Abstract
Viral vaccine vectors have emerged as an attractive strategy for the development of a human immunodeficiency virus (HIV) vaccine. Recombinant Newcastle disease virus (rNDV) stands out as a vaccine vector since it has a proven safety profile in humans, it is a potent inducer of both alpha interferon (IFN-α) and IFN-β) production, and it is a potent inducer of dendritic cell (DC) maturation. Our group has previously generated an rNDV vector expressing a codon-optimized HIV Gag protein and demonstrated its ability to induce a Gag-specific CD8+ T cell response in mice. In this report we demonstrate that the Gag-specific immune response can be further enhanced by the targeting of the rNDV-encoded HIV Gag antigen to DCs. Targeting of the HIV Gag antigen was achieved by the addition of a single-chain Fv (scFv) antibody specific for the DC-restricted antigen uptake receptor DEC205 such that the DEC205 scFv-Gag molecule was encoded for expression as a fusion protein. The vaccination of mice with rNDV coding for the DC-targeted Gag antigen induced an enhanced Gag-specific CD8+ T cell response and enhanced numbers of CD4+ T cells and CD8+ T cells in the spleen relative to vaccination with rNDV coding for a nontargeted Gag antigen. Importantly, mice vaccinated with the DEC205-targeted vaccine were better protected from challenge with a recombinant vaccinia virus expressing the HIV Gag protein. Here we demonstrate that the targeting of the HIV Gag antigen to DCs via the DEC205 receptor enhances the ability of an rNDV vector to induce a potent antigen-specific immune response.
Almost 3 decades since the identification of HIV as the causative agent of AIDS, the development of a prophylactic vaccine against the virus is still proving to be a daunting task. Two phase III HIV-1 vaccine trials, Merck's STEP trial and VaxGen's AIDSVAX B/E vaccine trial, failed to prevent HIV-1 infection and had no effect on viral load in trial participants who later became infected (2, 28). A phase III clinical trial employing Aventis Pasteur's canarypox virus vector, Alvac-HIV (vCP1521), as a prime and VaxGen's rgp120 (recombinant glycoprotein 120) (AIDSVax B/E) as a protein boost showed a modest reduction in HIV-1 infection (30). The combination of both of these immunogens was intended to elicit strong HIV-specific humoral and cellular immune responses; it is widely believed that an effective HIV vaccine will have to activate both the humoral and cellular arms of the adaptive immune system (2). Although the underpinnings of the observed degree of protection remain undetermined, this study emphasized the importance of viral vaccine vectors like the Alvac-HIV canarypox virus vector as a major component of any HIV vaccine regimen, since a phase III trial of VaxGen bivalent rgp120 alone showed no effect on HIV-1 acquisition (28). The Alvac-HIV canarypox virus vector was shown previously to induce a low level of HIV-specific CD8+ T cells in a phase II clinical trial (32). The optimization of viral vectors to enhance the induction of HIV-specific mucosal and systemic immunity will likely further increase the efficacy of virus-based HIV vaccine constructs.
Newcastle disease virus (NDV) is a member of the genus Avulavirus of the family Paramyxoviridae, a genus that does not include any known natural pathogens of humans. The NDV genome consists of a small, single-stranded, negative-sense RNA genome containing six transcriptional units, NP, P, M, F, HN, and L (1). Several characteristics make NDV a promising live vaccine vector candidate. First, NDV is well tolerated by humans. Previously reported clinical trials that employed NDV for its oncolytic activity demonstrated that repeated intravenous dosing is associated with few adverse side effects (11). Second, in contrast to viral vectors used in the design of HIV vaccines, such as adenoviruses (Ad5) or human paramyxoviruses (measles virus and mumps virus), there is no preexisting immunity to NDV in the majority of the adult human population (1). Preexisting antibodies in the host can reduce the efficacy of a viral vector by diminishing levels of infection. A reverse genetics system for NDV has been developed and used to generate recombinant viruses stably expressing foreign antigens (4, 22, 26, 27). Recombinant NDV (rNDV) grows to high titers in embryonated chicken eggs and cell culture and generates a strong immune response in murine and nonhuman primate models, making this vector an attractive vaccine candidate (4, 9, 22, 23). Finally, NDV codes for fewer proteins that may compete for immunodominant epitopes between the viral vector and the expressed foreign antigens.
Our group has previously generated an rNDV vector expressing a synthetic codon-optimized HIV-1 Gag protein and demonstrated its ability to induce a potent Gag-specific CD8+ T cell response in mice (5). In order to increase the immunogenicity of the rNDV vaccine, we sought to target the encoded antigen to dendritic cells (DCs). DCs are the most efficient antigen-presenting cells (APCs) described to date; however, only a fraction of the antigen-pulsed DCs reach regional lymph nodes, where cell-to-cell contact between DCs and T cells is required for effective T cell induction (5, 8). To overcome this limitation, antigens have been targeted to DCs by the conjugation/fusion of the antigen to DC receptor-specific monoclonal antibodies (MAbs) or single-chain Fv (scFv) molecules (6). Among the surface molecules expressed by dendritic cells, DEC205 may be particularly suitable for DC targeting. DEC205 is an endocytic receptor of the macrophage mannose receptor family and is expressed on DCs in the T cell area of lymphoid organs (15). More importantly, in addition to cross-presenting antigens to CD8+ T cells, DEC205 targets antigens to the major histocompatibility complex (MHC) class II-positive late endosomal compartment, resulting in superior antigen presentation compared with pinocytosis or phagocytosis (20). In addition, DEC205 was shown previously to access the cross-presentation pathway of many human HLA haplotypes (3). A DNA vaccine encoding an HIV-1 Gag-scFv DEC205 fusion protein increased both Gag antibody titers and the numbers of Gag-specific gamma interferon (IFN-γ)-producing T cells compared to a nontargeted vaccine in mice (25). In addition, the Gag-specific immune response was further enhanced when the targeted DNA vaccine was combined with synergistic Toll-like receptor (TLR) ligands that induce interferon production and consequently induce the maturation of DCs (16). In this study, using a murine model, we confirm the efficacy of rNDV as a vector for the expression and delivery of the HIV Gag protein and demonstrate that the targeting of the Gag protein to DCs through the DEC205 receptor enhances the anti-Gag immune response.
MATERIALS AND METHODS
Cells, viruses, and animals.
Vero, A549, CHOneo, CHOmDEC205, and CV-1 cells where maintained in Dulbecco modified Eagle medium containing 10% fetal bovine serum (FBS) and 1% penicillin streptomycin. Wild-type New York City Board of health vaccinia virus (Vac-Wt) was kindly provided by Gomez Yafal at Therion Biologicals, and recombinant vaccinia virus expressing HIV-1 Gag (Vac-gag) (vP1287) was kindly provided by Matthias J. Schnell. Both vaccinia viruses were grown and titrated in CV-1 cells. Six- to eight-week old female BALB/c mice and CxB6 F1 mice were obtained from Charles River. All animal care and procedures were in accordance with the animal experimentation guidelines of the National Institutes of Health (NIH).
