cells. Importantly, cells expressing EGFP-DPV022 were also protected from the induction of apoptosis, with only 3% of transfected cells exhibiting a loss of mitochondrial membrane potential (Fig. 2B). To ensure that differences in expression levels did not affect the inhibition of apoptosis, cell lysates were analyzed (Fig. 2C). Although EGFP-FPV039 and EGFP-F1L were expressed at lower levels than the other EGFP-tagged proteins, both were potent inhibitors of apoptosis. EGFP alone was expressed at the highest level, but it was not protective (Fig. 2C). Together, these data demonstrate that DPV022 is a mitochondrion-localized protein capable of inhibiting apoptosis induced by the activation of the death receptor pathway in HeLa cells, presumably by interfering with events at the mitochondria.
DPV022 interacts with Bak and inhibits Bak-induced apoptosis.
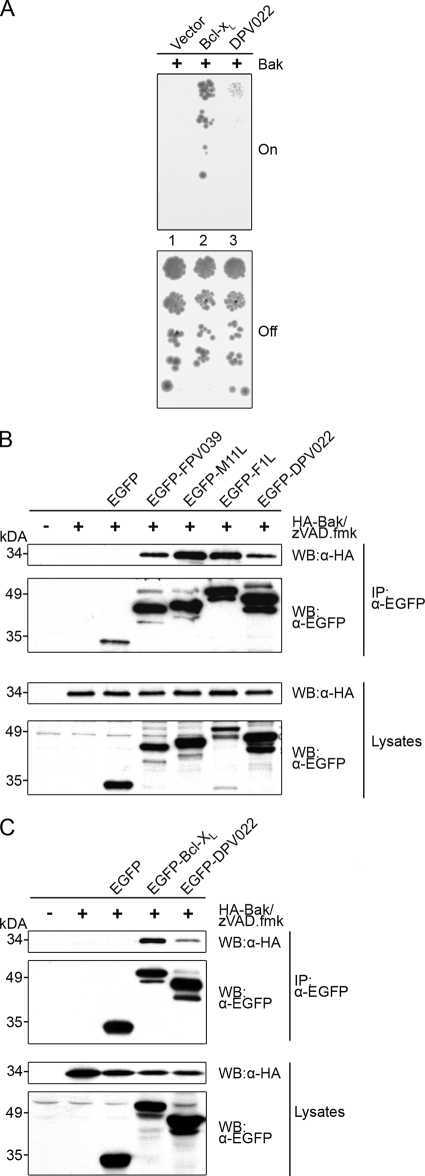
The intrinsic apoptotic pathway is ultimately regulated at the mitochondria by the proapoptotic proteins Bak and Bax (44, 67, 77). The activation of Bak results in the release of cytochrome c from the mitochondria and the subsequent induction of apoptosis (66). Bak activity is inhibited by constitutive interactions with the cellular antiapoptotic protein Bcl-xL (70) as well as several viral inhibitors of apoptosis, including FPV039, F1L, and M11L (5, 61, 63). To determine if DPV022 was capable of interacting directly with Bak, we used a heterologous system based on the yeast Saccharomyces cerevisiae, which lacks endogenous Bcl-2 family proteins. Because S. cerevisiae cells do not have the equivalent components of the mammalian cell death machinery, they do not undergo apoptosis in a manner similar to that of mammalian cells. Nonetheless, the overexpression of proapoptotic Bax or Bak results in growth arrest and the loss of yeast colony formation, potentially due to a disruption of mitochondrial function (32, 36, 51). Yeast cells can be rescued from this growth arrest by the overexpression of mammalian antiapoptotic proteins, such as Bcl-xL, which leads to robust colony growth (32, 51). Thus, in this well-established functional assay (23, 29, 37, 76), the expression of Bak blocks yeast growth, which can be rescued by the overexpression of Bcl-xL (Fig. 3A, “on”). Yeast cells were serially diluted and duplicate plated vertically onto medium containing either galactose, to induce the expression of Bak (Fig. 3A, on), or glucose, to repress the expression of Bak (Fig. 3A, “off”). As expected, yeast cells expressing only Bak did not produce any colonies (Fig. 3A, lane 1, on) (57); however, the coexpression of Bcl-xL, which interacts with Bak, efficiently rescued yeast growth and resulted in colony formation (Fig. 3A, lane 2, on). Likewise, the coexpression of DPV022 neutralized Bak and allowed the growth of the yeast cells (Fig. 3A, lane 3, on). Compared to Bcl-xL, DPV022 appeared less potent, possibly because it is expressed at lower levels. Regardless, these data suggest that DPV022 can directly engage Bak and neutralize its activity in the absence of any other mammalian Bcl-2 proteins. Yeast cells plated onto glucose-containing medium produced a similar number of colonies in each case, indicating that the expression of Bak was being repressed and that equivalent concentrations of yeast were plated (Fig. 3A, off). To further confirm the ability of DPV022 to interact with Bak, HEK 293T cells were transfected with pEGFP, pEGFP-FPV039, pEGFP-M11L, pEGFP-F1L, pEGFP-Bcl-xL, or pEGFP-DPV022 and pcDNA-HA-Bak, and cells were lysed in buffer containing 2% CHAPS, a detergent that does not artificially affect the conformation of Bcl-2 family proteins (34, 35). Cell lysates were immunoprecipitated with an anti-EGFP antibody, which was followed by Western blotting with either an anti-HA or an anti-EGFP antibody. Consistent with previous data, an interaction between HA-Bak and EGFP-FPV039, EGFP-M11L, and EGFP-F1L was observed (Fig. 3B) (5, 61, 63). Bcl-xL, a cellular Bcl-2 family protein, also interacted with HA-Bak, as expected (Fig. 3C) (70). Similarly, DPV022 precipitated Bak, suggesting that the two proteins interact (Fig. 3B and C). EGFP-tagged proteins were precipitated from cell lysates at equal levels (Fig. 3B and C, second panel), and all constructs were expressed (Fig. 3B and C, bottom two panels).
FIG. 3.
