Abstract
Aberrant activation of the B-cell compartment and hypergammaglobulinemia were among the first recognized characteristics of HIV-1-infected patients in the early 1980s. It has been demonstrated previously that HIV-1 particles acquire the costimulatory molecule CD40L when budding from activated CD4+ T cells. In this paper, we confirmed first that CD40L-bearing virions are detected in the plasma from untreated HIV-1-infected individuals. To define the biological functions of virus-associated CD40L and fully characterize its influence on the activation state of B cells, we conducted a large-scale gene expression analysis using microarray technology on B cells isolated from human tonsillar tissue. Comparative analyses of gene expression profiles revealed that CD40L-bearing virions induce a highly similar response to the one observed in samples treated with a CD40 agonist, indicating that virions bearing CD40L can efficiently activate B cells. Among modulated genes, many cytokines/chemokines (CCL17, CCL22), surface molecules (CD23, CD80, ICAM-1), members of the TNF superfamily (FAS, A20, TNIP1, CD40, lymphotoxin alpha, lymphotoxin beta), transcription factors and associated proteins (NFKB1, NFKBIA, NFKBIE), second messengers involved in CD40 signaling (TRAF1, TRAF3, MAP2K1, phosphatidylinositol 3-kinase), and the activation-induced cytidine deaminase (AID) were identified. Moreover, we show that soluble factors induced upon the exposure of B cells to CD40L-bearing virions can exert chemoattractant properties toward CD4+ T cells. We thus propose that a positive feedback loop involving CD40L-bearing HIV-1 particles issued from CD4+ T cells productively infected with HIV-1 play a role in the virus-induced dysfunction of humoral immunity by chronically activating B cells through sustained CD40 signaling.
Human immunodeficiency virus type 1 (HIV-1) is the causal agent of AIDS, which is characterized by a slow but relentless deterioration of the immune system. The virus-mediated detrimental effects on the various cell populations that participate in the normal immune response are numerous, resulting in a generalized deficiency in the ability to respond to immunological threats adequately. One of the major hallmarks of HIV-1 infection is a progressive loss of CD4+ T cells, a primary target of the virus. Interestingly, B-lymphocyte functions also are severely disrupted in the context of a natural infection, even though there is little evidence that this cell subset is productively infected by HIV-1 in vivo. For example, original reports often describe the elevated production of type G immunoglobulins (IgG) (referred to as hypergammaglobulinemia), increased B-cell turnover, polyclonal B-cell activation, and differentiation coupled with poor responses against novel and common recall antigens in samples from HIV-1-infected patients (19, 25, 30, 31, 40-42, 49). These effects are correlated with elevated viral loads and usually disappear over time in patients treated with highly active antiretroviral therapy (12, 19, 46). However, the exact mechanisms responsible for B-cell abnormalities still are ill defined but might have a multifactorial origin (reviewed in reference 39).
B-cell activation is a tightly regulated process that necessitates multiple spatially and temporally regulated signal transduction events. One of the most important secondary signals following the binding of the B-cell receptor to a cognate antigen is the interaction between CD40 on the surface of a B cell and CD40L (CD154) expressed by an activated T cell (4, 7, 24, 54). This event culminates in the formation of a germinal center, B-cell proliferation, plasma cell differentiation, and the induction of somatic hypermutation (SHM) and class switch recombination (CSR) processes to generate highly specific IgG, IgA, or IgE antibodies, which depends on the activation-induced cytidine deaminase (AID) (26).
It has been established previously that host-derived CD40L can be inserted at the surface of HIV-1 particles produced by peripheral blood and tissue CD4+ T cells in vitro (34). Importantly, the attachment of CD40L-bearing virions onto primary human B cells is sufficient to induce potent cellular activation, which demonstrates the functionality of such host-derived molecules (33). The capacity of CD40L to interact with its natural cognate ligand CD40 on the surface of B cells was confirmed by showing that CD40L-bearing viruses induce homotypic cell aggregation, the nuclear translocation of NF-κB, and IgG secretion (33). We report herein that CD40L incorporation is a physiological phenomenon, since this cell surface molecule is detected when using an in vivo source of virus (i.e., plasma virus from patients). We demonstrate also that virus-associated host CD40L is capable of inducing signal transduction events in human primary tissue B cells through CD40. Data from DNA microarray experiments revealed that the transcriptomic profile of B cells exposed to CD40L-bearing virions resembles the one seen with a CD40 agonist. Therefore, it can be proposed that the insertion of host-derived CD40L into the HIV-1 envelope is responsible for some of the multiple functional alterations detected in the B-cell compartment of HIV-1-infected individuals.
(This study was performed by M.I. in partial fulfillment of a Ph.D. degree in the Microbiology-Immunology Program, Faculty of Medicine, Laval University.)
MATERIALS AND METHODS
Ethics statement.
Samples from tonsillar tissues were obtained from patients in accordance with the guidelines of the Bioethics Committee from the Centre Hospitalier de l'Université Laval. All parents signed an ethics board-approved informed consent form.
Isolation and purification of tonsillar B and CD4+ T cells.
Tonsillar tissues were obtained from 2- to 4-year-old patients undergoing routine tonsillectomy at the Centre Hospitalier de l'Université Laval (Québec, Canada). Briefly, tonsillar tissue was chopped into small pieces and minced. The resulting cell suspension was washed in culture medium containing Fungizone (250 ng/ml), filtered through a 30-μm nylon mesh cell strainer (Miltenyi Biotec Inc., Auburn, CA), and separated using a StemSep human B-cell enrichment kit (StemCell Technologies, Vancouver, Canada). Isolated B cells (CD19+) were maintained at a density of 10 × 106 cells/ml in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), streptomycin (100 μg/ml), Fungizone (250 ng/ml), recombinant human interleukin-4 (IL-4) (400 U/ml), and IL-10 (50 ng/ml) (both from R&D Systems Inc., Minneapolis, MN). It should be noted that the isolated B cells were rested for 48 h before the start of the experiment. Therefore, the vast majority of germinal center B cells and plasma cells are likely to die in the tonsillar sample, leaving primarily memory and naïve B cells for our studies. Primary human tonsillar CD4+ T cells were purified using a StemSep human CD4+ T cell enrichment kit according to the manufacturer's instructions (StemCell Technologies) and maintained in complete culture medium (i.e., RPMI-1640 supplemented with 10% FBS and 1% penicillin-streptomycin) at a density of 2 × 106 cells/ml. Experiments were performed with cell preparations that were highly enriched in the studied cell subpopulations (e.g., B-cell purity, >95% CD19+; CD4+ T-cell purity, >98% CD3+/CD4+).
Flow cytometry.
Before antibody staining, tonsillar B cells were treated with 10% pooled human sera for 30 min on ice and washed with cold phosphate-buffered saline (PBS) to block nonspecific binding sites and Fc receptors. Cells then were pelleted and resuspended at a density of 10 × 106 cells per 100 μl of cold sterile endotoxin-free PBS (pH 7.4) containing a saturating amount of either PE-coupled mouse anti-human CD19, fluorescein isothiocyanate (FITC)-coupled mouse anti-human CD54 (ICAM-1), FITC-coupled mouse anti-human CD80, or an appropriate isotype-matched control antibody (i.e., PE- or FITC-coupled IgG1) (all from BD Biosciences, Mississauga, Canada) for 20 min on ice. The purity of isolated B and CD4+ T cells was assessed using antibodies directed against CD19 for B lymphocytes or CD3 and CD4 for CD4+ T cells, followed by appropriate secondary antibodies. Cells were washed three times in cold sterile endotoxin-free PBS, fixed in 2% paraformaldehyde for 30 min at 4°C, and analyzed on a cytofluorimeter (EPICS XL; Beckman Coulter, Miami, FL).
Detection of CD40L in circulating virions from patient plasma.
