Abstract
Certain murine leukemia viruses (MLVs) can induce progressive noninflammatory spongiform neurodegeneration similar to that caused by prions. The primary MLV determinants responsible have been mapped to within the env gene; however, it has remained unclear how env mediates disease, whether non-Env viral components are required, and what central nervous system (CNS) cells constitute the critical CNS targets. To address these questions, we examined the effect of transplanting engraftable C17.2 neural stem cells engineered to pseudotype, disseminate, and trans-complement neurovirulent (CasBrE, CasE, and CasES) or non-neurovirulent (Friend and SFF-FE) env sequences (SU or SU/TM) within the CNS using either the “non-neurovirulent” amphotropic helper virus, 4070A, or pgag-polgpt (a nonpackaged vector encoding Gag-Pol). These studies revealed that acute MLV-induced spongiosis results from two separable activities of Env. First, Env causes neuropathology through unique viral targeting within the CNS, which was efficiently mediated by ecotropic Envs (CasBrE and Friend), but not 4070A amphotropic Env. Second, Env induces spongiosis through a toxin activity that is MLV-receptor independent and does not require the coexpression of other viral structural proteins. CasBrE and 4070A Envs possess the toxin activity, whereas Friend Env does not. Although the identity of the critical viral target cell(s) remains unresolved, our results appear to exclude microglia and oligodendrocyte lineage cells, while implicating viral entry into susceptible neurons. Thus, MLV-induced disease parallels prionopathies in that a single protein, Env, mediates both the CNS targeting and the toxicity of the infectious agent that manifests itself as progressive vacuolar neurodegeneration.
Murine leukemia viruses (MLVs) are capable of causing severe progressive noninflammatory spongiform motor neuron disease when inoculated into susceptible neonatal mice. The prototypic virus of this class, referred to as CasBrE, was first isolated from wild mice and shown to cause a paralytic wasting disease with low incidence and a long incubation period, which is highly reminiscent of certain prion diseases and amyotrophic lateral sclerosis (ALS) (1, 17-19). Subsequent studies manipulating either the host or the virus have demonstrated that this paralytic disease can be reproducibly induced with complete penetrance within 3 weeks of neonatal inoculation, making it an attractive model for studying mechanisms of infectious spongiform neurodegeneration (6, 14, 48).
Early genetic mapping studies with CasBrE and other neurovirulent MLVs have shown that the neurovirulence determinants reside within the viral env gene, specifically to the surface expressed subunit (SU) of the Env protein (see, for example, references 14, 23, 40, 46, 50, and 62). Although much is known about how MLV SU is involved in retroviral binding and entry, very little is known about how it is involved in precipitating spongiosis. It has been speculated that central nervous system (CNS) expression of neurovirulent MLV Env protein alone might be sufficient for inducing neurodegeneration (14). Attempts to address this issue through the generation of Env transgenic mice have yet to effectively recapitulate the acute disease process (25, 65). To circumvent these limitations, our laboratory has explored the use of neural stem cell (NSC)-based brain chimeras in order to reconstitute restricted aspects of the fastest viral models. These studies (reviewed in reference 31) demonstrated that transplantation of C17.2 NSCs expressing CasBrE Env, either the surface subunit alone (SU) or combined with the transmembrane subunit (SU/TM), did not induce spongiosis despite widespread engraftment and abundant Env expression from the NSCs. Moreover, NSC-mediated CNS dissemination of CasBrE viruses that were restricted at a postentry/preintegration stage of the virus life cycle by the host resistance gene Fv-1 also failed to cause acute neurodegeneration. In contrast, NSC delivery of a weakly neuropathogenic but nonrestricted CasBrE virus induced severe acute spongiform neurodegeneration (34). These experiments suggested that neither exogenous Env protein nor Env-mediated particle fusion was sufficient for disease, but rather that spongiform pathology required viral integration and host cell expression. To assess whether CNS delivery of env alone could induce spongiform disease, NSCs were engineered into retroviral packaging cells capable of producing infectious but replication-incompetent virus encoding the CasBrE Env protein (SU/TM). Transplantation of these packaging NSCs failed to induce acute spongiosis, despite env dissemination to, and expression within host microglia (33), a major CNS target of MLVs (3, 4, 20, 21, 30, 52). These latter experiments suggested that MLV-induced spongiform neuropathogenesis might require host cell expression of certain non-Env retroviral proteins or, alternatively, the disease might require CasBrE env virus delivery to, and protein expression within, host cells other than, or in addition to, microglia.
Therefore, in order to more thoroughly explore the requirements for MLV-induced spongiform neurodegeneration, we revisited the NSC-based chimera approach to identify the retrovirus components and life cycle events are required for inducing spongiosis. Specifically, we sought to determine whether NSCs engineered with retroviral or mammalian expression vectors encoding Env from either the neurovirulent CasBrE or non-neurovirulent Friend ecotropic viruses could induce acute spongiform neuropathology when trans-complemented by either (i) a “non-neurovirulent” amphotropic helper virus, 4070A (14, 61), or (ii) a selectable gag-pol expression vector (pgag-polgpt) that is not packaged (35). The results of these experiments indicate that the induction of acute spongiform neuropathology is dependent upon (i) unique receptor-mediated virus targeting within the CNS and (ii) the expression of a neurovirulent env gene alone within host target cells. However, our findings suggest that we reconsider the identity the critical viral target cell(s) since neurovirulent virus delivery to and expression within microglia and oligodendroglial lineage cells was not sufficient for disease. Preliminary evidence for viral entry into neurons was observed, rekindling the debate as to whether Env-induced neuropathology is mediated directly or indirectly.
MATERIALS AND METHODS
DNA constructs.
The CasBrE Env expression vectors CasE (SU/TM) and CasES (SU), described previously (34), contain the env gene derived from the CasBrE molecular clone 15.1 (34) (GenBank accession number X57540) inserted into the pSFF vector (27). The p15-1EIH expression construct was assembled using the pCDNA3.1-Hygro(−) vector (Invitrogen) into which the CasBrE SU/TM from clone 15-1 (48) was inserted, along with a chimeric intron from pCI (Promega). The intron was situated between the cytomegalovirus immediate-early promoter and the env start to increase CasBrE env gene expression levels. The pgag-polgpt plasmid (a gift from A. Bank) has been described previously (35) and contains MLV gag and pol gene sequences and the selection gene gpt (encoding xanthine-guanine phosphoribosyltransferase). The Friend env expression vector, pSFF-FE, was generated by introducing Friend env from Friend 57E into multiple cloning sites in the pSFF vector (51). In order to assess effects associated with vector alone, a pSFF-based vector encoding humanized green fluorescent protein (GFP) from Renilla (hrGFP; Stratagene) was generated by introducing a XhoI and SalI fragment from pFB-hrGFP (Promega) into the multiple cloning site of pSFF.
Cells.
C17.2 neural stem cell lines with or without expression of CasBrE SU or SU/TM expression vectors have been described previously (34, 53) and were grown on Primaria dishes (Falcon) in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, penicillin, and streptomycin (100 U/ml). Mus dunni, NIH 3T3 fibroblasts, and PT67 packaging cells (Invitrogen) were maintained in DMEM with 10% newborn calf serum, penicillin, and streptomycin (100 U/ml) on Nunclon D dishes (Nunc).
The CasBrE Env-expressing-only NSC line, 15-1EIH NSC, was generated by transfecting the pCDNA3.1-Hygro-based vector encoding the CasBrE env gene (p15-1EIH) into C17.2 NSCs, followed by hygromycin selection and subsequent selection for high cell surface expression of CasBrE Env by fluorescence-activated cell sorting (FACS). hrGFP NSCs were generated by exposing C17.2 cells to hrGFP virus at a multiplicity of infection (MOI) of 1 in the presence of 8 μg of Polybrene/ml. Positive NSCs were sorted by flow cytometry with the brightest 10% selected and used for subsequent analysis. C17.2 NSCs with or without SU, SU/TM, hrGFP, or 15-1EIH were infected with the 4070A virus at an MOI of 1 in the presence of 8 μg of Polybrene/ml and passaged four times prior to analysis for CasBrE Env and hrGFP expression and 4070A virus pseudotyping.
The packaging NSC cell line (gpgpt NSCs) was generated by transfecting the pgag-polgpt plasmid into naive C17.2 NSCs, followed by selection in HXM medium as described previously (35). To introduce env-encoding vectors into packaging gpgpt NSCs, they were infected by spinoculation (43) with replication-defective retrovirus collected from PT67 packaging cells transfected with pCasE or pSFF-FE.
Viruses.
4070A amphotropic and Friend ecotropic virus stocks were generated by transfecting the permuted proviral clone p4070A (37) or the unpermuted pFr57E proviral plasmid (15), respectively, into NIH 3T3 cells. Virus encoding hrGFP was generated by cotransfection of PT67 packaging cells (Invitrogen) with pCDNA3.1-Hygro and phrGFP, followed by collection of viral supernatants from hygromycin-selected cultures. Similarly, CasE, CasES, and SFF-FE virus stocks were generated by transfection of PT67 cells with the corresponding plasmid.
Virus titration assay (VTA).
Virus titers of tissue culture supernatants, tissue extracts, and serum samples were determined by a focus-forming assay on Mus dunni (dunni) cells (11). Supernatants collected from confluent cell cultures were frozen at −80°C before analysis. Virus production in the CNS was determined using a homogenate comprised of 10 20-μm fresh frozen brain sections in 100 μl of phosphate-buffered saline (PBS) by trituration as described previously (34). Viral Env-positive foci were detected by using the CasBrE Env-specific monoclonal antibody (MAb) 697, rat MAb 83A25 (16), which recognizes an epitope common to all known endogenous and most exogenous MLV Env, or the polytropic specific antibodies Hy7, 514, and 516 (8). To assess infection via the ecotropic receptor, 4070A-infected dunni cells were used as a means to interfere with virus entry via the amphotropic receptor, PiT-2. CasBrE Env+ foci were detected using MAb 697, and hrGFP foci were detected by direct examination of dunni cells for GFP by fluorescence microscopy. Assays were run in triplicate, and error bars represent the standard deviations of the logs of the vector titers.
Infectious center assay.
In order to assess NSCs' ability to disseminate viral vectors by cell-to-cell contact, infectious center assays were performed as outlined by Czub et al. (12) with minor modifications. Briefly, transduced C17.2 NSCs were treated with mitomycin C (10 μg/ml; Fisher) in DMEM at 37°C for 2 h, washed with PBS three times, treated with trypsin, and resuspended in DMEM plus 10% fetal bovine serum. Treated NSCs were seeded into TC-6 tissue culture plates using serial dilutions yielding from 100 to 2,000 cells per well. Wells were also seeded with 105 dunni target cells per well. Once the dunni cells reached confluence, MAbs 697, R187, and 720/500/48 were used to detect CasBrE Env, amphotropic Gag, and Friend Env expression, respectively. Primary antibodies were detected with species-specific secondary antibodies coupled to Alexa Fluor dyes (Molecular Probes). Foci were then examined by using epifluorescence microscopy.
Immunoblotting.