DPV022 interacts with Bak. (A) S. cerevisiae cells were cotransformed with the indicated plasmids, and standardized concentrations were spotted in serial dilutions vertically down the plate. Galactose-containing media (on) induced the expression of Bak, whereas glucose-containing media (off) repressed the expression of Bak. Colony growth reflects resistance to Bak-induced growth suppression. (B and C) HEK 293T cells were cotransfected with pEGFP, pEGFP-FPV039, pEGFP-M11L, pEGFP-F1L, or pEGFP-DPV022 and pcDNA3-HA-Bak (B) or pEGFP, pEGFP-Bcl-xL, or pEGFP-DPV022 and pcDNA3-HA-Bak (C). zVAD.fmk was added after transfection to prevent caspase activation. Following lysis in 2% CHAPS buffer, lysates were immunoprecipitated (IP) with an anti-EGFP antibody and subjected to Western blotting (WB) with anti-EGFP or anti-HA antibodies to detect an interaction.
The transient overexpression of Bak results in its activation and the subsequent induction of apoptosis (14). Given that DPV022 interacted with Bak, we investigated whether DPV022 could inhibit Bak-induced apoptosis. HeLa cells were transfected with pEGFP, pEGFP-DPV022, pEGFP-FPV039, pEGFP-M11L, or pEGFP-Bcl-2 and cotransfected with HA-Bak. Apoptosis was then quantified via TMRE staining and flow cytometric analysis. EGFP was unable to protect against the apoptosis triggered by Bak overexpression, with 39% of cells exhibiting a loss of mitochondrial membrane potential. Conversely, the two viral proteins EGFP-FPV039 and EGFP-M11L as well as the antiapoptotic cellular protein EGFP-Bcl-2 prevented Bak-induced apoptosis. Likewise, EGFP-DPV022 was a potent inhibitor of Bak-induced apoptosis, with fewer than 2% of cells exhibiting a loss of mitochondrial membrane potential (Fig. 4A). HA-Bak and all EGFP-tagged proteins were expressed in each case (Fig. 4B). Thus, DPV022 is capable of interacting with Bak and inhibiting apoptosis induced by Bak overexpression.
FIG. 4.

DPV022 inhibits Bak-induced apoptosis. (A) HeLa cells were cotransfected with pEGFP, pEGFP-DPV022, pEGFP-FPV039, pEGFP-M11L, or pEGFP-Bcl-2 and pcDNA3-HA-Bak. Apoptosis was assessed by quantifying TMRE fluorescence via flow cytometry, and the percentage of cells that demonstrated a loss of mitochondrial membrane potential (ΔΨm) is given on the y axis. Standard deviations were calculated from three independent experiments. (B) HeLa cells were transfected, and the expression level of each EGFP-tagged construct was assessed by Western blotting (WB).
DPV022 interacts with Bax and inhibits Bax-induced apoptosis.
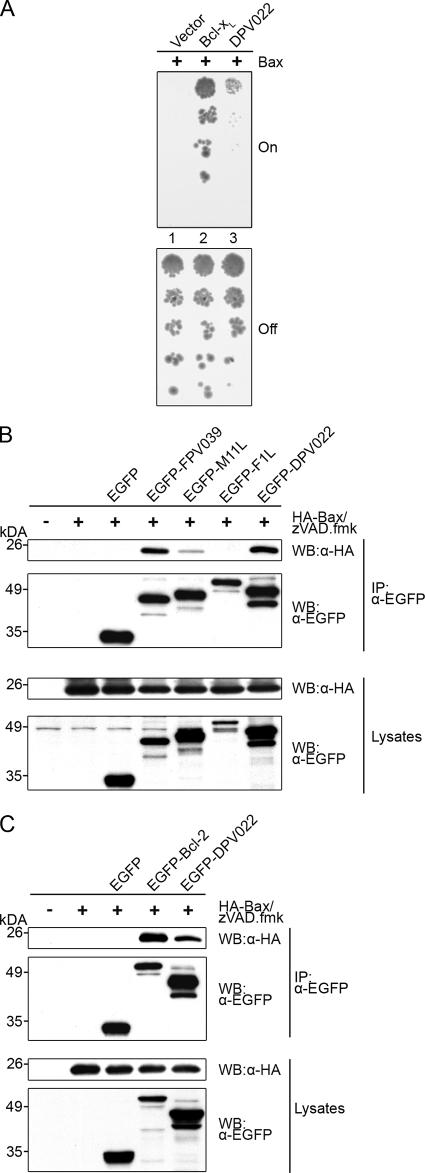
Both Bak and Bax are independently able to facilitate the release of cytochrome c and induce apoptosis (44, 67, 77). Therefore, to effectively inhibit apoptosis, Bax must also be inactivated. To determine whether DPV022 is able to engage and neutralize Bax directly, we used an approach identical to that described above for Bak (Fig. 3A). The expression of Bcl-xL rescued yeast from growth arrest induced by Bax expression (Fig. 5A, lane 1 versus lane 2, “on”), as did the expression of DPV022 (Fig. 5A, lane 3, on), suggesting that DPV022 directly interacts with and inhibits Bax. The duplicate plating of yeast cells onto glucose-containing medium indicated that Bax expression was repressed and that equivalent concentrations of yeast were being plated (Fig. 5A, “off”). The interaction between DPV022 and Bax was then confirmed by coimmunoprecipitation (Fig. 5B and C). As expected, EGFP-FPV039 and EGFP-M11L both precipitated HA-Bax, whereas EGFP-F1L did not (Fig. 5B) (6, 54, 59). The cellular antiapoptotic protein EGFP-Bcl-2, which is known to interact with Bax, also precipitated Bax in this assay (Fig. 5C) (73, 75). Importantly, EGFP-DPV022 robustly precipitated Bax, indicating an interaction between the two proteins (Fig. 5B and C). In each case, EGFP-tagged proteins were precipitated, and all proteins were expressed (Fig. 5B and C).
FIG. 5.
DPV022 interacts with Bax. (A) S. cerevisiae cells were cotransformed with the indicated plasmids, and standardized concentrations were spotted in serial dilutions vertically down the plate. Galactose-containing media (on) induced the expression of Bax, whereas glucose-containing media (off) repressed the expression of Bax. Colony growth reflects resistance to Bax-induced growth suppression. (B and C) HEK 293T cells were cotransfected with pEGFP, pEGFP-FPV039, pEGFP-M11L, pEGFP-F1L, or pEGFP-DPV022 and pcDNA3-HA-Bax (B) or pEGFP, pEGFP-Bcl-2, or pEGFP-DPV022 and pcDNA3-HA-Bax (C). zVAD.fmk was added after transfection to prevent caspase activation. Following lysis in 2% CHAPS buffer, lysates were immunoprecipitated (IP) with an anti-EGFP antibody and subjected to Western blotting (WB) with anti-EGFP or anti-HA antibodies to detect an interaction.
