Abstract
Several viral genome-linked proteins (VPgs) of plant viruses are intrinsically disordered and undergo folding transitions in the presence of partners. This property has been postulated to be one of the factors that enable the functional diversity of the protein. We created a homology model of Potato virus A VPg and positioned the known functions and structural properties of potyviral VPgs on the novel structural model. The model suggests an elongated structure with a hydrophobic core composed of antiparallel β-sheets surrounded by helices and a positively charged contact surface where most of the known activities are localized. The model most probably represents the fold induced immediately after binding of VPg to a negatively charged lipid surface or to SDS. When the charge of the positive surface was lowered by lysine mutations, the efficiencies of in vitro NTP binding, uridylylation reaction, and unspecific RNA binding were reduced and in vivo the infectivity was debilitated. The most likely uridylylation site, Tyr63, locates to the positively charged surface. Surprisingly, a Tyr63Ala mutation did not prevent replication completely but blocked spreading of the virus. Based on the localization of Tyr119 in the model, it was hypothesized to serve as an alternative uridylylation site. Evidence to support the role of Tyr119 in replication was obtained which gives a positive example of the prediction power of the model. Taken together, our experimental data support the features presented in the model and the idea that the functional diversity is attributable to structural flexibility.
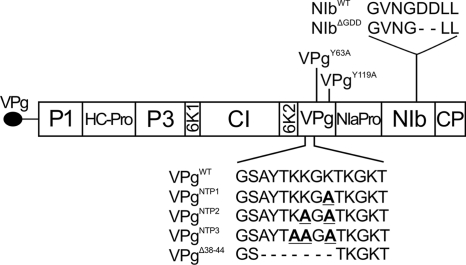
Potyviral genomes are expressed as a single polyprotein which is subsequently cleaved by three virus-encoded proteinases to yield up to 10 mature proteins (27, 48) (Fig. 1). Recently, a bioinformatic approach revealed that an 11th protein, PIPO (pretty interesting Potyviridae ORF), is translated from a +2 frame (5). The first study investigating the functions of this protein revealed its role in virus movement (56). Most of the potyviral proteins are shown to have multiple functions, and the viral genome-linked protein (VPg) is one of the most versatile of them. Potyviral VPg has been shown to take part in RNA replication, cell-to-cell and long-distance movement, translation, gene silencing suppression, and phloem loading of the virus (4, 22, 24, 37, 45, 47).
FIG. 1.
Potato virus A genome organization and mutations used in the study. Boldfaced and underlined letters indicate lysines mutated to alanines.
Uridylylation is one of the key events in the RNA synthesis of the Picornaviridae family (30, 32). In this reaction, the viral RNA polymerase attaches UMP covalently to a tyrosine residue within VPg which then serves as a primer for RNA synthesis. In potyviruses the link between the protein and the RNA is formed between UMP and a conserved tyrosine corresponding to Tyr63 in Potato virus A (PVA) VPg (1, 29, 31). Consequently, mutations of this residue have been shown to inhibit potyvirus infection. Amino acids 38 to 44 of PVA VPg are responsible for nucleotide triphosphate (NTP) binding, and their deletion impairs uridylylation of VPg (35). Furthermore, this region contains the second part of the bipartite nuclear localization signal (NLS) of PVA VPg (amino acids 41 to 50) (38). Replication of positive-sense RNA viruses takes place in the cytoplasm in compartments formed from host-derived membranes (7, 28). The best known membrane-bound protein of potyviruses is the short 6K2 transmembrane protein. It induces the formation of vesicles from endoplasmic reticulum (ER) membranes of the host, later leading to formation of active replication compartments that contain all components required for virus replication, including VPg (46, 55). PVA VPg can be found in association with the membrane fraction of infected plants (18). We recently showed that PVA VPg possesses a capacity to bind anionic phospholipids in vitro and provided evidence for a structural change associated with the binding (39).
The structure of plant virus VPg has been difficult to obtain. However, several recent studies have shown the intrinsic structural disorder of these proteins. The resulting structural flexibility has been proposed to enable VPg to carry out the variety of functions (16, 20, 40, 44). The N terminus, NTP-binding region, and a short segment in the middle of the protein were speculated to be part of a natively unstructured stretch of amino acids, which may allow these regions to be multifunctional and structurally flexible and thereby facilitate participation of VPg in various interactions. Disorder, however, is not a homogeneous state but comes in different structural flavors, ranging from random coil through premolten globule to a rather compact structural state denoted as molten globule (10, 51). The native structure of PVA VPg is a partially disordered molten globule-like type (39, 40). Our previous observations with circular dichroism (CD) spectroscopy, ANS (1-anilo-8-naphthalene sulfonate) binding, and disorder prediction are all consistent with this.
The process of structural adaptation in the presence of interacting molecules is essential in the case of many intrinsically disordered proteins (IDPs), which very often function by molecular recognition when they undergo induced folding (disorder-to-order transition) to a more ordered state (57). IDPs can adapt to the structure of different partners and thus fulfill different functions, a phenomenon termed functional promiscuity or moonlighting (23, 52). It is generally held that this capacity of IDPs is important for their ability to act as hub proteins and to increase functional complexity (8), but the structural underpinning of this phenomenon is little studied so far. For viruses, structural disorder, together with other means such as using polyprotein intermediates, offers a way to control a wealth of functions with a low number of proteins. The question about the generality of structural disorder in viral proteins has been recently addressed by Tokuriki et al. (50). It seems that the structure of virus proteins in general is unusual, being enriched in coil regions and depleted in orderly secondary-structure elements and well-formed hydrophobic cores. It is argued by Tokuriki et al. that viral proteins have a lot of flexibility and a high level of disorder and that they apparently are rapidly evolving proteins, stabilized only in the presence of partners. Accordingly, structural disorder together with polyprotein intermediates and various posttranslational modifications of VPg (18) as well as formation of VPg dimers (16, 31, 40) give rise to a great variety of functions and complex targets for structure-function studies.
We have earlier characterized the partially disordered, molten globule-like state of PVA VPg and observed induced folding in the presence of synthetic anionic vesicles (39, 40). Here we continue the study of the structure-function relationship and suggest a model for potyviral VPg structure attained upon induced folding which is consistent with several structural observations, mutagenesis, and functional studies. The suggested structure shows the general biophysical features of viral proteins (50) and enables interpretation of several of VPg's observed functions.
MATERIALS AND METHODS
Recombinant proteins.
The pQE30 (Qiagen) construct was used to express wild-type (WT) VPg (VPgWT) (26) and as a template for production of the mutants. Mutations were introduced using standard PCRs with Phusion DNA polymerase (Finnzymes). All mutations were verified by sequencing. Viral recombinant polymerase NIb was purified under native conditions as described earlier (35) with the following modifications. All buffers for purification under native conditions contained 50 mM NaH2PO4, pH 8.0, 1 M NaCl, 10% glycerol, 0.1% Tween 20, and 20, 30, or 500 mM imidazole. Lysozyme (1 mg/ml) and proteinase inhibitor cocktail were added to the cell lysis buffer. One hour of lysis on ice was followed by six 10-s bursts with a tip sonicator. All purified proteins were dialyzed overnight against water.