Tissue culture cells, brain tissue samples, and virus extracts were generated as previously outlined (29, 34), and equivalent samples were separated by 9% SDS-PAGE, transferred to nitrocellulose membrane, and blocked with 5% nonfat milk in TBS. CasBrE SU/TM and SU were detected with mouse MAb 697 or goat anti-Friend Env serum. Friend Env was detected with goat anti-Friend Env antisera. Gag proteins were detected with anti-capsid rat MAb R187 or rabbit anti-p30 Gag antisera R3. 4070A Env and Gag proteins were detected with swine anti-AMLV serum (NCI). The primary antibodies were followed by species-specific secondary antibodies coupled to horseradish peroxidase and then detected using enhanced chemiluminescence reagents (Pierce). Equivalent amounts of proteins were loaded for each tissue or cell sample, and this was confirmed by Coomassie blue staining of a gel run in parallel with each experiment.
Mice and C17.2 NSC transplantation.
All mice used were IRW strain, previously characterized to be highly susceptible to the neurodegenerative effects of neurovirulent N- and NB-tropic MLVs (48). Mice were bred, housed, and cared for in a specific-pathogen-free animal facility at Northeastern Ohio Universities College of Medicine and Pharmacy (NEOUCOM/P). All animal procedures were carried out in accordance with National Institutes of Health and American Association for the Accreditation of Laboratory Animal Care guidelines and were approved by the Animal Care and Use Committee at NEOUCOM/P. Appropriate procedures were used in all experiments to ameliorate or eliminate any suffering of the mice used in these studies.
Engineered C17.2 cells were transplanted into the lateral cerebral ventricles of P0 mice as previously outlined (34). NSC transplants into the brain stem were performed freehand, using a Nanofil syringe fitted with a retinal pigment epithelium injection kit employing a 34-gauge needle (World Precision Instruments). C17.2 NSCs (5 × 107 cells/ml) were transplanted bilaterally at up to three sites per side (200 nl/injection), 1 to 2 mm lateral to the midline starting at the level of the superior colliculus moving caudally toward the cerebellum. Control and experimental mice were evaluated daily for clinical signs of neurological disease as previously described (13, 30, 48). Animals were deeply anesthetized by using isoflurane and killed by decapitation at 3 to 4 weeks postinoculation. Brains were dissected from the animals and either immersion fixed in 4% formalin for later histological evaluation or snap-frozen over liquid nitrogen for subsequent biochemical and virological analysis. Serum was obtained and evaluated for the presence of circulating virus by VTA.
No overt disease was noted despite evidence of severe spongiform neuropathological changes in some animals. These findings were consistent with previous reports (33, 34) and are likely due to the circumscribed nature of the NSC engraftment and the capacity of spared neurons to compensate for the progressive changes.
Histopathology.
Blinded histological assessment of spongiform neuropathology was carried out on hematoxylin and eosin (H&E)-stained brain sections as outlined by Czub et al. (13). The brains of all mice in the present study were screened from the forebrain through the medulla for evidence of vacuolar changes. Pathological changes were only reported if they were observed in two adjacent sections, and the scores presented for individual animals represent an average of the two highest scores noted for each animal.
Immunostaining.
Paraffin-embedded sections were deparaffinized, rehydrated, and then were subjected to antigen retrieval in citrate buffer (10 mM sodium citrate, 0.05% Tween 20 [pH 6.0]). Immunohistochemistry staining was performed as described previously (33, 34). Double immunofluorescence staining for viral and cell-type-specific markers was carried out sequentially, and cell-type-specific localization was determined by confocal microscopy after z-stack analysis. A M.O.M kit (Vector Labs) was used when primary antibodies from mouse were used (e.g., CNPase and 697). The primary antibodies used were mouse MAb 697, swine anti-4070A serum, rat MAb R187, goat anti-Friend Env serum, rat MAb 83A25, rabbit anti-β-galactosidase (Cappel), rabbit anti-Iba-1 (Wako), rabbit anti-GFAP (Dako), rabbit anti-carbonic anhydrase II (Chemicon), rabbit anti-Olig2 (Chemicon), mouse anti-CNPase (Chemicon), and mouse anti-HuC/D (Invitrogen). The secondary antibodies included biotinylated horse anti-mouse IgG (Vector), biotinylated donkey anti-goat IgG (Jackson Immunoresearch), biotinylated donkey anti-rat IgG (Jackson Immunoresearch), biotinylated donkey anti-rabbit (Jackson Immunoresearch), biotinylated goat anti-swine IgG (RDI), biotinylated goat anti-rabbit IgG, Alexa Fluor 488-goat anti-mouse IgG, Alexa Fluor 594-donkey anti-mouse, Alexa Fluor 594- donkey anti-rabbit IgG, Alexa Fluor 488-donkey anti-rat IgG, Alexa Fluor 488-streptavidin, and Alexa Fluor 594-streptavidin. Images were collected by epifluorescence (Olympus BX51) and confocal microscopy (Olympus IX70).
FACS analysis.
NSCs were removed from tissue culture plates by using 0.05% trypsin with EDTA (Gibco), quenched with DMEM plus 10% NCS, washed with PBS, and incubated at 4°C for 30 min with the mouse MAb 697 for detecting CasBrE Env (36) or mouse MAb 48 for Friend 57 Env. Cells were washed three times with PBS and incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG (Molecular Probes) for another 30 min before analysis and/or sorting. hrGFP expression within NSCs was analyzed directly. Analysis and sorting was carried out on an Altra flow cytometer (Beckman-Coulter).
RESULTS
NSC-mediated 4070A pseudotyping and trans-complementation of CasBrE env induces acute spongiform neurodegeneration.
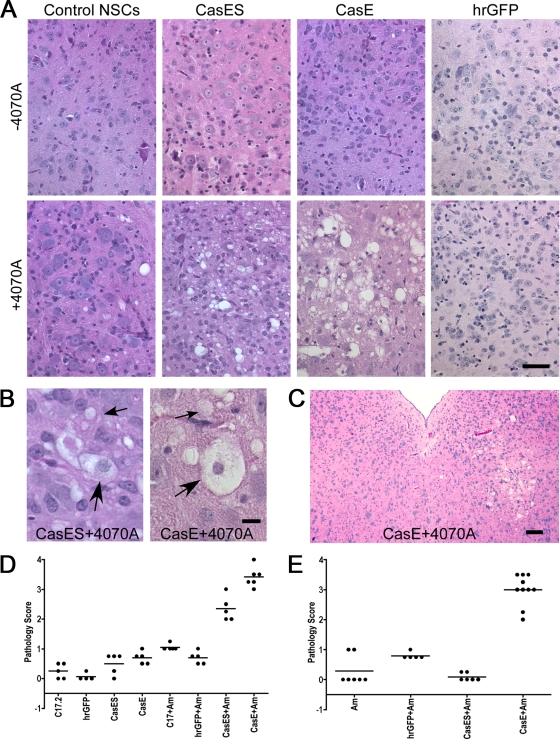
We have recently reported that NSCs can be transduced with the amphotropic virus 4070A and pSFF-based retroviral vectors encoding either the CasBrE Env SU subunit alone (CasES), CasBrE SU/TM (CasE), or hrGFP, which facilitates the trans-complementation, pseudotyping, and dissemination of viral and vector genes to naive and ecotropic receptor-restricted targets in culture, in the absence of detectable replication-competent recombinant CasBrE Env-encoding virus (28). Given the ability of these NSCs to disseminate viral and vector genes, we investigated whether they could induce neurodegenerative changes via this complementation since previous studies from our lab have demonstrated that neither the 4070A virus nor the CasE vector dissemination alone from these cells could induce disease. Thus, control, viral and vector-transduced NSCs were transplanted into either the brain stems or lateral cerebral ventricles of neonatal IRW mice, followed by examination for spongiform changes at 3 and 4 weeks posttransplantation. No spongiform pathology was noted for control, CasE, CasES, or hrGFP NSCs (Fig. 1A and D), a finding consistent with previous studies (33, 34, 61). Similarly, transplantation of 4070A NSCs (61) or hrGFP+4070A NSCs did not induce significant spongiosis through 4 weeks posttransplantation. In contrast, transplantation of 4070A-infected CasES or CasE NSCs induced significant spongiform neurodegeneration when the cells were transplanted directly into the brain stem. Interestingly, 4070A+CasES cells failed to induce significant neuropathogenic changes after injection into lateral ventricles (Fig. 1E and 2A), a site from which the NSCs must migrate to infect susceptible cells within the parenchyma. No similar loss of neurotoxicity was noted for 4070A+CasE NSCs, suggesting that the reduced efficiency of trans-complementation of CasBrE SU by 4070A noted in culture (28) was restricting neuropathogenesis after ventricle transplant. In all animals, spongiosis included vacuolation within the neuropil and cell bodies that showed seemingly intact nuclei but variable loss of cytoplasmic structure (Fig. 1B), which is consistent with wild-type CasBrE virus-induced changes (33). Spongiform neurodegeneration often appeared asymmetrically, as shown in Fig. 1C, consistent with the variable placement of the NSCs, rather than from virus entering the CNS from the circulation (13).
FIG. 1.
4070A NSCs pseudotyping CasBrE SU or SU/TM induce acute spongiform neurodegeneration. (A) Representative H&E-stained sections are shown indicative of the histological consequences associated with brain stem transplantation of control, CasES, CasE, and hrGFP NSCs with or without 4070A virus infection (+4070A and −4070A, respectively), at 4 weeks posttransplantation. Bar, 40 μm. (B) High-magnification examples of the spongiform pathology seen in mice receiving CasES+4070A and CasE+4070A NSCs illustrate characteristic vacuolation within the neuropil (small arrows) and the presence of cells possessing intact nuclei but translucent cytoplasm (large arrows). Bar, 10 μm. (C) Example of a focal asymmetric lesion indicative of virus/vector-transducing NSC-mediated changes rather than from recombinant virus entering the CNS through the circulation (see reference 13). Bar, 100 μm. (D and E) A summary of the pathology scores observed for mice receiving NSC transplants within the brain stem (D) or lateral cerebral ventricles (E) is shown for 4 weeks postinjection. 4070A virus-infected NSCs are denoted by “+Am”.
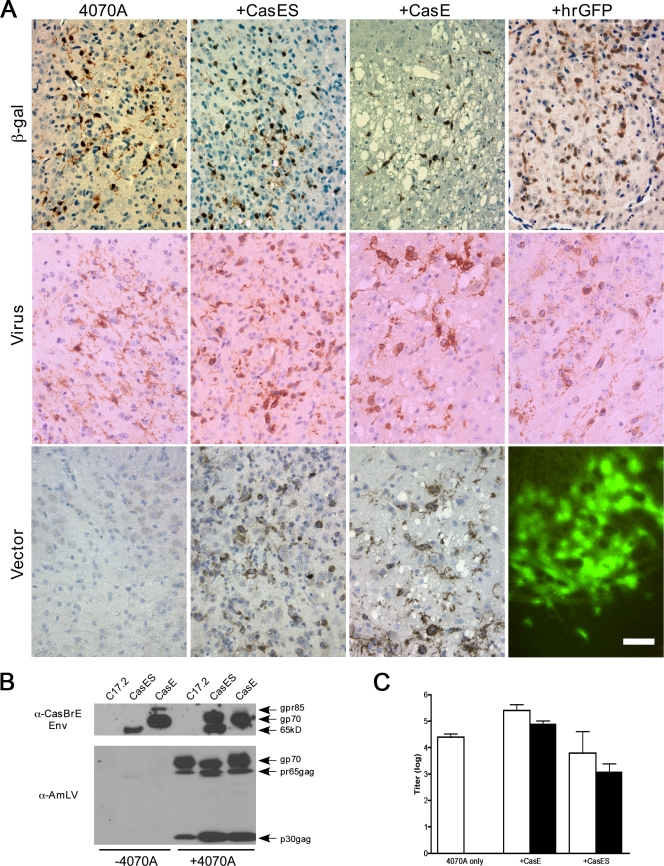
FIG. 2.