Similar to Bak overexpression, the overexpression of Bax also induces apoptosis (38, 72). In order to assess whether DPV022 could inhibit Bax-induced apoptosis, HeLa cells were transfected with pEGFP, pEGFP-DPV022, pEGFP-FPV039, pEGFP-M11L, or pEGFP-Bcl-2 and HA-Bax. Bax induced almost 50% apoptosis in cells expressing EGFP alone (Fig. 6A). However, cells expressing EGFP-FPV039, EGFP-M11L, or EGFP-Bcl-2 were all protected from apoptosis, each with fewer than 5% of transfected cells exhibiting a loss of mitochondrial membrane potential (Fig. 6A). Fewer than 1% of cells expressing EGFP-DPV022 exhibited a loss of mitochondrial membrane potential, indicating that DPV022 was a potent inhibitor of Bax-induced apoptosis (Fig. 6A). HA-Bax and all EGFP-tagged proteins were expressed in each case (Fig. 6B).
FIG. 6.
DPV022 inhibits Bax-induced apoptosis and Bax oligomerization. (A) HeLa cells were cotransfected with pEGFP, pEGFP-DPV022, pEGFP-FPV039, pEGFP-M11L, or pEGFP-Bcl-2 and pcDNA3-HA-Bax. Apoptosis was assessed by quantifying TMRE fluorescence via flow cytometry, and the percentage of cells that demonstrated a loss of mitochondrial membrane potential (ΔΨm) is given on the y axis. Standard deviations were calculated from three independent experiments. (B) HeLa cells were transfected, and the expression level of each EGFP-tagged construct was assessed by Western blotting (WB). (C) HEK 293T cells were cotransfected with pEGFP, pEGFP-Bcl-2, or pEGFP-DPV022 and pcDNA3-HA-Bax. zVAD.fmk was added after transfection to prevent the activation of caspases. Following lysis in 2% CHAPS buffer, cells were treated with 1 μM BMH and Western blotted (WB) with anti-HA to detect Bax oligomers (top). A portion of the lysates (input) was treated with dimethyl sulfoxide (DMSO) alone and subjected to Western blotting with either anti-HA or anti-EGFP antibodies to determine expression levels of the transfected proteins (middle and bottom). ×1, Bax monomer; ×2, Bax dimer; ×N, higher-order Bax oligomers.
Following the activation of Bax and prior to the induction of apoptosis, Bax forms homo-oligomers in the outer mitochondrial membrane that are thought to assemble a pore sufficiently large to release cytochrome c (2-4). To confirm the ability of DPV022 to inhibit Bax-induced apoptosis, we assessed the formation of Bax homo-oligomers in the presence of DPV022. HEK 293T cells were transfected with pEGFP, pEGFP-Bcl-2, or pEGFP-DPV022 and HA-Bax. Following lysis in buffer containing 2% CHAPS, cell lysates were treated with the covalent cross-linker BMH. Bax oligomers could thus be preserved and visualized by Western blotting after SDS-PAGE. Because the overexpression of Bax induces apoptosis, HA-Bax expression alone was sufficient to induce the formation of Bax homo-oligomers, and 44-kDa Bax dimers, 66-kDa Bax trimers, and multiple higher-order Bax oligomers were clearly visible (Fig. 6C). The coexpression of EGFP alone was unable to prevent Bax oligomerization, whereas the expression of either EGFP-Bcl-2 or EGFP-DPV022 completely prevented Bax oligomerization. Cell lysates confirmed that HA-Bax and each EGFP-tagged construct were expressed (Fig. 6C). Together, these data demonstrate that DPV022 is able to interact with Bax, inhibit Bax oligomerization, and prevent Bax-induced apoptosis.
DPV022 replaces the cellular antiapoptotic Bcl-2 proteins.
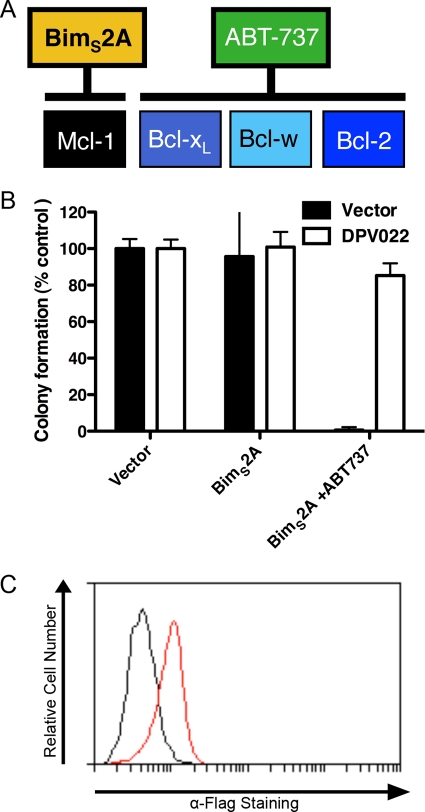
Given that DPV022 appeared to rescue Bax- and Bak-induced growth arrest in yeast cells by directly engaging both proapoptotic Bcl-2 proteins, we sought to determine if this was also the case in a mammalian system. MEFs stably expressing DPV022 were retrovirally transduced to express the BH3-only protein BimS2A, a Bim variant that engages only Mcl-1 (Fig. 7A) (43). Additionally, cells were treated with the Bad BH3 mimetic ABT-737, which neutralizes the antiapoptotic proteins Bcl-2, Bcl-xL, and Bcl-w (Fig. 7A) (48, 60). Thus, the combination of ABT-737 and BimS2A potently neutralizes all endogenous antiapoptotic Bcl-2 proteins (43). Apoptosis was measured as a function of cell colony formation, with the colonies produced by MEFs transduced with a pMIG empty vector retrovirus arbitrarily set to 100% (Fig. 7B). MEFs expressing the pMIH empty vector or DPV022 and BimS2A, which neutralizes only Mcl-1, showed no significant defect in colony formation, as expected. The treatment of MEFs expressing DPV022 and BimS2A with ABT-737, to neutralize all endogenous antiapoptotic Bcl-2 proteins, resulted in only a small decrease in colony formation. Conversely, the treatment of the MEFs expressing the empty vector and BimS2A with ABT-737 resulted in the complete loss of colony formation (Fig. 7B). These data suggest that even when all cellular antiapoptotic Bcl-2 proteins are neutralized, DPV022 is still able to promote colony growth and, therefore, inhibit apoptosis. The expression of Flag-DPV022 was confirmed by flow cytometry (Fig. 7C).