Plant expression.
Viral constructs used in the Agrobacterium-mediated infection experiments of this study were based on the full-length infectious cDNA (icDNA) copies of PVA-B11 (34) tagged with the Renilla luciferase (Rluc) gene (35S-PVA::rluc [15]) under the control of the Cauliflower mosaic virus (CaMV) 35S promoter and nos terminator. In order to prevent bacterial expression, the rluc gene contained intron 1 from ribulose-1,5-bisphosphate carboxylase/oxygenase (RBC-1I; Rubisco). Construction of 35S-PVAWT::rlucInt-nos and 35S-PVAΔGDD::rlucInt-nos is described in detail in reference 12. Site-directed mutagenesis of the VPg-encoding sequence was carried out in a vector containing a 1,898-nucleotide HindIII-ApaI fragment of PVA-B11 (37) using appropriate nucleotide primers. The same amino acids were mutated in plant expression vectors and in recombinant protein expression constructs (Fig. 1). Mutations were verified by sequencing, and the fragment was transferred into the icDNA using unique SwaI and ApaI sites. The gene cassettes containing 35S-PVA::rlucInt-nos were cloned with the aid of SalI and KpnI into the binary vector pRD400 as described earlier (12).
Agroinfiltration, plants, and luciferase activity determination.
Plant infections and luciferase measurements were essentially done as described earlier (12). A minimum of three plants were Agrobacterium infiltrated with each of the gene constructs. All samples in a particular experiment were analyzed concurrently, using the same set of reagents. Firefly luciferase (Fluc) and Rluc activities were determined with the Dual Luciferase assay kit (Promega), according to the supplier's instructions. The luciferase activities (photons/second) were measured with a Luminoscan TL Plus device (Thermo Labsystems). Rluc activities were normalized with Fluc activities. The mean values for Rluc and Fluc activities were calculated from parallel samples and were used for calculating the Rluc/Fluc activity relation. Averages were calculated from a minimum of three samples collected at 3 and 4 days postinoculation (dpi). Each sample consisted of a pool of three leaf disks. The standard deviation of the mean is given for each result.
NTP-binding and uridylylation assays.
Incorporation of [α-32P]UTP or [α-33P]UTP was quantified in both assays. In the NTP-binding assay, oxidized [α-33P]UTP was cross-linked to WT and mutant VPgs using methods described previously (6, 35), with the following modifications. The protein (3 μg) was cross-linked to 0.002 μCi of oxidized label, in a buffer containing 10 mM HEPES (pH 7.4), 5 mM MnCl2, and 3 mM sodium cyanoborohydride (NaCNBH3) in a final volume of 30 μl. The reaction mixtures were incubated for 60 min on ice and stopped by the addition of SDS-PAGE sample buffer. The uridylylation assay was done using the enzymatic activity of recombinant polymerase as described earlier (35) with the following modifications. The final reaction volume was 15 μl containing 0.5 μg of wild-type or mutant VPg, ∼0.5 μg of NIb, and 0.2 μCi of [α-32P]UTP in reaction buffer (10 mM HEPES, pH 7.5, 2.5 mM MnCl2). Reactions were stopped after 30 min incubation by 5 min boiling in the presence of SDS-PAGE sample buffer. Samples from NTP-binding and uridylylation assays were subjected to electrophoresis on 12% or 15% SDS-PAGE gels, and covalently bound label was visualized and quantified using an FLA-5100 imaging system and Image Gauge v3.46 software (FUJI Photo Film Co.). All gels were stained with Coomassie bio-safe stain (Bio-Rad Laboratories) to monitor the amount of protein loaded in each lane.
RNA-binding assay.
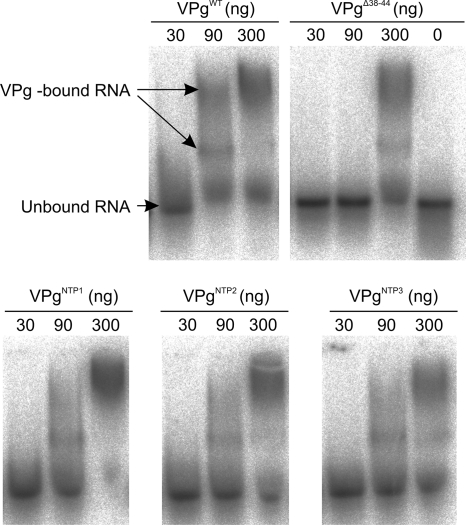
Labeled RNA was produced for an RNA-binding assay with the MEGAscript kit (Applied Biosystems) using the same label as in the uridylylation assay and the kit control DNA as the template. Labeled RNA was purified with NICK columns (Amersham Biosciences). The binding assay was done essentially as described earlier (21). The presence of the labeled RNA on purified fractions was verified on 1% agarose gels in TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8.0). The highest activity fraction was selected and diluted 1:100 for the binding assay. VPg (30, 90, and 300 ng) and 1 μl of diluted RNA were used in a reaction mixture containing 10 mM HEPES, pH 7.4, 1 mM EDTA, 50 mM NaCl, 1 mM dithiothreitol (DTT), 1 mg/ml bovine serum albumin (BSA), 10% (vol/vol) glycerol, and 0.3 μl Ribolock RNase inhibitor (Fermentas) in a final volume of 15 μl. The mixtures were incubated for 30 min on ice and subjected to electrophoresis on a 1% agarose gel in TAE buffer as described above.
Bioinformatic analysis and homology modeling.
The structural disorder of mutant VPgs were estimated with PONDR software (Molecular Kinetics) and the VL-XT algorithm as described earlier (25, 40). Homology modeling of PVA VPg was done with the LOMETS metaserver (58). The server gives the 10 best models. After initial evaluation, few different types of overall folds were noted and the model that was ranked first was selected for further evaluation. This model was provided by the SP3 algorithm (59). The template was the C-terminal catalytic domain of restriction endonuclease FokI from Flavobacterium okeanokoites (Protein Data Bank [PDB] identification, 2fok; domain identification, 2foka4). The model was evaluated by assessing the localization of known functional amino acids, hydrophobic amino acids, surface charge profile, and predicted structural disorder and by the localization of known limited trypsin digestion sites. Hydrophobic clusters in the primary sequence were assessed with hydrophobic cluster analysis (HCA) software and positioned to the model (14). Clusters of three or more hydrophobic amino acids were considered potential core-forming units. DeepView software was used for visualization of the model (17) and Persistence of Vision Raytracer (POV-Ray) software was used for rendering and enhancing the visual appearance of the structure images (http://www.povray.org/).
Circular dichroism spectroscopy.
