Abstract
We recently developed a novel multiplex reverse transcription (RT)-PCR assay that allows rapid and sensitive detection of transcripts corresponding to all 68 unique varicella-zoster virus (VZV) open reading frames (ORFs) in only five amplification reactions (M. A. Nagel, D. Gilden, T. Shade, B. Gao, and R. J. Cohrs, J. Virol. Methods 157:62-68, 2009). Herein, we applied multiplex RT-PCR analysis to mRNA extracted from 26 trigeminal ganglia latently infected with VZV and one control trigeminal ganglion negative for VZV DNA that were removed from 14 men and women, 16 to 84 years of age, within 24 h after death. Analysis identified VZV transcripts mapping to VZV ORFs 29, 62, and 63, previously detected and sequence verified; VZV ORFs 4 and 40, previously detected by in situ hybridization; and VZV ORFs 11, 41, 43, 57, and 68, not previously detected. VZV ORF 63 transcripts were the most prevalent. Comparison of the 10 VZV ORFs transcribed during latency to their herpes simplex virus type 1 homologues reveals that the latently transcribed VZV genes encode immediate-early, early, and late transcripts.
Varicella-zoster virus (VZV) is an exclusively human, neurotropic alphaherpesvirus. Primary infection typically produces chickenpox (varicella), after which virus becomes latent in cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis (16). Decades later, virus reactivation results in shingles (zoster), frequently complicated by chronic pain (postherpetic neuralgia) as well as stroke (VZV vasculopathy), paralysis and incontinence (VZV myelopathy), and blindness (VZV progressive outer retinal necrosis).
Knowledge of the full extent of VZV gene transcription and translation during latency is a prerequisite to hypotheses concerning how virus establishes latency and reactivates. The exact extent of VZV transcription in latently infected ganglia is unknown. Sequence analysis of the 3′ terminus and its polyadenylated tracts in cDNA synthesized from RNA extracted from latently infected human ganglia identified transcripts mapping to VZV genes 21, 29, 62, 63, and 66 (8, 11), of which VZV gene 63 transcripts were the most prevalent and abundant (12). In situ hybridization (ISH) also detected expression of VZV genes 4, 18, 21, 29, 40, 62, and 63 (13, 16, 17). Using multiplex reverse transcription (RT)-PCR and the GenomeLab genetic analysis system (GeXPS), we detected as few as 20 copies of all 68 predicted VZV open reading frames (ORFs) in VZV-infected cells in tissue culture (33). Herein, we applied the same strategy and technology to analyze the extent of VZV transcription in latently infected human ganglia.
MATERIALS AND METHODS
Virus and cells.
VZV (Ellen strain) was isolated from a zoster lesion and propagated by cocultivation of uninfected and VZV-infected human malignant melanoma (MeWo) cells (17) as described previously (10).
Human tissue.
The right and left trigeminal ganglia were removed less than 24 h after death, cleaned, flash-frozen in liquid nitrogen, and powdered individually in liquid nitrogen before nucleic acid extraction. At the time of death, subjects did not exhibit cutaneous signs of herpesvirus infection. An extensive review of hospital records revealed that none of the 14 subjects had received any immunomodulatory drugs or corticosteroids the month before they died (Table 1). Treatment of metastatic cancer patients with chemotherapy and interstitial lung disease patients with steroids is usually started at the time of diagnosis. In our subjects, treatment was discontinued when disease became end stage (subjects 6, 8, and 12); the same applied to our subjects with terminal sepsis (subjects 5 and 9).
TABLE 1.
Clinical features of humans from whom trigeminal ganglia were removed
| Subject identifier | Age | Gendera | Cause of death | No. of h from death to autopsy | Treated with immunomodulatory drugs or steroids 1 mo before death |
|---|---|---|---|---|---|
| 1 | 72 | F | Pulmonary hypertension | 15 | No |
| 2 | 75 | F | Myocardial infarction | 22 | No |
| 3 | 53 | F | Respiratory failure | 14 | No |
| 4 | 52 | M | Myocardial infarction | 18 | No |
| 5 | 58 | F | Sepsis | <24 | No |
| 6 | 30 | M | Interstitial lung disease | 12 | No |
| 7 | 84 | F | Alzheimer's disease | 18 | No |
| 8 | 37 | M | Metastatic lung cancer | 18 | No |
| 9 | 83 | M | Sepsis | 11 | No |
| 10 | 16 | F | Hanging suicide | 23 | No |
| 11 | 84 | F | Intracerebral hemorrhage | 23 | No |
| 12 | 55 | M | Metastatic lung cancer | 9 | No |
| 13 | 52 | F | Pulmonary embolism | 14 | No |
| 14 | 46 | F | Hanging suicide | 17 | No |
F, female; M, male.
DNA extraction.
DNA was extracted from one-fifth of each powdered human trigeminal ganglion using the Qiagen DNeasy blood and tissue kit (Valencia, CA) and quantitated by optical adsorbance (Nanodrop Technologies, Wilmington, DE).
RNA extraction and cDNA synthesis.
VZV-infected MeWo cells (2 × 107) were harvested at the peak of a virus-induced cytopathic effect 3 days postinfection. Uninfected MeWo cells (2 × 107) were processed in parallel. Infected and uninfected cells in culture were released with trypsin, collected by low-speed centrifugation (1,000 × g for 5 min at 4°C), washed with phosphate-buffered saline (20 mM phosphate [pH 7.4], 150 mM NaCl), and resuspended in 1 ml cell lysis buffer (1% SDS in high-salt buffer; μMACS mRNA isolation kit; Miltenyi Biotec, Gladbach, Germany). Poly(A+) RNA was hybridized to an oligo(dT) polynucleotide conjugated to paramagnetic particles (μMACS mRNA isolation kit; Miltenyi Biotec, Gladbach, Germany) and collected by column chromatography in a high-density magnetic field. Residual DNA on the column was digested with RNase-free DNase (amplification-grade DNase I; Invitrogen, Carlsbad, CA). mRNA was eluted with 1.5 ml nuclease-free water and quantitated by optical adsorbance (Nanodrop Technologies, Wilmington, DE).
The remaining four-fifths of powdered human trigeminal ganglia was thawed in TRI Reagent (Molecular Research Center, Cincinnati, OH) and disrupted by Polytron sonication (Brinkmann, Westbury, NY), and total RNA was extracted as described previously (6). Aliquots (1.5 μl) of total RNA were used for quantitation (Nanodrop Technologies, Thermo Scientific, Wilmington, DE), and 200 to 300 ng was analyzed for RNA integrity by microfiltration on BioAnalyzer chips (Agilent Technologies, Quantum Analytics, Inc., Foster City, CA). Poly(A+) RNA was isolated, DNase treated using the μMACS mRNA isolation kit, and quantitated as described above.
Poly(A+) RNA (100 ng) from uninfected MeWo cells, VZV-infected MeWo cells, and trigeminal ganglia was used for first-strand cDNA in 20-μl reaction mixtures containing 50 mM Tris-HCl (pH 8.5), 30 mM KCl, 8 mM MgCl2, 20 U of RNase inhibitor, 0.5 mM each dNTP, and 10 μM oligo(dT18) (Transcriptor first-strand cDNA synthesis kit, Roche Diagnostics, Mannheim, Germany). Reaction components in 96-well microtiter plates were sealed, mixed, and centrifuged at 1,000 × g for 5 min, followed by incubation at 25°C for 10 min, 50°C for 60 min, 85°C for 5 min, and 4°C for 20 min. Control cDNA reaction mixtures lacking reverse transcriptase were processed in parallel.
qPCR and data analysis.