FIG. 7.
DPV022 can replace the endogenous antiapoptotic Bcl-2 proteins. (A) Binding and neutralization of the endogenous mammalian antiapoptotic proteins by BimS2A and ABT-737 (11, 43, 60). (B) Wild-type MEFs expressing the empty pMIH vector or Flag-DPV022 were infected with a retrovirus expressing the empty pMIG vector or BimS2A. EGFP-positive MEFs were sorted and incubated for 5 days. MEFs infected with the retrovirus expressing Bim2SA were cultured with or without 1 μM ABT-737. The number of colonies formed by MEFs infected with the empty virus represents 100% colony formation. Standard deviations were calculated from two independent experiments. (C) Flow cytometric histogram indicating the expression of Flag-DPV022 (red line) compared to cells mock transfected with pMIH (black line).
DPV022 interacts with endogenous Bak and Bax during virus infection.
Although DPV022 interacted with Bak and Bax, we wanted to confirm these interactions by assessing the ability of DPV022 to interact with endogenous Bak and Bax in the context of virus infection. We therefore made use of the recombinant vaccinia viruses VVΔF1L, which lacks the antiapoptotic F1L (63), and VVΔF1L-Flag-DPV022, which lacks F1L but expresses a Flag-tagged DPV022. Following infection, HeLa cells were lysed in buffer containing 2% CHAPS, and lysates were immunoprecipitated with either an anti-Bak or an anti-Bax antibody. Lysates were subsequently Western blotted with an anti-Flag antibody to detect interactions (Fig. 8). The immunoprecipitation of Bak pulled down Flag-DPV022, indicating that DPV022 interacts with endogenous Bak in the context of infection (Fig. 8, top). Similarly, DPV022 coprecipitated with Bax, suggesting that DPV022 and endogenous Bax also interact (Fig. 8, second panel). Endogenous Bak and Bax were expressed at equal levels in all cases, as were the Flag-tagged viral proteins (Fig. 8, bottom two panels). Together, these data suggest that DPV022 retains its ability to interact with endogenous Bak and Bax in the context of virus infection.
FIG. 8.

DPV022 interacts with endogenous Bak and Bax during virus infection. HeLa cells were mock infected or infected at an MOI of 2 with VVΔF1L or VVΔF1L-Flag-DPV022. Ten hours postinfection, cells were lysed in 2% CHAPS buffer, immunoprecipitated (IP) with either anti-Bak or anti-Bax antibodies, and Western blotted (WB) with an anti-Flag antibody to detect interactions. Lysates were Western blotted with anti-Bak, anti-Bax, and anti-Flag antibodies to detect expression levels.
DPV022 inhibits the conformational activation of Bak and Bax during virus infection.
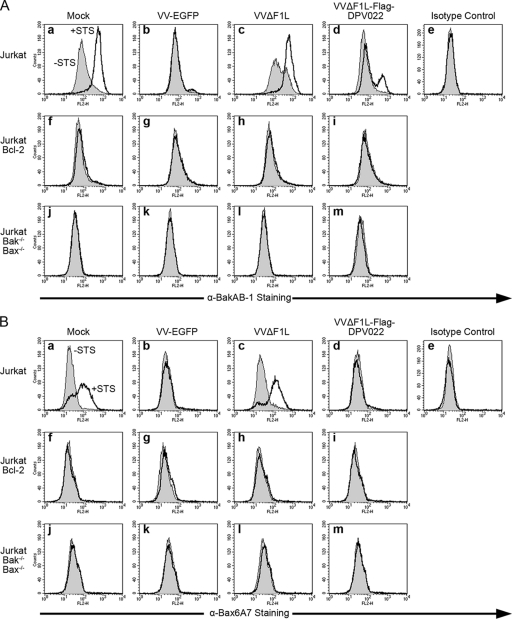
When transiently overexpressed, DPV022 was a potent inhibitor of apoptosis induced by TNF-α treatment or Bak and Bax overexpression. To determine if DPV022 expressed by vaccinia virus could inhibit the activation of Bak, we used a flow cytometry-based assay to detect the activation-associated conformational change of Bak. Jurkat cells were mock infected or infected with VV-EGFP, VVΔF1L, or VVΔF1L-Flag-DPV022 and treated with staurosporine (STS), a potent apoptotic stimulus (56). Prior to Bak homo-oligomerization and the release of cytochrome c, Bak undergoes a series of conformational changes that result in the exposure of an N-terminal epitope that can be detected by staining with anti-Bak Ab-1 and quantified by flow cytometry (Fig. 9A) (30, 31). Mock-infected Jurkat cells not treated with STS displayed a basal level of fluorescence, likely as a result of nonspecific antibody binding (Fig. 9A, panel a). After STS treatment, however, the fluorescence intensity of mock-infected Jurkat cells increased substantially, indicating that Bak was activated and had undergone a conformational change exposing its N terminus to binding by anti-Bak Ab-1 (Fig. 9A, panel a). Cells infected with VV-EGFP, which expresses F1L, completely prevented the activation of Bak during virus infection alone and after STS treatment (Fig. 9A, panel b), as previously observed (5, 63). Conversely, VVΔF1L, which lacks F1L, in combination with STS treatment showed robust Bak activation, whereas infection with VVΔF1L alone resulted in a less robust activation of Bak, presumably due to the slower induction of apoptosis during vaccinia virus infection (Fig. 9A, panel c) (64). Infection with VVΔF1L-DPV022 inhibited the activation of Bak; however, DPV022 was unable to completely prevent the activation of Bak upon treatment with STS (Fig. 9A, panel d). No increase in fluorescence intensity was observed for Jurkat cells stained with an isotype control antibody, confirming the specificity of anti-Bak Ab-1 (Fig. 9A, panel e). Additionally, Jurkat cells overexpressing the cellular antiapoptotic protein Bcl-2 were resistant to Bak activation in all cases (Fig. 9A, panels f to i), and activated Bak was undetectable in Jurkat cells devoid of both Bak and Bax (Fig. 9A, panels j to m), again confirming the specificity of our assay. These data demonstrate that, like F1L, DPV022 can inhibit the activation of Bak induced by virus infection alone. Unlike F1L, however, DPV022 was incapable of inhibiting all Bak activation following the additional apoptotic stimulus provided by STS.