Circular dichroism (CD) spectra revealing the SDS-induced changes in VPg secondary-structure element proportions were recorded with a Jasco 715 spectropolarimeter equipped with a Jasco programmable Peltier element to control the sample temperature. Spectra were recorded in the far-UV region (190 to 250 nm) in 1-mm cuvettes. A 0.1-mg/ml portion of VPg was used in 10 mM MES buffer, pH 6.0. Three spectra were averaged, and buffer background was subtracted from all measurements before the values were converted to mean molar ellipticity. Structural change was induced by the addition of 2 mM SDS. To enable the recording of induced folding, the structural change was delayed by cooling the sample to +8°C and increasing the temperature in 4- to 8°C increments until 72°C was reached. A 2-min incubation time was allowed after each temperature increment raise. Conversion of CD data to secondary structure element proportions was made with the CDPro package as described earlier using reference set SDP42 for deconvolution (40, 49).
RESULTS
The structural model shows a positively charged contact surface and supports the presence of a hydrophobic core.
A structural model of PVA VPg was achieved using the LOMETS metaserver and SP3 program (Fig. 2). LOMETS uses the MODELLER software to build the final, full-length models (13). The best template for the structural model of VPg was the C-terminal cleavage domain of restriction endonuclease FokI. FokI is a bacterial enzyme that recognizes a specific DNA sequence and cleaves the DNA strand at a nonspecific site a short distance away from the recognition site (3, 53). FokI has an N-terminal DNA recognition domain and a C-terminal cleavage domain. The latter contains a dimerization interface which is required for FokI to cleave both DNA strands. A very similar overall fold was later modeled with the MUSTER software, which is also part of the LOMETS server package (template domain PDB identification, 1fokA2; see Fig. S1F in the supplemental material). It should be noted that the ranking of the rest of the individual models altered between runs (see Fig. S1 for the five first-ranked VPg models). This reflects the rapid development of algorithms, an increasing number of template structures, and maybe also the inherent ambiguity of the VPg structure.
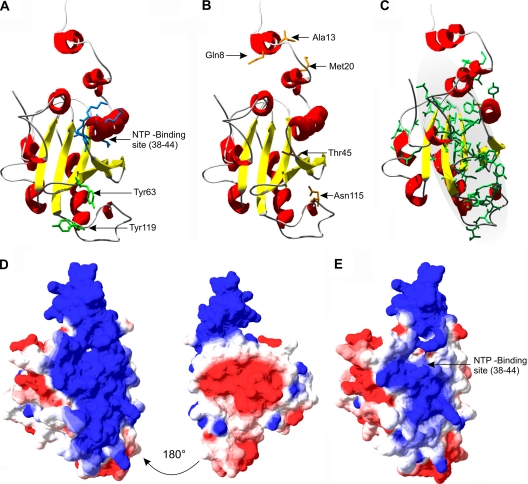
FIG. 2.
Structural model of PVA VPg created with the X-ray structure of the C-terminal catalytic domain of restriction endonuclease FokI as a template (PDB identification, 2fok; domain identification, 2foka4). (A and B) NTP-binding site and putative uridylylation sites are located on the same surface of the structure as all limited trypsin digestion sites described earlier (39, 40). (C) Location of hydrophobic clusters based on HCA plotting (see Materials and Methods for details). The gray highlighted area represents the proposed hydrophobic core. (D) Surface charge profile of the model. The view at the left is in the same orientation as in panels A and B. Blue and red colors mark positively and negatively charged surfaces, respectively. (E) Change in surface charge when three lysines of the nucleotide-binding site are mutated (figure corresponds to the VPgNTP3 mutant).
Dominant features of the model are a protruding N terminus, an ∼22-Å-wide core composed of three parallel β-strands, and a positively charged surface on one side. The calculated proportions of the secondary structures of the modeled state are ∼30% α-helix, ∼15% β-sheet, and ∼50% coil and random structures. The core is surrounded by α-helices and two antiparallel β-sheets. The NTP-binding site, the putative uridylylation site Tyr63 (Fig. 2A), the trypsin-accessible sites studied earlier (40) (Fig. 2B), and the positively charged surface that spans the length of the whole model (Fig. 2D) are all on the same side of the model. The hydrophobic clusters are mostly located within the core of the model with the exception of the lower right part of the structure (amino acids 101 to 112) (Fig. 2C). Lys44 of the NTP-binding site and Arg114, which are exposed in the disordered form (40) and protected in the vesicle-interacting form of the protein (39), are also part of the proposed core domain.
VPg was considered a “hard target” by the software, and the confidence of the model was estimated as “low” by the server. This is partly caused by the low sequence identity of ∼18% between FokI and VPg and probably the exposed positioning of part of the hydrophobic amino acids which argues for a limited compatibility of the VPg sequence with a stable, well-folded structural state. Therefore, the validity of this model needed to be carefully assessed on the basis of the known structural and functional features of potyviral VPgs and by further experiments to test it.
Disruption of the putative positively charged contact surface affects NTP-binding, uridylylation, and RNA-binding activities.
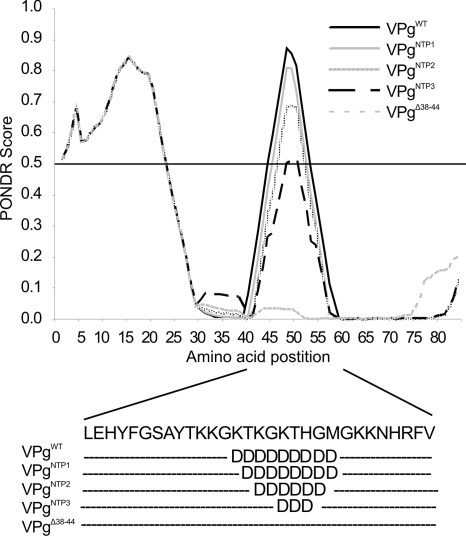
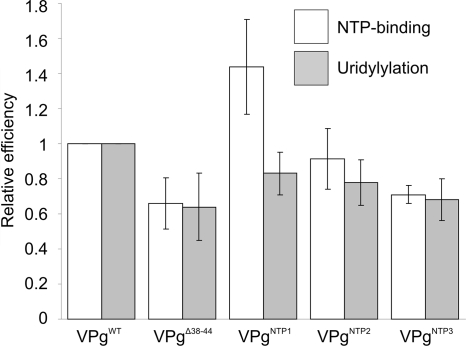
High local charge, a bioinformatic analysis (Fig. 3), and sensitivity to limited trypsin digestion (40) indicate a loosely folded NTP-binding domain of VPg. The lysines in this region were mutated to reduce surface charge (see Fig. 2D and E), and the effect on NTP binding and uridylylation was examined. A gradual decrease was observed in both reactions, with increasing numbers of lysines mutated (Fig. 4), with the exception of VPgNTP1 binding more UTP than VPgWT. A triple Lys mutation in the region lowered both activities to ∼65% of that of the VPgWT. NTP binding and uridylylation occurred to some extent also with the VPgΔ38-44 mutant, indicating that although they are not essential for NTP coordination, these lysines or the overall charge and flexibility of the NTP-binding region affect this reaction in vitro.
FIG. 3.
PONDR prediction of disorder of VPgWT and of NTP-binding site mutants. The predicted disordered region shortens with increasing number of lysine mutations; local positive charge is therefore likely to contribute to the flexibility of the region.
