Abstract
A hallmark of human immunodeficiency virus type 1 (HIV-1) pathogenesis is the rapid loss of CD4 T cells leading to generalized immune dysfunction, including an exhausted CD8 T cell phenotype. Understanding the necessary factors that govern the functional quality and protective potential of antiviral T cell responses would facilitate rational vaccine design and improve therapeutic strategies to combat persistent infections. Mouse models of chronic viral infection demonstrate that interleukin-21 (IL-21), produced primarily by CD4 T cells, is required for the generation and maintenance of functionally competent CD8 T cells and viral containment. We reasoned that preserved IL-21 production during HIV-1 infection would be associated with enhanced CD8 T cell function, allowing improved viral control. Here we analyzed the ability of CD4 and CD8 T cells to produce several cytokines in addition to IL-21 ex vivo following stimulation with overlapping HIV-1 peptides. Both CD4 and CD8 T cells were able to produce IL-21 in response to HIV-1 infection, with the latter cell type more closely associated with viral control. Furthermore, IL-21-producing HIV-1-specific CD4 T cells (compared to those producing other cytokines) were the best indicator of functional CD8 T cells. Our results demonstrate that HIV-1-specific IL-21-producing CD8 T cells are induced following primary infection and enriched in elite controllers, suggesting a critical role for these cells in the maintenance of viremia control.
Despite the induction of ample virus-specific CD8 T cell responses, ongoing antigenemia precludes human immunodeficiency virus type 1 (HIV-1) containment in the majority of patients. A hallmark of patients with primary HIV-1 infection (PHI) is the massive depletion of memory CD4 T cells (3, 24). This virus-induced destruction continues into chronic infection and is accompanied by the functional abrogation of robust HIV-1-specific CD4 T cell responses (19, 40). The impaired capacity of virus-specific CD4 T cells to provide cognate help to CD8 T cells compromises the protective immune responses necessary to control chronic viral infection (19). As such, HIV-1-specific CD8 T cells gradually become less functional and persist in an exhausted state, unable to elicit potent polyfunctional effector traits or effectively eradicate infected targets. Remarkably, polyfunctional CD8 T cells, capable of cytokine secretion, proliferation, and degranulation, are maintained in HIV-1 controllers, chronically infected patients who spontaneously control viremia in the absence of antiretroviral therapy (ART) (1). Still, virus-specific T cell populations in these patients are considerably heterogeneous, and attributes that confer protection remain elusive. Understanding the necessary factors that regulate the induction, functional quality, and longevity of such responses is imperative for the rational design of preventative and therapeutic interventions against HIV-1.
The underlying mechanisms for T cell dysfunction were first recognized in mouse models of chronic viral infection, including lymphocytic choriomeningitis virus (LCMV), which results in defects similar to those seen in HIV-1 infection. From these studies, the molecular signatures of exhausted T cells are being elucidated. Alterations in expression of costimulatory common gamma-chain (γc) cytokines, such as interleukin-2 (IL-2), IL-7, and IL-15, are a common feature of many viral infections and have been shown to correlate with T cell nonresponsiveness (18, 33). Notably, T cell exhaustion is exacerbated under conditions of chronic antigenic stimulation and reduced CD4 T cell help, as seen in progressive HIV-1 infection (12, 13, 22, 34).
Several lines of evidence suggest that early CD4 T cell-mediated CD8 T cell priming events are crucial for programming vigorous effector and memory CD8 T cell responses. CD4 T cells are principal producers of IL-21, a γc cytokine referred to as a “third signal” (in addition to major histocompatibility complex class I [MHC-I] engagement and costimulation) for the differentiation, expansion, and phenotype of antigen-specific CD8 T cells (5, 30). IL-21/IL-21 receptor (IL-21R)-deficient hosts infected with LCMV exhibit exhausted CD8 T cells unable to clear virus, thereby resembling CD4 T cell-deficient hosts (9, 11, 37, 38). Strikingly, IL-21 administration reverses CD8 T cell exhaustion and reduces viral titers (37).
Early in vitro studies of bulk CD8 T cells from HIV-1-infected patients suggested a role for IL-21 in upregulating perforin expression in memory CD8 T cells, protecting CD8 T cells from undergoing apoptosis, and restoring cytotoxicity, the latter requiring IL-15. In contrast, IL-21 was not necessary to augment proliferation or increase degranulation (35). Definitive links to protective immunity came from subsequent studies showing enhanced suppression of HIV-1 replication following IL-21 exposure to CD8 T cells (6). In vivo evidence of the importance of IL-21 is demonstrated by reduced serum levels in HIV-1-infected patients and the finding that IL-21 production from total CD4 T cells correlates with disease progression (16, 17). However, the only study that comprehensively analyzed CD4 T cell responses by intracellular cytokine staining (ICS) demonstrated no major differences in HIV-1-specific IL-21 production when comparing chronically infected patients with various levels of viral control (39). These discordant results highlight the importance of further studies examining HIV-1-specific CD4 and CD8 T cells in well-characterized cohorts.
Here we present a comprehensive analysis demonstrating that HIV-1 controllers and primary infected patients have qualitatively superior HIV-1-specific T cells capable of IL-21 secretion, which is associated with enhanced CD8 T cell functionality and viral containment. Using peripheral blood mononuclear cells (PBMC) from primary and chronically infected patients, we directly assess several attributes of HIV-1-specific IL-21-producing CD4 and CD8 T cells ex vivo, including the functional profile, frequency, and protein targeting of these populations. These data clearly indicate a relationship between the aforementioned parameters and the ability to curtail the infection, providing additional insights into complexity and qualitative requirements of immune protection against HIV-1 disease progression, thus aiding in vaccine design.