4070A virus-pseudotyping NSCs engraft and disseminate CasBrE Env vectors within the CNS. (A) Representative paraffin brain sections from mice transplanted with control, CasES, CasE, and hrGFP NSCs plus 4070A after immunostaining for β-galactosidase (β-gal; brown), a genetic marker engineered into C17.2 NSCs (53); total virus expression (Virus; brown); or CasBrE Env expression (Vector; brown/black). hrGFP vector expression was assessed in frozen brain sections by direct examination under epifluorescence illumination (lower right panel). Bar, 40 μm. (B) Semiquantitative immunoblot assessment of the 4070A virus/CasBrE Env protein expression levels from equivalent brain extracts of freshly frozen brain sections from mice transplanted with control, CasES, and CasE NSCs with or without 4070A. Samples were separated on 8% SDS-PAGE gels and immunoblotted with MAb 697 (top) and pig anti-AmLV antiserum (bottom). The results are representative of at least three separate mouse brains for each group and at least three separate samples per brain. (C) Virus titration analysis on 4070A-infected control, CasES, and CasE NSC-transplanted brains for total virus (white bars) and CasBrE Env-encoding virus (black bars). Virus titers were determined on samples taken from at least three separate transplanted brains in each group. Error bars indicate the standard deviations. All analyses were performed on mice sacrificed at 4 weeks after NSC transplantation.
To examine how the neuropathology observed correlated with NSC engraftment, virus, and vector expression, brain sections were immunostained for β-galactosidase (a marker engineered into the NSCs), MLV Env, and CasBrE Env. Figure 2A shows representative examples of β-galactosidase expression (top row) typical of the observed NSC engraftment. Similar levels of engraftment were noted for vector-expressing NSCs without 4070A infection (data not shown), an observation consistent with previous reports from our laboratory (33, 34). Viral Env expression (middle row) was similar in all mice transplanted with 4070A-infected NSCs, with or without vector. Staining of NSC-transplanted brains for CasBrE Env expression (bottom row) demonstrated that both the CasE and the CasES vectors were robustly expressed compared to the 4070A NSC controls. No overt differences in immunostaining were noted between mice receiving intraventricular versus brain stem transplants other than the distribution of the engrafted cells.
Similarly, examination of mice transplanted with 4070A+hrGFP NSCs showed that the hrGFP vector was also expressed in vivo (Fig. 2A, bottom row, far right panel). However, mice transplanted with hrGFP NSCs without 4070A infection only rarely showed GFP+ cells persisting in these brains through 4 weeks posttransplantation, despite the presence of β-galactosidase-positive cells (data not shown). These results were consistent with our observations made in culture showing the loss of GFP marker expression in C17.2 NSCs in the absence of 4070A (28).
Engrafted brain regions were assessed by immunoblotting for viral proteins to provide a qualitative and semiquantitative comparison of Env and Gag expression. As shown by the representative samples in Fig. 2B, CasBrE Env expression in CasE+4070A and CasES+4070A NSC-transplanted brains did not show obvious quantitative differences in CasBrE or 4070A Env loads. However, significant qualitative differences were noted with regard to the Env isoforms being expressed. Interestingly, the CasBrE SU isoforms expressed appeared to influence the 4070A Env isoform being made, which is consistent with our findings showing 4070A Env-CasBrE SU interactions in culture (28). Gag expression was similar between CasE+4070A and CasES+4070A NSC transplants but was elevated over 4070A NSC transplants alone, as was observed in culture (28).
Assessment of brain section homogenates for total virus and CasBrE Env encoding virus (Fig. 2C) showed that the total virus titers (white bars) were similar between animals injected with 4070A, CasE+4070A, and CasES+4070A NSCs, whereas CasE virus titers were 1 to 2 logs higher than CasES titers, an observation consistent with the culture findings. No evidence of recombinant CasBrE virus was detected within the sera or brain extracts when examined by focus assay (28, 33), although evidence of circulating polytropic virus was occasionally observed in animals transplanted with 4070A NSCs (Table 1). Although polytropic viruses can be neuropathogenic (7, 49), their appearance was not consistently associated with the presence the CasBrE vectors or the induction of spongiform neuropathogenesis.
TABLE 1.
Incidence of polytropic virus in mice transplanted with pseudotyping NSCs
| 4070A infection of NSCs | NSC viral vector | Serum MCF incidencea |
|---|---|---|
| No | None | 0/6 |
| Yes | None | 1/13 |
| No | CasE | 0/7 |
| Yes | CasE | 2/21 |
| No | CasES | 0/8 |
| Yes | CasES | 1/15 |
| No | hrGFP | 0/7 |
| Yes | hrGFP | 0/17 |
Polytropic virus was detected in the sera using a focus assay with a mixture of MAbs specific for polytropic Env proteins, including MAbs 514, 516, and Hy7 (8). The titers for polytropic virus-positive animals were greater than 102 to 103 FFU/ml. The incidence values are expressed as the number of mice with detected virus/total number of mice examined.
Pseudotyping NSCs facilitate 4070A and CasBrE env delivery to multiple CNS cell types.
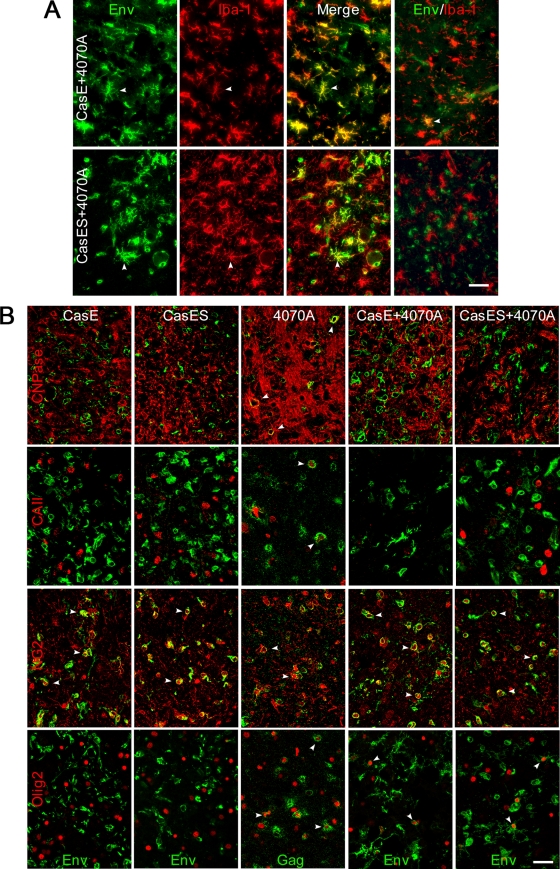
To assess the ability of the transplanted 4070A NSCs to deliver CasE and CasES to host cells, CasBrE Env expression in microglia was evaluated, since the transplanted NSCs do not differentiate into this cell type (57). As shown in Fig. 3A (and summarized in Table 2), CasBrE Env expression was readily identified in microglia (Iba-1+ cells) in 4070A+CasE and 4070A+CasES brains when sections were taken from brain regions showing neuropathological changes. However, in regions with little or no pathology, microglial cell infection was less obvious or absent (Fig. 3A, far right). Moreover, examination of brains with CasES+4070A NSCs transplanted into the ventricles failed to show colocalization of CasBrE Env and Iba-1, suggesting that vector delivery was restricted after these NSCs migrated from the ventricles into the brain parenchyma.
FIG. 3.
Pseudotyping NSCs facilitate CasBrE Env vector expression in host microglia and OPCs, but not mature oligodendrocytes. (A) Representative brain stem sections from mice transplanted with CasE+4070A and CasES+4070A NSCs immunostained for CasBrE Env (Env; green) and the microglial marker Iba-1 (Iba-1; red) and then merged to show colocalization. Env-positive microglia were readily detected in areas with or without spongiform pathology, as indicated by arrowheads; however, there were also areas where Env expression was observed with limited or no colocalization to microglia (far right images). Images were obtained by using epifluorescence optics. Bar, 40 μm. (B) Confocal double-immunofluorescence staining of CasE, CasES, 4070A, CasE+4070A, and CasES+4070A NSC-transplanted brains with antibodies specific for the mature oligodendrocyte markers CNPase (red; top row) and carbonic anhydrase II (red; second row); the oligodendrocyte progenitor (OPC) markers NG2 (red; third row) and Olig2 (red; bottom row); and either CasBrE Env (Env; green) in CasE, CasES, CasE+4070A, and CasES+4070A NSC transplants or Gag (Gag; green) in 4070A NSC transplants. Arrowheads (white) indicate examples of cells showing Env or Gag colocalization with an oligodendrocyte cell type-specific marker. Bar, 40 μm.
TABLE 2.
Glial expression of CasBrE Env or 4070A Gag in brain stems transplanted with pseudotyping NSCs
| CNS cell type | Marker | No. of cells positive for marker/total no. of Env- or Gag-positive cells (%)a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CasE (MAb 697)b | CasES (MAb 697) | 4070A (MAb R187)c | CasE + 4070A |
CasES + 4070A |
15-1EIH + 4070A (MAb R187)d | ||||
| MAb 697 | MAb R187 | MAb 697 | MAb R187 | ||||||
| Microglia | Iba-1 | ND | ND | 19/133 (14.3) | 50/230 (21.7) | 29/276 (10.5) | 23/185 (12.4) | 13/186 (7) | 35/168 (20.8) |
| Oligodendrocyte | CAII | 0/259 | 0/318 | 49/219 (22.4) | 0/46 | 3/168 (1.8) | 0/360 | 2/322 (0.62) | Pathology: 2/81 (2.5); Other: 33/217 (15.2) |
| CNPase | 0/607 | 0/439 | 45/245 (18.4) | 0/258 | 1/285 (0.35) | 0/234 | 3/426 (0.7) | Pathology: 4/115 (3.5); Other: 42/176 (23.9) | |
| Astrocyte | GFAP | 0/422 | 0/382 | 0/187 | 0/325 | ND | 0/266 | ND | 0/47 |
| Olig2+ cell | Olig2 | 0/644 | 0/585 | 126/410 (30.7) | 29/317 (9.1) | 41/600 (6.8) | 20/626 (3.2) | 14/371 (3.8) | 62/256 (24.2) |
| NG2+ cell | NG2 | 388/1,168 (33.2) | 154/791 (19.5) | 65/243 (27.8) | 174/453 (38.4) | ND | 116/507 (22.9) | ND | 108/359 (30.1) |
Values are expressed as the number of cells positive for the glial cell marker/the total number of Env- or Gag-positive cells observed in the brain stem sections surveyed, with the corresponding percentage given in parentheses. Analysis was performed by using z-stack analysis of confocal images. ND, not determined.
MAb 697 (36) was used to detect CasBrE Env protein, which represents cells expressing the env vector.
MAb R187 (8) was used to detect Gag protein, which represents cells expressing the 4070A virus.
“Pathology” refers to the incidence of double-positive cells within or directly adjacent to brain regions expressing spongiform changes; “Other” refers to the incidence of double-positive cells away from areas expressing spongiosis.
Transplanted brains were also examined for 4070A infection of microglia by assessing Gag and Iba-1 colocalization. The results of these studies showed Gag expression in microglia in either the presence or the absence of CasBrE Env encoding retroviral vectors (Table 2), a finding consistent with previous results for 4070A NSC transplantation (61).