Generation of full-length rNDV-LaSota cDNA.
To generate full-length rNDV-LaSota cDNA, the LaSota genome was divided to 9 segments, and each segment was obtained by reverse transcription (RT)-PCR using RNA purified from the NDV-LaSota virus, followed by cloning into the pGEM-T vector (Promega) and sequencing. Each segment was then digested, ligated, and inserted into the HindIII and BssHII positions of vector pSL1180 (22). Finally, a segment which contained the sequence of the hepatitis delta virus (HDV) ribozyme and T7 terminator was digested from an rNDV-Hitchner B1 cDNA plasmid (22) using a BssHII enzyme and ligated into the BssHII position of the plasmid containing full-length rNDV-LaSota cDNA. The primer sequences are available upon request.
Generation of rNDV vaccine vectors.
scFv-Gagp41 open reading frame (ORF) sequences were obtained by PCR using plasmids pE-mISO-olla-P41 and pE-mDEC-olla-p41 as templates, which code for scCont-Gagp41 and scDEC-Gagp41, respectively (25). The green fluorescent protein (GFP) ORF sequence was obtained by PCR using pEGFP-C1 (BD Biosciences-Clontech) as a template. The amplified cDNAs were cloned into NDV transcriptional units containing NDV-specific gene start and gene end sequences. An optimal Kozak translation sequence was also included just upstream of the ATG. In order to follow the rule of six, necessary for NDV replication, nucleotides were inserted after the stop codon when needed. The scFv-Gagp41 and GFP transcriptional units were inserted into the SacII site in the NDV-LaSota genome between the P and M genes. The obtained plasmids were used to rescue the recombinant viruses by using previously described methods (22). The rescued viruses, rNDV-L-scDEC-Gagp41, rNDV-L-scCont-Gagp41, and rNDV-L-GFP, were passaged three times in eggs, and viral clones were isolated by limiting dilution in eggs. Clones were used to generate vector stocks from which the inserted segments were amplified using RT-PCR and sequenced. scFV-Gagp41-expressing rNDV vectors were titrated by indirect immunofluorescence in Vero cells infected with different dilutions of the vectors and by using an anti-NDV rabbit serum followed by incubation with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit immunoglobulin G (IgG) (Sigma-Aldrich). rNDV-L-GFP virus was titrated by direct fluorescence on Vero cells.
rNDV growth kinetics in embryonated chicken eggs.
Ten-day-old eggs were infected with 100 fluorescence-forming unit (FFU) of each of the different rNDV vectors. Allantoic fluids were harvested at 24, 48, 72, and 96 h after infection. Viral titers were measured by indirect immunofluorescence on Vero cells for the scFv-Gagp41-expressing vectors and by direct fluorescence for rNDV-L-GFP.
Transgene expression profile.
Vero cells were inoculated with each rNDV vector at a multiplicity of infection (MOI) of 1. Cell lysates and cell supernatant were harvested 12, 24, and 36 h after infection. Proteins were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Membranes were then incubated with anti-NDV rabbit serum followed by incubation with anti-rabbit IgG peroxidase-labeled antibody (Amersham) to detect NDV proteins. For the detection of scFv-Gagp41, membranes were incubated with an anti-HIV1 p24 monoclonal antibody (Abcam) followed by anti-mouse IgG peroxidase-labeled antibody (Amersham).
DEC205 binding.
Vero cells were infected with either rNDV-scDEC-Gagp41 or rNDV-scCont-Gagp41 at an MOI of 5. At 24 h postinfection, the supernatant was harvested and cleared from cell debris by centrifugation at 10,000 rpm for 30 min. Supernatants were then incubated with either CHOneo or CHOmDEC205 cells for 30 min at 4°C. The cells were washed and incubated with an FITC-labeled mouse anti-E. coli OmpF linker and mouse langerin fusion sequence (anti-OLLAS) antibody for 30 min at 4°C, and cells were then washed and analyzed by flow cytometry.
Immunizations of mice.
Groups of 4- to 6-week-old female CxB6 F1 mice where intranasally immunized with 5 × 105 FFU of rNDV-L-scDEC-Gagp41, rNDV-L-scCont-Gagp41, or rNDV-L-GFP. Four weeks later mice were boosted with 106 FFU of the same vector.
Challenge infections with Vac-gag and Vac-Wt.
Four weeks after the boost, groups of CxB6 F1 mice (5 mice per group) were challenged with 106 PFU of either an HIV-1 Gag-expressing vaccinia virus (Vac-gag) or a wild-type vaccinia virus (Vac-Wt). Six days after the challenge, mice were sacrificed, and their lungs were extracted and homogenized for vaccinia virus titration on CV-1 cells. Two days after the infection CV-1 cells were fixed and stained with 0.1% crystal violet solution to count the number of vaccinia virus plaques.
Dose-response experiments.
Groups of 6- to 8-week-old female BALB/c mice (n = 10) were intranasally immunized in a homologous prime-and-boost vaccine regimen with graded doses (10-fold dilutions) of either rNDV-L-scDEC-Gagp41 or rNDV-L-scCont-Gagp41. The mice were allowed to rest for 3 weeks between the prime and boost. The following groups were used: mice primed with 5 × 105 FFU and boosted with 106 FFU of NDV-L-scDEC-Gagp41 (DEC A group), mice primed with 5 × 105 FFU and boosted with 106 FFU of NDV-L-scCont-p41 (Cont A group), mice primed with 5 × 104 FFU and boosted with 105 FFU of NDV-L-scDEC-Gagp41 (DEC B group), mice primed with 5× 104 FFU and boosted with 105 FFU of NDV-L-scCont-p41 (Cont B group), mice primed with 5 × 103 FFU and boosted with 104 FFU of NDV-L-scDEC-Gagp41 (DEC D group), and mice primed with 5 × 103 FFU and boosted with 104 FFU of NDV-L-scCont-p41 (Cont D mice). Negative-control mice (GFP group) were primed with 5 × 105 FFU of rNDV-L-GFP and boosted with 106 FFU of the same vector. Three weeks after the last immunization, mice (n = 5) were challenged intranasally with 106 PFU of either Vac-gag or Vac-Wt. Five days after the challenge, lungs were extracted and homogenized, and vaccinia virus titers were determined by using a plaque assay on CV-1 cells.
Serum antibody titers.