FIG. 9.
DPV022 inhibits the conformational activation of endogenous Bak and Bax during vaccinia virus infection. Wild-type Jurkat cells (a to e), Jurkat cells overexpressing Bcl-2 (f to i), and Jurkat cells devoid of Bak and Bax (j to m) were infected with VV-EGFP, VVΔF1L, or VVΔF1L-Flag-DPV022 at an MOI of 10 for 4 h (A) or 6 h (B) and then treated with 250 nM (A) or 2 μM (B) staurosporine (STS) for 2 h to induce apoptosis. Bak and Bax activations were quantified by flow cytometry using anti-Bak Ab-1 (A) and anti-Bax 6A7 (B) antibodies, respectively, or an isotype control antibody (NK1.1). Shaded histograms, untreated cells; open histograms, STS-treated cells. Data are representative of at least three independent experiments.
Similar to Bak, Bax also undergoes a series of activation-associated conformational changes that result in the relocalization of Bax from the cytoplasm to the mitochondria and the exposure of an N-terminal epitope. To determine if DPV022 could inhibit Bax activation, we performed an assay similar to that described above, using an antibody specific for the activation-associated N terminus of Bax, anti-Bax 6A7 (Fig. 9B) (35). Mock-infected Jurkat cells displayed a basal level of fluorescence that increased after treatment with STS, indicating that Bax had been activated and that the N-terminal epitope had been exposed to binding by anti-Bax 6A7 (Fig. 9B, panel a). VV-EGFP completely prevented the activation of Bax during infection alone and following treatment with STS (Fig. 9B, panel b). Although VVΔF1L infection alone did not induce the activation of Bax, perhaps because of its slower activation kinetics, VVΔF1L was unable to prevent Bax activation induced by STS treatment (Fig. 9B, panel c). Importantly, infection with VVΔF1L-Flag-DPV022 completely prevented the activation of Bax following treatment with STS, indicating that DPV022, like F1L, can inhibit Bax activity (Fig. 9B, panel d). Staining with an isotype control antibody confirmed the specificity of anti-Bax6A7 (Fig. 9B, panel e), and no Bax activation was observed for Jurkat cells overexpressing Bcl-2 or devoid of both Bak and Bax, further validating our assay (Fig. 9B, panels f to m). Thus, even in the context of virus infection, DPV022 is able to inhibit the activation of Bax.
DISCUSSION
Apoptosis presents a significant barrier to viral replication, and many viruses encode proteins to prevent infected cells from committing suicide (16, 58). Here we have completed the first functional characterization of a deerpox virus protein, DPV022, a poxviral inhibitor of apoptosis. DPV022 localized to the mitochondria, where it interacted directly with both Bak and Bax, the critical proapoptotic members of the Bcl-2 family, and potently inhibited apoptosis induced by TNF-α stimulation as well as Bak or Bax overexpression. Furthermore, DPV022 was able to inhibit apoptosis even when all cellular antiapoptotic Bcl-2 proteins were neutralized. In the context of virus infection, DPV022 retained its ability to interact with Bak and Bax, and it inhibited the conformational activation of Bak and Bax in response to staurosporine treatment. Thus, DPV022 represents another unique viral protein that has evolved to inhibit apoptosis at the mitochondria.
Poxviruses are renowned for their ability to manipulate the apoptotic pathway, and they encode a variety of distinct proteins that interfere with the Bcl-2 family of proteins (58). Vaccinia virus, the prototypical member of the genus Orthopoxvirus, and myxoma virus, the prototypical member of the genus Leporipoxvirus, both encode proteins, F1L and M11L, respectively, that inhibit apoptosis by inactivating Bak and Bax (54, 59, 61, 63). However, F1L and M11L lack obvious amino acid homology with each other or cellular Bcl-2 proteins, despite the fact that both M11L and F1L fold like a Bcl-2 protein (18, 41, 42). In fact, members of the genus Avipoxvirus are the only poxviruses that encode proteins with obvious Bcl-2 sequence homology, and FPV039, encoded by fowlpox virus, is the only antiapoptotic protein of this genus characterized to date (5, 6). DPV022, encoded by deerpox virus, the type species of the newly described genus Cervidpoxvirus, has no obvious sequence homology with the BH domains of cellular Bcl-2 proteins (Fig. 1B), but it does share regions of amino acid identity with both M11L and F1L (Fig. 1A). Much of the homology is concentrated within the regions of DPV022 that correspond to the M11L and F1L BH domains. Strikingly, the “LAT” motif (corresponding to amino acids 147 to 149 in F1L) within the BH1 region is conserved among all three proteins, highlighting the importance of the BH1 domain to the function of these proteins. In fact, A148 in F1L and the corresponding amino acid A82 in M11L occupy structurally important positions in the F1L and M11L binding grooves, and a mutation of A82 in M11L abrogates its interaction with Bak and Bax (41, 42). The corresponding amino acid A93 in DPV022 is therefore likely to play a critical role in the ability of DPV022 to inhibit apoptosis. Interestingly, however, the majority of identical amino acids shared between M11L and DPV022 are not the same amino acids shared between F1L and DPV022. Besides A82 of M11L, DPV022 shares M11L amino acids I37, Y41, and F122, all of which have been implicated to play key roles in the ability of M11L to inhibit apoptosis (41). Except for M11L Y41, none of these critical amino acids are shared with F1L. Conversely, F1L F152 was previously suggested to be critical to the ability of F1L to inhibit apoptosis, and, along with both flanking residues, F152 is shared between F1L and DPV022 but not M11L (42). Thus, the sequence of DPV022 is intermediate between M11L and F1L, which themselves share very little sequence identity. Together with the fact that DPV022, M11L, and F1L are all encoded in the same position on the poxvirus genome, these data suggest that DPV022 is evolutionarily related to both M11L and F1L (1, 9, 26).