MATERIALS AND METHODS
Study subjects.
Forty-three HIV-1-infected patients were recruited from the University of Alabama at Birmingham (UAB) Adult AIDS 1917 clinic after informed consent and UAB institutional review board approval were obtained. PBMC were isolated from anticoagulated peripheral blood by Histopaque density gradient centrifugation and cryopreserved for later use. Subjects were stratified according to immune control of virus and categorized as follows: those with primary infection (n = 17), based on identification less than 6 months after infection (Fiebig stages II to VI); elite controllers (EC; n = 7), based on spontaneous control of viremia without ART initiation and undetectable viral loads (VL) (<50 RNA copies/ml plasma); viremic controllers (VC; n = 8), based on VL of >50 but <2,000 RNA copies/ml in the absence of ART; and progressors (P; n = 11), based on VL of >10,000 RNA copies/ml in the absence of ART. All patients with chronic infection were not on ART, while 11/17 patients with primary infection began ART 2 to 4 weeks prior to sample collection.
Viral load and CD4 T cell count determination.
HIV-1 RNA levels were quantified in plasma samples using the Amplicor ultrasensitive HIV-1 monitor assay, in accordance with the manufacturer's protocol (Roche Diagnostics). The absolute CD4 T cell count was measured by flow cytometry using the flow count method and analyzed on a FACScan/FACSort instrument using MultiSET software (BD Biosciences).
Peptides.
Synthetic peptides (15-mers overlapping by 11 amino acids; clade B consensus sequence; courtesy NIH AIDS Research and Reference Reagent Program) spanning the entire HIV-1 proteome were used to enumerate antigen-specific CD4 and CD8 T cell responses. Lyophilized peptides were reconstituted at 50 μg/ml in dimethyl sulfoxide or distilled water. Five pools were prepared—one corresponding to each HIV-1 antigen (Gag, Pol, Env, and Nef and accessory proteins Rev, Tat, Vif, Vpr, and Vpu were combined to form the final pool)—and used at final concentrations of 2 μg/ml. Optimized HLA class I-restricted epitopes (New England Peptide) were alternatively used to confirm CD8 T cell specificity.
Intracellular cytokine staining assay.
PBMC were thawed, washed twice (in RPMI medium supplemented with 10% human AB sera), and resuspended at 2 × 106 cells/ml. Cells were then pulsed with the appropriate peptide pool (2 μg/ml) in the presence of costimulatory monoclonal antibodies (anti-CD28 and anti-CD49d; each at 1 μg/ml), Benzonase (50 U/ml; Novagen), GolgiStop (10 μg/ml; BD Biosciences), GolgiPlug (10 μg/ml; BD Biosciences), and anti-CD4-Qdot 655 (BD Biosciences). An unstimulated and positive control (phorbol 12,13-dibutyrate [PDBu]-ionomycin [25 ng/ml and 500 ng/ml, respectively; Sigma-Aldrich]) was included in each assay. Following a 5-h incubation period at 37°C in 5% humidified CO2, cells were harvested and washed twice with phosphate-buffered saline (PBS) containing 5% fetal bovine serum (FBS) prior to being labeled with a fluorescent LIVE/DEAD fixable dead cell dye (Molecular Probes, Invitrogen). Fluorochrome-conjugated monoclonal antibodies anti-CD3-Pacific Blue, CD8-Qdot 605, and CD45RO-allophycocyanin (APC) (BD Biosciences) were used for surface labeling. Following fixation and permeabilization with Caltag Fix and Perm reagents (Invitrogen), cells were washed and phenotypically identified by staining with monoclonal antibodies against intracellular markers IL-21-phycoerythrin (PE) (eBioscience), gamma interferon (IFN-γ)-Alexa Fluor 700 (BD Biosciences), IL-2-fluorescein isothiocyanate (FITC) (BD Biosciences), IL-17-peridinin chlorophyll protein (PerCP)-Cy5.5 (eBioscience), and tumor necrosis factor alpha (TNF-α)-PE-Cy7 (BD Biosciences). Following staining, cells were washed, fixed in 2% paraformaldehyde (Sigma-Aldrich), and analyzed on an LSRII flow cytometer within 24 h (BD Biosciences).
Flow cytometric analysis.
At least 100,000 CD3+ events were acquired from each sample. Data analysis was performed using FlowJo version 8.1.1 software (Tree Star). CD3+ CD4+ or CD3+ CD8+ lymphocytes were gated based on forward and side scatter properties after the exclusion of dead cells and doublets. Gates were set relative to the media control and subsequently applied to all samples per individual analyzed. Contemporaneous assays were performed using PBMC obtained from HIV-1-uninfected individuals (n = 5) to derive strict criteria for a positive IL-21 response. A positive response was determined to be (i) any value greater than or equal to the mean plus three standard deviations for all HIV-1-uninfected samples, as determined relative to medium control values, (ii) twice the medium control value for each individual, and (iii) greater than 0.05. A Boolean gating strategy was employed, and polyfunctionality was determined using PESTLE and SPICE software (courtesy of Mario Roederer, Vaccine Research Center, NIH).
Quantification of IL21 and IFNG mRNA transcripts in CD4 and CD8 T cells.