Plasma virus from three distinct HIV-1-infected individuals (untreated/viremic) (kindly provided by R.-P. Sékaly, Université de Montréal, Montréal, Canada) was isolated using the μMACS Streptavidin MicroBeads test by following the manufacturer's instructions (Miltenyi Biotec, Auburn, CA), with minor modifications. In brief, prior to virus capture, the samples were centrifuged at 13,000 × g for 1 min to remove particulate matter. Processed plasma (200 μl) first was incubated for 30 min at room temperature with one of the following biotinylated antibodies (1 μg): CD19 (clone HIB19 from eBioscience, San Diego, CA) (negative control), HLA-DR (clone 2.06 from the American Type Culture Collection, Manassas, VA) (positive control), or CD40L (clone hCD40L-M91 from BD Biosciences, Mississauga, Canada). Thereafter, magnetic beads were added and the antibody-bound virus was captured by magnetic separation. Viral RNA then was purified with the MagMAX-96 viral RNA isolation kit (Applied Biosystems/Ambion, Austin, TX) with slight modifications. Briefly, virus lysis was performed directly on the columns with a total of 180 μl of lysis buffer (50 μl with an incubation at room temperature for 5 min, followed by an additional 130 μl). An additional 50 μl of endotoxin-free 1× PBS was added to the lysis buffer, and viral RNA was eluted in 25 μl of elution buffer. Purified viral RNA was quantified with the TaqMan RNA-to-CT 1-step kit (Applied Biosystems Inc., Foster City, CA). The samples were amplified in triplicate using primers LTR S4 and LTR As3 and probes LTRP1 and LTRP2 as described previously (16). The HIV-1 RNA used for the standard curve was obtained from the NIH AIDS Research and Reference Reagent Program (Germantown, MD) (i.e., HIV-1 VQA RNA quantification standard) and purified with the MagMax-96 viral RNA isolation kit as described above for the plasma samples. Thermal cycling was performed in a Rotor-Gene RG-3000 (Corbett Research, Sydney, Australia).
Preparation of virus stocks and appropriate controls.
pNL4-3 is a full-length infectious molecular clone of HIV-1 (X4 tropic) (1) that was obtained through the NIH AIDS Research and Reference Reagent Program. Progeny viruses were produced by the calcium phosphate transfection of 293T cells as previously described (5). Briefly, 293T cells were cotransfected with pNL4-3 and pcDNA3.1-CD40L, a plasmid coding for the complete human CD40L molecule (23) (a kind gift from R. S. Kornbluth, UCSD, CA), to generate CD40L-bearing virions (virus preparation called NL4-3/CD40L). Viruses lacking host-derived CD40L were obtained by transfecting 293T cells with pNL4-3 only (virus preparation called NL4-3). It should be noted that parental 293T cells do not constitutively express CD40L. Controls consisted of 293T cells transfected with pCDNA3.1 (called mock) or pcDNA3.1-CD40L (called mock/CD40L). The latter control was used to eliminate the possible contribution of exosomes and/or microvesicles loaded with CD40L. Supernatants from such transfected 293T cells were filtered through a 0.22-μm cellulose acetate filter (Millipore Corporation, Billerica, MA), ultracentrifuged to eliminate nonviral vesicular bodies, and resuspended in endotoxin-free PBS (Invitrogen, Mississauga, Canada). An enzyme-linked immunosorbent assay (ELISA) developed in our laboratory was used to estimate the amount of p24 in each viral preparation (5). For all virus stocks, the absence or presence of host-derived CD40L was confirmed by performing our virus capture assay using an initial virus input of 2 ng of p24, as we described previously (34).
Microarray experiments.
Purified tonsillar B cells were incubated for 8 and 24 h at 37°C with either NL4-3, NL4-3/CD40L, or an anti-human CD40 (clone G28.5 from the American Type Culture Collection, Manassas, VA). A virus input of 10 ng of p24 per 1 × 105 target cells was used in all studies. Samples from two different healthy donors were used. Cell pellets were frozen at −80°C until the isolation of total mRNA was performed using the RNeasy Mini kit according to the manufacturer's protocol (Qiagen Inc., Mississauga, Canada). All samples were processed at the same time and using the same kit. Gene expression profiles were analyzed using commercial oligonucleotide microarrays (HG Focus GeneChips; Affymetrix, Santa Clara, CA), which contain probe sets representing more than 8,500 transcripts. A total of 12 microarrays were used. Affymetrix standard protocols were followed throughout these experiments. Data were globally normalized, and “present” calls were determined using MAS 5.0. Results were analyzed using GeneSpring 6.0. Signal intensity was normalized for each microarray, and genes with a signal below 100 were ignored. In addition, genes having a present call in all samples or genes going from an absent call to a present call following treatment were included in the analysis. Fold changes of twice the control and higher were considered significant. Gene ontology (GO) and pathway overrepresentation analysis were performed with DAVID (http://david.abcc.ncifcrf.gov/).
qRT-PCR analysis.
The expression level of transcripts was determined using a Rotor-Gene RG-3000 (Corbett Research, Sydney, Australia). Total RNA was isolated using the Qiagen RNA extraction kit and then digested with DNase to remove any contaminating genomic DNA. RNA was reverse transcribed using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI). We then quantified transcripts by quantitative reverse transcription-PCR (qRT-PCR) using HotStart Taq DNA polymerase (Promega) and Sybr green detection (Applied Biosystems). Normalization on 18S mRNA levels was performed to obtain final expression values. A standard curve was drawn for each gene of interest by serial dilutions of a pool of RNA. The sequences of primers that were used in this study are shown in Table 1.
TABLE 1.
List of primer sequences used to confirm microarray data by qRT-PCR
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| TRAF-1 | 5-CTCCCCAGCCTTCTACACTG-3 | 5-GATCACGATGAAGAGCGACA-3 |
| ICAM-1 | 5-CAAGGCCTCAGTCAGTGTGA-3 | 5-CCTCTGGCTTCGTCAGAATC-3 |
| LTB | 5-AGGAGCCACTTCTCTGGTGA-3 | 5-TTCTGAAACCCCAGTCCTTG-3 |
| LTA | 5-AACCTGCTGCTCACCTCATT-3 | 5-TGCTCAAGGAGAAACCATCC-3 |
| A20 | 5-GCTGGCAACTGGAGTCTCTC-3 | 5-CATGGGTGTGTCTGTGGAG-3 |
| AID | 5-CGTAGTGAAGAGGCGTGACA-3 | 5-ATGTAGCGGAGGAAGAGCAA-3 |
| CCL22 | 5-GCCGTGATTACGTCCGTTAC-3 | 5-CGGCACAGATCTCCTTATCC-3 |
Western blots.
Total cell extracts were heated at 100°C for 7 min in 1× sample buffer made of 62 mM Tris-HCl (pH 6.8), 2% SDS, 5% β-mercaptoethanol, 9% glycerol, 0.002% bromophenol blue, and 1 mM phenylmethylsulfonyl fluoride (PMSF). The samples then were separated on a 7.5 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel and transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, MA). Immunoblotting was performed using antibodies specific for TRAF1 (clone H3; Santa Cruz Biotechnology Inc., Santa Cruz, CA), A20 (clone 59A426; Abcam, Cambridge, MA), or β-actin (clone I-19; Santa Cruz Biotechnology Inc.). Membranes were labeled with horseradish peroxidase-conjugated secondary anti-mouse or anti-goat antibodies (Jackson ImmunoResearch, Mississauga, Canada) at a respective final dilution of 1:10,000 and 1:20,000. Signals were revealed using the ECL Western blotting detection reagent (GE Healthcare, Piscataway, NJ).
CCL22 quantification.
Tonsillar B cells (5 × 106 cells/ml) were incubated for 24 h at 37°C with either NL4-3, NL4-3/CD40L, or anti-CD40 (clone G28.5 at a final concentration of 2 μg/ml) (positive control). Additional controls consisted of B cells exposed to similar volumes as the tested virus stocks of cell-free supernatants from 293T cells transfected with pCDNA3.1 or pCDNA3.1-CD40L. Thereafter, cell-free supernatants were collected and treated with 0.5% Triton X-100 before being subjected to a Milliplex CCL22 detection kit (Millipore). Finally, levels of the CCL22 chemokine were estimated with a Luminex 100 instrument according to the manufacturer's instructions (Luminex Corporation, Austin, TX).