Despite sharing little sequence identity with each other, M11L and F1L both have structures homologous to cellular Bcl-2 proteins (18, 41, 42). Moreover, FPV039 and the Orf virus inhibitor of apoptosis, ORFV125, both of which lack significant sequence homology with other poxviral inhibitors of apoptosis, are also predicted to fold like Bcl-2 proteins (5, 68). In fact, poxviruses have recently been shown to encode a variety of proteins that all adopt a Bcl-2-like fold (27, 28). A52R and B14R, both vaccinia virus-encoded proteins that share little sequence homology with each other, fold like Bcl-2 proteins and inhibit NF-κB signaling but not apoptosis (28). Additionally, Gonzalez and Esteban recently proposed that like A52R and B14R, vaccinia virus A46R, K7R, N1L, N2L, and C1L all possess a Bcl-2-like fold and should be grouped within the same protein family (27). Although some of these proteins have been implicated in interfering with Toll-like receptor (TLR) signaling at different levels, only N1L has been shown to have an ability to affect apoptosis (15). Indeed, A52R and B14R lack the hydrophobic binding groove required by other viral proteins, such as M11L and F1L, to interact with proapoptotic Bcl-2 proteins and inhibit apoptosis. Given the amino acid identity shared between DPV022, M11L, and F1L, it is likely that DPV022 also folds like a Bcl-2 family protein. The residues conserved among these three poxviral proteins, and the ability of DPV022 to interact with Bak and Bax, also suggests that DPV022 retains the hydrophobic binding groove necessary for the homo- and heterotypic interactions that characterize the antiapoptotic Bcl-2 family of proteins.
Viral inhibitors of apoptosis, including M11L and F1L, inhibit apoptosis by inactivating Bak and Bax, the critical proapoptotic Bcl-2 proteins. DPV022 interacted with both overexpressed and endogenous Bak and Bax in the presence or absence of vaccinia virus infection. M11L, F1L, and FPV039 interact constitutively with Bak to inhibit apoptosis; however, only M11L and FPV039 display an ability to interact with Bax (5, 6, 54, 59, 61, 63). Likewise, the adenoviral protein E1B 19K inhibits apoptosis by interacting with both Bak and Bax. Conversely, ORFV125 inhibits apoptosis by interacting with only active Bax and an array of BH3-only proteins (21, 55, 68). Similarly, the Bcl-2 homologue encoded by Kaposi's sarcoma-associated herpesvirus interacts with numerous BH3-only proteins but not with Bak or Bax, suggesting that the inhibition of BH3-only proteins alone may be sufficient to inhibit apoptosis (12, 22). Indeed, FPV039 and F1L inhibit the conformational activation of Bax, presumably by interacting with, and inhibiting the activity of the BH3-only protein Bim (6, 41, 59). Whether or not DPV022 has the ability to interact with BH3-only proteins and the functional consequences of such interactions are the subject of future research. Regardless, it is clear that interacting with BH3-only proteins is an effective way to neutralize Bak and Bax and inhibit apoptosis. In fact, BHRF1, an antiapoptotic protein encoded by Epstein-Barr virus, may inhibit apoptosis primarily through an interaction with Bim (17). Although these viral proteins interact with the network of cellular Bcl-2 proteins in slightly different ways, in each case the outcome is the same: the inhibition of apoptosis.
By interacting with Bak and Bax, DPV022 was able to prevent the activation of these critical proapoptotic proteins and circumvent cell death induced by virus infection. However, when virus-infected cells were stimulated with staurosporine, DPV022 was unable to completely inhibit the activation of Bak (Fig. 9). This result differs from those obtained previously for other poxviral inhibitors of apoptosis, namely, F1L and FPV039, which completely inhibited Bak activation induced by virus infection and staurosporine treatment (5, 63). Nonetheless, DPV022 was sufficient to prevent the activation of Bak and Bax when all cellular antiapoptotic Bcl-2 proteins were inactivated (Fig. 7). These data support the idea that DPV022 interacts with Bak and Bax to hold them in an inactive conformation, but they do not rule out the possibility that DPV022 interacts with a subset of BH3-only proteins to further preempt the activation of Bak and Bax. It is possible that the apoptotic stimuli provided by virus infection concomitant with staurosporine treatment overwhelmed the ability of DPV022 to inhibit apoptosis or perhaps induced apoptosis via an additional pathway not inhibited by DPV022. Moreover, DPV022, which presumably evolved to interfere with apoptosis in its natural host, mule deer, may be less efficient at interacting with the human Bak or human BH3-only proteins that were used in our experiments. While DPV022 seems to be an effective inhibitor of apoptosis, its ability to interact with BH3-only proteins and the affinity with which it does so remain to be assessed. It will be interesting to determine whether the intriguing differences in amino acid sequence between DPV022 and other poxviral inhibitors of apoptosis account for the mechanistic and functional differences observed in this study.
DPV022 represents yet another novel protein that poxviruses have evolved to inhibit cell death. Although DPV022 shares little homology with cellular Bcl-2 proteins, DPV022 effectively inhibits cell death. DPV022 possesses discrete regions of identity with both F1L and M11L, suggesting that DPV022 is evolutionarily related to F1L and M11L. It is clear from this study and others that poxviruses, and, to a larger extent, all viruses, have evolved multiple, yet subtly different, ways of achieving the same goal: keeping the infected cell alive. We anticipate that an understanding of precisely how these viral proteins differentially inhibit cell death will continue to provide important insights into the regulation of the apoptotic cascade.