Herein, we adhered to recently proposed guidelines concerning the minimum information for publication of quantitative real-time PCR experiments (MIQE) (2). Quantitative PCR (qPCR) using hydrolysis (TaqMan) probes was performed in 20-μl reaction mixtures containing Absolute fast qPCR ROX master mix (Thermo Fisher Scientific, Leicestershire, England) as described previously (8). Amplification conditions consisted of denaturation at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Fluorescence from probe degradation was recorded during the 60°C extension cycle and analyzed using the SDS program (Sequence Detection Software; Applied Biosystems). The SDS-determined cycle threshold (CT) is defined as the cycle number at which the fluorescence value intersects the threshold value, which is set 10-fold above background fluorescence and is within the logarithmic phase of PCR amplification. VZV DNA standards were included in each qPCR and yielded a consistent inverse linear relationship between the CT value and the amount of input template DNA. Data were analyzed graphically using SigmaPlot (Systat, Point Richmond, CA).
To analyze human trigeminal ganglia for virus DNA, 100 ng of each trigeminal ganglionic DNA sample was PCR amplified in duplicate using primers and probes (Integrated DNA Technologies, Coralville, IA) for glyceraldehyde-3-phosphate dehydrogenase (GAPdH) as described previously (8): VZV (VZV forward, 5′-CGA ACA CGT TCC CCA TCA A-3′; VZV reverse, 5′-CCC GGC TTT GTT AGT TTT GG-3′; VZV probe, 5′-FAM-TCC AGG TTT TAG TTG ATA CCA-/BkFQ/-3′ [where FAM is 6-carboxyfluorescein]) and herpes simplex virus type 1 (HSV-1) (HSV-1 forward, 5′-TGG TAT TGC CCA ACA CTT TCC-3′; HSV-1 reverse, 5′-GCG CCA GGC ACA CAC AT-3′; HSV probe, 5′-FAM-CGT GTC GCG TGT GGT-BHQ-3′).
To analyze human trigeminal ganglia for cellular transcripts, 6% of the cDNA reaction was amplified in duplicate using primers and probes for GAPdH and neurofilament heavy subunit (NFH-200) (12). Residual cellular DNA present in mRNA samples was quantified by PCR analysis of the control cDNA sample (first-strand cDNA reaction without reverse transcriptase).
Multiplex reverse transcription-linked PCR.
For each human trigeminal ganglionic mRNA sample, five multiplex RT-PCRs using multiplex primer sets A, B, C, D, and E were performed as described previously (33). Each 20-μl cDNA synthesis reaction mix contained 100 ng trigeminal ganglia mRNA or 2 ng uninfected or VZV-infected MeWo mRNA, 0.1 μM reverse oligonucleotide primer set mix, 20 U reverse transcriptase, 0.1 U/ml RNase inhibitor, 250 fM kanamycin-resistant (Kanr) RNA in RT master mix buffer (10 mM HCl, 50 mM KCl, 2.5 mM MgCl2, 10 mM dithiothreitol [DTT], 1 mM each deoxynucleoside triphosphate [dNTP], and 0.5 μM Kanr reverse primer). Reverse transcriptase, RNase inhibitor, Kanr RNA, and RT master mix buffer were supplied in kit form (GenomeLab GeXP start kit; Beckman Coulter, Fullerton, CA). RT reaction mixtures were incubated at 48°C for 1 min, 37°C for 5 min, 42°C for 60 min, and 95°C for 5 min.
Each subsequent 20-μl multiplex PCR mixture contained 9.3 μl of RT reaction mix, 0.02 μM the corresponding forward primer PCR oligonucleotide set mix, 5 mM MgCl2, and 3.5 U Thermo-Start Taq DNA polymerase (Thermo Fisher Scientific, Leicestershire, England) in PCR master mix buffer containing 10 mM HCl, 50 mM KCl, 0.3 mM each dNTP, 0.025 μM Kanr forward primer, 1 μM universal reverse primer, and 1 μM D4-labeled universal forward primer (GenomeLab GeXP start kit; Beckman Coulter). Amplification conditions included initial denaturation at 95°C for 15 min, followed by 35 cycles of 94°C for 30 s and 55°C for 30 s, ending in a single extension cycle of 70°C for 1 min.
Ten percent of each PCR product from multiplex primer sets A to E was added to a 37.75-μl sample loading solution and 0.25 μl DNA size standard-400 (GenomeLab GeXP start kit; Beckman Coulter, Coralville, CA). The GeXPS system was used to separate PCR products based on size by capillary electrophoresis and to measure dye signal strength in arbitrary units (AU) of optical fluorescence, defined as fluorescent signal minus background.
SYBR green qPCR and data analysis.
Each of the 68 VZV gene-specific primer pairs comprising the multiplex RT-PCR analyses (33) was used to amplify increasing amounts of VZV DNA (1, 5, 10, 100, 500, 1,000, and 5,000 copies), using SYBR green to detect the amplified DNA. The 25-μl PCR mixture contained 0.8 μM each forward and reverse primer and VZV DNA in 1× Maxima SYBR green/ROX qPCR master mix (solution of Taq DNA polymerase, qPCR buffer, SYBR green, ROX passive reference dye, and dUTP; Fermentas Inc., Glen Burnie, MD). Amplification conditions were initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min, and ending in a thermal dissociation stage (repeated cycles of denaturation at 95°C for 15 s and a stepwise increase from 60°C to 95°C in the temperature of the 1-min optical density recording). The fluorescence signal was analyzed using the SDS program as described above.
DNA sequence confirmation of VZV transcripts.
Table 2 describes the primer sequences for RT-PCR amplification and DNA sequence determination. mRNA extracted from the left trigeminal ganglion of subject 14 was used to verify VZV ORF 63 transcripts. mRNA extracted from the right trigeminal ganglion of subjects 5 and 14 and from both trigeminal ganglia of subjects 8 and 13 was used to verify VZV ORF 11 transcripts. mRNA extracted from the left trigeminal ganglion of subject 12 was used to verify VZV ORF 41 transcripts. mRNA extracted from both trigeminal ganglia of subject 6 was used to verify VZV ORF 43 transcripts. mRNA extracted from the right trigeminal ganglion of subject 1 was used to verify VZV ORF 57 transcripts.
TABLE 2.
Primers for sequence verificationa
| Primer name | ORF | Binding regionb | Sequence (5′ to 3′) | Use |
|---|---|---|---|---|
| Lut7Hind3 | NA | NA | GTAAAACGACGGCCAGTTAATACGACTCACTATAGGGAAGCTTTTTTTTTTTTTTTTTT | Nest RT |
| P1 | NA | NA | GTAAAACGACGGCCAGTT | Nest PCR 1 |
| P2 | NA | NA | TAATACGACTCACTATAGGG | Nest PCR 2, 3 |
| 63F1 | 63 | 314-333 | GTGCTGGGAGGAATTGTTAC | Nest PCR 1 |
| 63F2 | 63 | 412-431 | GGTTCATTGAGGCGCCGAAT | Nest PCR 2, 3 |
| 63F3 | 63 | 512-531 | GCCATCGGATGTAATTGAAT | Sequencing |
| 11F1 | 11 | 1928-1947 | ATATGAGGCTGTTCGCACAG | Nest PCR 1 |
| 11F2 | 11 | 2036-2055 | ATTGTTACAACGGGTGTTGG | Nest PCR 2, 3 |
| 11F3 | 11 | 2126-2146 | CCGAGGTATTTTAGATGCATA | Sequencing |
| 68F1 | 68 | 1346-1365 | GCCTAGCTTTGGTCTAATCT | Nest PCR 1 |
| 68F2 | 68 | 1441-1460 | ATTTTAACGGGCATGTTGAA | Nest PCR 2, 3 |
| 68F3 | 68 | 1551-1570 | GCGACTACTAAACCCAAGGA | Sequencing |
| 41 forward | 41 | 421-440 | AGGTGACACTATAGAATACCCAGACCTTAATGCGAGAA | Multiplex PCR, sequencing |
| 41 reverse | 41 | 595-616 | GTACGACTCACTATAGGGAAAACCAATGTGCGAATAGCC | Multiplex RT-PCR |
| 43 forward | 43 | 228-247 | AGGTGACACTATAGAATACCCGATAACGCCTTAACAAA | Multiplex PCR, sequencing |
| 43 reverse | 43 | 344-363 | GTACGACTCACTATAGGGAATCAAAGAATAAACCGGGGC | Multiplex RT-PCR |
| 57 forward | 57 | 10-31 | AGGTGACACTATAGAATACGAGAACGTAATGTGTTTGGAA | Multiplex PCR, sequencing |
| 57 reverse | 57 | 194-212 | GTACGACTCACTATAGGGAACGTTGATGAGCCTTGCAG | Multiplex RT-PCR |
| Universal forward | NA | NA | AGGTGACACTATAGAATA | Multiplex PCR |
| Universal reverse | NA | NA | GTACGACTCACTATAGGGA | Multiplex PCR |
NA, not applicable.