CD4 and CD8 T cells from cryopreserved patient PBMC were obtained by negative selection (CD4+ and CD8+ T cell isolation kits; Miltenyi Biotec) and cocultured in RPMI medium containing 10% FBS with HIV-1 peptide-pulsed monocytes (stimulated with Gag, Env, Nef, or accessory peptide pools or HLA-A*03-, -B*07-, and -B*57-restricted [QVPLPPMTYK {Nef}, HPVHAGPIA {Gag}, and KAFSPEVIPMF {Gag}, respectively] CD8 T cell epitopes) for 6 h. Total RNA was extracted from cell pellets using the AllPrep DNA/RNA/protein minikit (Qiagen). mRNA transcripts (500 ng) were converted to cDNA with the RT2 First Strand kit (SABiosciences) before being tested by real-time, quantitative PCR (custom RT2 PCR array, catalog no. CAPH09425; SABiosciences). The abundance of IL21 and IFNG mRNA was normalized to that of the housekeeping gene, beta-2 microglobulin (β2M), after 40 cycle reactions in an ABI 7500 fast system (Applied Biosystems). Upregulation of IL21 and IFNG mRNA expression was determined against cells cultured in RPMI medium-10% FBS alone using the ΔΔCT method, where CT is the cycle threshold for SYBR green-based detection of gene-specific PCR amplicons (SABiosciences).
Statistical analysis.
Nonparametric Mann-Whitney U, Spearman rank correlation, and Fisher's exact tests were performed using GraphPad Prism software version 5.0. All tests were two tailed, and P values of <0.05 were considered significant.
RESULTS
Induction of antigen-specific IL-21 T cells during HIV-1 infection.
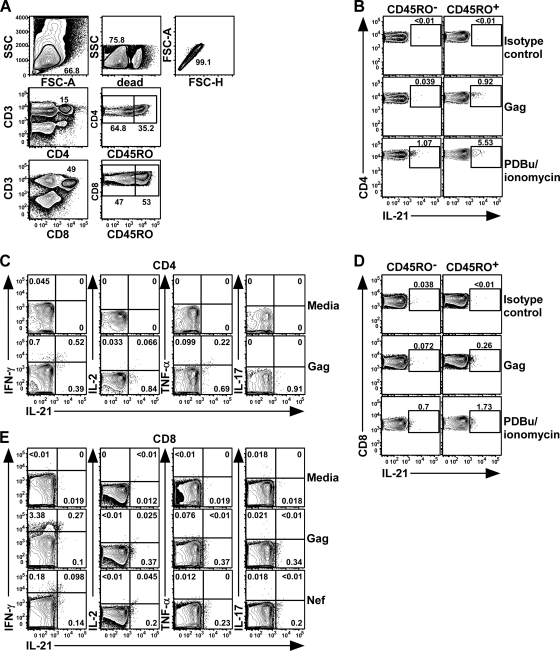
Previous studies examining IL-21 in humans have shown its selective expression by CD45RO+ but not CD45RO− T cells (4, 26). To assess HIV-1-specific T cell IL-21 production, we stimulated patient PBMC with overlapping peptide pools (HIV-1 clade B consensus sequence Gag, Pol, Env, Nef, and accessory proteins), followed by intracellular cytokine staining for IL-21, IFN-γ, IL-2, IL-17, and TNF-α. Using multiparametric flow cytometry, IL-21 was readily detected in the CD45RO+ CD4 T cell compartment (Fig. 1 A and B), and a subset of responding cells displayed coproduction of IFN-γ and, to a lesser extent, IL-2 and TNF-α (Fig. 1C). However, virus-specific T cells coexpressing IL-21 and IL-17 or IL-17 alone were not observed, as previously reported (2, 11).
FIG. 1.
HIV-1-specific IL-21 T cell responses are detected in human peripheral blood. Representative flow cytometric analysis of intracellular staining for IL-21, IFN-γ, IL-2, TNF-α, and IL-17 production by HIV-1-specific CD4 and CD8 T cells. PBMC from an elite controller were stimulated for 5 h ex vivo with a peptide pool spanning Gag (15-mers overlapping by 11 amino acids; consensus B sequence) in the presence of anti-CD28, CD49d, GolgiStop, and GolgiPlug. (A) Cells were gated based on light scatter properties, followed by dead cell exclusion (fluorescent dye) and gating for singlets and CD3+ CD4+ or CD3+ CD8+ T lymphocytes. SSC, side scatter; FSC-A, forward scatter area; FSC-H, forward scatter height. (B and D) Elaboration of IL-21-producing Gag-specific or PDBu-ionomycin-activated CD4 (B) or CD8 (D) T cells in the CD45RO− and CD45RO+ compartments after staining for IL-21 or the isotype control (post-cell permeabilization). (C and E) Frequencies of IL-21+ Gag-specific CD45RO+ CD4 (C) or Gag or Nef-specific CD8 (E) T cells that coproduced IFN-γ, IL-2, TNF-α, or IL-17.
IL-21 production by polyclonal CD8 T cells from psoriasis patients and LCMV-infected mice has been demonstrated (11, 27). We therefore analyzed whether HIV-1-specific CD8 T cells were cellular sources of IL-21, responsible for sustained CD8 T cell functionality. Indeed, a modest but consistently reproducible population of IL-21-producing CD8 T cells, capable of IFN-γ production, was discernible after antigenic stimulation (Fig. 1D and E). Similar to CD4 T cells, IL-21-producing CD8 T cells did not coexpress IL-17.
Differential IL-21 secretion from HIV-1-specific T cells across an HIV-1 cohort.