Chemotaxis assay.
Tonsillar B cells (CD19+) (5 × 106 cells/ml) were incubated for 16 h at 37°C with either NL4-3, NL4-3/CD40L, anti-CD40 (clone G28.5 at a final concentration of 2 μg/ml; used as a positive control), or similar volumes as the tested virus stocks of cell-free supernatants from 293T cells transfected with pCDNA3.1 or pCDNA3.1-CD40L. Purified CD4+ T cells originating from the same tonsillar tissue were kept in a separate cell culture flask at a density of 2 × 106 cells/ml in complete culture medium. Prior to the chemotaxis assay, CD4+ T cells were stained with the fluorogenic dye calcein-AM (5 μM; Molecular Probes, Eugene, OR). Cell migration was measured in Transwell systems with polycarbonate membranes of 3 μM pore size (Corning Life Sciences, Lowell, MA). In brief, stained CD4+ T cells (2 × 105/300 μl) were deposited onto the upper migration chamber. The lower migration chamber contained 400 μl of culture medium or cell-free supernatants from purified tonsillar B cells subjected to the above-mentioned treatments. After 150 min of migration at 37°C, the content of the lower chamber was harvested by repeated washings with PBS supplemented with 2% FBS, and labeled cells were lysed with 0.5% Triton X-100 at room temperature for 30 min. Finally, cell migration was estimated by measuring fluorescence with a fluorescence microplate reader (Perkin Elmer Victor 1420) at 485-nm excitation and 535-nm emission wavelengths. Results were normalized against total migrating cells (2 × 105 stained cells/400 μl medium) and subtracted from background signal.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism software version 5. One-way analysis of variance (ANOVA) with correction for multiple testing (Bonferroni or Dunnett's) was performed, and a threshold of P < 0.05 was considered statistically significant.
RESULTS
CD40L is acquired by HIV-1 in vivo.
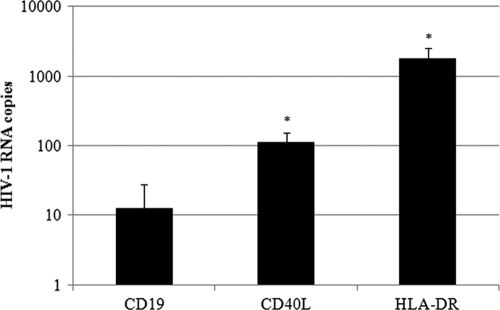
It has been shown previously that host-derived CD40L molecules can be acquired in vitro by HIV-1 replicating in mitogen-activated peripheral blood mononuclear cells and human tonsil histocultures (34). In an attempt to determine whether the CD40L incorporation process also can occur under physiological conditions, we assessed the presence of host-derived CD40L in viruses directly from patient plasma. Clinical plasma samples from three different treatment-naive viremic individuals infected with HIV-1 first were subjected to a virus capture assay relying on the use of streptavidin-coated magnetic beads coupled with biotin-labeled antibodies specific for human CD19, HLA-DR, or CD40L. In this series of investigations, the HLA-DR determinant was used as a positive control because this cell surface constituent is efficiently inserted within emerging virions, while CD19 was used as a negative control, since it is a marker specific for B cells, which are known to be refractory to productive HIV-1 infection. The total amount of captured virus was monitored using a previously described qRT-PCR specific for HIV-1 (16). Data shown in Fig. 1 indicate that host-derived CD40L is found at the surface of virions circulating in patient plasma. Indeed, the level of captured virus with magnetic beads coated with anti-CD40L is an order of magnitude above the basal noise level (i.e., anti-CD19). Moreover, the efficiency of viral capture with anti-HLA-DR is about 10-fold more important than that with anti-CD40L, which was expected because HLA-DR has been characterized as one of the most abundant host molecules acquired by HIV-1 (9, 27, 34). Taken together, these results validate studies aimed at assessing the possible involvement of virus-associated CD40L in B-cell abnormalities that afflict HIV-1-infected persons.
FIG. 1.
CD40L is acquired by HIV-1 in vivo. Plasma samples from three distinct treatment-naive viremic HIV-1-infected persons were subjected to an immunomagnetic precipitation assay using antibodies specific for CD19 (negative control), HLA-DR (positive control), or CD40L. The quantification of captured virions was achieved using a qRT-PCR test. Results are expressed as the mean number of RNA copies ± standard deviations on a logarithmic scale. The asterisks denote a P value lower than 0.05 relative to the negative control as determined by a two-tailed paired t test analysis.
Transcriptome analysis reveals that CD40L-bearing virions efficiently activate B cells.
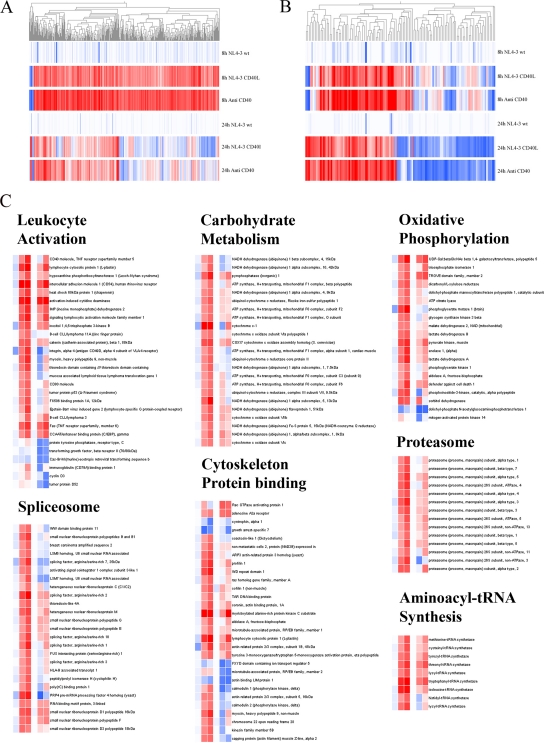
We next investigated the possible function and consequences of virus-anchored CD40L on normal B lymphocyte biology. To do so, we performed a large-scale gene expression analysis through the use of human genome focus arrays from Affymetrix. We used B cells purified from human tonsils, because this anatomical organ constitutes a rich source of B cells exhibiting a wide variety of phenotypes and activation states. Experiments were conducted with isogenic viral particles either lacking (NL4-3) or bearing host-derived CD40L (NL4-3/CD40L). The former virus preparation was used as a control based on previous work indicating that such virus stocks could not induce significant phenotypic changes in B cells (33). An agonistic antibody directed against human CD40 (clone G28.5) was used as a positive control (13, 28, 29). Data displayed in Fig. 2 demonstrate that the exposure of tonsillar B cells to either CD40L-bearing virions or the anti-CD40 antibody induced dramatic changes in gene expression profiles compared to those of viruses lacking host-derived CD40L. The CD40L-mediated induction in host cell gene expression peaked early, as we found 559 genes modulated more than 2-fold at the 8-h time point compared to 128 genes at 24 h (see supplemental data). Moreover, both CD40L-bearing viruses and the anti-CD40 antibody altered the expression of a comparable array of cellular genes, as illustrated by the hierarchical clustering of the genes modulated at 8 and 24 h (Fig. 2A and B, respectively). Gene expression analysis was achieved using a clustering approach instead of a Venn diagram comparison, as it appears that CD40L-bearing virions and anti-CD40 induce a roughly similar host cell gene expression pattern, although the modulation induced by the agonistic antibody is slightly stronger overall. This could simply reflect a difference in the kinetics of induction between these experimental sets. As most changes are near the 2-fold threshold, the Venn diagram approach did not adequately represent the high level of similarity between the two datasets (data not shown). For this reason, the list of genes modulated by either CD40L-bearing viruses or anti-CD40 compared to those of the control is provided in the same table (see Tables S1 and S2, 8 and 24 h time points, respectively, in the supplemental material).