Because several studies have implicated oligodendrocytes as important viral targets in MLV-induced neurodegeneration (3, 10, 20, 38, 41, 44), we examined NSC-transplanted mice for MLV Gag and CasBrE Env expression in cells expressing the mature oligodendroglial markers carbonic anhydrase II (CAII) and 2′,3′-cyclic nucleotide 3′-phosphohydrolase (CNPase). As summarized in Table 2, no CasBrE Env-positive cells showed CNPase coexpression in mice receiving brain stem transplants of CasE or CasES NSCs with or without 4070A virus infection. However, in animals transplanted with CasE+4070A or CasES+4070A NSCs, a small number of Gag+ cells expressed CNPase and CAII. Moreover, animals transplanted with 4070A NSCs showed abundant Gag+ cells expressing CNPase or CAII (Fig. 3B and Table 2). Whether Gag expression within oligodendrocytes represents infection of endogenous cells or was simply differentiation of the transplanted NSCs could not be discerned owing to the variable loss of β-galactosidase expression after NSC transplantation (see references 33 and 34). Interestingly, immunostaining for CAII was decreased in brain stem areas showing engraftment of CasE+4070A NSCs (Fig. 3B) and coincided with spongiosis. Whether reduced CAII expression was due to pseudotyped CasE or was instead a result of cellular degeneration remains to be investigated. Nonetheless, the lack of CasBrE Env expression in mature oligodendrocytes suggests either that CasBrE Env expression is restricted in these cells or that Env is toxic during the differentiation of infected oligodendrocyte progenitor cells (OPCs).
To assess the potential involvement of OPCs in pathogenesis, we examined CasBrE Env and 4070A Gag expression in cells expressing NG2 or Olig2, markers that identify OPCs at different developmental stages. CasBrE Env and Gag colocalized with NG2 at similar levels in all transplants where the C17.2 cells contained either CasBrE Env or 4070A (Fig. 3B and Table 1). These results indicate that the transplanted NSCs themselves expressed NG2 upon transplantation, since they did not express NG2 in culture (data not shown). Because host CNS cells did not possess a genetic marker, we could not determine to what extent the 4070A virus or CasBrE Env vectors were delivered to host NG2 cells. However, preliminary data collected from mice infected by intraperitoneal inoculation with either FrCasE or Fr57E (isogenic neurovirulent and non-neurovirulent ecotropic viruses, respectively) indicated that host NG2+ cells are targets of ecotropic viruses (unpublished observations). Whether NG2 infection is related to pathogenesis is not yet clear; however, given that CasBrE Env expression from NG2+ C17.2 NSCs does not cause vacuolar neuropathology, it would seem less likely.
In mice transplanted with CasE or CasES NSCs or NSCs alone, no coexpression of CasBrE Env and Olig2 was noted (nor β-galactosidase and Olig2 [data not shown]). In contrast, both CasBrE Env and 4070A Gag proteins were readily found within Olig2+ cells when transplanted NSCs contained the 4070A virus (Fig. 3B and Table 1). Again, C17.2 NSCs in culture did not express Olig2 in either the presence or the absence of CasBrE Env vectors and/or 4070A. These findings suggested that because control, CasE, or CasES NSCs did not express Olig2 upon transplantation, the Env/Olig2 double-positive cells observed with 4070A represent infected host target cells rather than C17.2 NSCs that had differentiated into OPCs. Intriguingly, the percentage of Env+ Olig2+ or Gag+ Olig2+ cells in mice transplanted with CasE+4070A and CasES+4070A NSCs was considerably lower than the percentage of Gag+ Olig2+ cells observed in mice transplanted with 4070A NSCs. This could result from an ongoing loss of this cell type due to virus plus vector expression, it could be a secondary consequence of neurodegeneration, or it could reflect a reduced OPC susceptibility to CasBrE Env-containing viruses.
For completeness, we also examined whether CasBrE Env expression could be localized to neurons or astrocytes using antibodies directed toward HuC/D and GFAP, respectively. No coexpression was noted regardless of whether the brains developed spongiosis or not (Table 2). The lack of astrocyte or neuronal infection in the degenerating brain stem was consistent with previous studies from this and other laboratories that have only rarely observed neurovirulent Env expression within these cells (3, 4, 26, 30).
4070A pseudotyping/trans-complementation by the non-neurovirulent Friend env induces acute spongiform neurodegeneration.
The experiments described above were initiated based on the idea that the non-neurovirulent 4070A virus would serve to pseudotype and trans-complement the vectors encoding CasBrE SU and SU/TM in vivo and, in doing so, provide support for the idea that spongiform neurodegeneration required Env and other viral structural proteins. However, studies from Munk et al. demonstrated that the chimeric amphotropic virus, MoAmphoV (containing the env gene from 4070A), induces spongiform neurodegeneration in certain mouse strains, albeit with an extended latency. It was further shown that disease could be accelerated by coinfection with a second virus of ecotropic or polytropic host range (39). Although the mechanism by which MoAmphoV induced disease was not clear, the results raised the possibility that spongiosis might arise by ecotropic or polytropic Env pseudotyping of an amphotropic virus whose neurovirulence properties were otherwise silent. Thus, the possibility existed that in our NSC-based trans-complementation system, spongiform neuropathogenesis could result from either CasBrE Env-mediated ecotropic targeting of an entry restricted 4070A virus, involve genetic complementation of the neurovirulent CasBrE Env, or result from 4070A Env-CasBrE Env interactions, such as those observed in vitro (28), that might not occur with either Env alone.
Therefore, to test whether pathogenesis was specific to the neurovirulence determinants in CasBrE env or 4070A, NSCs were transduced with SFF-FE (Fig. 4A), a vector identical to CasE except that it encodes the Env from the non-neurovirulent ecotropic Friend virus, clone 57 (51). Friend Env-expressing NSCs (SFF-FE NSCs) were infected with 4070A, followed by transplantation into the lateral cerebral ventricles or brain stems of IRW mice for comparison with the CasBrE env pseudotyping analysis. As shown in Fig. 4B, 4070A+SFF-FE NSCs induced severe spongiosis by 3 weeks posttransplantation. Neuropathology was characterized by neuropil vacuolation and cytoplasmic effacement, similar to that noted for CasE+4070A NSC transplants. Ventricular NSC transplant resulted in a broad CNS distribution of spongiosis involving the cerebral cortex, thalamus, pons, and medulla, whereas neuropathology associated with brain stem transplants was confined to the pons and medulla. Importantly, SFF-FE NSCs alone did not cause overt neuropathology (Fig. 4C) regardless of the transplantation site, nor did NSCs infected with the replication-competent Fr57E virus (data not shown). Immunohistochemical analysis showed abundant Friend Env expression in brains transplanted with NSCs expressing SFF-FE with or without 4070A (data not shown). Virus and vector titers in the serum were substantial for both 4070A (2.8 × 105 ± 2 × 105 FFU/ml; n = 4) and Friend Env (9.3 × 104 ± 1.1 × 105 FFU/ml; n = 5) in SFF-FE+4070A transplants, indicating that vector pseudotyping was efficient for this virus-vector combination. No evidence for Friend Env recombination was observed in these samples when foci were examined for cell-to-cell spread (28, 33). Moreover, because neuropathology was restricted to brain stem nuclei after focal brain stem transplants, it suggests that spongiosis was NSC-mediated rather than arising from a recombinant virus entering the CNS from the circulation.
FIG. 4.
4070A NSC pseudotyping of a vector encoding the non-neurovirulent ecotropic Friend Env induces acute spongiform neurodegeneration. (A) Structure of the pSFF retroviral vector encoding the Env from Friend virus clone 57, SFF-FE (51). (B) Representative H&E-stained section from a mouse transplanted with NSCs expressing both the 4070A virus and SFF-FE in the brain stem, at 3 weeks posttransplantation, indicative of the severe spongiosis induced with this virus vector combination. (C) Example of a mouse brain stem transplanted with expressing Friend Env (SFF-FE) alone that was not neuropathogenic. (D) Summary of the neuropathology scores for all of the SFF-FE and SFF-FE+4070A NSC mice receiving brain stem transplants (BS) or cerebral ventricle transplants (V). NSC infection with the 4070A virus is indicated by “Am” in the graph labels. Bar, 40 μm.
CasBrE Env particle pseudotyping reveals the inherent neuropathogenic potential of the 4070A virus.
The results presented up to this point cannot distinguish whether neurodegeneration results from ecotropic targeting of the 4070A virus or from the interaction between the amphotropic and ecotropic Envs within host targets. To resolve this issue, C17.2 NSCs were engineered with a CasBrE env expression vector, p15-1EIH (Fig. 5A), that would allow pseudotyping of 4070A encoding virus particles, but whose mRNA is not packaged and therefore could not trans-complement 4070A in host targets. As shown in Fig. 5B, the 15-1EIH NSCs expressed CasBrE Env on the plasma membrane with or without 4070A infection. Western blot analysis of these cells for CasBrE Env and 4070A Gag (Fig. 5C) indicated no qualitative changes in viral protein expression; however, 15-1EIH expression of CasBrE Env facilitated higher Gag expression levels than were seen with 4070A infection alone, a finding consistent with what was observed for the CasE vector (28). Sedimentation analysis of tissue culture supernatants (Fig. 5D) demonstrated that CasBrE Env pelleted with virions for 15-1EIH+4070A NSCs, which is consistent with CasBrE Env incorporation into viral particles. As illustrated in Fig. 5E, a high titer virus encoding 4070A was obtained from 4070A NSCs and 15-1EIH+4070A NSCs, with the latter cell type producing higher levels of infectious virus, reflective of the increased Gag noted by immunoblotting. Importantly, no CasBrE env gene-transducing virus was detected, which is consistent with the expectation that the 15-1EIH expression vector would not be incorporated into virus particles.
FIG. 5.
CasBrE Env viral particle pseudotyping reveals hidden 4070A neurovirulence. (A) Structure of the p15-1EIH vector used to facilitate CasBrE SU/TM expression without CasBrE env gene packaging into virions. (B) Cell surface expression of CasBrE Env by FACS analysis on C17.2 NSCs after p15-1EIH transfection and selection that persists upon infection with the 4070A virus (right). (C) Immunoblotting for CasBrE Env (top) and Gag (bottom) of postnuclear cell extracts from C17.2 NSCs with (+) or without (−) 15-1EIH and 4070A shows enhanced Gag expression with CasBrE Env. (D) To demonstrate that the CasBrE Env protein was effectively incorporated into virions, culture supernatants from C17.2 NSCs with (+) or without (−)15-1EIH and 4070A were subjected to ultracentrifugation, and the sedimented fraction was evaluated by immunoblotting for CasBrE Env (top) and Gag (bottom). (E) Capacity of 15-1EIH, 4070A, and 15-1EIH+4070A NSCs to produce virus, as assessed by VTA, using a broadly reactive anti-MLV Env antibody. No CasBrE Env-positive foci were observed when VTAs were specifically assessed. (F) Representative example of a 15-1EIH+4070A NSC-transplanted brain stem showing severe spongiform neuropathology at 3 weeks postinjection. Bar, 40 μm. (G) Example of a 15-1EIH+4070A NSC-transplanted brain stem double-immunostained for 4070A viral Gag (green) and the microglial marker Iba-1 (red). Cells exhibiting colocalization (yellow/orange) are identified with arrowheads. Bar, 40 μm. (H) Neuropathology scores for animals receiving control, 15-1EIH, 4070A, and 15-1EIH+4070A NSCs at 3 weeks after brain stem transplantation. (I) Neuropathology scores at 4 weeks for animals receiving control, 15-1EIH, 4070A, and 15-1EIH+4070A NSCs introduced into lateral brain ventricles. (J) Immunoblot assessment of viral protein expression levels in equivalent brain stem lysates from mice receiving 15-1EIH, 4070A, or 15-1EIH+4070A NSCs using anti-AMLV antiserum. Note the 4070A Env, precursor (pr65), and processed (p30) Gag proteins were expressed in brains transplanted with 4070A virus-expressing NSCs with or without 15-1EIH. The data are representative of samples taken from at least three separate brains in each group. No CasBrE Env protein was detected in samples processed in parallel and immunoblotted with CasBrE Env-specific antibody (MAb 697 [data not shown]).