For the detection of HIV Gagp41-specific antibodies, high-protein-binding enzyme-linked immunosorbent assay (ELISA) plates were coated overnight at 4°C with 0.2 μg/ml of Gagp41 protein in phosphate-buffered saline (PBS). Plates were washed three times with PBS-Tween 20 (0.02%) and blocked for 2 h using PBS-1% bovine serum albumin (BSA) at room temperature. Plates were washed with PBS-Tween 20 and incubated with serial dilutions of mouse sera for 3 h at 37°C. Plates were washed five times with PBS-Tween 20 (0.02%) and incubated with alkaline phosphatase-labeled anti-mouse IgG (Southern Biotech) antibody for 3 h at 37°C. Plates were washed, and the optical density (OD) was determined at 405 nm with a plate reader after the addition of p-nitrophenyl phosphate solution (Sigma). For the detection of NDV-specific antibody, the same protocol was followed, with the exception that medium-protein binding plates were coated with sucrose cushion (30%)-purified rNDV-LaSota at a concentration of 0.5 μg/ml, and bound anti-NDV antibodies were detected by alkaline phosphatase-labeled anti-mouse heavy-plus-light-chain (H+L) antibody (Southern Biotech).
Intracellular cytokine staining.
Bulk splenocytes were restimulated with either 0.2 μg/ml of an HIV Gagp41 pool mix of 15-mer peptides staggered every 4 amino acids along the HIV-1 Gagp41 sequence or 2 μg/ml of a nonreactive peptide (HIV Gag p17 pool 1) as a negative control (Proteomic Resource Center, the Rockefeller University) in the presence of 2 μg/ml of anti-CD28 antibody (eBioscience) for 6 h, adding brefeldin A (Sigma-Aldrich) the last 4 h. After blocking Fcγ receptors (rat anti-mouse CD16/CD32; BD Biosciences), cells were stained with the antibodies CD3-Pacific Blue, CD8-FITC, and CD4-peridinin chlorophyll protein (PerCP) (eBioscience) for 20 min at 37°C. Dead cells were excluded by using Aqua Live/Dead stain (Invitrogen). Cells were washed, fixed (Cytofix/Cytoperm Plus; BD Biosciences), permeabilized with PermWash, and stained with antibodies to IFN-γ (Alexa Fluor 700), tumor necrosis factor alpha (TNF-α) (CY7-phycoerythrin [PE]), and interleukin-2 (IL-2) (allophycocyanin) (eBioscience) for 15 min at room temperature. Stained samples were analyzed by flow cytometry using a BD LSRII instrument, and Flowjo software (Tree Star) was used for data analysis. Data are representative of three experiments where spleens from two mice were pooled in each experiment. We acquired 100,000 events in the live-cell/CD3+ gate; the x axis indicates CD8+/CD4+ cells, and the y axis indicates cytokine expression (IFN-γ, TNF-α, and IL-2). To examine multiple-cytokine-expressing T cells, we gated on either CD3+ CD4+ or CD3+ CD8+ T cells that are also positive for one cytokine and examined the total number of cells that expressed either one or both of the other two cytokines. The mean total number of cytokine-forming cells was calculated as the difference between Gag-stimulated samples and samples stimulated with the unreactive peptide.
Tetramer staining.
Isolated splenocytes and lung cells were blocked with Fc block (rat anti-mouse CD16/CD32; BD Bioscience) for 1 h at room temperature. Cells were then washed with fluorescence-activated cell sorter (FACS) buffer (2% BSA-PBS) and stained for 30 min using a PE-Gag tetramer (H-2Kd-restricted AMQLMLKETI epitope; Beckman Coulter) and antibodies to CD3 (Pacific Blue), CD8 (FITC), and CD4 (PerCP) (eBioscience). Dead cells were excluded by using Aqua Live/Dead stain (Invitrogen). Stained samples were analyzed by flow cytometry using a BD LSRII instrument, and Flowjo software (Tree Star) was used for data analysis.
Statistical analysis.
An unpaired two-tailed t test was used to deduce the difference between groups of vaccinated mice. A P value of <0.05 indicates significance between two groups (indicated with an asterisk in the figures).
RESULTS
Characterization of the viral vaccine constructs.
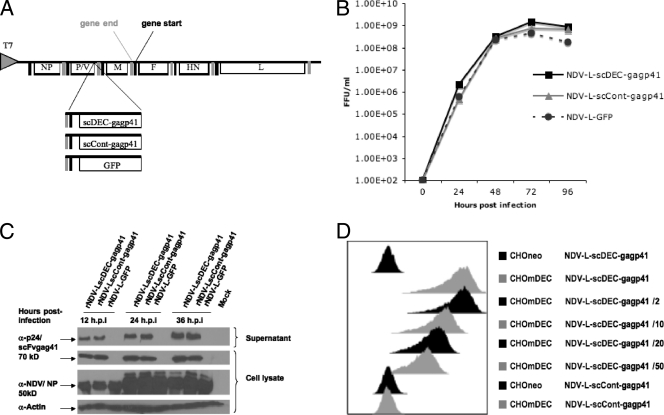
An rNDV expressing the HIV Gag protein was previously generated, and the optimal insertion site in the NDV genome to achieve the highest level of Gag expression that consequently induced the most potent Gag-specific cellular immune response was determined (5). Using previously defined reverse genetics techniques, rNDV vectors expressing either a targeted or untargeted HIV Gagp41 protein were generated in the lentogenic LaSota background (Fig. 1 A). For the vector expressing DEC205-targeted HIV Gagp41, rNDV-L-scDEC-Gagp41, HIV Gagp41 conjugated to the variable regions of the light and heavy chains of the parental anti-DEC205 antibody (NLDC145) (25) was inserted between the P and M genes of the rNDV genome. The Gagp41 gene expresses a truncated version of the strain NL4-3 Gag protein. The inserted construct presented a typical scFv structure. The variable domain of the immunoglobulin heavy chain (VH) was linked to light chain 1 via an interchain linker in a VH-VL orientation, and scFv was fused in frame to the Gagp41 sequence. In addition, a 14-amino-acid sequence corresponding to the OLLAS epitope tag was inserted between scFv and the Gagp41 sequence. Similarly, for the untargeted vector, HIV Gagp41 fused to the variable regions of the light and heavy chains of a nonreactive antibody (the same isotype as NLDC145) was inserted between the P and M genes of the NDV genome, rNDV-L-scCont-Gagp41. The negative-control vector rNDV-L-GFP consisted of an rNDV vector with a GFP gene inserted between the P and M genes (Fig. 1A). Rescued viral clones were isolated by limiting dilution in eggs, and the inserted segments were amplified by reverse transcription-PCR and sequenced in order to confirm the integrity of the vaccine constructs.
FIG. 1.
Characterization of the viral vaccine constructs. (A) Schematic diagram of the different recombinant NDVs expressing either the DC-targeted or the untargeted HIV Gag protein. A transcriptional unit containing either scDEC-Gagp41, scCont-Gagp41, or GFP was inserted between the P and M genes of NDV. (B) Growth kinetics of NDVs in embryonated chicken eggs. (C) Cell lysates and supernatants from Vero cells infected for 12, 24, or 36 h with rNDV-L-scDECGagp41, rNDV-scCont-Gagp41, or rNDV-L-GFP viruses (MOI of 1) were subjected to SDS-PAGE and were transferred onto nitrocellulose membranes. Membranes were immunoblotted by using anti-Gag p24 antibody, anti-NDV rabbit serum, or anti-actin as a loading control. (D) Binding to the murine DEC205 receptor. Binding to CHOmDEC205 or CHOneo control cells of supernatants from Vero cells infected for 36 h with recombinant NDV-L-scDEC-Gagp41 or NDV-L-scCont-Gagp41 at an MOI of 5. Supernatants from NDV-L-scDEC-Gagp41-infected cells were either undiluted or diluted 1/2, 1/10, 1/20, or 1/50 in PBS. scFV-Gagp41 fusion proteins were detected by an OLLA-specific, FITC-labeled antibody.