FIG. 2.
B cells exposed to CD40L-bearing virions and anti-CD40 display comparable gene expression profiles. Tonsillar B cells from two donors were exposed for 8 or 24 h at 37°C to NL4-3, NL4-3/CD40L, or anti-CD40, and the total mRNA was extracted. The oligonucleotide array analysis was performed using the Affymetrix oligonucleotide microarray HG Focus GeneChips, and results were analyzed with GeneSpring. The figure shows the hierarchical clustering of genes modulated by either NL4-3/CD40L or anti-CD40 compared to that for NL4-3. (A) The 559 genes modulated at the 8-h time point and (B) the 128 genes modulated after 24 h; both time points are represented for both lists of genes for a better visualization of gene expression patterns. Upregulated genes are shown in red, while downregulated genes are depicted in blue; the most intense color on each side corresponds to a 5-fold change. (C) The hierarchical clustering of selected gene ontology categories or cellular pathways identified as having overrepresented number of genes according to DAVID. The same color scheme described above applies, as well as the nature of the sample (from left to right: NL4-3 at 8 h, NL4-3/CD40L at 8 h, anti-CD40 at 8 h, NL4-3 at 24 h, NL4-3/CD40L at 24 h, and anti-CD40 at 24 h).
We next used the DAVID (Database for Annotation, Visualization, and Integrated Discovery) clustering engine to obtain an overview of the intracellular metabolic pathways affected by either virus bearing host-derived CD40L or anti-CD40. This tool identifies, from a list of genes, enriched biological themes through the clustering of multiple features, such as gene ontology (GO) categories, pathways, protein domains, and known protein interactions, among many others (14). The highest scoring categories for our data are associated with biological processes and molecular functions involved in B-cell activation. These categories include the proteasome, glycolysis, oxidative phosphorylation, mRNA splicing, cytoskeleton-related proteins, tRNA synthetases, and lymphocyte activation. A hierarchical clustering view of the genes contained in these specific categories confirms that the gene expression profile induced by CD40L-bearing HIV-1 particles is analogous to the transcriptional program triggered by the anti-CD40 antibody (Fig. 2C).
CD40L-bearing virions induce modulation of genes involved in CD40-related early signal transduction events.
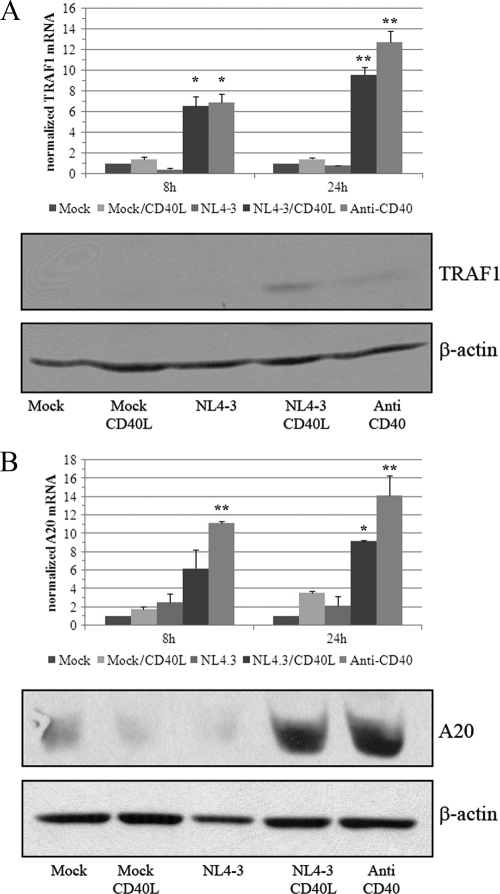
One of the best-described biochemical events following signaling through CD40 remains NF-κB induction (2, 8). The activation of this transcription factor is regulated at the posttranslational level by phosphorylation, polyubiquitinylation, and the subsequent proteasome-dependent degradation of a natural inhibitor, mainly IκBα. Following the degradation of its inhibitor, the NF-κB complex can translocate to the nucleus, bind specific DNA sequences, drive the assembly of RNA polymerase II-dependent transcription machinery, and further promote RNA polymerase II processivity. It already has been established that CD40L-bearing viruses can induce NF-κB activation and nuclear translocation (33). While microarray studies may not directly monitor events occurring at the protein level, such as phosphorylation and ubiquitinylation, they can provide indirect insight into these processes, as numerous signaling pathways feature feedback loops that require the transcriptional regulation of some of their components. Indeed, microarray data indicate that many key components of the CD40/NF-κB signaling pathway are modulated following the engagement of cell surface CD40 by either CD40L-bearing viruses or anti-CD40. Among the regulated genes, we found NF-κB1, IκBα, IκBɛ, A20 (also called TNFAIP3), TNIP1, and cIAP2 to be upregulated. The expression of CD40 mRNA itself, as well as that of the regulatory signal adapter TRAF1 and kinases such as MAP2K1 and phosphatidylinositol 3-kinase (PI3K), also were found to be augmented. Confirmatory qRT-PCR and Western blot analyses demonstrate that TRAF1 and A20 are increased at both mRNA and protein levels (Fig. 3 A and B, respectively). We selected these two genes because they both are implicated in negative retroaction feedback loops following CD40 binding.
FIG. 3.
CD40L-bearing virions drive the expression of signal transduction regulators TRAF1 and A20. Tonsillar B cells were exposed for 8 or 24 h at 37°C to supernatants from 293T cells transfected with an empty control vector (mock), supernatants from 293T cells transfected with a vector coding for CD40L (mock/CD40L), NL4-3, NL4-3/CD40L, or anti-CD40. mRNA and protein levels of TRAF1 (A) and A20 (B) were assessed by qRT-PCR and Western blot assays, respectively. Results for mRNA amounts are expressed as fold changes above the mock control and are normalized on 18S expression. β-Actin was used as a loading control in the protein assay. The data shown are the means ± standard deviations for triplicate samples and are representative of two separate experiments performed with different donors. Asterisks denote statistically significant data (ANOVA test, Bonferronni-corrected P values; *, P < 0.05; **, P < 0.01).
CD40L-bearing virions upregulate expression of surface activation markers on B cells.
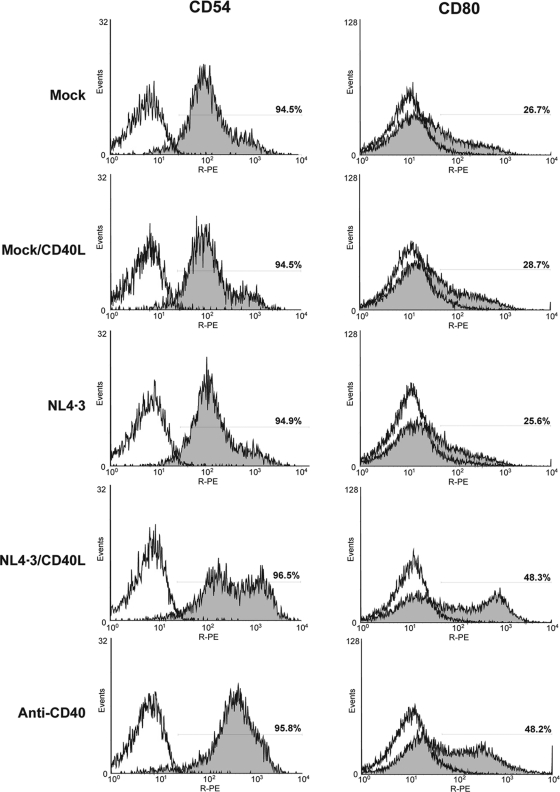
Proper signaling through CD40 is a prerequisite for B-cell activation and differentiation, two interlinked events characterized by phenotypic changes and the increased expression of numerous cell surface receptors and adhesion molecules. Our microarray data reveal that exposure to virus carrying host-derived CD40L or the agonistic antibody induces higher levels of genes coding for membrane proteins that are ontologically related to B-cell activation. Among these, we note the alpha chain of the IL-13 receptor (IL13RA), the low-affinity receptor for the Fc portion of IgE (CD23), the adhesion molecule ICAM-1 (CD54), the costimulatory molecule CD80, and the death receptor FAS. ICAM-1 and CD80 were selected as interesting candidates for the confirmation of microarray findings at the protein level, since simple flow cytometry analysis could yield fast and highly relevant information on the B-cell activation status. Results depicted in Fig. 4 corroborate at the protein level that ICAM-1 and CD80 both are enhanced upon the attachment of CD40L-bearing virions onto tonsillar B cells.