FIG. 4.
NTP binding and uridylylation of VPgWT and mutants. A gradual decrease was seen in both NTP-binding and uridylylation efficiency with increasing number of lysines mutated, with the exception of VPgNTP1, which bound more labeled UTP than VPgWT.
A sequence-independent RNA-binding function of VPg was first demonstrated by Merits et al. (26) and was here found to rely on the integrity of the putative contact surface. According to the model, the NTP-binding site mutations reduced the extent of the positively charged surface (Fig. 2E). In an RNA-binding experiment, free, nonretarded RNA and two separate species of the VPg-RNA complex were seen (Fig. 5). For VPgWT, 90 ng of protein was enough to retard the migration of the labeled RNA, whereas the same amount of VPgΔ38-44 did not result in any retardation. At 300 ng, all of the VPgs tested retarded the migration of at least part of the RNA. An increasing amount of free RNA was found to be migrating in the front when two or three lysines were mutated, suggesting that the capability of VPg to bind RNA was reduced due to the reduction of charge at the NTP-binding site. Again the VPgNTP1 showed an unexpected behavior and bound RNA even more efficiently than VPgWT. At 300 ng, VPgNTP1 bound almost all labeled RNA, in contrast to the result with VPgWT, where the free RNA was clearly visible.
FIG. 5.
Sequence-independent binding of RNA by recombinant VPgs. The smallest amount of VPgWT (90 ng) bound part of the labeled RNA, whereas 90 ng VPgΔ38-44 did not show any retardation of RNA. A gradual increase of the free RNA was seen from VPgNTP1 to VPgNTP3 when 300 ng of the proteins was added into the assay.
In order to study the in vivo effect of the NTP-binding site mutations to virus infection, an infection assay was used (12). For the infiltration experiments, Agrobacterium carrying either PVAWT icDNA, replication-deficient PVAΔGDD mutant icDNA, or the VPg mutants of PVAWT icDNA in the 35S-PVA::rlucInt-nos construct was mixed with Agrobacterium carrying the 35S-fluc construct. Rluc-based detection of PVA gene expression followed by subsequent normalization with Fluc was used to observe the progress of infection in the infiltrated N. benthamiana leaves. Viral gene expression from PVAWT and its VPg mutants was compared to the gene expression from the replication-deficient polymerase mutant PVAΔGDD, which was set to a relative value of 1 (Table 1). Clearly, the development of infection is impaired by a single lysine mutation in VPgNTP1, although this mutant was able to cause systemic infection. The capacity of the PVANTP2-3 RNAs to produce Rluc activity was severely reduced, and no systemic infection was developed. This is a clear indication that the NTP-binding site is functionally essential for virus infection.
TABLE 1.
Viral gene expression from PVA mutantsa
| Mutant | Expression (mean ± SD) at: |
|
|---|---|---|
| 3 dpi | 4 dpi | |
| Nucleotide binding site mutants | ||
| PVAΔGDD | 1.0 ± 0.4 | 1.0 ± 0.3 |
| PVAWT | 5.2 ± 2.5 | 9.8 ± 4.4 |
| PVANTP1 | 1.5 ± 0.5 | 2.5 ± 1.2 |
| PVANTP2 | 1 × 10−3 ± 9 × 10−5 | 1 × 10−3 ± 6 × 10−4 |
| PVANTP3 | 1.1 ± 0.9 | 1.4 ± 1.2 |
| Tyrosine mutants | ||
| PVAΔGDD | 1.0 ± 0.3 | 1.0 ± 0.6 |
| PVAWT | 34.8 ± 9.0 | 91.7 ± 18.4 |
| PVACPmut | 5.6 ± 1.5 | 10.3 ± 4.8 |
| PVAY63A | 7.3 ± 1.5 | 5.0 ± 1.3 |
| PVAY119A | 30.5 ± 6.3 | NDb |
| PVAY63A-Y119A | 1.9 ± 1.0 | ND |
Rluc activity expressed from PVA genome was used to quantify the gene expression of mutated viruses. N. benthamiana leaves were infiltrated with a mixture of Agrobacterium containing one of the 35S-PVA::rlucInt-nos constructs (OD600, 0.05) and 35S-fluc-nos (OD600, 0.01). Rluc was normalized with Fluc. Rluc activity from PVAΔGDD was set to a relative value of one.
ND, not determined.
An alternative uridylylation site predicted from the model allows replication but does not support cell-to-cell movement.
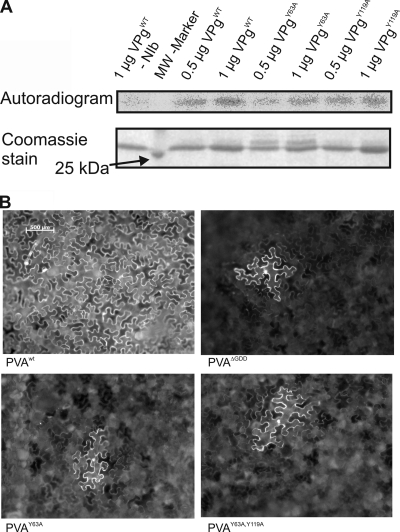
Based on previous literature (1, 29, 31) Tyr63 is the most likely site for PVA VPg uridylylation. At variance with this expectation, recombinant VPgY63A mutant was uridylylated in the in vitro assay (Fig. 6A). This suggests that an alternative uridylylation site exists. Tyr119 was regarded as the most likely candidate because of its proximity to Tyr63 in the model (Fig. 2A) and the similarities in the surrounding amino acid sequence (NMY for Tyr63 and NMVHY for Tyr119). Recombinant VPgY119A was found to be uridylylated to the same level as recombinant VPgWT (Fig. 6A), showing that Tyr119 is not the preferred site for in vitro uridylylation. In spite of several trials, we could not express the recombinant VPg with the double mutation Y63A and Y119A for in vitro assays. This may reflect drastic changes in the biophysical properties of the mutant protein. As a consequence, it could not be proven that Tyr119 is the alternative uridylylation site in vitro.
FIG. 6.
(A) In vitro uridylylation efficiencies of VPgWT, VPgY63A, and VPgY119A. Proteins were separated with SDS-PAGE and stained with Coomassie blue. Uridylylation was detected by autoradiography. VPgY63A did not abolish but only reduced the level of uridylylation. (B) Unlike PVAWT, the replication-deficient mutants PVAΔGDD, PVAY63A, and PVAY63A,Y119A were unable to move from cell to cell. At 4 days after inoculation, all of the mutants showed the same defective phenotype, with GFP fluorescence restricted to single cells.