In order to investigate whether the insertion of the foreign antigens in the NDV genome might have differentially affected the growth of one vector versus the other, we inoculated 10-day-old eggs with 100 FFU of the different rNDV vectors. The inoculated eggs were incubated at 37°C, and allantoic fluids were harvested every 24 h. The presence of infectious virus was determined by indirect immunofluorescence on Vero cells, probing for the scFv-Gagp41-expressing vectors, and by direct fluorescence for the GFP-expressing rNDV vector (Fig. 1B). At each time point, we found similar virus titers in the harvested allantoic fluids, indicating that all three rNDV vectors had similar replication and growth kinetics.
To determine the level of scFv-Gagp41 expression driven by the rNDV vectors, Vero cells were infected at an MOI of 1 with rNDV-L-scDEC-Gagp41, rNDV-L-scCont-Gagp41, or rNDV-L-GFP as a negative control. Cell lysates as well as supernatants were harvested at 12, 24, and 36 h postinfection, and a Western blot analysis was performed (Fig. 1C). At each time point we observed similar NP levels, suggesting comparable replication kinetics of the viral vectors in Vero cells. In addition, cells infected with the scFv-Gagp41-expressing rNDV vectors showed equivalent scFv-Gagp41 levels in the cell lysates as well as in the supernatants.
In order to confirm that scDEC-Gagp41 expressed by cells infected with rNDV-L-scDEC-Gagp41 retained DEC205 binding activity, Chinese hamster ovary cells stably expressing murine DEC205 (CHOmDEC205) or control CHO cells (CHOneo) were incubated with the supernatant of Vero cells infected with either rNDV-L-scDEC-Gagp41 or rNDV-L-scCont-Gagp41. scFv-Gagp41 binding was detected by flow cytometry analysis using an OLLAS-specific, FITC-labeled antibody (Fig. 1D). Supernatants from rNDV-L-scDEC-Gagp41-infected Vero cells showed binding activity toward CHOmDEC205 but not toward CHOneo cells; these supernatants retained binding activity even when diluted 1/50 in PBS. As expected, supernatants from cells infected with rNDV-L-scCont-GagP41 showed binding to neither CHOmDEC205 nor CHOneo cells. In summary, both the rNDV-L-scDEC-Gagp41 and rNDV-L-scCont-Gagp41 vectors had similar growth kinetics and equivalent transgene expression profiles; however, only supernatants from rNDV-L-scDEC-Gagp41-infected cells demonstrated DEC205 binding activity.
rNDV expressing a DEC205-targeted HIV-1 Gag antigen improves Gag-specific but not vector-specific antibody responses.
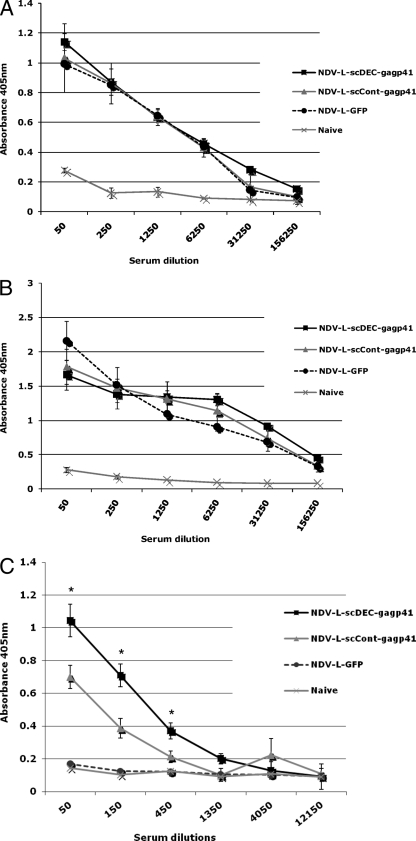
DEC205-targeted antigens were previously shown to enhance antigen-specific antibody responses when encoded by a plasmid DNA vaccine (25). To examine the antibody profile induced by rNDV vaccination, groups of six CxB6 F1 mice were immunized with 5 × 105 FFU of rNDV-L-scDEC-Gagp41, rNDV-L-scCont-Gagp41, or rNDV-L-GFP. Four weeks later mice were boosted with 106 FFU of the same vaccine vector. rNDV-specific antibodies were quantified by using an ELISA for the detection of total antibody against sucrose cushion-purified rNDV-LaSota (Fig. 2A and B). There were no significant differences in NDV-specific antibody titers in any of the 3 groups of vaccinated mice either at 30 days postprime (Fig. 2A) or at 30 days postboost (Fig. 2B). As expected, boosting with the rNDV vaccine vectors increased vector-specific antibody responses within each group (Fig. 2A and B). Next, an ELISA was performed to detect total serum IgG specific for the Gagp41 protein. Gag-specific total IgG antibodies were below our detection limit 30 days postprime (data not shown); however, 30 days after the boost, serum from mice vaccinated with rNDV-L-scDEC-Gagp41 showed a significant increase in levels of Gag-specific IgG compared to that in serum obtained from mice immunized with the nontargeted vector (Fig. 2C). These data indicate that even though vector-specific antibody responses were similar for all 3 groups of vaccinated mice (implying comparable replication kinetics in mice), the rNDV expressing a DEC205-targeted HIV Gagp41 antigen did enhance the antigen-specific humoral immune response compared to an rNDV expressing a nontargeted antigen.
FIG. 2.
Serum antibody titer after vaccination. (A and B) Total anti-NDV serum antibody titer in CxB6 F1 mice (6 mice per group) was determined 30 days after the first immunization or prime (A) and 30 days after the boost (B). (C) Anti-Gag IgG antibodies were determined 30 days postboost.
Induction of HIV Gagp41-specific cellular immune responses.