With respect to the first nucleotide within the initiation codon.
VZV transcripts detected in trigeminal ganglia by multiplex RT-PCR amplification were confirmed by DNA sequence analysis as described previously (11) with modifications. First-strand cDNA was synthesized from ∼200 ng mRNA using LuT7Hind3 primer in 20 μl RT mix (Transcriptor first-strand cDNA synthesis kit; Roche Applied Science). Parallel (control) reactions were done in the absence of reverse transcriptase. Five microliters of cDNA was used for PCR 1 in separate 50-μl reaction mixtures containing 0.4 μM either 63F1, 11F1, or 68F1 primer along with 0.4 μM P1 primer in 1× PCR buffer (Thermo Scientific Thermo-Start Taq DNA polymerase [Surrey, United Kingdom]). All PCR assays were supplemented with dimethyl sulfoxide (3% final concentration). After PCR (95°C for 15 min; 40 cycles of 95°C for 1 min, 45°C for 1 min, and 70°C for 1 min), DNA was extracted (MinElute PCR purification kit; Qiagen, Valencia, CA) and concentrated to 10 μl. Half of the PCR 1 product was amplified (PCR 2) in separate reaction mixtures containing 0.4 μM either 63F2, 11F2, or 68F2 primer along with 0.4 μM P2 primer in the same conditions as that used for PCR 1. PCR 2 DNA product was extracted and concentrated to 10 μl as above, and 5 μl was amplified in PCR 3 using the same conditions and primers as those used for PCR 2. The final PCR 3 DNA products were isolated from 2% agarose gels (MinElute gel extraction kit; Qiagen). DNA sequence analysis was obtained by an automated dideoxy-chain termination (35) using 1 pmol of the appropriate 63F3, 11F3, or 68F3 primer.
RESULTS
Identification of human trigeminal ganglia latently infected with VZV and HSV.
Quantitative PCR showed that all 28 ganglia contained amplifiable GAPdH DNA (Table 3), with CT values ranging from 20.5 to 23.0 (mean ± standard deviation [SD], 21.9 ± 0.7). VZV DNA was detected in 27 of 28 (96%) trigeminal ganglia, with a range of 9 to 3,100 copies of VZV DNA per 100 ng total DNA (mean ± SD, 592 ± 766 copies). HSV-1 DNA was detected in 14 of 28 (50%) trigeminal ganglia, with a range of 3 to 7,590 copies of HSV-1 DNA per 100 ng total DNA (mean ± SD, 1,981 ± 2,476 copies).
TABLE 3.
Prevalence of VZV and HSV-1 DNA in human trigeminal ganglia
| Subject identifier | Trigeminal gangliaa | GAPdH (CT) | No. of virus DNA copies/100 ng ganglionic DNA) |
|
|---|---|---|---|---|
| VZV | HSV-1 | |||
| 1 | R | 20.7 | 22 | 0 |
| L | 21.0 | 16 | 0 | |
| 2 | R | 20.8 | 9 | 0 |
| L | 21.6 | 0 | 0 | |
| 3 | R | 20.9 | 9 | 214 |
| L | 20.5 | 14 | 747 | |
| 4 | R | 21.1 | 1,063 | 3 |
| L | 21.7 | 1,302 | 11 | |
| 5 | R | 21.4 | 266 | 1,044 |
| L | 21.7 | 45 | 702 | |
| 6 | R | 22.6 | 319 | 956 |
| L | 22.3 | 291 | 1,560 | |
| 7 | R | 21.5 | 1,058 | 940 |
| L | 22.4 | 742 | 640 | |
| 8 | R | 22.2 | 348 | 0 |
| L | 22.0 | 485 | 0 | |
| 9 | R | 22.6 | 719 | 0 |
| L | 22.7 | 16 | 0 | |
| 10 | R | 22.2 | 208 | 7,073 |
| L | 22.6 | 96 | 7,590 | |
| 11 | R | 21.9 | 136 | 2,580 |
| L | 21.8 | 60 | 3,673 | |
| 12 | R | 22.6 | 201 | 0 |
| L | 22.0 | 213 | 0 | |
| 13 | R | 23.0 | 3,100 | 0 |
| L | 22.5 | 1,690 | 0 | |
| 14 | R | 23.0 | 1,495 | 0 |
| L | 22.0 | 2,055 | 0 | |
| Frequency (%) | 100 | 96 | 50 | |
R, right; L, left.
Quantitative RT-PCR analysis of human trigeminal ganglionic RNA for GAPdH and NFH-200 transcripts.
Figure 1 A shows CT values obtained by qPCR for GAPdH sequences in each trigeminal ganglion mRNA sample before and after reverse transcription. For trigeminal ganglia in which no amplifiable GAPdH DNA was detected in the poly(A+) RNA fraction, the CT value was set at 40 (the maximum qPCR cycle number). The mean ± SD CT for reverse-transcribed trigeminal ganglionic mRNA was 18.0 ± 2.2 compared to 38.1 ± 0.6 in the absence of reverse transcription. The difference between the mean CT values indicates the presence of 1.1 × 106-fold more GAPdH mRNA than residual chromosomal GAPdH DNA. Similarly, the NFH-200-dependent CT values obtained from each trigeminal ganglionic cDNA sample were 19.0 ± 3.6 in the absence and 36.6 ± 3.7 in the presence of reverse transcriptase. Overall, 2.0 × 105-fold more NFH-200 mRNA than residual chromosomal NFH-200 DNA was detected (Fig. 1B).
FIG. 1.
Quantitative PCR analysis of human trigeminal ganglia. Poly(A+) RNA was untreated (•) or reverse transcribed (▴) and analyzed using primers and probes specific for GAPdH (A) or neurofilament heavy 200-kDa protein (B). When no target sequences were detected, the cycle threshold (CT) was set at 40 (○). Data are mean (±SD) CT values.
Efficiency of multiplex RT-PCR.
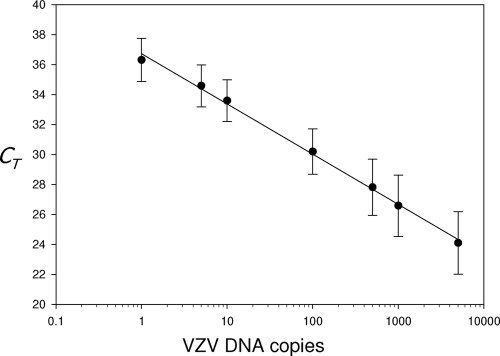
To determine the sensitivity of the multiplex RT-PCR primer pairs and to confirm that each primer pair amplified a single VZV-specific product, replicate copies of VZV DNA (1 to 5,000 genome equivalents in 100 ng carrier DNA) were amplified with each of the 68 VZV ORF multiplex primer pairs in separate reactions. Linear regression was applied to each amplification reaction and for each primer pair. Table 4 lists the linear range of DNA copies amplified, the regression line slope, the intercept, the R2 value, and the PCR efficiency, along with the PCR product dissociation temperature and temperature range. The lower limit of VZV DNA detected for each PCR primer pair was 1 to 500 copies, and all primer pairs amplified VZV DNA with a linear response to at least 5,000 VZV DNA copies. The slope of the linear regression lines obtained for each amplification reaction indicated that all primer pairs amplified target VZV DNA with efficiencies of 1.0 ± 0.1. Melting point analysis of VZV DNA amplified with each of the 68 VZV-specific primer pairs produced a single discrete peak, indicating that the primer pairs amplified a single product. No PCR product was detected when VZV DNA was omitted from the amplification reaction. Importantly, all primer pairs amplified VZV DNA with similar linear regression slopes, as seen when >1,080 individual qPCR data points were combined (Fig. 2). The subsequent regression line gave an overall slope of −3.4 with an R2 value of 0.99, indicating an overall PCR efficiency of 97%.