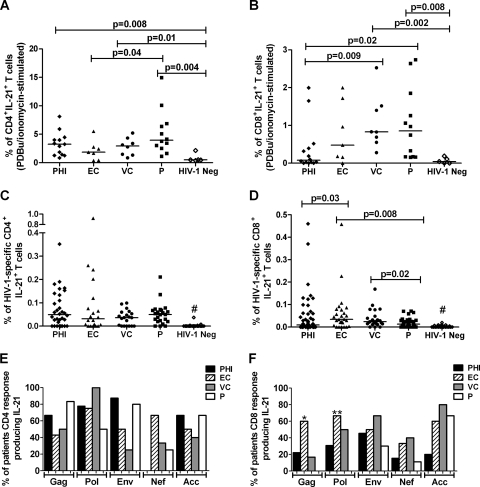
Given the data supporting the obligatory role of IL-21-producing T cells in immune-mediated viral containment, we reasoned that these cells represent at least one contributor to the differences in HIV-1 control observed among patients. Having established that CD45RO surface expression consistently marked IL-21+ T cells, we examined polyclonal and HIV-1-specific CD45RO+ T cell IL-21 production across HIV-1 cohorts consisting of patients with primary HIV-1 infection (PHI), chronic infection with undetectable or low viremia (elite controllers [EC] and viremic controllers [VC], respectively), and chronic infection with uncontrolled viremia (progressors [P]) (Table 1). In marked contrast to uninfected persons, the proportion of polyclonal CD4 and CD8 T cells producing IL-21 in response to PDBu/ionomycin was substantially greater in HIV-1 patients, and this coincided with the degree of viral persistence (Fig. 2 A and B). Notably, these responses were significantly increased in all cohorts except EC compared to those of seronegative subjects (Fig. 2 B). A comparative analysis of HIV-1-specific responses revealed similar CD4 T cell IL-21 production across the cohorts (Fig. 2C). Furthermore, neither the frequency of individuals demonstrating responses to at least one peptide pool nor the total number of positive responses distinguished the patient groups (data not shown). In contrast, EC and VC maintained significantly higher percentages of HIV-1-specific IL-21-producing CD8 T cells than P, with PHI displaying appreciable response heterogeneity albeit an overall lower median response (EC, 0.035 [interquartile range {IQR}, 0.006 to 0.078]; VC, 0.024 [IQR, 0.014 to 0.048]; P, 0.013 [IQR, 0.004 to 0.024]; PHI, 0.009 [IQR, 0 to 0.044]) (Fig. 2D). This decreased fraction of IL-21-competent CD8 T cells in P was paralleled by a 3- to 4-fold reduction in frequencies of positive responses in these subjects (P = 0.001 and 0.008 versus EC and VC, respectively) (data not shown). As expected, HIV-1-specific IL-21-producing T cells were not seen in seronegative controls (Fig. 2C and D). Therefore, the magnitude and frequency of the HIV-1-specific IL-21 CD8 T cell response is a better correlate of viral control than the IL-21 CD4 T helper response. However, in the progressor cohort, the magnitude of CD8 T cells producing IL-21 did not correlate with the viral load or CD4 T cell count, suggesting that a threshold level of this cytokine may be needed for enhanced viral control (data not shown).
TABLE 1.
Clinical characteristics of study participants
| Cohort | No. of patients | Plasma VLa |
CD4 T cell count (no. of cells/μl) |
ART | ||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| PHI | 17 | 10,841 | 1,531-15,300,000 | 521 | 164-964 | Yesb |
| EC | 7 | <50 | <50 | 769 | 368-1,741 | No |
| VC | 8 | 236 | 63-847 | 985 | 722-1,620 | No |
| P | 11 | 86,900 | 18,501-484,600 | 514 | 334-874 | No |
Viral load (VL) is shown as the number of HIV-1 RNA copies/ml at the time of sample collection.
A total of 11/17 patients were on antiretroviral therapy (ART) for 2 to 4 weeks.
FIG. 2.
Magnitude and responder frequency of IL-21-producing HIV-1-specific CD4 and CD8 T cells. PBMC obtained from patients with primary HIV-1 infection (PHI), elite controllers (EC), viremic controllers (VC), and progressors (P) were stimulated with HIV-1 consensus B peptide pools (15-mers overlapping by 11 amino acids) spanning the entirety of the expressed proteome (pooled to represent Gag, Pol, Env, Nef, and the remaining accessory proteins). The magnitudes of CD45RO+ CD4 (A) and CD8 (B) T cells producing IL-21 are shown, with horizontal bars indicating median values. The percentages of patients with HIV-1-specific CD45RO+ CD4 (C) and CD8 (D) T cells producing IL-21 in response to peptides from the indicated proteins are shown. Statistical comparisons were made using the two-tailed Fisher's exact test. *, P = 0.03; **, P = 0.006 (compared to results for P) by Fisher's exact test. #, P < 0.0001 compared to results for each group of HIV-1-seropositive patients by the Mann-Whitney U test.
To further define an optimal antiviral IL-21 response, we evaluated the extent to which viral control correlated with IL-21 responses directed against different viral proteins. Despite similar protein targeting by CD8 T cells, as measured by IFN-γ production (data not shown), Gag- and Pol-specific IL-21-producing CD8 T cells were completely ablated in P, concomitant with their exclusive presence in PHI and persistence in EC and VC (Fig. 2F). In contrast, no protein-specific differences in IL-21 production by CD4 T cells were apparent (Fig. 2E). These results are consistent with increasing evidence demonstrating that preferentially targeting Gag, and possibly Pol, is strongly associated with improved clinical markers of disease progression (8, 14, 21, 23) and suggest that CD8 T cell IL-21 responses directed toward these proteins may contribute to the maintenance of viremia control.
HIV-1-specific IL21 mRNA analysis mirrors protein expression by ICS.