Acknowledgments
This research was supported by grants and fellowships from the Canadian Institutes of Health Research, the Howard Hughes Medical Institute, and Alberta Innovates—Health Solutions to M.B.; the Australian National Health and Medical Research Council (program grant 461221, project grant 637336, fellowships to M.K. and D.C.H., and IRIISS grant 361646); the Australian Research Council (fellowship to T.O.); the Leukemia and Lymphoma Society (SCOR grant 7413-07); the Australian Cancer Research Foundation; and the Victorian State Government (OIS grant). L.B. was supported by a Canada graduate doctoral scholarship from the National Sciences and Engineering Council of Canada (NSERC-CGSD) and an Alberta Innovates—Health Solutions studentship. M.B. is a tier I Canada research chair, a senior scholar of Alberta Innovates—Health Solutions, and a Howard Hughes Medical Institute scholar in infection and parasitology.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1.Afonso, C. L., et al. 2005. Genome of deerpox virus. J. Virol. 79:966-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annis, M. G., et al. 2005. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 24:2096-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonsson, B., S. Montessuit, S. Lauper, R. Eskes, and J. C. Martinou. 2000. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 345(Pt. 2):271-278. [PMC free article] [PubMed] [Google Scholar]
- 4.Antonsson, B., S. Montessuit, B. Sanchez, and J. C. Martinou. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276:11615-11623. [DOI] [PubMed] [Google Scholar]
- 5.Banadyga, L., J. Gerig, T. Stewart, and M. Barry. 2007. Fowlpox virus encodes a Bcl-2 homologue that protects cells from apoptotic death through interaction with the proapoptotic protein Bak. J. Virol. 81:11032-11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banadyga, L., K. Veugelers, S. Campbell, and M. Barry. 2009. The fowlpox virus BCL-2 homologue, FPV039, interacts with activated Bax and a discrete subset of BH3-only proteins to inhibit apoptosis. J. Virol. 83:7085-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry, M., et al. 2000. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol. Cell. Biol. 20:3781-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borgese, N., S. Colombo, and E. Pedrazzini. 2003. The tale of tail-anchored proteins: coming from the cytosol and looking for a membrane. J. Cell Biol. 161:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron, C., et al. 1999. The complete DNA sequence of myxoma virus. Virology 264:298-318. [DOI] [PubMed] [Google Scholar]
- 10.Campbell, S., B. Hazes, M. Kvansakul, P. Colman, and M. Barry. 2010. Vaccinia virus F1L interacts with Bak using highly divergent Bcl-2 homology domains and replaces the function of Mcl-1. J. Biol. Chem. 285:4695-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, L., et al. 2005. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 17:393-403. [DOI] [PubMed] [Google Scholar]
- 12.Cheng, E. H., et al. 1997. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. U. S. A. 94:690-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chipuk, J. E., and D. R. Green. 2008. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 18:157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chittenden, T., et al. 1995. Induction of apoptosis by the Bcl-2 homologue Bak. Nature 374:733-736. [DOI] [PubMed] [Google Scholar]
- 15.Cooray, S., et al. 2007. Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein. J. Gen. Virol. 88:1656-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuconati, A., and E. White. 2002. Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev. 16:2465-2478. [DOI] [PubMed] [Google Scholar]
- 17.Desbien, A. L., J. W. Kappler, and P. Marrack. 2009. The Epstein-Barr virus Bcl-2 homolog, BHRF1, blocks apoptosis by binding to a limited amount of Bim. Proc. Natl. Acad. Sci. U. S. A. 106:5663-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas, A. E., K. D. Corbett, J. M. Berger, G. McFadden, and T. M. Handel. 2007. Structure of M11L: a myxoma virus structural homolog of the apoptosis inhibitor, Bcl-2. Protein Sci. 16:695-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earl, P. L., B. Moss, L. S. Wyatt, and M. W. Carroll. 1998. Generation of recombinant vaccinia viruses, p. 16.17.1-16.17. 19. In F. M. Ausubel et al. (ed.), Current protocols in molecular biology. John Wiley & Sons Inc., New York, NY.
- 20.Ehrenberg, B., V. Montana, M. D. Wei, J. P. Wuskell, and L. M. Loew. 1988. Membrane potential can be determined in individual cells from the Nernstian distribution of cationic dyes. Biophys. J. 53:785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrow, S. N., et al. 1995. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature 374:731-733. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan, A. M., and A. Letai. 2008. BH3 domains define selective inhibitory interactions with BHRF-1 and KSHV BCL-2. Cell Death Differ. 15:580-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher, J. I., et al. 2008. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc. Natl. Acad. Sci. U. S. A. 105:18081-18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Galluzzi, L., C. Brenner, E. Morselli, Z. Touat, and G. Kroemer. 2008. Viral control of mitochondrial apoptosis. PLoS Pathog. 4:e1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goebel, S. J., et al. 1990. The complete DNA sequence of vaccinia virus. Virology 179:247-266, 517-563. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez, J. M., and M. Esteban. 2010. A poxvirus Bcl-2-like gene family involved in regulation of host immune response: sequence similarity and evolutionary history. Virol. J. 7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham, S. C., et al. 2008. Vaccinia virus proteins A52 and B14 share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis. PLoS Pathog. 4:e1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenhalf, W., C. Stephan, and B. Chaudhuri. 1996. Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in Saccharomyces cerevisiae. FEBS Lett. 380:169-175. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths, G. J., et al. 2001. Cellular damage signals promote sequential changes at the N-terminus and BH-1 domain of the pro-apoptotic protein Bak. Oncogene 20:7668-7676. [DOI] [PubMed] [Google Scholar]
- 31.Griffiths, G. J., et al. 1999. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 144:903-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanada, M., C. Aime-Sempe, T. Sato, and J. C. Reed. 1995. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem. 270:11962-11969. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins, C. J., S. L. Wang, and B. A. Hay. 1999. A cloning method to identify caspases and their regulators in yeast: identification of Drosophila IAP1 as an inhibitor of the Drosophila caspase DCP-1. Proc. Natl. Acad. Sci. U. S. A. 96:2885-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu, Y. T., and R. J. Youle. 1998. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 273:10777-10783. [DOI] [PubMed] [Google Scholar]
- 35.Hsu, Y. T., and R. J. Youle. 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272:13829-13834. [DOI] [PubMed] [Google Scholar]
- 36.Ink, B., et al. 1997. Human Bak induces cell death in Schizosaccharomyces pombe with morphological changes similar to those with apoptosis in mammalian cells. Mol. Cell. Biol. 17:2468-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jabbour, A. M., et al. 2006. Human Bcl-2 cannot directly inhibit the Caenorhabditis elegans Apaf-1 homologue CED-4, but can interact with EGL-1. J. Cell Sci. 119:2572-2582. [DOI] [PubMed] [Google Scholar]
- 38.Kitanaka, C., et al. 1997. Caspase-dependent apoptosis of COS-7 cells induced by Bax overexpression: differential effects of Bcl-2 and Bcl-xL on Bax-induced caspase activation and apoptosis. Oncogene 15:1763-1772. [DOI] [PubMed] [Google Scholar]
- 39.Koo, G. C., and J. R. Peppard. 1984. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma 3:301-303. [DOI] [PubMed] [Google Scholar]
- 40.Reference deleted.