Given the evidence that 15-1EIH+4070A NSCs were releasing viral particles incorporating the CasBrE Env protein and the 4070A genome, we next assessed their capacity to induce acute spongiform neuropathology. As shown in Fig. 5F and H, by 21 days postinjection severe spongiform neurodegeneration was observed within 15-1EIH+4070A NSC-transplanted brain stems in all of the mice examined. The neuropathology included both neuropil vacuolation and cells exhibiting vacant cytoplasm but intact nuclei, a picture indistinguishable from the CasBrE virus. In contrast, control, 15-1EIH, and 4070A NSCs induced little or no pathology at 3 and 4 weeks posttransplantation (Fig. 5H). To further assess whether the site of NSC injection influenced the pathogenic process, we examined the motor cortex in mice receiving control, 15-1EIH, 4070A, or 15-1EIH+4070A NSC injections in lateral brain ventricles. These experiments showed that spongiform neuropathology was limited after intraventricular injection (Fig. 5I), despite the presence of abundant engrafted NSCs (as detected by β-galactosidase immunostaining [data not shown]). This finding was consistent with the CasES+4070A trans-complementation experiments indicating that intraventricular NSC transplants were less effective at inducing spongiform neurodegeneration.
To assess the relationship of viral protein to neuropathogenesis, brains from mice transplanted with 15-1EIH NSCs with or without 4070A were subjected to immunohistochemistry, immunofluorescence, and immunoblotting for CasBrE Env and 4070A Gag proteins. Surprisingly, no CasBrE Env expression was detected in the brains of mice transplanted with 15-1EIH NSCs with or without 4070A when assessed at 3 and 4 weeks postimplantation, regardless of where the NSCs were transplanted or whether they induced spongiosis (not shown). Because earlier time points were not examined, it was unclear when the 15-1EIH NSCs lost detectable CasBrE Env expression. In contrast, 4070A Env (gp70) and Gag (pr65 and p30) were readily noted by immunoblotting brain extracts from mice receiving 4070A NSCs with or without 15-1EIH, with mice transplanted with 4070A NSCs showing more robust gp70 expression (Fig. 5J).
The induction of acute spongiosis by brain stem-transplanted 15-1EIH+4070A NSCs suggested that CasBrE Env-pseudotyped 4070A possessed an expanded tropism for cells that were not otherwise efficient targets. To address this idea, we performed double immunofluorescence staining on mouse brain stems for Gag and CNS cell type-specific markers. As illustrated in the example shown in Fig. 5G, 4070A Gag protein expression was observed in Iba-1+ microglia, indicating delivery to endogenous host cells. In addition, Gag was observed to be expressed in NG2 cells (NG2+) and immature (Olig2+) and mature (CAII+, CNPase+) oligodendrocytes at similar frequencies for transplants with or without 15-1EIH (Table 2). However, no Gag expression was seen in GFAP-positive astrocytes (data not shown). Neurons were not specifically examined in this analysis. Although there appeared to be a small increase in the percentage of Gag+ cells expressing Iba-1 in mice transplanted with 4070A+15-1EIH NSCs, it was not feasible to do a reliable statistical assessment due to the variability associated with both the extent and the location of the engrafted cells in each mouse brain.
It was of interest that despite the fact that many Gag+ cells expressed mature oligodendrocyte markers after transplantation with 4070A or 15-1EIH+4070A NSCs (15 to 24% of the total Gag+ cells), Gag+ cells coexpressing CAII or CNPase comprised just 2 to 4% of the total when brain stem areas within or proximal to spongiosis were assessed separately in 4070A+15-1EIH NSC-transplanted brains (Table 2). This result suggests that loss of Gag+ oligodendroglia was a secondary consequence of 4070A-induced neurodegeneration rather than specific 4070A viral targeting of these cells.
Packaging NSCs disseminating the CasBrE env alone induce acute spongiosis.
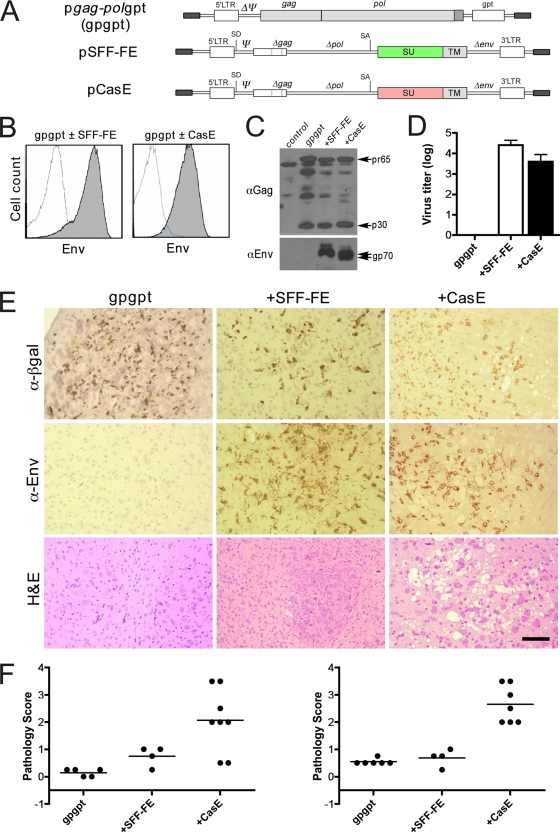
Because the CasBrE Env protein could pseudotype the 4070A virus to reveal otherwise silent 4070A neurovirulence, the initial trans-complementation experiments undertaken to assess the minimal viral elements required for CasBrE Env-induced disease were obscured. Therefore, to explore this issue in a more direct fashion, we revisited the idea of using NSCs as in vivo packaging cells to deliver env only encoding vectors. Toward this end, C17.2 NSCs were engineered with the Gag-Pol expression plasmid pgag-polgpt (gpgpt) (35), and either CasE or SFF-FE viral vectors (Fig. 6A). These cells showed cell surface CasBrE and Friend Env expression (Fig. 6B) of the appropriate molecular sizes (Fig. 6C, lower panel), as well as Gag protein expression (Fig. 6C, top panel). However, despite the abundant Gag and Env proteins, free virus production from these cells was relatively low (Fig. 6D). Nonetheless, their ability to transduce their respective Env genes via cell-to-cell contact was quite high, with >80% of these NSCs showing delivery of CasBrE or Friend env to target fibroblasts (Table 3).
FIG. 6.
Infectious CasBrE env alone is sufficient for inducing spongiform neurodegeneration. (A) Schematic representation of the molecular constructs used to produce packaging C17.2 NSCs that encode either neurovirulent (CasE) or non-neurovirulent (SFF-FE) MLV env's. The pgag-polgpt vector (gpgpt) encodes Gag and Gag-Pol polyproteins but because the psi sequence has been deleted (ΔΨ) the RNA is not incorporated into virions. The pCasE and pSFF-FE vectors possess the psi sequence allowing for packaging of the transcribed RNA into particles. (B) FACS analysis of NSCs transfected with pgag-polgpt (open traces), followed by transduction with viruses encoding SFF-FE (left panel) or CasE (right panel) vectors (gray traces). (C) Gag (αGag) and Env (αEnv) immunoblots of equivalent extracts from the cells shown in panel B. The results are representative of three different analyses. (D) VTAs for CasBrE and Friend Env vectors from control, SFF-FE, and CasE-gpgpt NSCs, respectively. (E) Representative sections from mice receiving P0 brain stem transplants of control (gpgpt, left), SFF-FE (+SFF-FE), and CasE+gpgpt (+CasE) NSCs, taken 3 weeks postinjection, immunostained for β-galactosidase (α-β-gal; brown), Env (α-Env; brown), or stained with H&E. Bar, 100 μm. (F) Summary of the neuropathology scores for mice transplanted with the gpgpt-based packaging NSCs after 3 weeks (left) and 4 weeks (right).
TABLE 3.
Infectious center assay for env vector transmission by gpgpt-based packaging NSCs
| NSC type | Mean ± SD |
IC efficiency (%)c | |
|---|---|---|---|
| IC focia | Mean plating efficiency (%)b | ||
| gpgpt | <0.05d | NDe | ND |
| CasE-gpgpt | 15.3 ± 2.1 | 17.8 ± 2.0 | 86.0 |
| FE-gpgpt | 10.3 ± 3.8 | 12.2 ± 2.0 | 83.3 |
Mitomycin C-treated and trypsin-EDTA-resuspended engineered NSCs were seeded with 105 naive Mus dunni cells at different ratios. The numbers of Env-expressing foci were evaluated when target cells reached confluence. Numbers are expressed as IC foci/100 NSCs seeded.
To determine the efficiency of plating, mitomycin C-treated NSCs were seeded alone, and the vector-expressing cells present on these plates were assessed at the same time as those seeded with targets. Plating efficiency indicates the percentage of seeded cells that attached.
IC efficiency is the number of foci/100 NSCs seeded divided by the plating efficiency and is expressed as a percentage.
No retroviral Env-positive cells were detected with both virus-specific and broadly reactive antiviral antibodies in assays where 2,000 NSCs were seeded into each assay.
ND, not determined.
Because the pseudotyping experiments described above indicated that brain stem transplantation of NSCs provided the most sensitive means for assessing viral neuropathogenesis, our analysis of the packaging NSCs was confined to this transplant paradigm, with analyses at 21 and 28 days after NSC implantation. Transplanted β-galactosidase-positive gpgpt NSCs were observed dispersed within the brain stem, regardless of whether they possessed an Env encoding vector or not (Fig. 6E). Both SFF-FE-gpgpt and CasE-gpgpt NSCs showed that Friend and CasBrE Env protein expression was readily detected in many ovoid and ramified cells, a finding consistent with persistent NSC expression and virus dissemination to host cells. H&E staining of engrafted brain stem sections showed that gpgpt and SFF-FE-gpgpt NSCs induced little spongiform change through 4 weeks posttransplantation (n = 11 and 8, respectively), whereas, CasE-gpgpt NSC-transplanted brains showed significant spongiosis in six of eight animals examined at 21 dpi and in seven of seven animals examined at 28 dpi. Importantly, brain stem homogenates obtained from mice treated with CasE, SFF-FE, or control-gpgpt NSCs (n = 5, 5, and 4, respectively) did not show evidence of replication-competent virus when assessed for contiguous Env expression foci on dunni cells, nor were foci detected upon serial passage of homogenates (not shown).
To assess whether the induction of spongiform pathology may have been simply due to the binding and fusion of viral particles containing CasBrE Env, we generated gpgpt NSCs that expressed CasBrE Env from the 15-1EIH vector and transplanted those into neonatal brain stems. Although no spongiform neurodegenerative changes were observed at 21 and 28 days posttransplantation (n = 6 and 5, respectively), detection of CasBrE Env-expressing cells in these mice was infrequent, although the β-galactosidase-positive cells were more common (not shown). However, because it was not clear whether these NSCs expressed CasBrE Env for a sufficient period or even persisted long enough and to generate fusogenic particles, it could not be determined whether this approach would address the issue of whether particle entry could induce neuropathogenesis.