Next, the tyrosine mutants were tested in the infection assay together with PVAWT, PVAΔGDD, and PVACPmut, which is movement deficient but replicates normally (12). Interestingly, PVAY63A produced higher Rluc activities in the infected leaves than the replication-deficient PVAΔGDD at 3 and 4 dpi, respectively (Table 1). Furthermore the Rluc activities were similar to those of the replicating PVACPmut, implying that PVAY63A was able to replicate. Systemic infection was followed at 6, 14, and 21 dpi by measuring Rluc from upper systemic leaves and by following the appearance of symptoms. In accordance with an earlier report (38), PVAY63A did not spread systemically. PVAY119A-derived Rluc gene expression was on the same level as PVAWT in infiltrated leaves, and it was able to cause systemic infection, whereas the Rluc activity from PVAY63A-Y119A double mutant was on the level of PVAΔGDD, and no systemic infection developed. Comparison of Rluc activities from PVAY63A and PVAY63A-Y119A mutants to those from PVAΔGDD and PVACPmut strongly suggested that PVAY63A was able to replicate but probably did not move from cell to cell. Low Rluc activities measured from the PVAY63A-Y119A double mutant suggested that this mutant neither replicated nor moved. Cell-to-cell movement of these mutants was studied with versions of the corresponding icDNAs in which the rluc gene was replaced with the GFP gene. Nicotiana benthamiana leaves were infiltrated with Agrobacterium suspension at an optical density at 600 nm (OD600) of 0.001, and green fluorescent protein (GFP) fluorescence was monitored in the infiltrated leaves until 6 dpi. Both mutants showed GFP fluorescence in single cells that was similar to that of PVAΔGDD, whereas at 4 dpi the PVAWT already had infected the whole infiltrated region (Fig. 6B). Thus, the mutants were not able to move from cell to cell.
The SDS-induced fold of PVA VPg resembles that observed in the presence of anionic lipids.
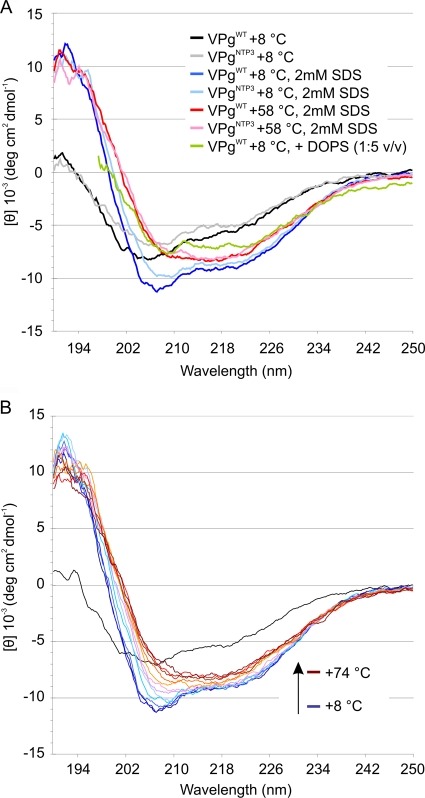
By CD analysis, the folding of VPg in the presence of 2 mM SDS closely resembled that observed in the presence of anionic phospholipids (DOPS [1,2-dioleoyl-sn-glycero-3-phospho-l-serine] vesicles in Fig. 7A), which can interact with PVA VPg (39). While the high background signal caused by the presence of DOPS vesicles complicated further analysis of the data (39), the buffer background signal in the presence of SDS remained well in the range of acceptable values throughout the far-UV region. Consequently, this allowed us to collect enough data to deconvolute the CD spectra into proportions of secondary-structure elements. The result of this process should not be considered an accurate measurement of the secondary-structure amounts, but it can give a clear indication of the direction of the induced structural change, i.e., whether the structure is stabilized and what secondary-structure element gains most upon the transformation. The triple lysine mutation caused a slight shift in the curve minima, but eventually the VPgNTP3 reached the same stabilized form as VPgWT, suggesting that the lysine mutations delay the induced folding but do not prevent it. Addition of lipid or SDS induced a rapid conformational change in VPgWT. Therefore, the folding process was followed by lowering the starting temperature of measurements to 8°C and then increasing the temperature in small increments (Fig. 7B). This revealed an α-helical folding intermediate which gradually over time and by increasing temperature stabilized to a spectrum typical for β-amyloids (Table 2) (19, 43, 54). When the fractions of secondary structures were calculated from the model and compared to those deconvoluted from CD data (Fig. 7A and Table 2), the model corresponded most to the intermediate state that is formed immediately after SDS addition. As the folding progressed, the proportion of β-sheets increased at the expense of α-helices and random structures.
FIG. 7.
(A) After the addition of SDS, VPg folds to a more structured conformation. (B) SDS-induced folding of VPgWT was followed by heating the sample from +8°C to +74°C. The final conformation was reached also at room temperature, but the process of folding could not be followed without controlling the temperature. v/v, vol/vol.
TABLE 2.
Composition of structures calculated by algorithms and modelinga
| Algorithm and measurement conditions | Proportions (%) of secondary structures by type |
|||
|---|---|---|---|---|
| Helix | Sheet | Turn | Random | |
| CONTINLL | ||||
| VPgWT +8°C | 12.2 | 24.0 | 16.8 | 47.0 |
| VPgWT +8°C, 2 mM SDS | 28.0 | 18.8 | 20.5 | 32.6 |
| VPgWT +58°C, 2 mM SDS | 20.8 | 28.8 | 21.2 | 29.2 |
| CDSSTR | ||||
| VPgWT +8°C | 8.0 | 23.2 | 16.5 | 52.3 |
| VPgWT +8°C, 2 mM SDS | 28.1 | 16.1 | 17.8 | 37.7 |
| VPgWT +58°C, 2 mM SDS | 20.0 | 26.5 | 26.6 | 32.8 |
| SELCON3 | ||||
| VPgWT +8°C | 12.2 | 25.7 | 17.1 | 43.4 |
| VPgWT +8°C, 2 mM SDS | 27.4 | 19.4 | 21.7 | 31.5 |
| VPgWT +58°C, 2 mM SDS | 20.6 | 27.6 | 21.3 | 28.1 |
Secondary-structure proportions of VPgWT at +8°C, VPgWT at +8°C with 2 mM SDS, and VPgWT at +58°C with 2 mM SDS were deconvoluted with three algorithms included in the CDPRO package. All algorithms showed that the VPg structure stabilizes into a β-sheet-dominant fold through an α-helical intermediate in the course of temperature-controlled folding. Folding leads to a gradual decrease in random coil structures. Secondary-structure proportions of homology-modeled VPg structure were calculated manually from the PDB file. The proportions of secondary structures calculated from the model were as follows: helix, 32.8%; sheet, 17.5%; coil, 49.7%.
DISCUSSION
The intrinsic disorder of plant viral VPg proteins has been confirmed in several studies (16, 20, 40, 44). Several observations suggest that PVA VPg is not fully disordered but falls in the “twilight zone” between order and disorder. For example, CD measurements show that it has significant secondary structure, and ANS-binding and nuclear magnetic resonance (NMR) experiments showed the presence of a discernible hydrophobic core in the protein (40). Bioinformatic predictors also disagree on the disorder status of this protein; PONDR shows it as rather disordered (40), whereas the IUPred prediction (9) falls slightly below the threshold for disorder (data not shown), which, in our experience, suggests a significant tendency to undergo folding under appropriate conditions. For an IDP, this usually means the presence of binding partners or translocation to a different compartment, where the protein undergoes induced folding and structurally adapts to the given function.