TABLE 4.
Multiplex RT-PCR primer efficiencies
| ORFa | No. of VZV DNA copiesb |
Slopec | Interceptc | (R2)d | Efficiency of qPCR | Tme | Lower Tm limit | Higher Tm limit | |
|---|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||||
| 1 | 100 | 5,000 | −3.0 | 39.8 | 0.99 | 1.10 | 79.2 | 78.7 | 79.6 |
| 2 | 5 | 5,000 | −3.6 | 39.1 | 0.98 | 0.90 | 80.4 | 79.9 | 80.9 |
| 2 | 1 | 5,000 | −3.6 | 36.7 | 0.98 | 0.90 | 80.2 | 79.7 | 80.6 |
| 3 | 1 | 5,000 | −3.5 | 38.3 | 0.98 | 0.93 | 83.2 | 82.7 | 83.6 |
| 4 | 1 | 5,000 | −3.5 | 37.4 | 0.99 | 0.93 | 78.2 | 76.5 | 79.9 |
| 5 | 1 | 5,000 | −3.6 | 36.1 | 0.99 | 0.90 | 78.0 | 77.5 | 78.5 |
| 6 | 1 | 5,000 | −3.3 | 36.9 | 0.89 | 1.00 | 80.3 | 79.7 | 80.9 |
| 7 | 5 | 5,000 | −3.6 | 37.9 | 0.99 | 0.90 | 80.9 | 80.3 | 81.5 |
| 7 | 1 | 5,000 | −3.5 | 0.2 | 0.99 | 0.93 | 81.7 | 81.2 | 82.1 |
| 8 | 100 | 5,000 | −3.3 | 38.1 | 0.99 | 1.01 | 78.8 | 78.4 | 79.1 |
| 9 | 1 | 5,000 | −3.2 | 36.6 | 0.99 | 1.05 | 76.4 | 75.9 | 76.8 |
| 10 | 100 | 5,000 | −3.7 | 38.6 | 0.99 | 0.97 | 79.6 | 79.3 | 79.9 |
| 11 | 1 | 5,000 | −3.6 | 38.4 | 0.99 | 0.90 | 79.8 | 79.3 | 80.3 |
| 12 | 1 | 5,000 | −3.4 | 37.8 | 0.99 | 0.97 | 79.9 | 79.3 | 80.5 |
| 13 | 1 | 5,000 | −3.7 | 37.0 | 0.99 | 0.86 | 79.7 | 79.3 | 80.0 |
| 14 | 1 | 5,000 | −3.5 | 37.9 | 0.99 | 0.93 | 78.0 | 77.5 | 78.4 |
| 15 | 1 | 5,000 | −3.3 | 34.5 | 0.99 | 1.01 | 78.5 | 78.0 | 78.9 |
| 16 | 1 | 5,000 | −3.6 | 37.8 | 0.99 | 0.90 | 77.8 | 77.5 | 78.1 |
| 17 | 10 | 5,000 | −3.3 | 37.4 | 0.99 | 1.01 | 80.6 | 80.3 | 80.8 |
| 18 | 1 | 5,000 | −3.4 | 35.8 | 0.97 | 0.97 | 76.3 | 76.0 | 76.6 |
| 19 | 100 | 5,000 | −3.4 | 38.4 | 0.98 | 0.97 | 77.2 | 76.9 | 77.5 |
| 20 | 1 | 5,000 | −3.4 | 34.1 | 0.98 | 0.97 | 82.5 | 82.0 | 82.9 |
| 21 | 1 | 5,000 | −3.5 | 38.9 | 0.99 | 0.93 | 77.1 | 76.6 | 77.5 |
| 22 | 10 | 5,000 | −3.6 | 41.3 | 0.99 | 0.90 | 79.1 | 78.4 | 79.7 |
| 23 | 1 | 5,000 | −3.6 | 47.4 | 0.88 | 0.90 | 84.6 | 84.0 | 85.2 |
| 24 | 1 | 5,000 | −3.4 | 34.7 | 0.99 | 0.97 | 78.3 | 78.0 | 78.6 |
| 25 | 10 | 5,000 | −3.6 | 39.2 | 0.99 | 0.90 | 77.8 | 77.5 | 78.1 |
| 26 | 1 | 5,000 | −3.5 | 37.6 | 0.99 | 0.93 | 81.1 | 80.6 | 81.5 |
| 26 | 500 | 5,000 | −3.8 | 39.7 | 0.99 | 0.90 | 80.2 | 79.7 | 80.6 |
| 27 | 10 | 5,000 | −3.6 | 38.8 | 0.99 | 0.90 | 80.0 | 79.7 | 80.3 |
| 28 | 5 | 5,000 | −3.1 | 33.8 | 0.99 | 1.10 | 79.6 | 78.9 | 80.2 |
| 29 | 1 | 5,000 | −3.6 | 35.8 | 0.98 | 0.90 | 81.0 | 80.5 | 81.4 |
| 30 | 10 | 5,000 | −3.5 | 37.0 | 0.99 | 0.93 | 82.1 | 81.6 | 82.5 |
| 31 | 1 | 5,000 | −3.5 | 37.3 | 0.95 | 0.93 | 81.0 | 80.4 | 81.6 |
| 32 | 500 | 5,000 | −3.3 | 39.9 | 0.99 | 1.01 | 81.1 | 80.8 | 81.4 |
| 33 | 1 | 5,000 | −3.4 | 35.7 | 0.98 | 0.97 | 77.6 | 77.0 | 78.2 |
| 34 | 1 | 5,000 | −3.5 | 37.4 | 0.89 | 0.93 | 81.0 | 80.7 | 81.3 |
| 35 | 1 | 5,000 | −3.3 | 36.3 | 0.96 | 1.01 | 79.4 | 79.2 | 79.5 |
| 36 | 1 | 5,000 | −3.3 | 37.7 | 0.98 | 1.01 | 81.9 | 81.6 | 82.2 |
| 37 | 1 | 5,000 | −3.2 | 34.6 | 0.99 | 1.05 | 79.5 | 78.8 | 80.1 |
| 38 | 1 | 5,000 | −3.6 | 36.6 | 0.99 | 0.90 | 80.4 | 80.1 | 80.7 |
| 39 | 1 | 5,000 | −3.4 | 36.0 | 0.98 | 0.97 | 77.1 | 76.8 | 77.4 |
| 40 | 1 | 5,000 | −3.5 | 35.5 | 0.99 | 0.93 | 79.3 | 79.0 | 79.6 |
| 41 | 100 | 5,000 | −3.0 | 39.1 | 0.94 | 1.05 | 83.0 | 82.7 | 83.3 |
| 42 | 10 | 5,000 | −3.2 | 35.3 | 0.99 | 1.05 | 79.3 | 78.8 | 79.7 |
| 42 | 1 | 5,000 | −3.5 | 37.1 | 0.99 | 0.93 | 79.3 | 78.8 | 79.7 |
| 43 | 1 | 5,000 | −3.3 | 34.6 | 0.99 | 1.01 | 77.5 | 76.9 | 78.1 |
| 44 | 10 | 5,000 | −3.5 | 36.4 | 0.99 | 0.93 | 80.7 | 80.4 | 80.9 |
| 45 | 1 | 5,000 | −3.5 | 35.9 | 0.99 | 0.93 | 78.4 | 78.1 | 78.7 |
| 46 | 5 | 5,000 | −3.6 | 36.2 | 0.99 | 0.90 | 81.1 | 80.8 | 81.4 |
| 47 | 1 | 5,000 | −3.4 | 36.2 | 0.99 | 0.97 | 80.4 | 79.9 | 80.8 |
| 48 | 500 | 5,000 | −2.7 | 32.6 | 0.98 | 1.10 | 77.2 | 74.3 | 80.1 |
| 49 | 100 | 5,000 | −3.3 | 37.8 | 0.98 | 1.01 | 79.4 | 78.9 | 79.8 |
| 50 | 1 | 5,000 | −3.6 | 37.6 | 0.99 | 0.90 | 81.5 | 81.2 | 81.8 |
| 50 | 10 | 5,000 | −3.3 | 39.0 | 0.99 | 1.01 | 80.6 | 80.1 | 81.0 |
| 51 | 1 | 5,000 | −3.4 | 37.8 | 0.98 | 0.97 | 77.1 | 76.7 | 77.4 |
| 52 | 100 | 5,000 | −3.4 | 36.6 | 0.99 | 0.97 | 78.7 | 78.4 | 79.0 |
| 53 | 10 | 5,000 | −3.2 | 38.1 | 0.99 | 1.05 | 79.0 | 74.9 | 83.1 |
| 54 | 1 | 5,000 | −3.6 | 36.2 | 0.99 | 0.90 | 80.6 | 80.0 | 81.2 |
| 54 | 1 | 5,000 | −3.3 | 37.2 | 0.98 | 1.01 | 78.4 | 74.9 | 81.9 |
| 55 | 1 | 5,000 | −3.5 | 36.4 | 0.99 | 0.93 | 79.3 | 78.8 | 79.7 |
| 56 | 10 | 5,000 | −3.4 | 35.8 | 0.98 | 0.97 | 78.2 | 77.5 | 78.8 |
| 57 | 1 | 5,000 | −3.1 | 39.1 | 0.97 | 1.10 | 80.8 | 80.5 | 81.1 |