Env packaging NSCs variably target brain stem glia and neurons.
The data presented above indicate that ecotropic Env targeting of a neurotoxic Env is sufficient for inducing neurodegeneration; however, the specific CNS targets responsible for disease induction remain obscure. Given that microglia constitute a major CNS target of MLVs (3, 4, 20, 21, 30, 32, 34), we examined these cells for the expression of CasBrE and Friend Envs to assess the capacity of the packaging NSCs to infect host targets. In this regard, transplanted SFF-FE-gpgpt NSCs resulted in significant Env expression in microglia by 21 dpi (Fig. 7A, top). However, in mice transplanted with CasE-gpgpt NSCs, CasBrE Env expression within microglia was essentially absent in vacuolated brain regions (Fig. 7A, bottom) and was infrequent in engrafted areas without spongiosis (inset images).
FIG. 7.
CasE-gpgpt packaging NSC transplants facilitate minimal CasBrE Env expression in host cells. (A) Double immunofluorescence staining for Env (green) and Iba-1 (red) to assess env vector dissemination to host microglia in brains transplanted with gpgpt+SFF-FE (top) and gpgpt+CasE NSCs (bottom), 3 weeks posttransplantation. Arrowheads indicate cells showing colocalization of the two markers. An arrow indicates an example of an Env+, Iba-1− cell in a gpgpt+SFF-FE brain with a morphology consistent with a transplanted NSC. CasBrE Env+ cells possess a similar morphology. CasBrE Env/Iba-1 double-positive cells were only occasionally noted in the CasE+gpgpt NSC-transplanted mice (inset); however, they were uncommon in areas exhibiting vacuolation, observed in the sections as areas devoid of background tissue fluorescence, denoted by the white circles in gpgpt+CasE. The sections were assessed by using epifluorescence optics. Bar, 40 μm. (B) Confocal images of NSC-transplanted brain sections double immunostained for viral protein (green; Gag or Env) and the cell type-specific markers NG2 and Olig2 (red). White arrowheads indicate examples of viral protein expression in NG2- and Oligo2-positive cells (confirmed by z-stack analysis [data not shown]) that minimally represent the engrafted packaging NSCs. Bar, 20 μm. (C) Confocal images of brain stems transplanted with gpgpt packaging NSCs, double immunostained for Env (top) or Gag (bottom) and the pan-neuronal marker HuC/D (red). Note that no Env expression was associated with HuC/D+ neurons; however, examples of Gag immunostaining coincident with HuC/D were detectable in brain stems transplanted with either FE or CasE+gpgpt NSCs, illustrating particulate staining for Gag that was often clustered within the neuron cell bodies (yellow). Bar, 20 μm.
Given the lack of CasBrE Env expression in microglia coincident with spongiform neurodegeneration, we examined what other cell types could be targeted by env-encoding virions. To assess the fate of the packaging C17.2 NSCs, Gag expression was utilized as a surrogate marker rather than β-galactosidase. As shown in Fig. 7B and summarized in Table 4, 34% of Gag+ cells in brains transplanted with gpgpt NSCs were positive for NG2, whereas almost 6% showed Olig2 expression. However, only rarely was Gag expression observed coincident with the mature oligodendroglial markers CAII or CNPase. In addition, no Gag+ cells were observed to express the astrocytic marker GFAP. Examination of mice transplanted with CasE and SFF-FE-gpgpt NSCs showed similar trends in cellular colocalization of Gag and macroglial markers (summarized in Table 4). These results indicate that the gpgpt NSCs were minimally capable of differentiation toward OPCs during the assessment period.
TABLE 4.
Cell type expression of retroviral proteins in brain stems transplanted with packaging NSCs
| CNS cell type | Marker | No. of cells positive for glial cell marker/total no. of cells (%) in transplantsa |
||||
|---|---|---|---|---|---|---|
| gpgpt (MAb R187)b | CasE-gpgpt |
FE-gpgpt |
||||
| MAb 697c | MAb R187 | FEd | MAb R187 | |||
| Oligodendrocyte | CAII | 4/325 (1.7) | 1/438 (0.2) | 2/278 (0.7) | 6/300 (2) | 5/376 (1.3) |
| CNPase | 0/262 | 0/220 | 0/25 | 1/371 (0.3) | 1/241 (0.4) | |
| Astrocyte | GFAP | 0/273 | 0/308 | 0/246 | 0/40 | 0/194 |
| Olig2+ cell | Olig2 | 14/234 (5.6) | 1/358 (0.3) | 5/92 (5.4) | 7/321 (2.2) | 10/233 (4.5) |
| NG2+ cell | NG2 | 60/176 (34.1) | 49/166 (29.5) | 47/159 (29.6) | 68/233 (29.2) | 62/363 (17.1) |
The numbers provided indicate the number of cells positive for the glial cell marker (numerator) out of the total number of Env or Gag positive cells observed in the brain stem sections surveyed (denominator). Colocalization was determined by z-stack analysis of confocal images.
MAb R187 (8) was used to detect Gag protein expression.
MAb 697 (36) was used to detect CasBrE Env protein expression.
Goat anti-Friend Env antiserum (FE) was used to detect Friend Env expression.
To assess the dissemination of env genes to host neural cells, Env protein colocalization with macroglial markers was undertaken as outlined for Gag, with the expectation that the frequency of colocalization would increase if env dissemination to host cells was robust. Figure 7B shows that colocalization of Friend and CasBrE Env+ cells with the early glial differentiation marker NG2 could be readily detected; however, only SFF-FE-gpgpt NSC transplants showed an increase in the number of NG2+ cells expressing Env (Table 4). Colocalization of Env and the OPC marker Olig2 was only rarely observed in mice transplanted with CasE-gpgpt NSCs despite upward of 5% Olig2 colocalization with Gag in these transplants. The frequency of Friend Env+ Olig2+ cells in SFF-FE-gpgpt NSCs was higher than for CasE-gpgpt NSC transplants. However, the numbers were still only half of those expressing Gag alone, suggesting that the presence of any Env gene may negatively impacted the survival of the gpgpt NSCs. Examination of transplants for Env and CAII or CNPase showed only rare colocalization, which could not be distinguished from Gag colocalization.
Although a number of studies have suggested that MLVs induce neurodegeneration indirectly through glial infection, other reports have suggested that susceptible neurons may permit neurovirulent MLV entry but do not fully support postentry replication events (54, 59). To explore the potential for MLV particle entry and replication in neurons, we examined brain stem regions transplanted with the gpgpt NSCs with or without CasE or SFF-FE for neuron-associated Env and Gag proteins. As shown in Fig. 7C, confocal imaging of CasBrE Env (green) and HuC/D (red) showed abundant Env protein immunoreactivity in non-neuronal cells but no staining within brain stem neuron cell bodies. Parallel sections assessed for Gag protein expression showed immunostaining in HuC/D-negative cells for all gpgpt NSC transplants, which presumably represent the packaging NSCs. However, in transplants where the gpgpt NSCs contained either SFF-FE or CasE vectors, Gag staining was also observed within the HuC/D+ cell bodies, sometimes coalesced as aggregates. These data are consistent with the idea that ecotropic Env-containing MLV particles fused to and entered into neurons within susceptible brain regions but failed to generate detectable Env expression. Although Gag+, HuC/D+ double-positive cells were observed, no specific association with neuronal vacuolation was noted; however, we did not undertake a kinetic analysis to explore whether such Gag+ neurons could transition to vacuolated cells since neuronal changes are initially associated with dendritic processes (30) and documenting a clear association would not be straightforward.
DISCUSSION
In this report we used an NSC-based viral pseudotyping and trans-complementation strategy to reconstitute specific aspects of neurovirulent MLV infection within the brain to understand what viral components and MLV life cycle events are responsible for the induction of spongiform neurodegeneration. The results support the idea that the Env protein mediates disease by acting at two levels. First, Env acts within the context of the viral particle to uniquely target the virus within the CNS. Second, neurovirulent env mediates vacuolation through its expression within the CNS targets to alter cellular function. With regard to the identity of the critical cellular targets, our findings challenge the idea that neuronal degeneration is mediated through infection of microglia, OPCs, or mature oligodendroglia, since these cells were efficiently targeted by the 4070A virus, without the appearance of neuropathology, when in fact, 4070A is a neurovirulent virus. Our results suggest that we consider the infection of other CNS cell types, including those where neurovirulent Env protein expression cannot be readily detected, which based on our observations here appear to include neurons and astrocytes.
The current experiments were undertaken because neither neurovirulent MLV Env transgenic mice (25, 65) nor NSC-based chimeras (33, 34) were capable of recapitulating the acute severe neurodegenerative disease phenotype associated with CNS virus infection. The gross differences suggested that either some critical viral element was missing or that the experimental approaches used were not recapitulating critical quantitative or qualitative aspects of the infectious process. The NSC-based pseudotyping strategies used here were designed to circumvent these limitations; however, they were initiated based on what turned out to be misguided assumptions. In this regard, several lines of evidence suggested that the 4070A amphotropic virus was neuroinvasive but not neurovirulent (14, 44, 61), and thus it was initially used by Jolicoeur et al. to map the primary MLV neurovirulence determinants to the env gene (14). The CasBrE Env pseudotyping findings here showed that 4070A was indeed neurovirulent, but only if an entry restriction was bypassed by ecotropic Env particle pseudotyping. Thus, the original genetic neurovirulence mapping studies can be reinterpreted to have identified the critical CNS cellular tropism determinants, rather than the neurotoxin determinants. Importantly, the findings for 4070A provide support for the idea that the neurotoxic Env elements are not encoded by sequences responsible for MLV receptor specificity, an idea consistent with the existence of neurovirulent ecotropic, polytropic, amphotropic, and 10A1-tropic MLVs, as well as a variety of other neurovirulent animal and human retroviruses that possess similar Env structure.
A second assumption made upon the initiation of these studies was that because our previous studies had demonstrated that NSC-expressed CasBrE SU possessed almost no ecotropic receptor binding activity (34), particle tropism would be limited to amphotropic receptors after 4070A pseudotyping of the CasES vector. Moreover, since the glycophosphatidylinositol membrane anchor on prion protein is required for prion-induced spongiosis (9), we expected that membrane association through TM would be required for Env neurovirulence. The lack of spongiosis noted after lateral cerebral ventricle transplantation of 4070A+CasES NSCs appeared to support this assumption; however, the discovery of spongiosis after brain stem transplantation of these same cells forced us to reassess this position. We have recently reported that 4070A Env-CasBrE SU interact within NSCs and that this interaction restores lost CasBrE SU ecotropic receptor binding activity and association with the plasma membrane (28). The studies here extend the 4070A Env complementation of CasBrE SU to in vivo, where restored ecotropic targeting facilitated spongiform neurodegeneration.