FIG. 4.
CD40L-bearing viruses promote the surface expression of ICAM-1 and CD80. Purified B cells from tonsils were exposed for 24 h at 37°C to the same agents as those described in the legend to Fig. 3. Thereafter, expression levels of ICAM-1 (CD54) and CD80 were assayed by flow cytometry. The percentage of positive cells is depicted in each flow cytometry histogram. The results shown are representative of two different donors.
CD40L-bearing viruses modulate critical components of B-cell maturation and activation.
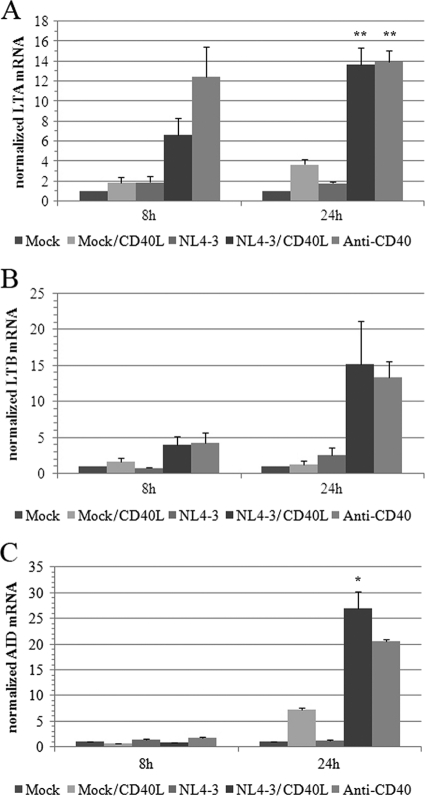
Lymphotoxin alpha (LTA), also known as tumor necrosis factor beta (TNF-β), is a crucial mediator of germinal center and lymphoid tissue organization that is regulated largely through CD40-CD40L signaling (10, 15, 35, 36, 50, 51). Interestingly, elevated levels of this proinflammatory cytokine have been correlated with progression to AIDS (37, 38, 43, 55), and the treatment of peripheral blood mononuclear cells with LTA has been shown to stimulate HIV-1 replication (56). LTA can be secreted or can form heterotrimers with the membrane-bound lymphotoxin beta (LTB) (15). Interestingly, microarray data suggest that both LTA and LTB transcripts were strongly enhanced following the exposure of tonsillar B cells to CD40L-bearing HIV-1 particles. This significant virus-mediated augmentation of LTA and LTB mRNAs was confirmed by qRT-PCR (Fig. 5 A and B, respectively).
FIG. 5.
CD40L-bearing virions promote mRNA expression of LTA, LTB, and AID. mRNA expression levels of LTA (A), LTB (B), and AID (C) were assessed by qRT-PCR in tonsillar B cells that were exposed for 8 or 24 h to the agents described in the legend of Fig. 3. Results are expressed as fold changes above the mock control and are normalized to 18S expression. The data shown are the means ± standard deviations for triplicate samples and are representative of two separate experiments performed with different donors. Asterisks denote statistically significant data (ANOVA test, Bonferroni-corrected P values; *, P < 0.05; **, P < 0.01).
The differentiation of resting B cells into plasma cells involves the induction of SHM of the variable region of immunoglobulins to generate highly specific antibodies and the activation of DNA rearrangement processes to allow immunoglobulin CSR (3, 21, 57). Those processes both depend on a cytidine deaminase that is induced following B-cell activation, known as AID (17, 26). Microarray data show that the transcription of AID is significantly upregulated at 8 and 24 h, going from an absent (could not be detected) to a present status call in samples treated with either CD40L-bearing virions or anti-CD40. These results were confirmed again by qRT-PCR (Fig. 5C) but could not be verified at the protein level, as commercially available antibodies were not sensitive enough to detect the protein in our samples by Western blotting (data not shown). Interestingly, the expression of other genes involved in somatic recombination, such as XRCC5 and p53, also were significantly increased.
CD40L-bearing viruses induce secretion of soluble factors involved in the recruitment of activated CD4+ T cells.
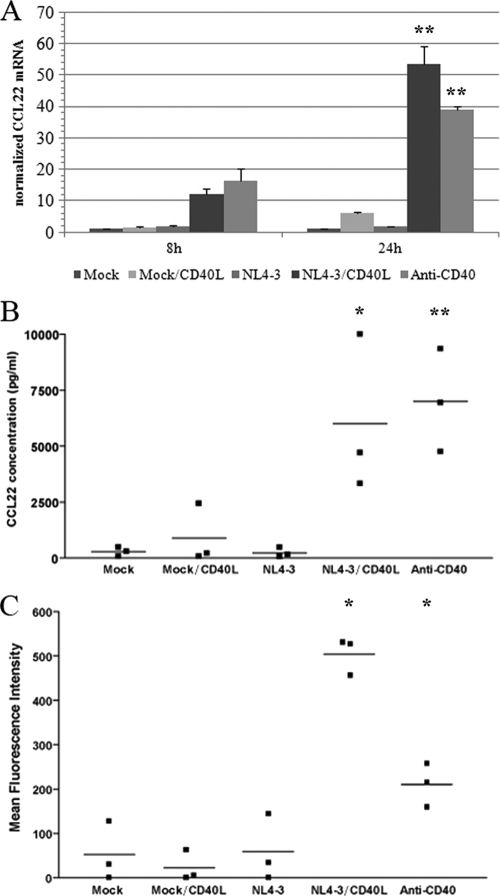
Among the genes that were found to be regulated by CD40 signaling in tonsillar B cells, transcripts for CCL17 and CCL22, two ligands of CCR4 found in the same genomic locus, were greatly upregulated as early as 8 h postexposure to CD40L-bearing virions or anti-CD40. CCR4 is a chemokine receptor expressed on various memory CD4+ T-cell subtypes, and both CCL17 and CCL22 have been shown to be potent chemoattractants for CCR4-expressing memory CD4+ T cells. The upregulation of CCL22 was confirmed at the mRNA level by qRT-PCR (Fig. 6 A) and at the protein level using the Luminex xMAP technology (Fig. 6B).
FIG. 6.
Exposure of tonsillar B cells to CD40L-bearing virions results in the production of the chemokine CCL22 and chemotaxis of CD4+ T cells. (A) Expression levels of CCL22 mRNA in B cells exposed to the listed agents for 8 or 24 h was measured by qRT-PCR. Results are expressed as fold changes above the mock control and are normalized to 18S expression. The data shown are the means ± standard deviations for triplicate samples and are representative of two separate experiments performed with different donors. Asterisks denote statistically significant data (ANOVA test, Bonferroni-corrected P values; *, P < 0.05; **, P < 0.01). (B) CCL22 protein levels produced by B cells exposed to the listed agents for 24 h at 37°C were estimated using a commercial multiplex assay. Experiments were performed using three different donors. Asterisks denote statistically significant data (ANOVA test, Bonferroni-corrected P values; *, P < 0.05; **, P < 0.01). (C) Chemotactic activity for autologous tonsillar CD4+ T cells of soluble factors released by B cells following a 16-h exposure to the listed agents has been monitored as described in Materials and Methods. These experiments have been performed with three independent biological replicates. The percentages of migration were determined from the original cell input and represent 2, 1, 2, 16, and 7% for mock, mock/CD40L, NL4-3, NL4-3/CD40L, and anti-CD40, respectively. The asterisks represent a P value lower than 0.05 obtained by a Dunnett's multiple-comparison test repeated-measure ANOVA relative to the mock control.