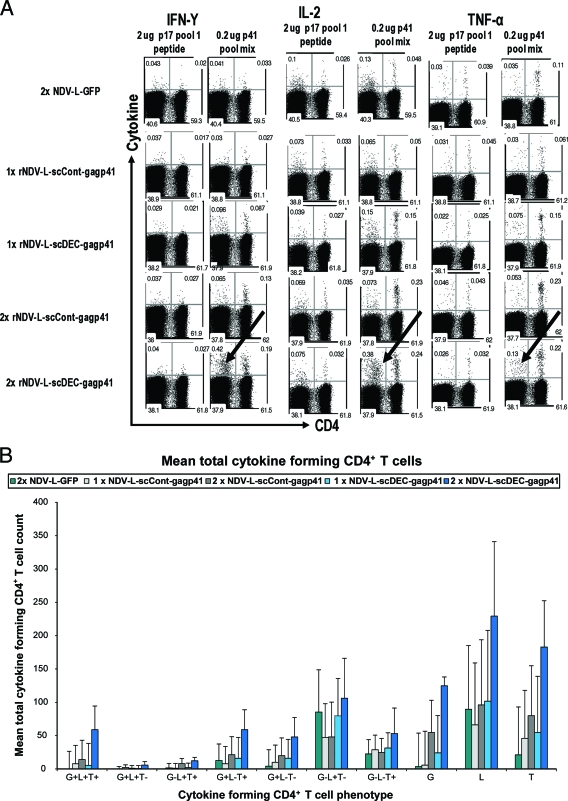
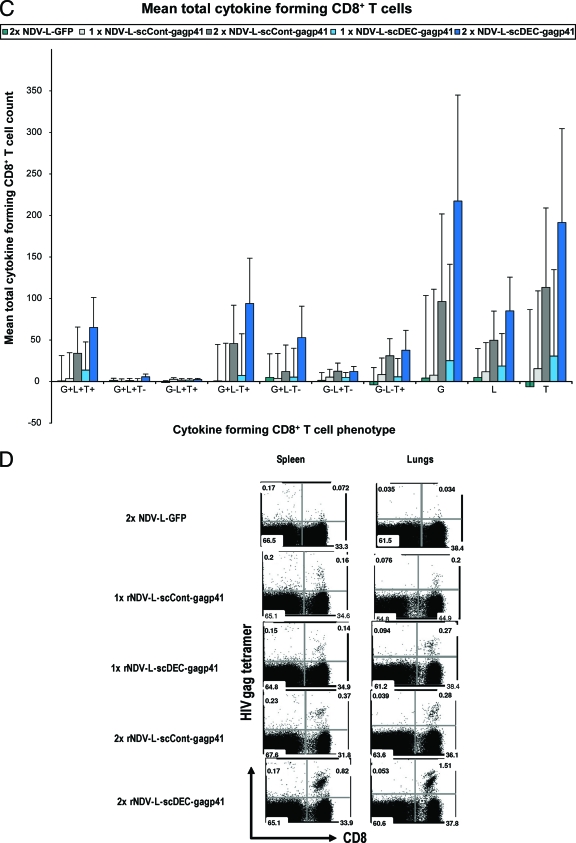
The hallmark of replication-competent viral vaccine vectors is the induction of potent antigen-specific cellular immune responses (31). To evaluate T cell immunity generated by an rNDV expressing a DEC205-targeted antigen, we measured HIV Gag-specific T cell responses induced by rNDV-L-scDEC-Gagp41 immunization and compared them to responses induced by rNDV-scCont-Gagp41 and rNDV-L-GFP. Groups of six CxB6 F1 mice were immunized in a homologous prime-boost vaccine regimen employing each of the above-mentioned vaccine vectors. At two time points, 7 days after the prime and 7 days after the boost, lungs and spleens were harvested. Splenocytes were subsequently used in a multiparametric intracellular cytokine assay that measured T cell cytokine responses against a library of 15-mer peptides representing the whole HIV-1 Gagp41 sequence; a mix of nonreactive peptides (P17 pool) was included as a negative control (Fig. 3A). As expected, mice vaccinated with rNDV-L-GFP showed only background levels of cytokine-expressing T cells. Seven days after the first immunization, mice vaccinated with the scFv-Gagp41-expressing vectors demonstrated similar levels of cytokine-producing T cells (Fig. 3A to C). However, 7 days after the boost, the numbers of Gag-specific CD4+ T cells and CD8+ T cells producing IFN-γ, TNF-α, and IL-2 were higher in the mice that received the DEC205-targeted vaccine than in the mice that received the nontargeted vaccine (Fig. 3A to C). rNDV-L-scDEC-Gagp41 vaccination induced a 2-fold increase of the amount of cytokine-producing CD8+ T cells compared to the nontargeted control vaccine (Fig. 3A and C, compare dark gray and dark blue bars). Splenocytes pulsed with the control peptide mix showed only background levels of cytokine production (Fig. 3A). Since T cells producing multiple cytokines are closely associated with protective immunity (17), we examined the capacity of the vaccine-induced T cells to secrete multiple cytokines such as IFN-γ, IL-2, and TNF-α (Fig. 3B and C). DEC205-targeted vaccination induced more polyfunctional CD4+ and CD8+ T cells that produced 2 or more cytokines than did the nontargeted vaccine. Triple-cytokine-producing CD4+ and CD8+ T cells (IFN-γ positive [IFN-γ+], IL-2+, and TNF-α+) were predominant in mice vaccinated with the DEC205-targeted vaccine 7 days after the boost (Fig. 3B and C). In addition, rNDV-L-scDEC-Gagp41 vaccination induced more CD4+ T cells that coexpressed TNF-α and IL-2 (Fig. 3B) and more CD8+ T cells that expressed both IFN-γ and TNF-α than the rNDV-scCont-Gagp41 vaccine 7 days after the boost (Fig. 3B and C). These data indicate that targeting the rNDV-expressed antigen to dendritic cells improves the induction of a potent and a broad antigen-specific T cell-mediated immune response. To examine the accumulation of vaccine-induced Gag-specific CD8+ T cells, we stained single-cell suspensions prepared from the lungs and the spleens of vaccinated mice with an MHC class I Gag tetramer (H-2Kd-restricted epitope). Mice vaccinated with rNDV expressing the DEC205-targeted Gagp41 antigen showed considerably more CD3+ CD8+ Gag tetramer-positive lymphocytes in the spleen and in the lungs 7 days after the boost (Fig. 3D). Mice vaccinated with rNDV-L-GFP showed low background levels of tetramer-positive cells (Fig. 3D). The Gag tetramer staining results were consistent with the cytokine expression analysis where we observed minimal differences in the ability scFv-Gagp41-expressing vectors to induce antigen-specific T cells at 7 days after the prime, whereas the DEC205-targeted vector performed considerably better than did the nontargeted vector after the boost. These data demonstrate that the DC-targeted vaccine enhanced both the quantity and the quality of the antigen-specific cellular immune response.
FIG. 3.
Vaccine-induced HIV Gag-specific immune response. (A) Bulk splenocytes were assessed for T cell immunity 1 week after prime and 1 week after boost. Splenocytes were restimulated for 6 h either with unreactive peptides (p17 pool 1) or with an HIV Gagp41 peptide mix, and IFN-γ, TNF-α, and IL-2 production was evaluated by intracellular cytokine staining in CD3+ CD4+ T cells and CD3+ CD8+ T cells. Arrows indicate CD8+ T cells. (B and C) Cytokine profile of CD3+ CD4+ T cell (B) and CD3+ CD8+ T cell (C) responses in vaccinated mice 1 week after prime and 1 week after boost. G, IFN-γ-expressing cells; T, TNF-α-expressing cells; L, IL-2-expressing cells. (D) Accumulation of Gag-specific CD8+ T cells in the spleen (left) and in the lungs (right) 1 week after prime and 1 week after boost. HIV Gag-specific T cell responses in the spleen and lungs of vaccinated CxB6 F1 mice were examined by using HIV Gag tetramer staining of total CD8+ CD3+ cells. Data are representative of 3 different experiments; data for 2 mice were pooled in each experiment.