- 41.Kvansakul, M., et al. 2007. A structural viral mimic of prosurvival bcl-2: a pivotal role for sequestering proapoptotic bax and bak. Mol. Cell 25:933-942. [DOI] [PubMed] [Google Scholar]
- 42.Kvansakul, M., et al. 2008. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 15:1564-1571. [DOI] [PubMed] [Google Scholar]
- 43.Lee, E. F., et al. 2008. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J. Cell Biol. 180:341-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindsten, T., et al. 2000. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell 6:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reference deleted.
- 46.Metivier, D., et al. 1998. Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunol. Lett. 61:157-163. [DOI] [PubMed] [Google Scholar]
- 47.Mikhailov, V., et al. 2003. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J. Biol. Chem. 278:5367-5376. [DOI] [PubMed] [Google Scholar]
- 48.Oltersdorf, T., et al. 2005. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435:677-681. [DOI] [PubMed] [Google Scholar]
- 49.Ow, Y. P., D. R. Green, Z. Hao, and T. W. Mak. 2008. Cytochrome c: functions beyond respiration. Nat. Rev. Mol. Cell Biol. 9:532-542. [DOI] [PubMed] [Google Scholar]
- 50.Petros, A. M., E. T. Olejniczak, and S. W. Fesik. 2004. Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 1644:83-94. [DOI] [PubMed] [Google Scholar]
- 51.Sato, T., et al. 1994. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc. Natl. Acad. Sci. U. S. A. 91:9238-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schinzel, A., T. Kaufmann, and C. Borner. 2004. Bcl-2 family members: integrators of survival and death signals in physiology and pathology. Biochim. Biophys. Acta 1644:95-105. [DOI] [PubMed] [Google Scholar]
- 53.Stuart, D., K. Graham, M. Schreiber, C. Macaulay, and G. McFadden. 1991. The target DNA sequence for resolution of poxvirus replicative intermediates is an active late promoter. J. Virol. 65:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su, J., et al. 2006. Myxoma virus M11L blocks apoptosis through inhibition of conformational activation of Bax at the mitochondria. J. Virol. 80:1140-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sundararajan, R., and E. White. 2001. E1B 19K blocks Bax oligomerization and tumor necrosis factor alpha-mediated apoptosis. J. Virol. 75:7506-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamaoki, T., et al. 1986. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem. Biophys. Res. Commun. 135:397-402. [DOI] [PubMed] [Google Scholar]
- 57.Tao, W., C. Kurschner, and J. I. Morgan. 1997. Modulation of cell death in yeast by the Bcl-2 family of proteins. J. Biol. Chem. 272:15547-15552. [DOI] [PubMed] [Google Scholar]
- 58.Taylor, J. M., and M. Barry. 2006. Near death experiences: poxvirus regulation of apoptotic death. Virology 344:139-150. [DOI] [PubMed] [Google Scholar]
- 59.Taylor, J. M., D. Quilty, L. Banadyga, and M. Barry. 2006. The vaccinia virus protein F1L interacts with Bim and inhibits activation of the pro-apoptotic protein Bax. J. Biol. Chem. 281:39728-39739. [DOI] [PubMed] [Google Scholar]
- 60.van Delft, M. F., et al. 2006. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10:389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, G., et al. 2004. Myxoma virus M11L prevents apoptosis through constitutive interaction with Bak. J. Virol. 78:7097-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, G. Q., et al. 2001. Resistance to granzyme B-mediated cytochrome c release in Bak-deficient cells. J. Exp. Med. 194:1325-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wasilenko, S. T., L. Banadyga, D. Bond, and M. Barry. 2005. The vaccinia virus F1L protein interacts with the proapoptotic protein Bak and inhibits Bak activation. J. Virol. 79:14031-14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wasilenko, S. T., A. F. Meyers, K. Vander Helm, and M. Barry. 2001. Vaccinia virus infection disarms the mitochondrion-mediated pathway of the apoptotic cascade by modulating the permeability transition pore. J. Virol. 75:11437-11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wasilenko, S. T., T. L. Stewart, A. F. Meyers, and M. Barry. 2003. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl. Acad. Sci. U. S. A. 100:14345-14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei, M. C., et al. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14:2060-2071. [PMC free article] [PubMed] [Google Scholar]
- 67.Wei, M. C., et al. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westphal, D., et al. 2009. The orf virus inhibitor of apoptosis functions in a Bcl-2-like manner, binding and neutralizing a set of BH3-only proteins and active Bax. Apoptosis 14:1317-1330. [DOI] [PubMed] [Google Scholar]
- 69.Willis, S. N., and J. M. Adams. 2005. Life in the balance: how BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 17:617-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willis, S. N., et al. 2005. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19:1294-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilton, B. A., et al. 2008. Ectromelia virus BTB/kelch proteins, EVM150 and EVM167, interact with cullin-3-based ubiquitin ligases. Virology 374:82-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiang, J., D. T. Chao, and S. J. Korsmeyer. 1996. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc. Natl. Acad. Sci. U. S. A. 93:14559-14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin, X. M., Z. N. Oltvai, and S. J. Korsmeyer. 1994. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369:321-323. [DOI] [PubMed] [Google Scholar]
- 74.Youle, R. J., and A. Strasser. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9:47-59. [DOI] [PubMed] [Google Scholar]
- 75.Zha, H., C. Aime-Sempe, T. Sato, and J. C. Reed. 1996. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 271:7440-7444. [DOI] [PubMed] [Google Scholar]
- 76.Zha, H., et al. 1996. Structure-function comparisons of the proapoptotic protein Bax in yeast and mammalian cells. Mol. Cell. Biol. 16:6494-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zong, W. X., T. Lindsten, A. J. Ross, G. R. MacGregor, and C. B. Thompson. 2001. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]