As folded states of virus proteins all seem to look rather unusual, for example when looking at the amounts of residues in coil regions (50), the PVA VPg structure suggested by homology modeling fits with this trend. Viral proteins appear to have a lot of coils; they are loosely packed and do not have a solid hydrophobic core. They apparently have a lot of flexibility and evolve rapidly to escape host defenses. Structural modeling of plant virus VPg proteins has been problematic because of the lack of template structures. These difficulties have been reported, together with other problems in VPg structure determination (16, 40, 41). The structure of PVY VPg has been modeled based on a nonhomologous template (33), which was selected on the basis of similarity in the hydrophobic-hydrophilic residue distribution. Recent advances in homology-modeling algorithms and the increasing number of template structures enabled us to achieve a plausible model of PVA VPg structure in spite of its apparently being on the border between order and disorder. The current physicochemical data indicate that potyviral VPgs have an elongated tertiary structure dominated by many disordered regions (16, 21, 40). Apparently, the structure of VPg generated by homology modeling shows these features, lending further credit to this approach. In this study, we addressed the structural features predicted by the model with a combination of in vitro, in silico, and in planta methods, to demonstrate the importance of the intrinsic structural flexibility to its functionality.
The folding pathway of VPg was studied with a set of CD spectroscopy measurements. We noted that the lipid-induced structural stabilization can be mimicked by SDS (39) (Fig. 7). By slowly increasing the temperature of the sample, we were able to follow the folding of the induced structure from an α-helical intermediate to a β-sheet dominant state. Based on the secondary-structure proportions deconvoluted from the CD-spectrum data, the model represents a transient folding state of VPg that forms immediately after SDS addition. Since the activities studied here and in our earlier papers are done in the absence of SDS and lipids (35, 36, 39, 40), the interaction surface for these reactions should be present already in the disordered state or formed upon binding. Also, the hydrophobic core domain was detected in the partially disordered state of VPg studied earlier by NMR and ANS-binding experiments (40). Therefore, our assumption is that in the lipid- or SDS-interacting state, the core is stabilized further, as the hydrophobic regions exposed in the model are likely to be buried while in contact with lipids. At the same time, this process would lead to an increase in the proportion of β-sheets after prolonged incubation. It is also possible that the surface-exposed hydrophobic regions presented in the model are protected by interacting proteins or subunits of VPg multimer in the in vivo environment. The structural basis and functional reasoning for VPg multimerization, aggregation, and inclusion formation are an open question. The possibility of lipid-enhanced aggregation or pore formation was discussed on the basis of the electron microscopy images of anionic vesicle-bound VPg in our previous study (39). The CD spectrum of the SDS-induced fold of VPg resembles closely those of known β-amyloids (19, 43, 54). It should be noted that although protein aggregation and amyloidosis do have similarities, they are different processes (42).
We proposed earlier that the conserved G43XXXGXXXG51 motif of VPg could take part in the membrane-associated α-helix stabilization (39). In the model, this motif starts where the helix part of the NTP-binding site ends and spans through the first of the two following β-sheets (Fig. 2A). It is also exposed on the positively charged surface of the model and on the edge of the proposed hydrophobic core domain (Fig. 2C). Based on the model, membrane penetration could progress through electrostatic attraction between the positively charged VPg surface and negatively charged lipids, leading to a formation of an α-helical intermediate state of the G43XXXGXXXG51 motif. All limited trypsin digestion sites are on the positively charged surface of the model (Fig. 2 B). Lys44 and Arg114 are both readily cleaved in the absence of lipid vesicles but protected when associated with lipids where only the N terminus is available for trypsin cleavage. This supports the hydrophobic core localization presented in Fig. 2C, as these two cleavage sites would be buried inside the bilayer.
The central domain in LMV VPg, located between amino acids 89 and 105, was shown to take part in eIF4E and viral HcPro interactions (41). In the PVA VPg model presented here, this region locates on the protruding loop on the lower part of the model in Fig. 2. It is predicted to be α-helical and locates to the surface of the model and is therefore a likely candidate for a disordered loop that is stabilized into the α-helix in protein/protein interactions. In TuMV, the polyprotein precursor 6K2-VPg-Pro intermediate and eIF4E are shown to interact within virus-induced vesicles (2). Our model, together with the earlier result of trypsin digestion protection of the central helix in vesicle interaction, raises the possibility that a membrane-embedded VPg could still interact with, e.g., eIF4E in the inner leaflet of the vesicle through the central helix. In conclusion, the model presented likely captures the structure of VPg in a state of partial stability only to arise under appropriate conditions, e.g., in the presence of membranes, substrates, or perhaps interacting partners.
All lysine mutations in the NTP-binding site affected the viral gene expression. Although being a debilitated virus, PVANTP1 was capable of replication and of causing systemic infection. Our further quantitative PCR (qPCR) experiment (K. Eskelin and K. M. Mäkinen, unpublished results) showed that the defect was on the level of viral RNA synthesis. Therefore, the low level of gene expression from PVANTP1 was attributable to the low replication capability of the virus. For a reason still unknown, PVANTP2 RNA was hardly expressed and did not even achieve the Rluc expression level of the replication-deficient polymerase mutant PVAΔGDD. The PVANTP3 mutant behaved similarly to PVAΔGDD. This surprising phenomenon observed with PVANTP2 cannot be explained yet and needs further studies. Replacement of the VPgNTP2 gene with the VPgWT gene within 35S-PVANTP2::rlucInt-nos restored the normal PVAWT infection, which suggests that the construct itself was functional. In vitro assays showed a gradual decrease in NTP binding, uridylylation, and non-sequence-specific RNA binding. Local structural disorder analysis and the model of VPg structure offer a possible explanation for this decrease (Fig. 2 and 3). It seems that VPg has a loosely folded and positively charged contact surface on one edge of the protein structure (Fig. 2D and E). When lysines on this region are mutated, the surface charge drops and the attraction between opposite charges of RNA or UTP and the protein surface is reduced. Besides reducing positive charge, replacement of lysines may also decrease the tendency for local disorder. Because disorder-to-order transition may be a key element of molecular recognition by this region and lysine is one of the most potent disorder-promoting amino acids (11), this may also adversely affect functions related to NTP binding.