Considering that CD40L is acquired by HIV-1 emerging from activated CD4+ T cells and that CD40-activated B cells secrete chemoattractant factors, we assessed whether the levels of secreted chemokines, which are produced upon the binding of CD40L-bearing viruses onto B cells, were sufficient to attract CD4+ T cells. Cell migration experiments thus were performed by using a permeable cell support system separating B cells and CD4+ T cells isolated from the same tonsillar tissue. As depicted in Fig. 6C, soluble factors produced by B cells exposed to CD40L-bearing virions or anti-CD40 are able to induce a significant migration of autologous CD4+ T cells compared to that of controls. Although such cell migration is not necessarily mediated exclusively by CCL17 and/or CCL22, it is a clear demonstration that a simple physical contact between B cells and viral entities bearing host-derived CD40L is sufficient per se to attract activated CD4+ T cells that ultimately can serve as cellular targets for HIV-1.
DISCUSSION
In this work, we provide evidence that HIV-1 can incorporate CD40L under in vivo situations and that the molecule is functionally capable of transducing signal through its cognate receptor CD40 on tonsillar B cells, inducing transcriptomic changes consistent with activated and differentiated phenotypes. We first assessed the physical presence of CD40L on virus isolated from plasma of HIV-1-infected patients by an immunomagnetic capture step followed by a sensitive qRT-PCR technique. We report here the presence of host-derived CD40L on plasma virus derived from three different patients. The fact that we were able to detect CD40L-bearing viruses from plasma samples, even at relatively moderate levels, indicates that this cell surface constituent can be incorporated under natural conditions, which provide physiological significance to our observations. Circulating blood is known to harbor a low percentage of activated CD4+ T cells positive for CD40L, and higher levels of CD40L-expressing CD4+ T cells are present in lymphoid organs where antigenic presentation is taking place. Unfortunately, we were unable to obtain tonsillar tissues from HIV-1-infected patients to test if CD40L-bearing virions were found in greater amounts in this compartment, which is rich in activated CD4+ T cells. The fact that CD40L is an inducible molecule that is expressed very transiently only on T cells might help to explain why it is incorporated at lower levels than HLA-DR, which is constitutively expressed at relatively high levels on all natural cellular reservoirs of HIV-1 (i.e., CD4+ T cells, macrophages, and dendritic cells). It should be noted that a recent work by Epeldegui and colleagues reported the physical presence of host CD40L on the surface of virions circulating in the plasma from two out of three HIV-1-infected persons (18). Therefore, our observations validate this report and render experiments aimed at monitoring the biological activity of virus-associated CD40L highly important.
The dysregulation of the B-cell compartment has been recognized early on as a characteristic of HIV-1 infection and has been shown to be multifactorial, as some aberrant features can be correlated to higher viral loads, while others are maintained even under efficient antiretroviral therapy. Some of these unique clinical features include high serum levels of IgG and autoantibodies and an abnormal expression of B-cell markers associated with their activation, proliferation, and/or terminal differentiation (39). It is apparent that a complex process is in play, as many factors have been shown to possibly be involved in B-cell abnormalities, among which are the viral proteins gp120 (22) and Nef (11, 52), cytokines such as TNF-α (48), IFN-α (6, 32), IL-6 (58), IL-10 (44), and IL-7 (20, 45), and also ferritin (52). We now demonstrate that interactions between CD40L-bearing HIV-1 particles and B lymphocytes also result in the establishment of a hyperactivity state.
We have previously published that exposure of primary human B cells to CD40L-bearing HIV-1 can induce homotypic aggregation, the nuclear translocation of NF-κB, and the secretion of IL-6 and IgG (33). The detection of CD40L associated with HIV-1 directly from patient plasma prompted us to evaluate more precisely and extensively the level of B-cell activation induced by CD40L-bearing HIV-1 to determine whether such viruses could account, at least in part, for the various B-cell dysfunctions and hypergammaglobulinemia seen in infected patients. For this purpose, we devised a gene expression study involving purified tonsillar B cells that were exposed to isogenic HIV-1 particles either devoid or carrying host-derived CD40L. Virions were produced upon the transient transfection of 293T cells with the infectious molecular clone NL4-3 along with either a vector coding for human CD40L or an empty control vector. It is possible that the use of this transfection-and-expression system yields virus particles bearing amounts of host-derived CD40L higher than the levels found in virions circulating in patient plasma. However, the use of such an artificial system was necessary, as transcriptome analysis using microarrays requires a high degree of cellular and stimulus homogeneity to yield significant data (53). Although it is quite likely that circulating HIV-1 particles carry smaller amounts of incorporated CD40L molecules in the context of a natural infection, it also is true that the B-cell compartment will be exposed to CD40L-bearing viruses in a chronic manner during extended periods of time. Moreover, virions produced in anatomic sites containing a superior percentage of activated CD4+ T cells (e.g., secondary lymphoid organs) might incorporate larger amounts of CD40L. Interestingly, comparative analyses revealed that the levels of virus-associated CD40L in virions produced by peripheral blood mononuclear cells (PBMCs) and human lymphoid tissue culture ex vivo (i.e., tonsillar tissue) were only slightly lower than those from virions produced in transiently transfected 293T cells (34). It should be noted that PBMCs and tonsillar tissue were acutely infected with two different clinical isolates of HIV-1 (i.e., 92HT599/X4-tropic and 92US657/R5-tropic) to more closely parallel in vivo conditions.
We found a high degree of similarity between the transcriptome of B cells exposed to NL4-3/CD40L and anti-CD40, illustrating that both agents induced comparable CD40-mediated signal transduction pathways. The virus-mediated signal was sufficient to induce the transcription of a significant number of genes related to all aspects of B-cell activation and differentiation. Among these, we identified genes related to cell size and metabolism, immune functions, mRNA splicing and processing, protein production and degradation, as well as SHM and CSR. From crucial regulators of CD40/NF-κB signal transduction and apoptosis, such as TRAF1 and A20, to adhesion molecules (ICAM-1), costimulation molecules (CD80), and cytokines (LTA and LTB) associated with B-cell immune functions, to negative-feedback-loop transcripts (IκBα) and essential components involved in antibody gene recombination and hypermutation (AID), the resulting portrait is unequivocally akin to an activated, proliferating, and/or differentiating B-cell phenotype. The present findings extend beyond the study made by Epeldegui and coworkers who have assessed the capacity of CD40L-bearing HIV-1 particles to induce the activation of a single gene in B cells, namely, AID (18).
The increased surface expression of ICAM-1 is probably the main cause of the homotypic aggregation of B cells following incubation with CD40L-bearing virions or anti-CD40 stimulation (33). Enhanced ICAM-1 expression also could lead to much stronger B-lymphocyte-T-lymphocyte interactions, which could help antigen presentation but also could explain why CD40-stimulated B cells are better at transferring HIV-1 to activated CD4+ T cells (33).
Secondary lymphoid tissues are specialized organs in which activated T cells can interact with B cells and follicular dendritic cells and lead to the formation of germinal centers, the generation of highly specific antibodies, and their eventual recombination to produce other immunoglobulin forms. CD40 signaling not only is primordial for the response to thymus-dependent antigens by B cells but also is required for the generation of IgG and is a major regulator of long-lived antibody-mediated acquired immunity (reviewed in references 8, 24, 47, and 54). Thus, the expression of CD40L on the surface of activated T cells normally is tightly regulated at the transcriptional level and through rapid endocytosis following binding to its receptor (54). The eventual acquisition of host-derived CD40L by HIV-1 releases this crucial mediator of B-cell activation from the numerous cellular homeostatic controls regulating its spatial and temporal expression. Cell-free HIV-1 particles bearing CD40L can travel to other anatomic sites, where they possibly can disturb the normal B-cell activation process. Since CD40L expression cannot be regulated by endocytosis on the surface of the virus, CD40 stimulation could occur on repeated occasions, therefore amplifying B-cell differentiation signals and leading to chronic and generalized B-cell dysfunctions.