| 58 | 1 | 5,000 | −3.2 | 49.1 | 0.99 | 1.05 | 79.4 | 79.2 | 79.5 |
| 59 | 10 | 5,000 | −3.4 | 35.9 | 0.98 | 0.97 | 83.8 | 83.3 | 84.3 |
| 60 | 1 | 5,000 | −3.6 | 36.2 | 0.99 | 0.90 | 80.3 | 80.0 | 80.6 |
| 61 | 1 | 5,000 | −3.4 | 38.4 | 0.99 | 0.97 | 80.0 | 79.5 | 80.4 |
| 62 | 10 | 5,000 | −3.4 | 37.2 | 0.98 | 0.97 | 84.1 | 83.6 | 84.5 |
| 63 | 1 | 5,000 | −3.4 | 37.6 | 0.99 | 0.97 | 83.6 | 82.8 | 84.3 |
| 64 | 10 | 5,000 | −3.6 | 37.4 | 0.99 | 0.90 | 84.8 | 84.3 | 85.2 |
| 64 | 500 | 5,000 | −3.0 | 36.9 | 0.96 | 0.90 | 86.5 | 86.0 | 86.9 |
| 65 | 100 | 5,000 | −3.6 | 37.2 | 0.94 | 0.90 | 81.2 | 80.6 | 81.8 |
| 66 | 5 | 5,000 | −3.5 | 36.3 | 0.99 | 0.93 | 78.0 | 77.2 | 78.8 |
| 67 | 100 | 5,000 | −3.1 | 36.6 | 0.97 | 1.10 | 79.7 | 79.2 | 80.1 |
| 68 | 10 | 5,000 | −3.5 | 38.7 | 0.99 | 0.93 | 80.4 | 80.1 | 80.7 |
Primers for ORF 2 are found in primer sets B and E, respectively. Primers for ORF 7 are found in primer sets B and E, respectively. Primers for ORF 26 are found in primer sets C and E, respectively. Primers for ORF 42 are found in primer sets A and E, respectively. Primers for ORF 50 are found in primer sets B and E, respectively. Primers for ORF 54 are found in primer sets A and E, respectively. Primers for ORF 64 are found in primer sets B and E, respectively.
Lower limit of VZV DNA copies detected by qPCR and upper limit of VZV DNA copies added to qPCR samples detected.
Slope and intercept of the linear regression line calculated from the graph of VZV DNA copies and CT values.
Coefficient of determination obtained from the linear regression line calculated from the graph of VZV DNA copies and CT values.
Melting temperature of PCR product.
FIG. 2.
Efficiency of VZV DNA quantitation. All 68 VZV ORF-specific primer pairs were analyzed in duplicate by qPCR using multiple dilutions (1 to 5,000 copies) of VZV DNA. Data are mean (±SD) cycle threshold (CT) values obtained with each different VZV DNA copy number. PCR primers detected 1 to 500 copies of VZV DNA with 100% ± 10% efficiency.
Identification of VZV transcripts in trigeminal ganglia by multiplex RT-PCR.
From each trigeminal ganglion, 100 ng of mRNA was reverse transcribed and amplified in separate reactions with each of five multiplex primer sets corresponding to each predicted ORF. Included in each reaction were primers designed to amplify cellular (GAPdH, neurofilament, beta-actin, and cyclophilin) and control (Kanr) transcripts. Fluorescence-labeled amplicons were resolved by size exclusion capillary chromatography and detected by fluorescence spectrophotometry, which recorded optical density (AU). To eliminate background fluorescence, the peak detection limit was set at 5,000 dye signal AU. Five PCRs were performed on cDNA synthesized from mRNA extracted from each trigeminal ganglion with primer pairs specific for all 68 unique VZV ORFs and successfully used to amplify the VZV transcriptome in VZV-infected tissue culture cells (33).
As shown in Fig. 3, peaks corresponding to ORF 41 and 62 transcripts were detected by multiplex primer set A (Fig. 3A and B, respectively), while primer set B detected ORF 4, 57, and 68 transcripts (Fig. 3C, D, and E, respectively), primer set C detected ORF 43 transcripts (Fig. 3F), primer set D detected ORF 40 transcripts (Fig. 3G), and primer set E detected ORF 11, 29, and 63 transcripts (Fig. 3H).
FIG. 3.
The latent VZV transcriptome detected by multiplex analysis of human trigeminal ganglia. Poly(A+) RNA extracted from all trigeminal ganglia was analyzed. Electropherograms show VZV transcripts mapping to VZV ORF 41 (A), ORF 62 (B), ORF 4 (C), ORF 57 (D), ORF 68 (E), ORF 43 (F), ORF 40 (G), and ORFs 11, 29, and 63 (H). The area on each electropherogram corresponding to the expected size of the VZV ORF-specific amplification products is enlarged. The dotted horizontal line on each panel indicates the lower limit of detection. Occasional peaks above background that do not correspond to the size of any predicted VZV amplification product represent nonspecific peaks (NSP). Results shown were obtained from cDNA synthesized from poly(A+) RNA extracted from latently infected trigeminal ganglia (A to H), from a VZV DNA-negative trigeminal ganglion (I), from uninfected MeWo cells (J), from VZV-infected MeWo cells (K), and from VZV-infected MeWo cells in the absence of reverse transcription (L). Peaks corresponding to the cellular transcripts (G, GAPdH; A, beta-actin; C, cyclophilin; K, internal Kanr control) are shown in each electropherogram.