Protection from viral challenge.
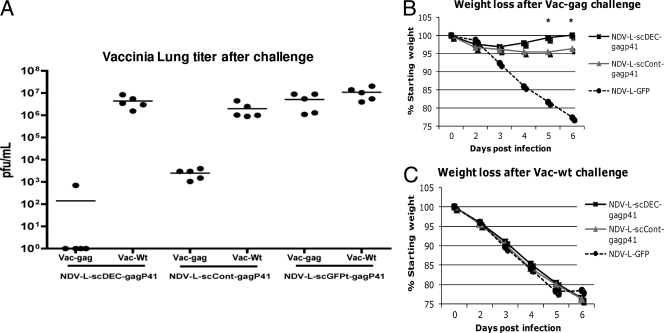
Next, we wanted to evaluate whether the vaccine-induced enhancement of the Gag-specific immune response correlated with better protective immunity. Since HIV-1 cannot infect mice, the ability of the different rNDV vectors to elicit a Gag-specific protective immune response at mucosal sites can be tested by infecting the vaccinated mice with a vaccinia virus expressing the HIV Gag protein (Vac-gag). A more effective vaccine should better control Vac-gag replication. Therefore, we challenged the previously vaccinated groups of CxB6 F1 mice (n = 5) with 106 PFU of either Vac-gag or wild-type vaccinia virus (Vac-Wt) as a background control via the intranasal route 4 weeks after the last immunization (Fig. 4). Four out of five mice vaccinated with rNDV-L-scDEC-Gagp41 were able to clear Vac-gag infection 6 days after challenge (Fig. 4A). Mice vaccinated with rNDV-L-scCont-Gagp41 were not able to clear the Vac-gag infection but demonstrated lower vaccinia virus lung titer loads than did rNDV-L-GFP-vaccinated mice or mice challenged with Vac-Wt (Fig. 4A). In addition, mice vaccinated with the DEC205-targeted vaccine stopped losing weight 3 days after Vac-gag challenge and were able to regain their initial weight by day 6 after the viral challenge, whereas mice that received the control vaccine rNDV-L-scCont-Gagp41 did not start to regain weight before the fifth day after Vac-gag challenge (Fig. 4B). As expected, all groups of mice challenged with Vac-Wt showed no protection against the viral challenge, indicating that the immune responses generated by vaccination were Gag specific (Fig. 4C). These data demonstrate that an rNDV expressing a DEC205-targeted antigen significantly enhanced antigen-specific cell-mediated immune responses compared to an rNDV expressing a nontargeted antigen, and importantly, this enhancement correlated with a superior ability to control viral infection.
FIG. 4.
Challenge with Vac-gag in mice immunized with the NDV-L-scFv-Gagp41 viruses. Groups of 10 female CxB6 F1 mice were immunized intranasally with 105 FFU of either NDV-L-scDEC-Gagp41 or NDV-L-scCont-Gagp41. Negative-control mice were inoculated with NDV-L-GFP. Four weeks later, animals were boosted with 106 FFU of the same virus. Four weeks after the boost, five mice per group were challenged with 106 PFU of Vac-Wt or Vac-gag. (A) Six days after the infection with vaccinia viruses, mice were sacrificed, lungs were homogenized in 1 ml of PBS, and vaccinia virus titers in CV-1 cells were determined. (B and C) Weight loss was monitored daily for 6 days after the Vac-gag (B) and Vac-Wt (C) challenges.
Dose-response efficacy of the DEC205-targeted vaccine versus the control.
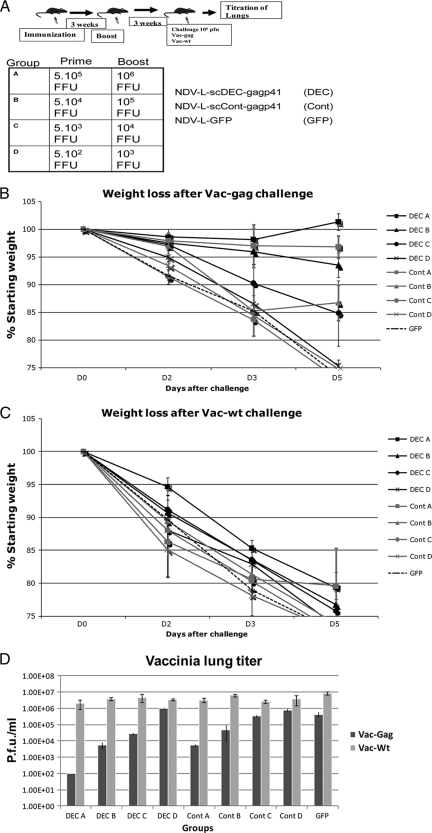
In order to better quantify the improvement conferred by the DEC205-targeted antigen in the context of an rNDV vaccine vector, we vaccinated groups of 6- to 8-week-old female BALB/c mice with graded doses (10-fold dilutions) of rNDV-L-scDEC-Gagp41 (DEC group) or rNDV-L-scCont-Gagp41 (Cont group). Negative-control mice (GFP group) received the highest dose of rNDV-L-GFP (Fig. 5 A). The rNDV-specific serum antibody titer was tested for all groups of mice to ensure the adequate inoculation of the vaccine vectors (data not shown). Three weeks after the boost, mice were challenged with either Vac-gag or Vac-Wt as a background control. As expected, the DEC A group was the most efficient in controlling Vac-gag infection, followed closely by the DEC B and Cont A groups (Fig. 5B to D), which showed a similar ability to control the Vac-gag infection. In addition, the DEC C group showed equivalent Vac-gag lung titers compared to the Cont B group (Fig. 5B). Weight loss postchallenge correlated closely with the ability of vaccinated mice to control the viral challenge. Significant vaccine-induced protection was lost for the groups of mice that received the lowest doses of the vaccine vectors (DEC D, Cont C, and Cont D groups) and the GFP negative control (Fig. 5B and D). All groups showed no protective effect when challenged with Vac-Wt virus, confirming the specificity of the immune response (Fig. 5C and D). These data indicate that the protective immune response induced by vaccination with rNDV-L-scDEC-Gagp41 is equivalent to the response generated by vaccination with a 10-times-more-concentrated dose of the control nontargeted vector rNDV-L-scCont-Gagp41. The DEC205-targeted vaccine is 10-fold better than the control vector in controlling vaccinia virus Gag infection.