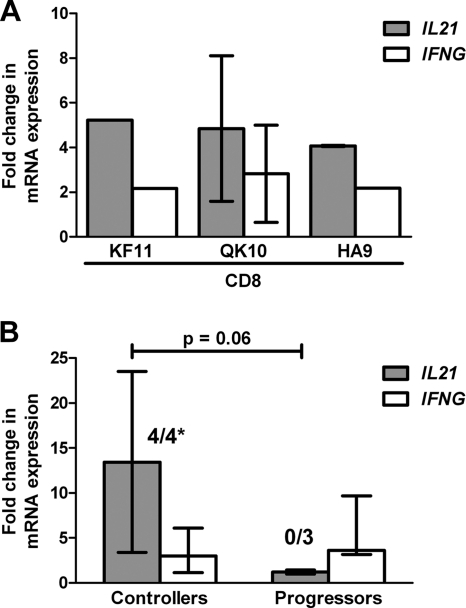
To further elucidate the specificity of IL-21 CD8 T cell secretion, we stimulated patient PBMC ex vivo with optimized HLA class I-restricted CD8 T cell epitopes. Notably, when CD4 T cells were depleted prior to stimulation (by negative selection [>99% purity]) (data not shown), mRNA analysis of isolated CD8 T cell populations demonstrated IL21 and IFNG upregulation in response to HLA-A*03-, -B*07-, and -B*57-restricted epitopes (QVPLPPMTYK [Nef], HPVHAGPIA [Gag], and KAFSPEVIPMF [Gag], respectively), further validating the foregoing results (Fig. 3A).
FIG. 3.
IL21 mRNA expression by HIV-1-specific CD8 T cells. CD8 T cells from controllers (n = 3) and progressors (n = 3) were negatively selected from PBMC by magnetic bead isolation (99% purity). T cells were cocultured with HIV-1 peptide-pulsed monocytes (stimulated with HLA class I A*03-, B*07-, or B*57-restricted epitopes [QVPLPPMTYK {Nef}, HPVHAGPIA {Gag}, or KAFSPEVIPMF {Gag}, respectively] (A) or HIV-1 peptide pools (comprising either Gag, Env, Nef, or accessory proteins) (B) and activated in the presence anti-CD28 and anti-CD49d for 6 h. The abundance of IL21 and IFNG mRNA was determined by real-time quantitative PCR and normalized to the housekeeping gene, beta-2 microglobulin (β2M). Upregulation of IL21 and IFNG mRNA expression was determined using the ΔΔCT method, where data are expressed as fold changes relative to cells cultured in RPMI-1640 alone. Numbers above the bars indicate the fraction of stimulations which resulted in IL21 fold change values of >2. *, 4/4 responses in controllers versus 0/3 responses in progressors; P = 0.03 by Fisher's exact test. Results are reported as median values, with error bars demonstrating the interquartile ranges, and are representative of at least two experiments. Comparison of the magnitudes of IL21 fold changes; P = 0.06 by the Mann-Whitney U test.
To confirm the observed differences in CD8 T cell IL-21 production across the cohorts (Fig. 2D), we next compared the transcriptional profiles of a subset of controllers and progressors either exhibiting or lacking these responses, respectively (as measured by flow cytometry). Following HIV-1 peptide pool stimulation, quantitative PCR analysis of purified CD8 T cell fractions revealed that controllers uniformly upregulated IL21 mRNA (4/4 responses), whereas none of the progressors substantially amplified transcripts (0/3 responses; P = 0.03) (Fig. 3B). Moreover, the frequency of CD8 T cells producing IL-21, as assessed by flow cytometry, directly correlated with the abundance of IL21 transcripts (P = 0.003; correlation coefficient [r] = 0.96).
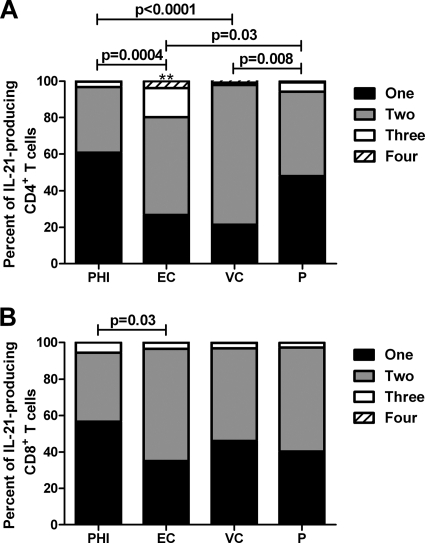
IL-21-producing CD4 T cells are more multifunctional in elite controllers.
Given the role of polyfunctional T cells in viral control, we speculated that IL-21 production in conjunction with other cytokines may be an important determinant of protective immunity (1, 20). To examine the relationship between the percentage of polyfunctional IL-21-producing T cells and viral load, a Boolean gating strategy was employed for HIV-1-specific CD4 and CD8 T cells, yielding 15 unique response patterns comprising every combination of the four potential measurements (IL-21, IFN-γ, IL-2, and TNF-α). Despite the lack of association between the magnitude of IL-21 CD4 T cell responses and viral control, we observed distinct differences in the functional quality of HIV-1-specific CD4 T cells that produced IL-21 (Fig. 4 A). Specifically, EC and VC maintained significantly greater proportions of multifunctional cells capable of simultaneously expressing two or more functions, whereas a largely monofunctional phenotype was observed in PHI and P (Fig. 4A). Overall, EC exhibited a superior functional profile comprised of greater than 20% of responding cells being positive for three or four functions compared to less than 5%, as seen in the other cohorts (P < 0.03) (Fig. 4A). Conversely, while the functionality of antiviral IL-21-producing CD8 T cells was not significantly enhanced in EC compared to that in VC and P, it was significantly higher than that in PHI (Fig. 4B).
