Abstract
The lifelong infection by varicelloviruses is characterized by a fine balance between the host immune response and immune evasion strategies used by these viruses. Virus-derived peptides are presented to cytotoxic T lymphocytes by major histocompatibility complex (MHC) class I molecules. The transporter associated with antigen processing (TAP) transports the peptides from the cytosol into the endoplasmic reticulum, where the loading of MHC-I molecules occurs. The varicelloviruses bovine herpesvirus 1 (BoHV-1), pseudorabies virus, and equid herpesviruses 1 and 4 have been found to encode a UL49.5 protein that inhibits TAP-mediated peptide transport. To investigate to what extent UL49.5-mediated TAP inhibition is conserved within the family of Alphaherpesvirinae, the homologs of another five varicelloviruses, one mardivirus, and one iltovirus were studied. The UL49.5 proteins of BoHV-5, bubaline herpesvirus 1, cervid herpesvirus 1, and felid herpesvirus 1 were identified as potent TAP inhibitors. The varicella-zoster virus and simian varicellovirus UL49.5 proteins fail to block TAP; this is not due to the absence of viral cofactors that might assist in this process, since cells infected with these viruses did not show reduced TAP function either. The UL49.5 homologs of the mardivirus Marek's disease virus 1 and the iltovirus infectious laryngotracheitis virus did not block TAP, suggesting that the capacity to inhibit TAP via UL49.5 has been acquired by varicelloviruses only. A phylogenetic analysis of viruses that inhibit TAP through their UL49.5 proteins reveals an interesting hereditary pattern, pointing toward the presence of this capacity in defined clades within the genus Varicellovirus.
Herpesviruses cause a lifelong infection in their host. The antiviral immune response of the host is counteracted by immune evasion strategies used by these viruses. CD8+ cytotoxic T lymphocytes (CTL) play an important role in immunity against viruses, recognizing viral peptides presented on major histocompatibility complex class I (MHC-I) molecules at the cell surface. The antigenic peptides originate from proteasomal degradation of virus-encoded proteins in the cytosol. The peptides are transported into the endoplasmic reticulum (ER) by the Transporter associated with Antigen Processing (TAP) in an ATP-dependent manner (1, 6, 32). TAP proteins are highly conserved among various species: for example, human, porcine, bovine and rodent TAP1 and TAP2 demonstrate 70 to 80% amino acid identity (15, 37). The transporter is thought to be involved in MHC-I-mediated antigen presentation in many different species, including Xenopus laevis (42).
The inhibition of peptide transport by TAP is often exploited by herpesviruses to prevent elimination by CTL. The first TAP inhibitor to be identified was the ICP47 protein of herpes simplex virus type 1 (HSV-1) (4). ICP47 of HSV-2 was later on found to have the same function (48). The ICP47 proteins of these viruses prevent peptide transport by obstructing the peptide binding site of the TAP complex (4, 5, 12, 23, 47). The US6 protein of the human cytomegalovirus (HCMV) interferes with ATP binding to TAP, thereby limiting its energy supply and, consequently, the transport of peptides (3, 19, 21, 22, 31). The Epstein-Barr virus (EBV) encodes the TAP inhibitor BNLF2a that blocks both the binding of peptides and ATP to TAP (24, 25). A fourth class of TAP-inhibiting proteins, encoded by the UL49.5 gene, has been identified in the varicelloviruses bovine herpesvirus 1 (BoHV-1), equid herpesvirus 1 (EHV-1) and EHV-4, and suid herpesvirus 1 or pseudorabies virus (PRV) (28, 30). The mechanisms by which UL49.5 homologs inhibit TAP demonstrate remarkable heterogeneity. All proteins block conformational changes within the complex that are required for peptide transport. In addition, BoHV-1 UL49.5 also induces degradation of TAP1 and TAP2 (28, 30). In contrast, EHV-1 and EHV-4 UL49.5 prevent ATP binding to TAP (30).