The ecotropic pseudotyping results using the nonpackaged 15-1EIH vector conclusively demonstrate that 4070A is neuropathogenic. Thus, when considering the trans-complementation experiments, it is possible that spongiosis could have been induced by the 4070A Env, CasBrE SU, or SU/TM. More detailed experiments examining the requirement for TM need to be undertaken to resolve the requirement for membrane association. Nonetheless, these results may help clarify why certain mouse strains are susceptible to the MoAmphoV amphotropic virus and why they develop more rapid and severe spongiform disease when coinfected with either an ecotropic or polytropic virus (39). Taken together, these findings suggest that the critical CNS target cells express significant levels of both ecotropic and polytropic receptors (5, 60, 63), whereas the numbers of amphotropic receptors (PiT-2 [24]) are either quite limited on the critical CNS cell type or PiT-2 is modified on these cells in a way that restricts amphotropic Env-mediated infection. The implication is that, once targeted, 4070A Env expression alone, like CasBrE Env, induces neuropathology by a mechanism of action that does not involve intracellular Env-receptor interactions. What feature(s) of Env is involved in precipitating disease remains unknown, but presumably this feature is shared by both CasBrE and 4070A Envs. In this regard, we have recently reported that 4070A Env can induce CasBrE SU protein refolding, analogous to the template-based protein refolding observed in prion formation (28). This finding raises the prospect that protein directed protein folding may be a critical feature of spongiogenic proteins. Alternatively, findings from Portis et al. suggest that the severity of MLV neurotoxicity correlates with protein folding instability associated with the induction of endoplasmic reticulum stress (47). Whether either or both of these possibilities are involved in the initiation of neuropathogenesis will require more detailed analysis.
A major question highlighted by these studies is what cells constitute the critical targets for Env-induced disease. Several early reports provided support for the idea that neurovirulent MLVs cause disease by directly infecting susceptible motor neurons (1, 45); however, these studies on protracted disease models could not rule out the possibility that the virus-like particles observed in neurons were the result of endogenous virus expression, rather than the input virus. Later studies of acute disease models, using specific nucleic acid and MAb probes were unable to confirm the presence of the exogenous input virus within the degenerating neurons. Instead, these later investigations demonstrated that infection in endothelia, microglia, oligodendroglia, and unaffected postnatally mitotic neurons occurred coincidently with disease development (10, 20, 26, 30, 38). These findings focused attention on the hypothesis that spongiform degeneration of neurons was mediated indirectly.
Findings from multiple groups have demonstrated a close correlation between microglial infection and spongiform neurodegeneration (3, 4, 20, 22, 30, 32, 52), suggesting that disease might be mediated through this cell type. However, our previous findings demonstrated that 4070A readily infects microglia (61). Although this was originally interpreted as indicating that 4070A was non-neurovirulent, the pseudotyping experiments presented here suggest otherwise and, by extension, that infection of this cell type does not play a critical role in spongiogenesis. This interpretation is consistent with our previous packaging NSC studies, wherein we observed that CasBrE env dissemination to and expression within microglia failed to induce spongiosis (33). It is also consistent with our gene array analyses indicating that neurovirulent MLVs do not induce overt microglial gene expression changes (15). Thus, while these cells appear to be targets for both neurovirulent and non-neurovirulent MLVs (2), their role in neurodegeneration appears to be ostensibly limited to serving as persistent sources for virus dissemination within the CNS parenchyma.
The observation of oligodendroglial infection coincident with glial vacuolation and myelin splitting (1, 10, 38, 45) has led to the suggestion that neurotoxicity may be initiated through oligodendrocyte infection (10). However, in the present study, we provide evidence for 4070A virus infection of mature (CAII+, CNPase+) and immature (Olig2+) oligodendroglia regardless of whether the 4070A virus was pseudotyped by an ecotropic Env protein. Since we only observed neurodegeneration under conditions where 4070A was pseudotyped, the results suggest that 4070A infection of oligodendroglia or OPCs was not sufficient to induce vacuolar changes directly in oligodendroglia or indirectly in neurons. The formal possibility exists that CasBrE Env binding to its receptor, mCAT-1, induced changes in oligodendroglial physiology that renders these cells susceptible to 4070A-induced changes, although Env-mCAT-1-mediated signal transduction has yet to be reported. Thus, the simplest interpretation of the pseudotyping data is that oligodendroglial degeneration is an indirect response to infection of another CNS cell type that is refractory to 4070A infection.
Another CNS cell type that we evaluated in the present study was the NG2 cell or polydendrocyte. These cells, which appear to constitute a fourth glial element in the CNS, exist in both white and gray matter of the mature and developing brain and possess the ability to proliferate and differentiate either toward oligodendroglia or persist as ramified cells capable of firing action potentials (reviewed in reference 42). Thus, it is interesting to speculate that their infection by neurovirulent MLVs could broadly affect CNS functioning and contribute to neurodegeneration. However, because we observed that the C17.2 NSCs express NG2 once they engraft, we could not reliably determine whether Env- or Gag-expressing NG2 cells were of host or donor origin. Thus, we were unable to evaluate whether infected NG2 cells were associated with neurodegeneration per se. However, given that NG2 cell infection in the CNS can be readily detected after peripheral virus inoculation with FrCasE (unpublished observations), it remains intriguing whether infection of this unique glial subpopulation could contribute to the development of spongiform neurodegeneration. In this regard, it is notable that CasBrE Env and NG2 are coexpressed in transplanted CasE-C17.2 NSCs without neuropathological changes, suggesting that neurovirulent Env expression in NG2 cells is not sufficient for disease induction. However, given that the C17.2 line was derived via the introduction of v-myc into cerebellar neural progenitor cells (53, 57), it is not clear whether these NSCs become fully integrated NG2 glia within the brain, or whether their v-myc expression could compensate for any adverse effect of neurovirulent Env expression. Thus, such experiments may require CNS reconstitution with infected primary NSCs to effectively explore whether NG2 cell expression of neurovirulent env is required for disease.
The lack of an obvious connection between glial infection and neuronal degeneration led us to revisit the idea that the degenerating neurons themselves might be MLV targets. Examination of mice transplanted with CasE and SFF-FE-gpgpt NSCs showed Gag but not Env immunoreactivity in cells expressing the neuronal marker HuC/D in regions where spongiosis develops. The punctate nature of the neuronal Gag staining suggested that it was due to the accumulation of Env-addressed particles in these cells, although we cannot rule out the possibility that such staining could represent endogenous Gag protein expression. Similar findings of Gag protein without Env expression in neurons was previously noted in mice infected with replication-competent CasBrE virus, prompting the suggestion that abortive infection of neurons was directly inducing neuropathology (54). It has also been demonstrated that the brains and spinal cords of MLV-infected mice contain high levels of unintegrated DNA (58, 59), although this may not be unique for neurovirulent viruses. The accumulation of unintegrated DNA is consistent with the lack of brain stem neuron cell division, since when MLVs enter the CNS gammaretroviruses require nuclear membrane dissolution for proviral DNA integration. Based on our examination of brains transplanted with the CasE and SFF-FE packaging cells, no obvious differences in Gag staining in the neurons were noted, suggesting that there were no obvious neuronal tropism differences between the Friend and CasBrE Envs that could account for the differences in spongiosis. This observation suggests that any direct neuropathology would have to be due to neurovirulent env expression rather than differential receptor targeting.
Because Env could not be detected in brain stem neurons containing Gag, it raises the possibility that disease could be induced, either directly or indirectly, by very low Env expression levels in target cells. One possibility is that such expression could arise from transcription of the viral RNA, as was recently demonstrated in culture with MLVs possessing mutant integrase (64). However, this idea runs counter to our earlier findings showing that restriction of proviral integration by the host resistance factor Fv-1 prevents the development of acute spongiform neurodegeneration (34). Alternatively, persistent exposure of susceptible neurons to infectious virions could lead to rare nuclear entry events with associated mRNA and protein expression that could alter neuronal function. Assessing whether the neurons exhibiting Gag accumulation ultimately express viral genes and undergo vacuolar changes will require the development of novel approaches for identifying the viral targets that are independent of Env expression levels, followed by physiologic and morphological assessment of those targets over time.
A major advance of the present study is the finding that CNS targeting of a neurovirulent env alone is sufficient to recapitulate MLV-induced spongiosis. The Gag and Pol viral components appear to be required simply to disseminate the env gene. Interestingly, the gpgpt-based packaging lines used here generated env virus titers that were 10- to 100-fold lower than those generated by an earlier packaging line (33). Nonetheless, these NSCs proved highly efficient at env gene transduction via cell-cell contact both in vivo and in vitro. We suspect that this facility was mediated through a mechanism similar to those described by Sherer et al. showing the formation of synaptonemal bridges between target cells and virus-producing cells that dramatically enhance retroviral infection (55, 56). Coupled with their capacity to engraft and persist in the perinatal CNS, these NSCs proved to be capable of inducing spongiosis where the previous CasBrE env packaging NSCs had failed (33). Another factor that appeared to be critical for demonstrating Env-only-induced spongiogenesis was the need for direct transplantation of the NSCs into the brain stem proper. This was first noted with NSCs pseudotyping 4070A with either CasES (CasBrE SU) or the 15-1EIH vectors. The presumption is that pseudotyping was more limited due to suppression of CasBrE SU/TM expression arising from either unique environmental cues in the ventricles, NSC transition to a migratory phenotype, or the time required for NSC migration and integration into susceptible parenchyma niches—events that would be circumvented by direct parenchymal transplantation and allow efficient env transduction.
In conclusion, the findings presented here using immortalized NSCs as gene delivery tools support the idea that a single retroviral protein, Env, is sufficient for recapitulating acute spongiform neurodegeneration within the CNS, but only when that Env contains neurotoxic sequences and is delivered to a unique CNS cell type. Ferreting out the identity of the critical target cells, what sequences are neurotoxic, and understanding the cellular and biochemical changes responsible for altering neuronal physiology will likely require further NCS chimera-based CNS reconstitution and analysis. Given the similarity of the neurodegenerative process induced by Env to other genetic, sporadic, and infectious diseases of the CNS associated with “abnormal” proteins, understanding this MLV model should provide critical insight into how diseases of protein folding are mediated.
Acknowledgments
This study was supported in part by a grant from the ALS Association (www.alsa.org) and by NIH grant RO1 NS37614 to W.P.L.
We thank Nianyuan (Hanna) Huang, Rochelle Cutrone, and Jaclyn Stenger for technical assistance with these studies.
Footnotes
Published ahead of print on 29 December 2010.