In addition to the peak for ORF 41 (Fig. 3A), a peak was detected between 236 to 258 nucleotides (nt) but only in the analysis of the left trigeminal ganglion of subject 12 and not in the right or any other trigeminal ganglia. Although such a peak would potentially obscure detection of transcripts mapping to ORFs 42 (237 nt), 44 (246 nt), and 29 (256 nt), primer sets A and E contained primers that would detect ORF 42 and 29 transcripts, yet those peaks were not found. Thus, the left trigeminal ganglion from subject 12 did not contain transcripts mapping to ORFs 42 or 29, and the peak is considered nonspecific (NSP). However, because ORF 44 primers were present exclusively in primer set A, we cannot exclude the possibility that the left trigeminal ganglion of subject 12 contained ORF 44 transcripts. Importantly, multiplex RT-PCR analysis of all remaining trigeminal ganglia showed no peak corresponding to ORF 44 transcripts. Figure 3 shows additional nonspecific peaks in panel B at 225 nt, in panel C at 222 nt, in panel E between 179 and 222 nt, in panel F at 166 nt, and in panel I at 187 nt, none of which corresponded to any size produced by PCR amplification of the entire VZV transcriptome in VZV-infected cells in tissue culture (24). Additionally, when each multiplex RT-PCR primer pair was tested separately, no NSPs were seen. Thus, while the origin of NSPs is unknown, they do not correspond to VZV-specific cDNA PCR products.
A control trigeminal ganglion that did not contain PCR amplifiable VZV DNA (subject 2, left trigeminal ganglion) as well as uninfected MeWo cells revealed no VZV transcripts using any primer sets (Fig. 3I and J, respectively), whereas transcripts corresponding to multiple VZV ORFs were found after reverse transcription of mRNA from VZV-infected MeWo cells using primer set A (Fig. 3K). No transcripts were detected when RT was omitted from the reaction (Fig. 3L).
Overall, 10 VZV transcripts were detected in 26 latently infected human trigeminal ganglia (Table 5) in the following order of decreasing prevalence: ORF 63 (69%), ORF 29 (38%), ORF 4 (31%), ORF 62 (27%), ORF 11 (27%), ORF 68 (23%), ORF 40 (15%), ORF 43 (8%), ORF 41 (4%), and ORF 57 (4%) (Table 4). Note that the left trigeminal ganglion of subject 9 was not analyzed due to insufficient RNA. Since the left trigeminal ganglion of subject 2 did not contain VZV DNA, it served as a negative control for multiplex RT-PCR.
TABLE 5.
Prevalence of VZV transcripts in latently infected human trigeminal gangliaa
| Subject identifier | Trigeminal ganglia | Presence of ORF: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 63 | 29 | 4 | 62 | 11 | 68 | 40 | 43 | 41 | 57 | ||
| 1 | R | × | |||||||||
| L | |||||||||||
| 2 | R | ||||||||||
| Lb | |||||||||||
| 3 | R | ||||||||||
| L | |||||||||||
| 4 | R | × | |||||||||
| L | × | × | × | ||||||||
| 5 | R | × | × | × | |||||||
| L | × | ||||||||||
| 6 | R | × | × | × | × | × | |||||
| L | × | × | × | ||||||||
| 7 | R | × | × | × | × | ||||||
| L | × | × | × | × | |||||||
| 8 | R | × | × | × | × | ||||||
| L | × | × | × | × | × | ||||||
| 9 | R | ||||||||||
| Lc | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 10 | R | × | × | × | × | ||||||
| L | × | × | × | ||||||||
| 11 | R | × | × | × | |||||||
| L | × | × | × | ||||||||
| 12 | R | × | × | × | |||||||
| L | × | × | |||||||||
| 13 | R | × | × | × | |||||||
| L | × | × | × | ||||||||
| 14 | R | × | × | × | |||||||
| L | × | × | × | ||||||||
| Frequency (%) | 69 | 38 | 31 | 27 | 27 | 23 | 15 | 8 | 4 | 4 | |
ND, not done; R, right; L, left.
Did not contain VZV DNA and was used as a negative control for multiplex RT-PCR analysis.
Insufficient RNA obtained; thus, multiplex RT-PCR analysis was not done.
Sequence verification of VZV-specific transcripts in latently infected trigeminal ganglia.
Trigeminal ganglia from subjects that contained multiplex RT-PCR peaks corresponding to ORFs 11, 63, and 68 were sequence verified (Fig. 4). The sequence obtained for ORF 63 spanned positions 618 to 836 of the coding region, ORF 11 spanned positions 2189 to 2459 of the coding region, and ORF 68 spanned positions 1602 to 1871 of the coding region. All three transcripts contained the respective termination codon, polyadenylation signal, and terminated in a poly(A) sequence. Due to the small amount of trigeminal ganglionic mRNA positive for VZV ORFs 41, 43, and 57, 3′-terminal sequences were unattainable. However, the presence of these three transcripts in latently infected human trigeminal ganglia was verified by reverse transcribing the pooled mRNA with the respective multiplex primer sets, followed by PCR amplification with VZV ORFs 41, 43, and 57 forward and reverse primers along with universal multiplex PCR primers (Table 2). Since forward primers were used to sequence each PCR product, sequence analysis revealed nucleotides located downstream of the forward PCR primer that continued and terminated within the reverse PCR primer: nucleotide locations 445 to 616 (VZV ORF 41), nucleotide locations 249 to 363 (VZV ORF 43), and nucleotide locations 75 to 201 (VZV ORF 57) (Fig. 4).
FIG. 4.
Sequence verification of VZV-specific transcripts in latently infected human trigeminal ganglia. Trigeminal ganglia from subjects containing ORF 11, 63, and 68 transcripts detected by multiplex RT-PCR analysis were sequence verified. The 3′-terminal sequences spanned positions 618 to 836 (ORF 63), positions 2189 to 2459 (ORF 11), and positions 1602 to 1871 (ORF 68) of the coding regions. All three transcripts contained the respective termination codon (boxed) and polyadenylation signal (underlined) and terminated in a poly(A) sequence (bolded). VZV ORF 41, 43, and 57 cDNA sequences mapped to nucleotide locations 445 to 616 (ORF 41), 249 to 363 (ORF 43), and 75 to 201 (ORF 57) and terminated within the reverse primers (italicized).
DISCUSSION
Our present analysis of 28 human trigeminal ganglia obtained within 24 h after death revealed VZV DNA and HSV-1 DNA in 96% and 50% of trigeminal ganglia, respectively, consistent with previous studies on the prevalence of these two neurotropic alphaherpesviruses in latently infected human ganglia (28). Intact RNA was obtained from 27 of the 28 trigeminal ganglia. In one of those 27 ganglia, VZV DNA was not detected, and RNA from that ganglion served as a VZV-negative control for multiplex RT-PCR analysis. Our genome-wide search of all 68 unique VZV transcripts in the 26 latently infected human trigeminal ganglia revealed transcripts corresponding to VZV ORFs 29, 62, and 63, as detected previously by PCR (12), as well as VZV ORFs 4 and 40, which confirmed previous in situ hybridization results (22). In addition, transcripts corresponding to VZV ORFs 11, 41, 43, 57, and 68 were found. Finally, transcripts corresponding to VZV ORFs 18, 21, and 66 have been found in latently infected human ganglia (7-9, 11, 12, 17) but were not detected in this study (Table 6).
TABLE 6.