FIG. 4.
IL-21-producing CD4 T cells are more functional in elite controllers. CD45RO+ CD4 (A) and CD8 (B) T cells that produced IL-21 were analyzed for their ability to coproduce IFN-γ, IL-2, or TNF-α using SPICE. The magnitudes of virus-specific mono- and multifunctional CD4 and CD8 T cells producing at least IL-21 from PHI, EC, VC, and P cohorts were assessed. Horizontal bars indicate the median percentages of IL-21+ T cells. Statistical comparisons were made using the two-tailed nonparametric Mann-Whitney U test. **, greater than 3 or 4 functions; P < 0.03 versus PHI, VC, and P cohorts.
Association of CD4 T cell IL-21 production with CD8 T cell functionality.
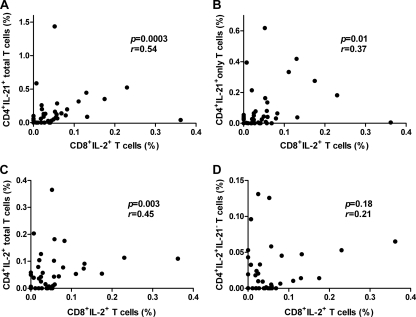
We hypothesized that the functional impairment of IL-21-producing CD4 T cells compromised CD8 T cell functional quality (i.e., IL-2 production) and memory potential, similar to those of IL-21-deficient mice (37, 38). Therefore, we assessed whether the total magnitude of HIV-1-specific IL-21 production from CD4 T cells correlated with the total magnitude of virus-specific CD8 T cell IL-2 secretion. When all patients were grouped together, we found that the percentage of IL-21 modestly correlated with the percentages of CD8 T cells expressing IL-2 (r = 0.54; P = 0.0003) (Fig. 5 A). This correlation remained significant even when the data were limited to CD4 T cells that produced IL-21 in the absence of the other cytokines (r = 0.37; P = 0.01) (Fig. 5B). However, the number of samples studied was too small to discern whether polyfunctional IL-21-producing CD4 T cells were better correlates of IL-2+ CD8 T cells compared with their monofunctional counterparts. Despite demonstrating a modest relationship between total IL-2+ CD4 and IL-2+ CD8 T cells (r = 0.45; P = 0.003) (Fig. 5C), this association was no longer significant when examining the fraction of CD4 T cells that produced IL-2 without coexpression of IL-21 (r = 0.21; P = 0.18) (Fig. 5D), suggesting that IL-2 and IL-21 act cooperatively to induce protective, polyfunctional CD8 T cell responses. Interestingly, when we restricted our analysis to the stratified cohorts, PHI sustained these associations, although trends were apparent in EC (Table 2, compare the r values). Hence, these findings further solidify that the link between IL-21-mediated T helper function and subsequent acquisition of CD8 T cell multifunctional traits is established in primary infection but rapidly lost in chronic, progressive infection.
FIG. 5.
IL-21-producing CD4 T cells predict functional CD8 T cells. Correlation between the magnitude of HIV-1-specific CD4 T cell cytokine production (summed across all peptide pools) and CD8 T cell IL-2 production. CD45RO+ CD4 T cells expressing IL-21 in combination with other cytokines (A), IL-21 alone (B), IL-2 in combination with other cytokines (C), or IL-2 without coexpression of IL-21 (D) were plotted against the corresponding proportion of HIV-1-specific IL-2+ CD45RO+ CD8 T cells. P and r values were calculated using the Spearman rank correlation test.
TABLE 2.
Association of HIV-1-specific CD4 T cell cytokine production with CD8 T cell IL-2 productiona
| Cytokine produced | Cohort value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All |
PHI |
EC |
VC |
P |
||||||
| P | r | P | r | P | r | P | r | P | r | |
| IL-21 total | 0.0003 | 0.54 | 0.01 | 0.63 | 0.11 | 0.67 | 0.08 | 0.66 | 0.82 | 0.08 |
| IL-21 alone | 0.01 | 0.37 | 0.03 | 0.54 | 0.14 | 0.63 | 0.70 | 0.16 | 0.95 | 0.03 |
| IL-21 without IL-2 | 0.02 | 0.37 | 0.03 | 0.53 | 0.14 | 0.64 | 0.50 | −0.27 | 0.61 | 0.17 |
| IL-2 total | 0.01 | 0.45 | 0.04 | 0.52 | 0.24 | 0.54 | 0.15 | 0.56 | 0.95 | 0.02 |
| IL-2 alone | 0.29 | 0.17 | 0.21 | 0.33 | 0.44 | 0.36 | 1.00 | 0.00 | 0.49 | 0.24 |
| IL-2 without IL-21 | 0.18 | 0.21 | 0.14 | 0.38 | 0.39 | 0.40 | 0.70 | 0.17 | 0.49 | 0.24 |
All CD4 T cell cytokine production was compared to total CD8 T cell IL-2 production. P and r values were calculated using the Spearman rank correlation test. Total, in combination with the production of other cytokines.