REFERENCES
- 1.Andrews, J. M., and M. B. Gardner. 1974. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J. Neuropathol. Exp. Neurol. 33:285-307. [DOI] [PubMed] [Google Scholar]
- 2.Askovic, S., F. J. McAtee, C. Favara, and J. L. Portis. 2000. Brain infection by neuroinvasive but avirulent murine oncornaviruses. J. Virol. 74:465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baszler, T. V., and J. F. Zachary. 1991. Murine retroviral neurovirulence correlates with an enhanced ability of virus to infect selectively, replicate in, and activate resident microglial cells. Am. J. Pathol. 138:655-671. (Erratum, 138:1058.) [PMC free article] [PubMed] [Google Scholar]
- 4.Baszler, T. V., and J. F. Zachary. 1990. Murine retroviral-induced spongiform neuronal degeneration parallels resident microglial cell infection: ultrastructural findings. Lab. Invest. 63:612-623. [PubMed] [Google Scholar]
- 5.Battini, J. L., J. E. Rasko, and A. D. Miller. 1999. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc. Natl. Acad. Sci. U. S. A. 96:1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks, B. R., J. R. Swarz, O. Narayan, and R. T. Johnson. 1979. Murine neurotropic retrovirus spongiform polioencephalomyelopathy: acceleration of disease by virus inoculum concentration. Infect. Immun. 23:540-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buller, R. S., K. Wehrly, J. L. Portis, and B. Chesebro. 1990. Host genes conferring resistance to a central nervous system disease induced by a polytropic recombinant Friend murine retrovirus. J. Virol. 64:493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesebro, B., et al. 1983. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology 127:134-148. [DOI] [PubMed] [Google Scholar]
- 9.Chesebro, B., et al. 2005. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308:1435-1439. [DOI] [PubMed] [Google Scholar]
- 10.Clase, A. C., et al. 2006. Oligodendrocytes are a major target of the toxicity of spongiogenic murine retroviruses. Am. J. Pathol. 169:1026-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czub, M., S. Czub, F. McAtee, and J. Portis. 1991. Age-dependent resistance to murine retrovirus-induced spongiform neurodegeneration results from CNS-specific restriction of virus replication. J. Virol. 65:2539-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czub, M., F. J. McAtee, and J. L. Portis. 1992. Murine retrovirus-induced spongiform encephalomyelopathy: host and viral factors which determine the length of the incubation period. J. Virol. 66:3298-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czub, S., W. P. Lynch, M. Czub, and J. L. Portis. 1994. Kinetic analysis of the spongiform neurodegenerative disease induced by a highly virulent murine retrovirus. Lab. Invest. 70:711-723. [PubMed] [Google Scholar]
- 14.DesGroseillers, L., M. Barrette, and P. Jolicoeur. 1984. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic virus. J. Virol. 52:356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimcheff, D. E., L. G. Volkert, Y. Li, A. L. DeLucia, and W. P. Lynch. 2006. Gene expression profiling of microglia infected by a highly neurovirulent murine leukemia virus: implications for neuropathogenesis. Retrovirology 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans, L. H., R. P. Morrison, F. G. Malik, J. Portis, and W. J. Britt. 1990. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J. Virol. 64:6176-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner, M. B. 1985. Retroviral spongiform polioencephalomyelopathy. Rev. Infect. Dis. 7:99-110. [DOI] [PubMed] [Google Scholar]
- 18.Gardner, M. B., et al. 1973. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J. Natl. Cancer Inst. 51:1243-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner, M. B., et al. 1976. Lower motor neuron disease in wild mice caused by indigenous type C virus and search for a similar etiology in human amyotrophic lateral sclerosis. UCLA Forum Med. Sci. 19:217-234. [PubMed] [Google Scholar]
- 20.Gravel, C., D. G. Kay, and P. Jolicoeur. 1993. Identification of the infected target cell type in spongiform myeloencephalopathy induced by the neurotropic Cas-Br-E murine leukemia virus. J. Virol. 67:6648-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen, R., et al. 2000. Abundant defective viral particles budding from microglia in the course of retroviral spongiform encephalopathy. J. Virol. 74:1775-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen, R., et al. 2001. Activation of microglia cells is dispensable for the induction of rat retroviral spongiform encephalopathy. J. Neurovirol. 7:501-510. [DOI] [PubMed] [Google Scholar]
- 23.Jolicoeur, P., C. Gravel, and D. G. Kay. 1992. Pathogenesis of murine spongiform myeloencephalopathy induced by a murine retrovirus, p. 199-224. In R. P. Roos (ed.), Molecular neurovirology. Humana Press, Inc., Totowa, NJ.
- 24.Kavanaugh, M., et al. 1994. Cell surface receptor for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. U. S. A. 91:7071-7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kay, D. G., et al. 1993. Neurological disease induced in transgenic mice expressing the env gene of the Cas-Br-E murine retrovirus. Proc. Natl. Acad. Sci. U. S. A. 90:4538-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kay, D. G., C. Gravel, Y. Robitaille, and P. Jolicoeur. 1991. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc. Natl. Acad. Sci. U. S. A. 88:1281-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak, S. L., and D. Kabat. 1990. Ping-pong amplification of a retroviral vector achieves high level gene expression: human growth hormone production. J. Virol. 64:3500-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, Y., and W. P. Lynch. 2010. Misfolding of CasBrE SU is reversed by interactions with 4070A Env: implications for gammaretroviral neuropathogenesis. Retrovirology 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch, W. P., W. J. Brown, G. J. Spangrude, and J. L. Portis. 1994. Microglial infection by a neurovirulent murine retrovirus results in defective processing of envelope protein and intracellular budding of virus particles. J. Virol. 68:3401-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch, W. P., S. Czub, F. J. McAtee, S. F. Hayes, and J. L. Portis. 1991. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron 7:365-379. [DOI] [PubMed] [Google Scholar]
- 31.Lynch, W. P., and J. L. Portis. 2000. Neural stem cells as tools for understanding retroviral neuropathogenesis. Virology 271:227-233. [DOI] [PubMed] [Google Scholar]
- 32.Lynch, W. P., S. J. Robertson, and J. L. Portis. 1995. Induction of focal spongiform neurodegeneration in developmentally resistant mice by implantation of murine retrovirus-infected microglia. J. Virol. 69:1408-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch, W. P., A. H. Sharpe, and E. Y. Snyder. 1999. Neural stem cells as engraftable packaging lines can mediate gene delivery to microglia: evidence from studying retroviral env-related neurodegeneration. J. Virol. 73:6841-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch, W. P., E. Y. Snyder, L. Qualtiere, J. L. Portis, and A. H. Sharpe. 1996. Late virus replication events in microglia are required for neurovirulent retrovirus-induced spongiform neurodegeneration: evidence from neural progenitor-derived chimeric mouse brains. J. Virol. 70:8896-8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markowitz, D., S. Goff, and A. Bank. 1988. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J. Virol. 62:1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAtee, F. J., and J. L. Portis. 1985. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J. Virol. 56:1010-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, A. D., M.-F. Law, and I. M. Verma. 1985. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol. Cell. Biol. 5:431-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morey, M. K., and C. A. Wiley. 1990. Immunohistochemical localization of the neurotropic ecotropic murine leukemia virus in moribund mice. Virology 178:104-112. [DOI] [PubMed] [Google Scholar]
- 39.Munk, C., et al. 1997. Amphotropic murine leukemia viruses induce spongiform encephalomyelopathy. Proc. Natl. Acad. Sci. U. S. A. 94:5837-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy, S. L., M. J. Honczarenko, N. V. Dugger, P. M. Hoffman, and G. N. Gaulton. 2004. Disparate regions of envelope protein regulate syncytium formation versus spongiform encephalopathy in neurological disease induced by murine leukemia virus TR. J. Virol. 78:8392-8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagra, R. M., P. G. Burrola, and C. A. Wiley. 1992. Development of spongiform encephalopathy in retroviral infected mice. Lab. Invest. 66:292-302. [PubMed] [Google Scholar]
- 42.Nishiyama, A., M. Komitova, R. Suzuki, and X. Zhu. 2009. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10:9-22. [DOI] [PubMed] [Google Scholar]
- 43.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oldstone, M. B. A., F. Jensen, F. J. Dixon, and P. W. Lampert. 1980. Pathogenesis of the slow disease of the central nervous system associated with the wild mouse virus. II. Role of virus and host gene products. Virology 107:180-193. [DOI] [PubMed] [Google Scholar]
- 45.Oldstone, M. B. A., P. W. Lampert, S. Lee, and F. J. Dixon. 1977. Pathogenesis of the slow disease of the central nervous system associated with WM 1504 E virus. I. Relationship of strain susceptibility and replication to disease. Am. J. Pathol. 88:193-212. [PMC free article] [PubMed] [Google Scholar]
- 46.Portis, J. L. 2001. Genetic determinants of neurovirulence of murine oncornaviruses. Adv. Virus Res. 56:3-38. [DOI] [PubMed] [Google Scholar]
- 47.Portis, J. L., P. Askovich, J. Austin, Y. Gutierrez-Cotto, and F. J. McAtee. 2009. The degree of folding instability of the envelope protein of a neurovirulent murine retrovirus correlates with the severity of the neurological disease. J. Virol. 83:6079-6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portis, J. L., S. Czub, C. F. Garon, and F. J. McAtee. 1990. Neurodegenerative disease induced by the Wild Mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J. Virol. 64:1648-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portis, J. L., S. Czub, S. Robertson, F. McAtee, and B. Chesebro. 1995. Characterization of a neurologic disease induced by a polytropic murine retrovirus: evidence for differential targeting of ecotropic and polytropic viruses in the brain. J. Virol. 69:8070-8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Portis, J. L., and W. P. Lynch. 1998. Dissecting the determinants of neuropathogenesis of the murine oncornaviruses. Virology 247:127-136. [DOI] [PubMed] [Google Scholar]
- 51.Robertson, M. N., et al. 1991. Production of monoclonal antibodies reactive with a denatured form of Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and Western blotting. J. Virol. Methods 34:255-271. [DOI] [PubMed] [Google Scholar]
- 52.Robertson, S. J., K. J. Hasenkrug, B. Chesebro, and J. L. Portis. 1997. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglial cells. J. Virol. 71:5287-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryder, E. F., E. Y. Snyder, and C. L. Cepko. 1990. Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J. Neurobiol. 21:356-375. [DOI] [PubMed] [Google Scholar]
- 54.Sharpe, A. H., J. J. Hunter, P. Chassler, and R. Jaenisch. 1990. Role of abortive retroviral infection of neurons in spongiform CNS degeneration. Nature 346:181-183. [DOI] [PubMed] [Google Scholar]
- 55.Sherer, N. M., et al. 2007. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol. 9:310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherer, N. M., and W. Mothes. 2008. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 18:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snyder, E. Y., et al. 1992. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68:33-51. [DOI] [PubMed] [Google Scholar]
- 58.Szurek, P. F., and B. R. Brooks. 1995. Development of physical forms of unintegrated retroviral DNA in mouse spinal cord tissue during ts1-induced spongiform encephalomyelopathy: elevated levels of a novel single-stranded form in paralyzed mice. J. Virol. 69:348-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szurek, P. F., and B. R. Brooks. 1996. ts1-Induced spongiform encephalomyelopathy: physical forms of high-mobility DNA in spinal cord tissues of paralyzed mice are products of premature termination of reverse transcription. J. Virol. 70:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tailor, C. S., A. Nouri, C. G. Lee, C. Kozak, and D. Kabat. 1999. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc. Natl. Acad. Sci. U. S. A. 96:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Traister, R. S., and W. P. Lynch. 2002. Re-examination of amphotropic murine leukemia virus neurovirulence: NSC-mediated microglial infection fails to induce acute neurodegeneration. Virology 293:262-272. [DOI] [PubMed] [Google Scholar]
- 62.Wong, P. K. Y., and P. H. Yuen. 1992. Molecular basis of neurologic disorders induced by a mutant ts1 of Moloney murine leukemia virus, p. 161-197. In R. Roos (ed.), Molecular neurovirology: pathogenesis of viral CNS infections. Humana Press, Inc., Totowa, NJ.
- 63.Yang, Y. L., et al. 1999. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nat. Genet. 21:216-219. [DOI] [PubMed] [Google Scholar]
- 64.Yu, S. S., et al. 2008. Transient gene expression mediated by integrase-defective retroviral vectors. Biochem. Biophys. Res. Commun. 368:942-947. [DOI] [PubMed] [Google Scholar]
- 65.Yu, Y. E., W. Choe, W. Zhang, G. Stoica, and P. K. Wong. 1997. Development of pathological lesions in the central nervous system of transgenic mice expressing the env gene of ts1 Moloney murine leukemia virus in the absence of viral gag and pol genes and viral replication. J. Neurovirol. 3:274-282. [DOI] [PubMed] [Google Scholar]