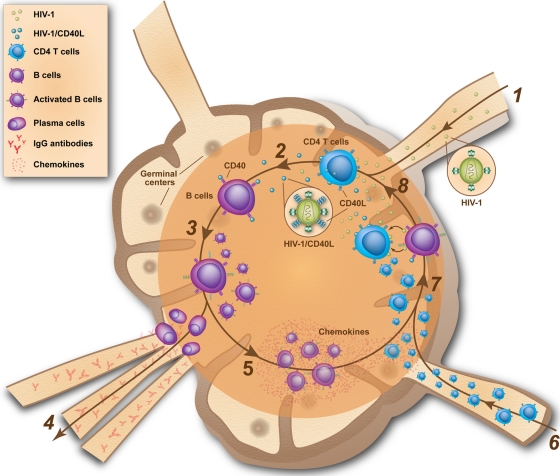
Taking results and conclusions from the current work together with those of previous studies, it can be proposed that CD40L is acquired by HIV-1 either replicating in vitro in primary human mononuclear cells and tonsil histocultures or in vivo in its natural cellular reservoirs. Our data further suggest that the engagement of cell surface CD40 by virus-associated CD40L can induce the polyclonal activation of tonsillar B cells, leading to cell growth and proliferation, activation, and terminal differentiation, resulting in class switch and IgG production, the expression of proinflammatory factors, and the secretion of memory CD4+ T-cell chemoattractants such as CCL17 and CCL22. Furthermore, CD40-stimulated B cells can transfer HIV-1 to activated CD4+ T cells in a more efficient manner than quiescent B lymphocytes (33). Based on these observations, we hypothesize that a positive feedback loop could be initiated whereby the attachment of CD40L-bearing HIV-1 onto B cells leads to polyclonal cell activation through CD40-mediated signal transduction (Fig. 7). Thereafter, CD40-stimulated B cells would secrete CCL17 and CCL22, attracting memory CD4+ T cells to the anatomic sites, where they would be exposed to both cell-free and B-cell-associated HIV-1 in a context of high levels of proinflammatory cytokines and costimulatory factors expressed by CD40-stimulated B cells. In such a specific environment, CD4+ T cells would be activated and thus become highly sensitive to productive HIV-1 infection, leading to the de novo synthesis of CD40L-bearing virions. As a by-product of polyclonal B-cell activation, proliferation, and terminal differentiation, the class switch recombination and somatic hypermutation of IgG would be induced, leading to the aberrant secretion of IgG and thus hypergammaglobulinemia.
FIG. 7.
Hypothetical model illustrating the possible implication of CD40L-bearing virions in B-lymphocyte phenotypic and functional alterations in HIV-1 disease. (1) Early after primary infection, HIV-1 concentrates in secondary lymphoid organs, where a high frequency of activated CD4+ T cells are located; (2) production of progeny viruses bearing host-derived CD40L on their surface from newly infected CD4+ T cells, (3) binding of CD40L-bearing virions to B lymphocytes residing within this microenvironment leads to polyclonal B-cell activation, proliferation, and terminal differentiation; (4) as a by-product of B-cell activation, class switch recombination and somatic hypermutation of IgG is induced, leading to the aberrant secretion of IgG and thus hypergammaglobulinemia; (5) CD40-stimulated B cells secrete soluble factors, among which are CCL17 and CCL22; (6) CD4+ T cells are recruited to the secondary lymphoid organs; (7) attracted CD4+ T cells are exposed to both cell-free and B cell-associated viruses in a context of high levels of proinflammatory cytokines and costimulatory factors expressed by CD40-stimulated B cells; (8) activated CD4+ T cells transiently express cell surface CD40L, which then can be acquired by de novo-produced virions, fuelling the feedback loop.
In conclusion, this report corroborates that HIV-1 does incorporate CD40L under in vivo conditions and demonstrates for the first time that the ligation of virion-associated host CD40L with cell surface CD40 is sufficient to efficiently activate B cells in a polyclonal fashion. Moreover, we demonstrate that this event can lead to the chemoattraction of CD4+ T cells, which could result in the efficient transfer/propagation of HIV-1 and enhanced production of CD40L-bearing HIV-1. We postulate that this phenomenon could contribute to some extent to the numerous B-cell dysfunctions associated with HIV-1 infection.
Supplementary Material
Acknowledgments
We thank Rafick-Pierre Sékaly for providing clinical plasma samples from HIV-1-infected patients. We are grateful to the Plateforme de Biopuces d'ADN of the Centre de Génomique du Québec for their assistance in scanning the Affymetrix microarrays.
This work was supported by an operating grant to M.J.T. from the Canadian Institutes of Health Research (CIHR) (HOP-14438). M.I. is the recipient of a CIHR Doctoral Award, while M.J.T. holds the Canada Research Chair in Human Immuno-Retrovirology (tier 1 level).
Footnotes
Published ahead of print on 22 December 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Adachi, A., et al. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, S. J., and E. P. Reddy. 1998. Modulation of life and death by the TNF receptor superfamily. Oncogene 17:3261-3270. [DOI] [PubMed] [Google Scholar]
- 3.Berek, C., and M. Ziegner. 1993. The maturation of the immune response. Immunol. Today 14:400-404. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, G. A., and B. S. Hostager. 2001. Signaling by CD40 and its mimics in B cell activation. Immunol. Res. 24:97-109. [DOI] [PubMed] [Google Scholar]
- 5.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4(+)-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., et al. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365-1371. [DOI] [PubMed] [Google Scholar]
- 7.Burstein, H. J., and A. K. Abbas. 1991. T-cell-mediated activation of B cells. Curr. Opin. Immunol. 3:345-349. [DOI] [PubMed] [Google Scholar]
- 8.Calderhead, D. M., Y. Kosaka, E. M. Manning, and R. J. Noelle. 2000. CD40-CD154 interactions in B-cell signaling. Curr. Top. Microbiol. Immunol. 245:73-99. [DOI] [PubMed] [Google Scholar]
- 9.Cantin, R., J. F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. [DOI] [PubMed] [Google Scholar]
- 10.Chaplin, D. D., and Y. Fu. 1998. Cytokine regulation of secondary lymphoid organ development. Curr. Opin. Immunol. 10:289-297. [DOI] [PubMed] [Google Scholar]
- 11.Chirmule, N., N. Oyaizu, C. Saxinger, and S. Pahwa. 1994. Nef protein of HIV-1 has B-cell stimulatory activity. AIDS 8:733-734. [DOI] [PubMed] [Google Scholar]
- 12.Chong, Y., et al. 2004. Downregulation of CXCR5 in CD27- B cells of HIV-1 infected patients. J. Med. Virol. 73:362-367. [DOI] [PubMed] [Google Scholar]
- 13.Cognasse, F., et al. 2005. Differential downstream effects of CD40 ligation mediated by membrane or soluble CD40L and agonistic Ab: a study on purified human B cells. Int. J. Immunopathol. Pharmacol. 18:65-74. [DOI] [PubMed] [Google Scholar]
- 14.Dennis, G., jr., et al. 2003. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 4:P3. [PubMed] [Google Scholar]
- 15.Drayton, D. L., et al. 2002. Lymphocyte traffic in lymphoid organ neogenesis: differential roles of Ltalpha and LTalphabeta. Adv. Exp. Med. Biol. 512:43-48. [PubMed] [Google Scholar]
- 16.Drosten, C., et al. 2006. Ultrasensitive monitoring of HIV-1 viral load by a low-cost real-time reverse transcription-PCR assay with internal control for the 5′ long terminal repeat domain. Clin. Chem. 52:1258-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durandy, A. 2003. Activation-induced cytidine deaminase: a dual role in class-switch recombination and somatic hypermutation. Eur. J. Immunol. 33:2069-2073. [DOI] [PubMed] [Google Scholar]
- 18.Epeldegui, M., et al. 2010. CD40 ligand (CD154) incorporated into HIV virions induces activation-induced cytidine deaminase (AID) expression in human B lymphocytes. PLoS One 5:e11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier, A. M., et al. 2002. Spontaneous secretion of immunoglobulins and anti-HIV-1 antibodies by in vivo activated B lymphocytes from HIV-1-infected subjects: monocyte and natural killer cell requirement for in vitro terminal differentiation into plasma cells. Clin. Immunol. 103:98-109. [DOI] [PubMed] [Google Scholar]