Surprisingly, mutation of the most likely uridylylation site in PVA VPg, amino acid Tyr63, to Ala neither abolished in vitro uridylylation nor prevented the replication of the virus in planta but completely inhibited viral movement. To explain these results, an alternative RNA attachment site on VPg, Tyr119, was predicted from the model. Both tyrosines are located at the lower end of the positively charged surface of the model (see Fig. 2A). The distance between the NTP-binding site and the uridylylation site suggests that a structural shift to allow formation of a covalent bond between UMP and either of the tyrosines is required. The mechanism of the uridylylation reaction and its structural demands still needs to be explored in further detail. Our study revealed that the presence of Tyr119 allowed viral replication in the absence of Tyr63 in N. benthamiana. This finding also makes an interesting link to a previous study (37), where it was reported that Tyr119 of VPg (numbered Tyr118 in reference 37) controls accumulation and phloem loading of PVA in a wild potato species, Solanum commersonii. In that study, the presence of Tyr118 instead of His led to a 7-fold increase in the accumulation level of PVA in the inoculated leaf and was required for systemic infection. Our results allow quite similar conclusions for Tyr63. Its presence leads to a 5-fold increase in viral gene expression in the initially infected leaves over that of a virus carrying Tyr63Ala mutation. Interestingly, Tyr63 is required for viral movement in N. benthamiana but differently from what was reported about Tyr118 in S. commersonii; in our case it was cell-to-cell movement that was blocked. The finding that Tyr119 can replace the replication functions of Tyr63 serves as a positive example of the prediction power of the structural model presented.
Supplementary Material
Acknowledgments
Liisa Holm (Institute of Biotechnology, University of Helsinki, Finland) is acknowledged for discussion of homology modeling. Mathilda Sjöberg is thanked for critical reading of the manuscript. Sini Lindström is thanked for excellent technical help at the greenhouse.
Financial support from the Academy of Finland (grants 115922 and 121622 to K.M. and grant 127969 to K.E.) is gratefully acknowledged.
Footnotes
Published ahead of print on 22 December 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Anindya, R., S. Chittori, and H. S. Savithri. 2005. Tyrosine 66 of Pepper vein banding virus genome-linked protein is uridylylated by RNA-dependent RNA polymerase. Virology 336:154-162. [DOI] [PubMed] [Google Scholar]
- 2.Beauchemin, C., N. Boutet, and J. F. Laliberte. 2007. Visualization of the interaction between the precursors of VPg, the viral protein linked to the genome of turnip mosaic virus, and the translation eukaryotic initiation factor iso 4E in planta. J. Virol. 81:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitinaite, J., D. A. Wah, A. K. Aggarwal, and I. Schildkraut. 1998. FokI dimerization is required for DNA cleavage. Proc. Natl. Acad. Sci. U. S. A. 95:10570-10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borgstrom, B., and I. E. Johansen. 2001. Mutations in pea seedborne mosaic virus genome-linked protein VPg after pathotype-specific virulence in Pisum sativum. Mol. Plant Microbe Interact. 14:707-714. [DOI] [PubMed] [Google Scholar]
- 5.Chung, B. Y., W. A. Miller, J. F. Atkins, and A. E. Firth. 2008. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. U. S. A. 105:5897-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clertant, P., and F. Cuzin. 1982. Covalent affinity labeling by periodate-oxidized [alpha-32P]ATP of the large-T proteins of polyoma and SV40 viruses. J. Biol. Chem. 257:6300-6305. [PubMed] [Google Scholar]
- 7.Denison, M. R. 2008. Seeking membranes: positive-strand RNA virus replication complexes. PLoS Biol. 6:e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dosztanyi, Z., J. Chen, A. K. Dunker, I. Simon, and P. Tompa. 2006. Disorder and sequence repeats in hub proteins and their implications for network evolution. J. Proteome Res. 5:2985-2995. [DOI] [PubMed] [Google Scholar]
- 9.Dosztanyi, Z., V. Csizmok, P. Tompa, and I. Simon. 2005. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 21:3433-3434. [DOI] [PubMed] [Google Scholar]
- 10.Dunker, A. K., et al. 2001. Intrinsically disordered protein. J. Mol. Graph. Model. 19:26-59. [DOI] [PubMed] [Google Scholar]
- 11.Dunker, A. K., et al. 2008. The unfoldomics decade: an update on intrinsically disordered proteins. BMC Genomics 9(Suppl. 2):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskelin, K., T. Suntio, S. Hyvarinen, A. Hafren, and K. Makinen. 2010. Renilla luciferase-based quantitation of potato virus A infection initiated with Agrobacterium infiltration of N. benthamiana leaves. J. Virol. Methods 164:101-110. [DOI] [PubMed] [Google Scholar]
- 13.Eswar, N., D. Eramian, B. Webb, M. Y. Shen, and A. Sali. 2008. Protein structure modeling with MODELLER. Methods Mol. Biol. 426:145-159. [DOI] [PubMed] [Google Scholar]
- 14.Gaboriaud, C., V. Bissery, T. Benchetrit, and J. P. Mornon. 1987. Hydrophobic cluster analysis: an efficient new way to compare and analyse amino acid sequences. FEBS Lett. 224:149-155. [DOI] [PubMed] [Google Scholar]
- 15.Gabrenaite-Verkhovskaya, R., et al. 2008. Cylindrical inclusion protein of potato virus A is associated with a subpopulation of particles isolated from infected plants. J. Gen. Virol. 89:829-838. [DOI] [PubMed] [Google Scholar]
- 16.Grzela, R., et al. 2008. Virulence factor of potato virus Y, genome-attached terminal protein VPg, is a highly disordered protein. J. Biol. Chem. 283:213-221. [DOI] [PubMed] [Google Scholar]
- 17.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 18.Hafren, A., and K. Makinen. 2008. Purification of viral genome-linked protein VPg from potato virus A-infected plants reveals several post-translationally modified forms of the protein. J. Gen. Virol. 89:1509-1518. [DOI] [PubMed] [Google Scholar]
- 19.Hamada, D., et al. 2009. Competition between folding, native-state dimerisation and amyloid aggregation in beta-lactoglobulin. J. Mol. Biol. 386:878-890. [DOI] [PubMed] [Google Scholar]
- 20.Hebrard, E., et al. 2009. Intrinsic disorder in viral proteins genome-linked: experimental and predictive analyses. Virol. J. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov, K. I., et al. 2003. Phosphorylation of the potyvirus capsid protein by protein kinase CK2 and its relevance for virus infection. Plant Cell 15:2124-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller, K. E., I. E. Johansen, R. R. Martin, and R. O. Hampton. 1998. Potyvirus genome-linked protein (VPg) determines pea seed-borne mosaic virus pathotype-specific virulence in Pisum sativum. Mol. Plant Microbe Interact. 11:124-130. [DOI] [PubMed] [Google Scholar]
- 23.Kriwacki, R. W., L. Hengst, L. Tennant, S. I. Reed, and P. E. Wright. 1996. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc. Natl. Acad. Sci. U. S. A. 93:11504-11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lellis, A. D., K. D. Kasschau, S. A. Whitham, and J. C. Carrington. 2002. Loss-of-susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF(iso)4E during potyvirus infection. Curr. Biol. 12:1046-1051. [DOI] [PubMed] [Google Scholar]