Summary of all VZV transcripts identified in human ganglia during latency
| ORF | Functiona | Presence of transcripts during latencyb |
|
|---|---|---|---|
| Current report | Previous report (reference) | ||
| 1 | Membrane protein | − | |
| 2 | − | ||
| 3 | − | ||
| 4 | Transactivator, tegument protein | + | + (22) |
| 5 | Glycoprotein K | − | |
| 6 | − | ||
| 7 | − | ||
| 8 | Deoxyuridine triphosphatase | − | |
| 9 | Syncytium formation, virion protein | − | |
| 10 | Transactivator, tegument protein | − | |
| 11 | + | ||
| 12 | − | ||
| 13 | Thymidylate synthetase | − | |
| 14 | Glycoprotein C | − | |
| 15 | − | ||
| 16 | − | ||
| 17 | Induces RNA cleavage | − | |
| 18 | Ribonucleotide reductase, small subunit | − | + (22) |
| 19 | Ribonucleotide reductase, large subunit | − | |
| 20 | − | ||
| 21 | Nucleocapsid protein | − | + (7, 8, 9, 22) |
| 22 | − | ||
| 23 | − | ||
| 24 | − | ||
| 25 | − | ||
| 26 | − | ||
| 27 | − | ||
| 28 | DNA polymerase | − | |
| 29 | Single-strand DNA binding protein | + | + (8, 9, 12, 22) |
| 30 | − | ||
| 31 | Glycoprotein B | − | |
| 32 | Probable substrate for ORF 47 kinase | − | |
| 33 | Protease, assembly protein | − | |
| 34 | − | ||
| 35 | Cell-to-cell fusion | − | |
| 36 | Thymidine kinase | − | |
| 37 | Glycoprotein H | − | |
| 38 | − | ||
| 39 | − | ||
| 40 | Major capsid protein | + | + (22) |
| 41 | + | ||
| 42 | − | ||
| 43 | + | ||
| 44 | − | ||
| 45 | − | ||
| 46 | − | ||
| 47 | Protein kinase, tegument protein | − | |
| 48 | − | ||
| 49 | − | ||
| 50 | − | ||
| 51 | Origin binding protein | − | |
| 52 | − | ||
| 53 | − | ||
| 54 | − | ||
| 55 | − | ||
| 56 | − | ||
| 57 | Cytoplasmic protein | + | |
| 58 | − | ||
| 59 | Uracil-DNA glycosylase | − | |
| 60 | Glycoprotein L, chaperone for glycoprotein H | − | |
| 61 | Transactivator, repressor | − | |
| 62 | Transactivator, tegument protein | + | + (8, 12, 22) |
| 63 | Transactivator, tegument protein | + | + (8, 9, 12, 22) |
| 64 | − | ||
| 65 | Virion protein | − | |
| 66 | Protein kinase | − | + (11, 12) |
| 67 | Glycoprotein I | − | |
| 68 | Glycoprotein E | + | |
Functions are from reference 5.
−, not detected; +, detected.
All of the five previously undetected VZV transcripts (ORFs 11, 41, 43, 57, and 68) were verified by DNA sequence analysis. As a control to confirm the sequence analysis protocol, transcripts corresponding to VZV ORF 63, the most prevalent VZV transcript detected by our multiplex RT-PCR analysis as well as by conventional real-time cDNA PCR analysis (12), including sequence verification (8), were also analyzed. The 3′ termini of VZV ORF 63, 11, and 68 cDNA products demonstrated a typical mRNA structure. Although PCR amplification of the entire pool of trigeminal ganglionic cDNA across the poly(A) stretch was not sufficiently sensitive to determine the 3′-terminal structure of VZV ORF 41, 43, and 57 transcripts in latently infected human ganglia, the VZV origin of the multiplex ORF 41, 43, and 57 product was verified by identifying the correct DNA sequences using their respective forward PCR primers.
Overall, VZV transcripts present in latently infected human ganglia verified by sequence analysis or confirmed by independent techniques (PCR amplification and in situ hybridization) are ORFs 4, 11, 21, 29, 40, 41, 43, 57, 62, 63, 66, and 68. Transcripts corresponding to VZV ORF 18 have been detected in latently infected ganglia but by only one technique, thus requiring further verification (Table 6).
We recognize that two of the VZV transcripts (ORFs 40 and 68) found by multiplex RT-PCR are putative late transcripts. ISH previously detected VZV ORF 40 transcripts in latently infected human ganglia (17). The presence of these transcripts does not change the operational definition of virus latency in which (i) infectious virus is not present in latently infected cells, but virus has the potential to reactivate, and (ii) viral gene expression is restricted. Although we have not previously detected late VZV transcripts, we are constantly striving to accurately determine the exact extent of VZV transcription in latently infected human ganglia, and that is why we developed a highly sensitive multiplex RT-PCR to screen for all 68 unique VZV transcripts in human ganglia. While the possibility of subclinical reactivation exists, we think it is unlikely for a number of reasons: (i) none of the subjects had been on immunosuppressive drugs or corticosteroids in their last month of life; (ii) if the presence of VZV ORF 40 and 68 transcripts reflected subclinical VZV reactivation, we would likely have detected a greater number of VZV transcripts; (iii) if the presence of these late virus transcripts represented a persistent VZV infection, the complete transcriptome would have been detected; and (iv) because VZV ORF 9 is an early viral gene that is the most abundant VZV gene transcribed during productive infection (11, 23) and is essential for virus growth (36), its transcription should precede transcription of late VZV genes, yet no VZV ORF 9 transcripts were detected in any of the 26 latently infected human ganglia.
Finally, the possibility also exists that subclinical reactivation might have occurred immediately after death. While studies of varicella gene expression in latently infected monkey ganglia have not yet investigated the entire transcriptome, and human and primates are not always comparable, we have shown that in the first 30 h after death of monkeys latently infected with simian varicella virus, spontaneous reactivation does not occur (30).
The proteins encoded by VZV genes transcribed during latency have diverse functions. VZV ORFs 4 and 62 encode regulatory proteins involved in activation of early and late gene transcription (20, 24). VZV ORF 63 encodes a highly phosphorylated protein (32) and regulates VZV gene transcription (25), most likely through epigenetic modification of the virus genome (1, 15, 19). VZV ORF 18 encodes the small subunit of ribonucleotide reductase, an enzyme critical to synthesis of DNA precursors, and also protects neurons from apoptotic cell death (34). VZV ORF 21 encodes a nucleocapsid protein (29), and VZV ORF 29 encodes a DNA binding protein that is essential for virus replication (4, 31). VZV ORFs 40 and 68 encode virus structural proteins. ORF 40 encodes the 155-kDa major capsid protein of VZV (18), and ORF 68 encodes glycoprotein E (14). VZV ORF 66 encodes one of the two VZV protein kinases. ORF 66 phosphorylates ORF 62 protein, thereby influencing its function and assembly into developing virus particles (26, 27). VZV ORFs 11 and 57 are dispensable, while ORFs 41 and 43 encode proteins essential for VZV growth in tissue culture (37). The function of VZV ORF 11, 41, and 43 proteins are unknown, but their HSV-1 homologues encode structural proteins of the virion (VZV ORF 11 and HSV-1 UL47; VZV ORF 41 and HSV-1 UL18; VZV ORF 43 and HSV-1 UL17). ORF 57 is one of six VZV genes with no HSV-1 homologue. The function of ORF 57, a protein located within the cytoplasm of infected cells, is unknown (3). On the whole, proteins encoded by VZV genes transcribed during latency are involved in gene regulation and DNA synthesis along with structural proteins of the virion.
In our current multiplex RT-PCR analyses, VZV ORF 18, 21, and 66 transcripts were not detected even though the sophisticated technology of multiplex RT-PCR detects as few as 33 copies of VZV ORF 21 and 20 copies of VZV ORF 66 (33). While VZV ORF 18 transcripts have been detected by ISH (22) and real-time PCR identified transcripts corresponding to VZV ORFs 21 and 66 (11), no direct comparison of the sensitivities of real-time PCR or ISH to multiplex RT-PCR exists. Of these three VZV transcripts, VZV ORF 66 transcripts are the most abundant, having been detected by real-time PCR at a prevalence of 21% (12). Thus, it could be predicted that five or six of our 27 latently infected ganglia might have contained VZV ORF 66 transcripts. There are a number of reasons VZV ORF 66 transcripts were not found. First, different primers were used for amplification. Real-time VZV ORF 66 primers are selected to optimize detection of the single transcript, while multiplex RT-PCR primers must simultaneously detect 18 to 24 cDNA targets. Second, not all VZV transcripts are present in all latently infected ganglia at all times. For example, while VZV ORF 63 transcripts are the most prevalent and abundant in latently infected human ganglia, there are no reports of its detection in 100% of latently infected human ganglia. Even before multiplex RT-PCR analysis, we learned that the prevalence and abundance of the five VZV transcripts verified by sequence analysis (VZV ORFs 21, 29, 62, 63, and 66) in latently infected ganglia were variable (9, 12).