- 20.Fry, T. J., et al. 2001. A potential role for interleukin-7 in T-cell homeostasis. Blood 97:2983-2990. [DOI] [PubMed] [Google Scholar]
- 21.Han, S., B. Zheng, Y. Takahashi, and G. Kelsoe. 1997. Distinctive characteristics of germinal center B cells. Semin. Immunol. 9:255-260. [DOI] [PubMed] [Google Scholar]
- 22.He, B., et al. 2006. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 176:3931-3941. [DOI] [PubMed] [Google Scholar]
- 23.Kornbluth, R. S., K. Kee, and D. D. Richman. 1998. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive beta-chemokines. Proc. Natl. Acad. Sci. U. S. A. 95:5205-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laman, J. D., E. Claassen, and R. J. Noelle. 1996. Functions of CD40 and its ligand, gp39 (CD40L). Crit. Rev. Immunol. 16:59-108. [DOI] [PubMed] [Google Scholar]
- 25.Lane, H. C., et al. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453-458. [DOI] [PubMed] [Google Scholar]
- 26.Larson, E. D., and N. Maizels. 2004. Transcription-coupled mutagenesis by the DNA deaminase AID. Genome Biol. 5:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn, S. D., and S. T. Butera. 2000. Incorporation of HLA-DR into the envelope of human immunodeficiency virus type 1 in vivo: correlation with stage of disease and presence of opportunistic infection. J. Virol. 74:10256-10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledbetter, J. A., et al. 1997. Agonistic activity of a CD40-specific single-chain Fv constructed from the variable regions of mAb G28-5. Crit. Rev. Immunol. 17:427-435. [PubMed] [Google Scholar]
- 29.Ledbetter, J. A., L. S. Grosmaire, D. Hollenbaugh, A. Aruffo, and S. G. Nadler. 1994. Agonistic and antagonistic properties of CD40 mAb G28-5 are dependent on binding valency. Circ. Shock 44:67-72. [PubMed] [Google Scholar]
- 30.Levesque, M. C., et al. 2009. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 6:e1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malaspina, A., et al. 2005. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J. Infect. Dis. 191:1442-1450. [DOI] [PubMed] [Google Scholar]
- 32.Mandl, J. N., et al. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14:1077-1087. [DOI] [PubMed] [Google Scholar]
- 33.Martin, G., et al. 2007. Human immunodeficiency virus type 1-associated CD40 ligand transactivates B lymphocytes and promotes infection of CD4+ T cells. J. Virol. 81:5872-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin, G., and M. J. Tremblay. 2004. HLA-DR, ICAM-1, CD40, CD40L, and CD86 are incorporated to a similar degree into clinical human immunodeficiency virus type 1 variants expanded in natural reservoirs such as peripheral blood mononuclear cells and human lymphoid tissue cultured ex vivo. Clin. Immunol. 111:275-285. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto, M. 1999. Role of TNF ligand and receptor family in the lymphoid organogenesis defined by gene targeting. J. Med. Investig. 46:141-150. [PubMed] [Google Scholar]
- 36.Matsumoto, M., Y. X. Fu, H. Molina, and D. D. Chaplin. 1997. Lymphotoxin-alpha-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers. Immunol. Rev. 156:137-144. [DOI] [PubMed] [Google Scholar]
- 37.Matsuyama, T., N. Kobayashi, and N. Yamamoto. 1991. Cytokines and HIV infection: is AIDS a tumor necrosis factor disease? AIDS 5:1405-1417. [DOI] [PubMed] [Google Scholar]
- 38.Medrano, F. J., et al. 1998. Tumor necrosis factor beta and soluble APO-1/Fas independently predict progression to AIDS in HIV-seropositive patients. AIDS Res. Hum. Retrovir. 14:835-843. [DOI] [PubMed] [Google Scholar]
- 39.Moir, S., and A. S. Fauci. 2009. B cells in HIV infection and disease. Nat. Rev. Immunol. 9:235-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moir, S., et al. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205:1797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moir, S., et al. 2001. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc. Natl. Acad. Sci. U. S. A. 98:10362-10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moir, S., et al. 2003. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc. Natl. Acad. Sci. U. S. A. 100:6057-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosmann, T. R. 1994. Cytokine patterns during the progression to AIDS. Science 265:193-194. [DOI] [PubMed] [Google Scholar]
- 44.Müller, F., P. Aukrust, I. Nordoy, and S. S. Froland. 1998. Possible role of interleukin-10 (IL-10) and CD40 ligand expression in the pathogenesis of hypergammaglobulinemia in human immunodeficiency virus infection: modulation of IL-10 and Ig production after intravenous Ig infusion. Blood 92:3721-3729. [PubMed] [Google Scholar]
- 45.Napolitano, L. A., et al. 2001. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7:73-79. [DOI] [PubMed] [Google Scholar]
- 46.Notermans, D. W., et al. 2001. Potent antiretroviral therapy initiates normalization of hypergammaglobulinemia and a decline in HIV type 1-specific antibody responses. AIDS Res. Hum. Retrovir. 17:1003-1008. [DOI] [PubMed] [Google Scholar]
- 47.Quezada, S. A., L. Z. Jarvinen, E. F. Lind, and R. J. Noelle. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307-328. [DOI] [PubMed] [Google Scholar]
- 48.Rieckmann, P., G. Poli, C. H. Fox, J. H. Kehrl, and A. S. Fauci. 1991. Recombinant gp120 specifically enhances tumor necrosis factor-alpha production and Ig secretion in B lymphocytes from HIV-infected individuals but not from seronegative donors. J. Immunol. 147:2922-2927. [PubMed] [Google Scholar]
- 49.Shirai, A., M. Cosentino, S. F. Leitman-Klinman, and D. M. Klinman. 1992. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J. Clin. Investig. 89:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spahn, T. W., and T. Kucharzik. 2004. Modulating the intestinal immune system: the role of lymphotoxin and GALT organs. Gut 53:456-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spahn, T. W., M. K. Muller, W. Domschke, and T. Kucharzik. 2006. Role of lymphotoxins in the development of Peyer's patches and mesenteric lymph nodes: relevance to intestinal inflammation and treatment. Ann. N. Y. Acad. Sci. 1072:187-193. [DOI] [PubMed] [Google Scholar]
- 52.Swingler, S., et al. 2008. Evidence for a pathogenic determinant in HIV-1 Nef involved in B cell dysfunction in HIV/AIDS. Cell Host Microbe 4:63-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szaniszlo, P., et al. 2004. Getting the right cells to the array: gene expression microarray analysis of cell mixtures and sorted cells. Cytometry A 59:191-202. [DOI] [PubMed] [Google Scholar]
- 54.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2-17. [DOI] [PubMed] [Google Scholar]
- 55.Vyakarnam, A., et al. 1991. Altered production of tumour necrosis factors alpha and beta and interferon gamma by HIV-infected individuals. Clin. Exp. Immunol. 84:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vyakarnam, A., J. McKeating, A. Meager, and P. C. Beverley. 1990. Tumour necrosis factors (alpha, beta) induced by HIV-1 in peripheral blood mononuclear cells potentiate virus replication. AIDS 4:21-27. [DOI] [PubMed] [Google Scholar]
- 57.Weill, J. C., and C. A. Reynaud. 1996. Rearrangement/hypermutation/gene conversion: when, where and why? Immunol. Today 17:92-97. [DOI] [PubMed] [Google Scholar]
- 58.Weimer, R., et al. 1998. HIV-induced IL-6/IL-10 dysregulation of CD4 cells is associated with defective B cell help and autoantibody formation against CD4 cells. Clin. Exp. Immunol. 111:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.