- 25.Li, X., P. Romero, M. Rani, A. K. Dunker, and Z. Obradovic. 1999. Predicting protein disorder for N-, C-, and internal regions. Genome Inform. Ser. Workshop Genome Inform. 10:30-40. [PubMed] [Google Scholar]
- 26.Merits, A., D. Guo, and M. Saarma. 1998. VPg, coat protein and five non-structural proteins of potato A potyvirus bind RNA in a sequence-unspecific manner. J. Gen. Virol. 79:3123-3127. [DOI] [PubMed] [Google Scholar]
- 27.Merits, A., et al. 2002. Proteolytic processing of potyviral proteins and polyprotein processing intermediates in insect and plant cells. J. Gen. Virol. 83:1211-1221. [DOI] [PubMed] [Google Scholar]
- 28.Miller, S., and J. Krijnse-Locker. 2008. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 6:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy, J. F., P. G. Klein, A. G. Hunt, and J. G. Shaw. 1996. Replacement of the tyrosine residue that links a potyviral VPg to the viral RNA is lethal. Virology 220:535-538. [DOI] [PubMed] [Google Scholar]
- 30.Murray, K. E., and D. J. Barton. 2003. Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for negative-strand RNA synthesis. J. Virol. 77:4739-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oruetxebarria, I., et al. 2001. Identification of the genome-linked protein in virions of Potato virus A, with comparison to other members in genus Potyvirus. Virus Res. 73:103-112. [DOI] [PubMed] [Google Scholar]
- 32.Paul, A. V., J. H. van Boom, D. Filippov, and E. Wimmer. 1998. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature 393:280-284. [DOI] [PubMed] [Google Scholar]
- 33.Plochocka, D., M. Welnicki, P. Zielenkiewicz, and W. Ostoja-Zagorski. 1996. Three-dimensional model of the potyviral genome-linked protein. Proc. Natl. Acad. Sci. U. S. A. 93:12150-12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puurand, U., K. Makinen, L. Paulin, and M. Saarma. 1994. The nucleotide sequence of potato virus A genomic RNA and its sequence similarities with other potyviruses. J. Gen. Virol. 75:457-461. [DOI] [PubMed] [Google Scholar]
- 35.Puustinen, P., and K. Makinen. 2004. Uridylylation of the potyvirus VPg by viral replicase NIb correlates with the nucleotide binding capacity of VPg. J. Biol. Chem. 279:38103-38110. [DOI] [PubMed] [Google Scholar]
- 36.Puustinen, P., M. L. Rajamaki, K. I. Ivanov, J. P. Valkonen, and K. Makinen. 2002. Detection of the potyviral genome-linked protein VPg in virions and its phosphorylation by host kinases. J. Virol. 76:12703-12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajamaki, M. L., and J. P. Valkonen. 2002. Viral genome-linked protein (VPg) controls accumulation and phloem-loading of a potyvirus in inoculated potato leaves. Mol. Plant Microbe Interact. 15:138-149. [DOI] [PubMed] [Google Scholar]
- 38.Rajamaki, M. L., and J. P. Valkonen. 2009. Control of nuclear and nucleolar localization of nuclear inclusion protein a of picorna-like potato virus A in Nicotiana species. Plant Cell 21:2485-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rantalainen, K. I., et al. 2009. Interaction of a potyviral VPg with anionic phospholipid vesicles. Virology 395:114-120. [DOI] [PubMed] [Google Scholar]
- 40.Rantalainen, K. I., et al. 2008. Potato virus A genome-linked protein VPg is an intrinsically disordered molten globule-like protein with a hydrophobic core. Virology 377:280-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roudet-Tavert, G., et al. 2007. Central domain of a potyvirus VPg is involved in the interaction with the host translation initiation factor eIF4E and the viral protein HcPro. J. Gen. Virol. 88:1029-1033. [DOI] [PubMed] [Google Scholar]
- 42.Rousseau, F., J. Schymkowitz, and L. Serrano. 2006. Protein aggregation and amyloidosis: confusion of the kinds? Curr. Opin. Struct. Biol. 16:118-126. [DOI] [PubMed] [Google Scholar]
- 43.Sabate, R., A. Espargaro, S. J. Saupe, and S. Ventura. 2009. Characterization of the amyloid bacterial inclusion bodies of the HET-s fungal prion. Microb. Cell Fact. 8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satheshkumar, P. S., P. Gayathri, K. Prasad, and H. S. Savithri. 2005. “Natively unfolded” VPg is essential for Sesbania mosaic virus serine protease activity. J. Biol. Chem. 280:30291-30300. [DOI] [PubMed] [Google Scholar]
- 45.Schaad, M. C., R. Haldeman-Cahill, S. Cronin, and J. C. Carrington. 1996. Analysis of the VPg-proteinase (NIa) encoded by tobacco etch potyvirus: effects of mutations on subcellular transport, proteolytic processing, and genome amplification. J. Virol. 70:7039-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaad, M. C., P. E. Jensen, and J. C. Carrington. 1997. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 16:4049-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaad, M. C., A. D. Lellis, and J. C. Carrington. 1997. VPg of tobacco etch potyvirus is a host genotype-specific determinant for long-distance movement. J. Virol. 71:8624-8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shukla, D. D., C. W. Ward, and A. A. Brunt. 1994. The Potyviridae. C.A.B. International, Wallingford, United Kingdom.
- 49.Sreerama, N., and R. W. Woody. 2000. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 287:252-260. [DOI] [PubMed] [Google Scholar]
- 50.Tokuriki, N., C. J. Oldfield, V. N. Uversky, I. N. Berezovsky, and D. S. Tawfik. 2009. Do viral proteins possess unique biophysical features? Trends Biochem. Sci. 34:53-59. [DOI] [PubMed] [Google Scholar]
- 51.Tompa, P. 2002. Intrinsically unstructured proteins. Trends Biochem. Sci. 27:527-533. [DOI] [PubMed] [Google Scholar]
- 52.Tompa, P., C. Szasz, and L. Buday. 2005. Structural disorder throws new light on moonlighting. Trends Biochem. Sci. 30:484-489. [DOI] [PubMed] [Google Scholar]
- 53.Wah, D. A., J. Bitinaite, I. Schildkraut, and A. K. Aggarwal. 1998. Structure of FokI has implications for DNA cleavage. Proc. Natl. Acad. Sci. U. S. A. 95:10564-10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wahlstrom, A., L. Hugonin, A. Peralvarez-Marin, J. Jarvet, and A. Graslund. 2008. Secondary structure conversions of Alzheimer's Abeta(1-40) peptide induced by membrane-mimicking detergents. FEBS J. 275:5117-5128. [DOI] [PubMed] [Google Scholar]
- 55.Wei, T., et al. 2010. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J. Virol. 84:799-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wen, R. H., and M. R. Hajimorad. 2010. Mutational analysis of the putative pipo of soybean mosaic virus suggests disruption of PIPO protein impedes movement. Virology 400:1-7. [DOI] [PubMed] [Google Scholar]
- 57.Wright, P. E., and H. J. Dyson. 2009. Linking folding and binding. Curr. Opin. Struct. Biol. 19:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, S., and Y. Zhang. 2007. LOMETS: a local meta-threading-server for protein structure prediction. Nucleic Acids Res. 35:3375-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou, H., and Y. Zhou. 2005. Fold recognition by combining sequence profiles derived from evolution and from depth-dependent structural alignment of fragments. Proteins 58:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.