DISCUSSION
Collectively, these data extend our understanding of the regulation of IL-21-producing antiviral T cell responses during primary and chronic HIV-1 infection. Previous reports of mice chronically infected with LCMV have demonstrated that virus-specific CD8 T cell responses elicited in the absence of IL-21 are functionally inferior, lacking the necessary attributes to fully clear virus-infected cells (9, 11, 37, 38). Indeed, in different cohorts of HIV-1-infected patients with distinct viral loads, we confirm that antigen-experienced CD4 T cells are a major cellular source of IL-21 and further define a previously unappreciated role for HIV-1-specific CD8 T cells—IL-21 secretion capacity, which is not detectable in the majority of patients with persistent viremia. Notably, IL-21-producing HIV-1-specific CD8 T cells responding against Gag and Pol epitopes were absent in progressors, while those producing IFN-γ and even IL-2 to these proteins were still present. Whereas the overall magnitude and frequency of CD4 T cell-derived HIV-1-specific IL-21 production did not correlate with viral load, elaboration of a polyfunctional IL-21 response reliably predicted HIV-1 containment. This poor induction of IL-21 by CD4 T cells seemed to translate into an inability of CD8 T cells to effectively produce IL-2, a parameter consistently linked with improved control of the virus (42). Thus, our findings are consistent with the essential role that IL-21 plays in containing murine viral infections.
Several studies have now convincingly demonstrated that IL-21 impacts HIV-1 disease chronicity, albeit with somewhat paradoxical findings (6, 16, 17, 39). It has been proposed that the stepwise and progressive impairment of IL-21 in HIV-1, from serum and ionomycin-activated CD4 T cells, occurs with disease progression, despite the lack of association with viral load (6, 16). This view is in contrast to studies by our group and others showing the sequential elevation of polyclonal IL-21-producing CD4 T cells into chronic infection, such that levels exceed those found in seronegative controls (39). This heightened IL-21 production was common to all patients with the exception of a few EC, a phenomenon reminiscent of the chronic immune activation prominently associated with this disease (28, 39). Indeed, this interpretation is consistent with the involvement of IL-21 in inflammatory bowel disease, triggering aberrant immune responses directed toward microbial constituents, thereby potentiating inflammation and damage to the mucosal barrier (25, 36).
While not demonstrated in this analysis, prior studies revealed the rapid loss of HIV-1-specific IL-21+ CD4 T cells following primary infection (6, 39). The basis for this apparent discrepancy is not clear but does not exclude the possibility that differences in PHI cohort characteristics (time of sample collection from date of infection, etc.) are a likely culprit. Nevertheless, the two studies that comprehensively analyzed IL-21 directly by flow cytometry were unable to show significant differences in the induction of these responses in long-term nonprogressors versus chronically infected patients with uncontrolled viremia, suggesting that compromised IL-21 secretion likely reflects early insults to the CD4 T cell compartment (39). The notion that HIV-1 uniformly disables CD4 T cell function following primary infection, regardless of controller or progressor classification, is further supported by a number of studies that argue against the assumption that elite controllers are exempt from a spectrum of immune alterations, namely, immune activation. The fact that the memory CD4 T cell pool is already depleted prior to the chronic phase, compounded by the limited reconstitution of the memory CD4 but not CD8 T cell pool, could provide an explanation as to why the CD4 fraction is damaged while the CD8 pool is left relatively intact (7). In line with these observations, levels of activated HIV-1-specific CD38+ HLA-DR+ CD4 T cells are similar among elite controllers and noncontrollers, whereas only elite controllers maintain lower levels of activated CD8 T cells (28). Thus, our findings are consistent with the foregoing observations whereby CD4 T cell function appears similar while CD8 T cell function is enhanced in the EC cohort.
Seminal studies established IL-21 as a principal product of CD4 T cells. However, increasing evidence supports that IL-21 production is not exclusively restricted to CD4 T cells. IL21 mRNA expression in CD4-depleted splenocytes of LCMV-infected mice suggested that natural killer T cells, B cells, macrophages, and dendritic cells are cellular sources (9). In accordance with the present findings, polyclonal CD8 T cell IL-21 production has been demonstrated in mice infected with LCMV via mRNA and protein expression, albeit at reduced levels compared to those of CD4 T cells (11). Furthermore, the latter cells have been implicated in the pathogenesis of psoriasis and immune thrombocytopenia in humans, suggesting a biological ability rather than a virus-specific modification (27, 41). Although it remains unresolved which cells are directly responsible for mediating viral control, our data favors the participation of both CD4 and CD8 T cell subsets during HIV-1 infection. Thus, it is tempting to speculate that CD8 T cells from HIV-1 controllers are endowed with IL-21 secretion capabilities to self-sustain CD8 T cell homeostasis (11). Indeed, activation of purified CD8 T cells differentiated in the presence of anti-CD3/CD28 and IL-21 results in robust IL21 mRNA expression (15).
A connecting hypothesis among the reports describing antigen-specific IL-21 is that this cytokine is a critical player in enhancing the functional quality and protective potential of CD8 T cell responses. It has been suggested that since IL-2 is rapidly abolished early in infection, IL-21 may compensate for the lack of IL-2 helper function during chronic infection (9). It is noteworthy that HIV-1-specific IL-21, but not IL-2 produced alone, from CD4 T cells correlates with CD8 T cell IL-2 secretion only during primary and possibly controlled chronic infection. From these data, it appears that although IL-21 responses are present in progressors, they fail to effectively program CD8 T cells to produce IL-2. It is plausible that the phenotypic alterations induced by HIV-1, including collapse of the lymph node architecture, could potentially disrupt CD4/CD8 T cell interactions and prevent crucial CD4 T cell-mediated costimulatory signaling required for optimal CD8 T cell function (31, 32). Moreover, the preferential preservation of lymph node architecture in long-term nonprogressors further supports this idea (10, 29). We propose that IL-21, along with IL-2 and other helper factors, act cooperatively to regulate CD8 T cell functional competence, which may be a consequence of the tightly clustered genomic organization of IL-21 and IL-2 (30).