FIG. 5.
Dose-response efficacy of the DEC205-targeted vaccine versus the control. (A) Immunization regimen. Groups of female BALB/c mice were immunized with graded doses of NDV-L-scDEC-Gagp41 or NDV-L-scCont-Gagp41. Negative-control mice were immunized with NDV-L-GFP. For the DEC A group, mice were primed with 5 × 105 FFU and boosted with 106 FFU of NDV-L-scDEC-Gagp41; for the Cont A group, mice were primed with 5 × 105 FFU and boosted with 106 FFU of NDV-L-scCont-Gagp41. The vaccination dose from group A (DEC and Cont) was serially diluted (10-fold) and administered to groups B, C, and D. Three weeks after the boost, mice were challenged with 106 PFU of either Vac-gag virus or wild-type vaccinia virus. Weight loss was monitored for 5 days postchallenge. (B) Weight loss following Vac-gag challenge. (C) Weight loss following Vac-Wt challenge. (D) Vaccinia virus lung titers 5 days postchallenge. Five days after challenge, lungs were harvested, and vaccinia virus titers in the lungs were determined by a plaque assay on CV-1 cells.
DISCUSSION
Live viral vaccine vectors have historically provided the most effective protection against viral infection and disease (31). These vaccines were paramount in the eradication efforts for devastating diseases like smallpox, poliomyelitis, yellow fever, and measles. Following the concept of a “Jennerian” immunization, a live attenuated viral vaccine elicited cross-protective immunity against a heterologous but closely related virus. Unfortunately, for safety reasons Jennerian immunization cannot be applied for the generation of HIV vaccines (18). Consequently, viral vectors such as adenovirus, canarypox virus, and others have been engineered to express HIV antigens and therefore elicit immune responses against one or more HIV proteins (31). These vectors were shown to be safe enough to be tested in human clinical trials; however, their effectiveness in eliciting a protective immune response remains in doubt. A live replicating viral vaccine vector has the ability to trigger innate and adaptive immune responses that usually become activated during the course of a natural infection. The problem lies in that the immune responses are typically directed against the vector's own protein and not the encoded HIV antigen, resulting in a somewhat weak HIV vaccine vector. Therefore, to achieve an effective HIV vaccine, it is essential to improve the transgene-specific immune response in the context of a live viral vaccine vector.
Previous studies have demonstrated that the targeting of an antigen to DCs through the DEC205 endocytic receptor by way of conjugating the antigen to an anti-DEC205 antibody increases the efficiency of antigen presentation on MHC class I and II molecules and enhances the magnitude, the breadth, and the quality of the antigen-specific immune response (3, 20, 25). In all of those studies, the addition of DC maturation signals, like TLR agonists, improved cell-mediated immunity due to the induction of high levels of type I interferon. For this reason, we sought to exploit the ability of NDV to induce a potent type I interferon response (22, 23). We therefore generated an rNDV vector expressing a secreted HIV Gagp41 protein that is targeted to DCs through coupling to a single-chain antibody (scFv) directed against DEC205. We compared the ability of the targeted vaccine to induce a potent Gag-specific immune response to that of an rNDV that expresses a nontargeted HIV Gagp41 protein. We have previously demonstrated that in the context of an rNDV vector, the potency of the antigen-specific immune response correlates with the expression level of the antigen itself (5). In order to exclude that possibility, we confirmed that both vectors have equivalent replication kinetics and transgene expression profiles. In addition, only the DC-targeted antigen showed binding to CHO cells expressing the murine DEC205 receptor. Next, we immunized mice with a homologous prime-and-boost vaccination regimen and demonstrated that even though the vector-specific humoral immune responses were equivalent in all groups of mice, we observed higher HIV Gag-specific IgG antibody titers in the serum of mice that received the DEC205-targeted vaccine.
The principal purpose of the rNDV vaccine vector is to elicit a strong antigen-specific T cell-mediated immune response. In order to examine whether DEC205 targeting of the HIV-1 Gag antigen would lead to enhanced cellular immunity in the context of an rNDV vector, we examined the accumulation of Gag-specific CD3+ CD8+ T lymphocytes in the lungs and in the spleen of vaccinated mice using Gag tetramer staining. In both the spleen and the lungs, we observed an increase in numbers of HIV Gag-specific CD8+ T cells in mice vaccinated with the DEC205-targeted vaccine compared to those in mice vaccinated with the nontargeted vaccine.
We subsequently analyzed the Gag-specific cytokine secretion profile generated by CD4+ T cells and CD8+ T cells in vaccinated mice. The DEC205-targeted vaccine induced more T cells that expressed IFN-γ, IL-2, and TNF-α than did the nontargeted vaccine. In addition, the DEC205-targeted vaccine produced more polyfunctional T cells that expressed 2 or more cytokines. Our results indicate that the enhancement of the cellular immune response was readily measurable after the vaccine boost. We did observe a trend where the DEC205-targeted vaccine improved vaccination efficiency after the first immunization; however, the levels of antigen-specific responses at that time point were low and not readily quantifiable. This observation is consistent with data from previous studies that indicated a requirement for a boost in the context of viral vaccine vectors (5, 23). Overall, the targeting of the HIV-1 Gag antigen to DCs in the context of an rNDV vector improved the quantity and the quality of the Gag-specific immune response.
Finally, in order to correlate the enhancement of the T cell-mediated immune response with protective immunity, we challenged the vaccinated groups of mice with a live vaccinia virus expressing the HIV-1 Gag protein. Mice vaccinated with the DEC205-targeted vaccine performed better in controlling the Vac-gag infection. In addition, dose-response experiments demonstrated that the targeting of the rNDV-encoded antigen to the DEC205 receptor on dendritic cells increased the efficiency of vaccination by 10-fold. Nchinda et al. (25) previously observed that a DNA vaccine expressing a DEC205-targeted antigen provided better protective immunity at low doses but not at high doses of DNA than did a DNA vaccine expressing a nontargeted antigen (25). In that study, dose-response experiments did not demonstrate a saturating effect when high doses of the rNDV vaccine were administered. This dichotomy can be attributed to the probability that we did not reach saturating doses in our experiments. However, this could also be explained by the superior ability of live viral vaccine vectors to recruit and activate dendritic cells compared to DNA vaccine vectors.
HIV-specific T lymphocytes play an essential role in the immune control of viral replication. The depletion of CD8+ T cells in simian immunodeficiency virus (SIV)-infected monkeys abrogated the control of viral replication (33); CD8+ lymphocytes have been shown to exert an immune pressure on HIV replication, inducing the virus to mutate within epitopes (29); and different genetic studies demonstrated a clear association between some HLA class I alleles and the control of HIV replication in humans (10, 13). In this study we describe a strategy to improve the vaccination-induced HIV-specific cell-mediated immune response using rNDV as a delivery vector. T cell-based vaccines are unlikely to induce sterilizing immunity against HIV but may control replication after infection (7). A potent immune response in a conserved region of an HIV protein may force escape mutants with a reduced viral replication capacity (21). HIV Gag is highly conserved among sequenced isolates. Previous studies provided evidence for a possible role of Gag-specific CD8+ T cells in the control of chronic HIV infection (12, 14, 19). Therefore, we chose HIV Gag as our model antigen. However, this strategy could be applied to any other HIV proteins, such as Nef, Pol, and Env, especially since NDV was shown previously to accommodate the whole Gagp55 gene but also larger genes such as the severe acute respiratory syndrome coronavirus (SARS-CoV) spike (S) glycoprotein gene (5, 9).