Overall, multiplex RT-PCR analysis has allowed screening of the entire VZV transcriptome in 27 human trigeminal ganglia. While VZV ORF 63 remains the most prevalent transcript, a distinct variability of VZV gene expression in latently infected ganglia was found.
Acknowledgments
This work was supported in part by the Public Health Service grants AG006127 (D.G.), AG032958 (D.G., R.J.C.), and NS067070 (M.A.N.) from the National Institutes of Health. The DNA samples were sequenced by the University of Colorado Cancer Center DNA Sequencing and Analysis Core, which is supported by the NIH/NCI Cancer Core Support Grant (P30 CA046934).
We thank Dana Griffith and Young Wu for technical assistance, Marina Hoffman for editorial assistance, and Cathy Allen for manuscript preparation.
Footnotes
Published ahead of print on 22 December 2010.
REFERENCES
- 1.Ambagala, A. P., et al. 2009. Varicella-zoster virus immediate-early 63 protein interacts with human antisilencing function 1 protein and alters its ability to bind histones h3.1 and h3.3. J. Virol. 83:200-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bustin, S. A., et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611-622. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, J. I. 2010. The varicella-zoster virus genome. Curr. Top. Microbiol. Immunol. 342:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, J. I., T. Krogmann, L. Pesnicak, and M. A. Ali. 2007. Absence or overexpression of the varicella-zoster virus (VZV) ORF29 latency-associated protein impairs late gene expression and reduces VZV latency in a rodent model. J. Virol. 81:1586-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, J. I., S. E. Straus, and A. M. Arvin. 2007. Varicella-zoster virus replication, pathogenesis, and management, p. 2773-2818. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 6.Cohrs, R., et al. 1992. Restricted transcription of varicella-zoster virus in latently infected human trigeminal and thoracic ganglia. J. Infect. Dis. 166(Suppl. 1):S24-S29. [DOI] [PubMed] [Google Scholar]
- 7.Cohrs, R. J., et al. 1994. Varicella-zoster virus (VZV) transcription during latency in human ganglia: construction of a cDNA library from latently infected human trigeminal ganglia and detection of a VZV transcript. J. Virol. 68:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohrs, R. J., M. Barbour, and D. H. Gilden. 1996. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J. Virol. 70:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohrs, R. J., et al. 2000. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J. Virol. 74:11464-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohrs, R. J., J. Wischer, C. Essman, and D. H. Gilden. 2002. Characterization of varicella-zoster virus gene 21 and 29 proteins in infected cells. J. Virol. 76:7228-7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohrs, R. J., D. H. Gilden, P. R. Kinchington, E. Grinfeld, and P. G. Kennedy. 2003. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J. Virol. 77:6660-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohrs, R. J., and D. H. Gilden. 2007. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J. Virol. 81:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croen, K. D., J. M. Ostrove, L. J. Dragovic, and S. E. Straus. 1988. Patterns of gene expression and sites of latency in human nerve ganglia are different from varicella-zoster and herpes simplex viruses. Proc. Natl. Acad. Sci. U. S. A. 85:9773-9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowler, W. J., et al. 1995. Identification of immunodominant regions and linear B cell epitopes of the gE envelope protein of varicella-zoster virus. Virology 214:531-540. [DOI] [PubMed] [Google Scholar]
- 15.Gary, L., D. H. Gilden, and R. J. Cohrs. 2006. Epigenetic regulation of varicella-zoster virus open reading frames 62 and 63 in latently infected human trigeminal ganglia. J. Virol. 80:4921-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilden, D. H., R. J. Cohrs, and R. Mahalingam. 2003. Clinical and molecular pathogenesis of varicella virus infection. Viral Immunol. 16:243-258. [DOI] [PubMed] [Google Scholar]
- 17.Grose, C., and P. A. Brunel. 1978. Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32 degrees C. Infect. Immun. 19:199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grose, C., W. E. Friedrichs, and G. C. Smith. 1983. Purification and molecular anatomy of the varicella-zoster virion. Biken J. 26:1-15. [PubMed] [Google Scholar]
- 19.Habran, L., et al. 2007. The varicella-zoster virus immediate-early 63 protein affects chromatin-controlled gene transcription in a cell-type dependent manner. BMC Mol. Biol. 8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inchauspe, G., S. Nagpal, and J. M. Ostrove. 1989. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology 173:700-709. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy, P. G., E. Grinfeld, and J. W. Gow. 1999. Latent varicella-zoster virus in human dorsal root ganglia. Virology 258:451-454. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy, P. G., E. Grinfeld, and J. E. Bell. 2000. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J. Virol. 74:11893-11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy, P. G., et al. 2005. Transcriptomal analysis of varicella-zoster virus infection using long oligonucleotide-based microarrays. J. Gen. Virol. 86:2673-2684. [DOI] [PubMed] [Google Scholar]
- 24.Kinchington, P. R., J. K. Hougland, A. M. Arvin, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinchington, P. R., D. Bookey, and S. E. Turse. 1995. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF 61, are associated with purified virus particles. J. Virol. 69:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinchington, P. R., K. Fite, and S. E. Turse. 2000. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J. Virol. 74:2265-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinchington, P. R., K. Fite, A. Seman, and S. E. Turse. 2001. Virion association of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, requires expression of the VZV open reading frame 66 protein kinase. J. Virol. 75:9106-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam, R., M. C. Wellish, A. N. Dueland, R. J. Cohrs, and D. H. Gilden. 1992. Localization of herpes simplex virus and varicella zoster virus DNA in human ganglia. Ann. Neurol. 31:444-448. [DOI] [PubMed] [Google Scholar]
- 29.Mahalingam, R., R. Lasher, M. Wellish, R. J. Cohrs, and D. H. Gilden. 1998. Localization of varicella-zoster virus gene 21 protein in virus-infected cells in culture. J. Virol. 72:6832-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahalingam, R., et al. 2010. Effect of time delay after necropsy on analysis of simian varicella-zoster virus expression in latently infected ganglia of rhesus macaques. J. Virol. 84:12454-12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier, J. L., and S. E. Straus. 1993. Varicella-zoster virus DNA polymerase and major DNA-binding protein genes have overlapping divergent promoters. J. Virol. 67:7573-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller, N. H., L. L. Graf, D. Orlicky, D. Gilden, and R. J. Cohrs. 2009. Phosphorylation of the nuclear form of varicella-zoster virus immediate-early protein 63 by casein kinase II at serine 186. J. Virol. 83:12094-12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagel, M. A., D. Gilden, T. Shade, B. Gao, and R. J. Cohrs. 2009. Rapid and sensitive detection of 68 unique varicella zoster virus gene transcripts in five multiplex reverse transcription-polymerase chain reactions. J. Virol. Methods 157:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkins, D., E. F. Pereira, M. Gober, P. J. Yarowsky, and L. Aurelian. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 76:1435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tischer, B. K., et al. 2007. A self-excisable infectious bacterial artificial chromosome clone of varicella-zoster virus allows analysis of the essential tegument protein encoded by ORF9. J. Virol. 81:13200-13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, Z., et al. 2010. Genome-wide mutagenesis reveals that ORF7 is a novel VZV skin-tropic factor. PLoS Pathog. 6:e1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]