While the studies presented here do not demonstrate causality, they nonetheless reveal a unique quality in elite controllers that may explain their superior viral control, namely, the ability to elicit IL-21-producing CD8 T cells. Furthermore, we provide evidence that HIV-1-specific CD8 T cells producing IL-21 are a better correlate of HIV-1 control than their CD4 T cell counterparts. These findings represent a key step toward improving our understanding of the composition of optimal HIV-1-specific T cell repertoires, which is directly relevant to strategies aiming to enhance vaccine efficacy or restore fatigued T cells.
Acknowledgments
We thank the study participants and Marion L. Spell for flow cytometric acquisition. We also thank Laurie Harrington and members of the Goepfert and Heath laboratories for discussions and review of the manuscript.
This work was supported by NIH grants R21 AI 73103 and R01 AI 084772 and Bill & Melinda Gates Foundation grant 37874 (all to P.A.G.), NIH grants T32 AI 007051 and F31 AI 085970 (to L.D.W.), and NIH grant K02 AI 076123 (to J.T.).
L.D.W., S.S., A.B., and S.L.H. contributed to the design of the experiments, analyses of T cell responses, and manuscript preparation. W.S. and J.T. provided expertise in performing mRNA analysis. A.J.Z. contributed to the conception and design of experiments as well as preparation of the manuscript. P.A.G. supervised the entire project, designed and coordinated experiments, and contributed to manuscript preparation.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1.Betts, M. R., et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenchley, J. M., et al. 2008. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112:2826-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenchley, J. M., et al. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caprioli, F., et al. 2008. Autocrine regulation of IL-21 production in human T lymphocytes. J. Immunol. 180:1800-1807. [DOI] [PubMed] [Google Scholar]
- 5.Casey, K. A., and M. F. Mescher. 2007. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J. Immunol. 178:7640-7648. [DOI] [PubMed] [Google Scholar]
- 6.Chevalier, M. F., et al. 2011. HIV-1-specific Interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J. Virol. 85:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, B. H., et al. 2002. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsaesser, H., K. Sauer, and D. G. Brooks. 2009. IL-21 is required to control chronic viral infection. Science 324:1569-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauci, A. S., G. Pantaleo, S. Stanley, and D. Weissman. 1996. Immunopathogenic mechanisms of HIV infection. Ann. Intern. Med. 124:654-663. [DOI] [PubMed] [Google Scholar]
- 11.Fröhlich, A., et al. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576-1580. [DOI] [PubMed] [Google Scholar]
- 12.Fuller, M. J., A. Khanolkar, A. E. Tebo, and A. J. Zajac. 2004. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J. Immunol. 172:4204-4214. [DOI] [PubMed] [Google Scholar]
- 13.Fuller, M. J., and A. J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477-486. [DOI] [PubMed] [Google Scholar]
- 14.Goepfert, P. A., et al. 2008. Transmission of HIV-1 Gag immune escape mutations is associated with reduced viral load in linked recipients. J. Exp. Med. 205:1009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber, M., et al. 2009. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 39:1716-1725. [DOI] [PubMed] [Google Scholar]
- 16.Iannello, A., et al. 2010. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J. Immunol. 184:114-126. [DOI] [PubMed] [Google Scholar]
- 17.Iannello, A., et al. 2008. Decreased levels of circulating IL-21 in HIV-infected AIDS patients: correlation with CD4+ T-cell counts. Viral Immunol. 21:385-388. [DOI] [PubMed] [Google Scholar]
- 18.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251-262. [DOI] [PubMed] [Google Scholar]
- 19.Kalams, S. A., et al. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannanganat, S., et al. 2007. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J. Virol. 81:12071-12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiepiela, P., et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. [DOI] [PubMed] [Google Scholar]
- 22.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews, P. C., et al. 2008. Central role of reverting mutations in HLA associations with human immunodeficiency virus set point. J. Virol. 82:8548-8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehandru, S., et al. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monteleone, G., et al. 2005. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn's disease. Gastroenterology 128:687-694. [DOI] [PubMed] [Google Scholar]
- 26.Onoda, T., et al. 2007. Human CD4+ central and effector memory T cells produce IL-21: effect on cytokine-driven proliferation of CD4+ T cell subsets. Int. Immunol. 19:1191-1199. [DOI] [PubMed] [Google Scholar]
- 27.Ortega, C., et al. 2009. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J. Leukoc. Biol. 86:435-443. [DOI] [PubMed] [Google Scholar]
- 28.Owen, R. E., et al. 2010. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS 24:1095-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantaleo, G., et al. 1995. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N. Engl. J. Med. 332:209-216. [DOI] [PubMed] [Google Scholar]
- 30.Parrish-Novak, J., et al. 2000. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408:57-63. [DOI] [PubMed] [Google Scholar]
- 31.Schacker, T. W., et al. 2006. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin. Vaccine Immunol. 13:556-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schacker, T. W., et al. 2002. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Invest. 110:1133-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White, L., et al. 2007. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV). Blood 109:3873-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto-Furusho, J. K., et al. 2010. Interleukin 21 expression is increased in rectal biopsies from patients with ulcerative colitis. Inflamm. Bowel Dis. 16:1090. [DOI] [PubMed] [Google Scholar]
- 37.Yi, J. S., M. Du, and A. J. Zajac. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi, J. S., J. T. Ingram, and A. J. Zajac. 2010. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 185:4835-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue, F. Y., et al. 2010. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J. Immunol. 185:498-506. [DOI] [PubMed] [Google Scholar]
- 40.Zajac, A. J., et al. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, X., et al. 2010. Elevated interleukin-21 correlated to Th17 and Th1 cells in patients with immune thrombocytopenia. J. Clin. Immunol. 30:253-259. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerli, S. C., et al. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 102:7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]