The immunological correlates for an effective HIV-1 vaccine are unknown; nevertheless, it is widely believed that protective vaccine-induced immune responses need to be more potent and durable in both systemic and mucosal compartments (2). To that end, a ′'heterologous prime-boost” vaccine regimen, consisting of a DC-targeted protein prime followed by a plasmid DNA boost, induced a more potent immune response than did a homologous prime-boost DNA vaccination (24). Each vaccine vector induced a different type of immune response (CD4+ T cells versus CD8+ T cells). This complementary vaccination regimen translated into a faster accumulation of antigen-specific CD8+ T cells to mucosal sites after challenge (24).Therefore, it will be interesting to test whether a heterologous vaccine regimen consisting of a DEC205-targeted antigen encoded by different vectors, such as rNDV or DNA, as well as a DC-targeted protein vaccine can elicit high-frequency, polyfunctional CD8+ T cells that efficiently traffic to both systemic and mucosal compartments.
Acknowledgments
This research project was partially supported by NIH HIVRAD grant sP0IAI08325 (to A.G.-S.) and U54AI057158 (to P.P.) and by a grant from the Bill and Melinda Gates Foundation (to P.P., R.M.S. and A.G.-S.).
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1.Alexander, D. J. 2000. Newcastle disease and other avian paramyxoviruses. Rev. Sci. Tech. 19:443-462. [DOI] [PubMed] [Google Scholar]
- 2.Barouch, D. H., and B. Korber. 2010. HIV-1 vaccine development after STEP. Annu. Rev. Med. 61:153-167. doi: 10.1146/annurev.med.042508.093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozzacco, L., et al. 2007. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc. Natl. Acad. Sci. U. S. A. 104:1289-1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukreyev, A., et al. 2005. Recombinant Newcastle disease virus expressing a foreign viral antigen is attenuated and highly immunogenic in primates. J. Virol. 79:13275-13284. doi: 10.1128/JVI.79.21.13275-13284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carnero, E., et al. 2009. Optimization of human immunodeficiency virus Gag expression by Newcastle disease virus vectors for the induction of potent immune responses. J. Virol. 83:584-597. doi: 10.1128/JVI.01443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demangel, C., et al. 2005. Single chain antibody fragments for the selective targeting of antigens to dendritic cells. Mol. Immunol. 42:979-985. doi: 10.1016/j.molimm.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Desrosiers, R. C. 2004. Prospects for an AIDS vaccine. Nat. Med. 10:221-223. doi: 10.1038/nm0304-221. [DOI] [PubMed] [Google Scholar]
- 8.De Vries, I. J., et al. 2003. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 63:12-17. [PubMed] [Google Scholar]
- 9.DiNapoli, J. M., et al. 2007. Immunization of primates with a Newcastle disease virus-vectored vaccine via the respiratory tract induces a high titer of serum neutralizing antibodies against highly pathogenic avian influenza virus. J. Virol. 81:11560-11568. doi: 10.1128/JVI.00713-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellay, J., et al. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317:944-947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman, A. I., et al. 2006. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 13:221-228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Fu, T. M., et al. 2007. Evaluation of cellular immune responses in subjects chronically infected with HIV type 1. AIDS Res. Hum. Retroviruses 23:67-76. doi: 10.1089/aid.2006.0114. [DOI] [PubMed] [Google Scholar]
- 13.Gao, X., et al. 2001. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N. Engl. J. Med. 344:1668-1675. doi: 10.1056/NEJM200105313442203. [DOI] [PubMed] [Google Scholar]
- 14.Gesprasert, G., et al. 2010. HLA-associated immune pressure on Gag protein in CRF01_AE-infected individuals and its association with plasma viral load. PLoS One. 5:e11179. doi: 10.1371/journal.pone.0011179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granelli-Piperno, A., et al. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 175:4265-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossmann, C., et al. 2009. Enhancement of the priming efficacy of DNA vaccines encoding dendritic cell-targeted antigens by synergistic Toll-like receptor ligands. BMC Immunol. 10:43. doi: 10.1186/1471-2172-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harari, A., et al. 2006. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211:236-254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann-Lehmann, R., et al. 2003. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. AIDS 17:157-166. doi: 10.1097/01.aids.0000042942.55529.01. [DOI] [PubMed] [Google Scholar]
- 19.Kiepiela, P., et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 20.Mahnke, K., et al. 2000. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol. 151:673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura, T., et al. 2009. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare Gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphotye [sic] recognition. J. Virol. 83:2743-2755. doi: 10.1128/JVI.02265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakaya, T., et al. 2001. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 75:11868-11873. doi: 10.1128/JVI.75.23.11868-11873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakaya, Y., et al. 2004. Induction of cellular immune responses to simian immunodeficiency virus Gag by two recombinant negative-strand RNA virus vectors. J. Virol. 78:9366-9375. doi: 10.1128/JVI.78.17.9366-9375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nchinda, G., et al. 2010. Dendritic cell targeted HIV gag protein vaccine provides help to a DNA vaccine including mobilization of protective CD8+ T cells. Proc. Natl. Acad. Sci. U. S. A. 107:4281-4286. doi: 10.1073/pnas.1000621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nchinda, G., et al. 2008. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J. Clin. Invest. 118:1427-1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, M. S., J. Steel, A. Garcia-Sastre, D. Swayne, and P. Palese. 2006. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc. Natl. Acad. Sci. U. S. A. 103:8203-8208. doi: 10.1073/pnas.0602566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peeters, B. P., O. S. de Leeuw, I. Verstegen, G. Koch, and A. L. Gielkens. 2001. Generation of a recombinant chimeric Newcastle disease virus vaccine that allows serological differentiation between vaccinated and infected animals. Vaccine 19:1616-1627. [DOI] [PubMed] [Google Scholar]
- 28.Pitisuttithum, P., et al. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661-1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 29.Price, D. A., et al. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. U. S. A. 94:1890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rerks-Ngarm, S., et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209-2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 31.Robert-Guroff, M. 2007. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 18:546-556. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell, N. D., et al. 2007. Phase 2 study of an HIV-1 canarypox vaccine (vCP1452) alone and in combination with rgp120: negative results fail to trigger a phase 3 correlates trial. J. Acquir. Immune Defic. Syndr. 44:203-212. doi: 10.1097/01.qai.0000248356.48501.ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz, J. E., et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]