Homologs of UL49.5 proteins are encoded by all herpesviruses sequenced (10, 38). The UL49.5 genes encode a type 1 transmembrane protein that is often N glycosylated and therefore known as glycoprotein N or gN. In several herpesviruses, UL49.5 has been demonstrated to be involved in virion maturation and infectivity: UL49.5 forms a heterodimeric complex with glycoprotein M (gM) and is necessary for proper glycosylation and maturation of the complex (14, 27, 35, 43, 50). Thus, for some viruses, UL49.5 possesses a dual role, functioning both as a molecular chaperone and as an immune evasion protein.
The family of Herpesviridae has been classified into three subfamilies: the Alpha-, Beta-, and Gammaherpesvirinae. Isolated expression of the UL49.5 homologs encoded by members of these subfamilies, including HSV-1 and -2 (alphaherpesvirus, genus Simplexviruses), HCMV (betaherpesvirus), and EBV (gammaherpesvirus), did not result in reduced TAP function (28). The TAP-inhibiting UL49.5 proteins of BoHV-1, EHV-1, EHV-4, and PRV all belong to the genus Varicellovirus of the Alphaherpesvirinae. However, the UL49.5 proteins of human herpesvirus 3 or varicella-zoster virus (VZV) and canid herpesvirus 1 (CaHV-1), which are members of the same genus, exhibit no or poor TAP inhibition, respectively, indicating that the TAP-inhibiting capacity of UL49.5 proteins is only found for a selection of varicelloviruses.
The Alphaherpesvirinae include two other genera, the Mardivirus and the Iltovirus. Gallid herpesvirus 2 or Marek's disease virus 1 (MDV-1) is a member of the mardiviruses. Serotype 1 of MDV is oncogenic, inducing T cell tumors in infected poultry. MHC-I downregulation has been observed on MDV-1-infected chicken cells (26, 33) and in epithelial and infiltrating cells derived from brain tissue of infected chickens (18). To date, the responsible viral protein(s) have not been identified. Gallid herpesvirus 1 or infectious laryngotracheitis virus (ILTV) is a member of the iltoviruses. At present, it is unclear whether MHC-I-restricted antigen presentation is affected by ILTV.
In the present study, immune evasion by UL49.5 homologs of various clades within the genus Varicellovirus was assessed. Human- and virus-specific host cell lines stably expressing the UL49.5 proteins were screened for MHC-I downregulation and TAP inhibition. If TAP inhibition was observed, the stability of TAP and ATP binding to the TAP complex were determined to investigate the mechanism utilized by the UL49.5 homologs to inhibit TAP function. The UL49.5 proteins of BoHV-5, water buffalo herpesvirus or bubaline herpesvirus 1 (BuHV-1), red deer herpesvirus or cervid herpesvirus 1 (CvHV-1), and feline rhinotracheitis virus or felid herpesvirus 1 (FeHV-1) were identified as potent TAP inhibitors. The UL49.5 homolog of cercopithecine herpesvirus 9 or simian varicella virus (SVV), which is closely related to VZV, only slightly reduced peptide transport by rhesus macaque TAP. UL49.5 proteins of the alphaherpesviruses MDV-1 (mardivirus) and ILTV (ilthovirus) fail to inhibit TAP. These and previous findings are discussed in the context of the phylogeny of these viruses.
MATERIALS AND METHODS
UL49.5 constructs.
Purified viral DNA from the clinical isolates BoHV-5 Evi 88/95 (9), BuHV-1 strain B6 (M. J. Studdert, Faculty of Veterinary Science, University of Melbourne, Melbourne, Australia) (45), CvHV-1 strain D2839 (P. F. Nettleton, Moredun Research Institute, Edinburgh, Great Britain), FeHV-1 strain B927 (R. de Groot, Department of Infectious Diseases and Immunology, Faculty of Veterinary Medicine, Utrecht University, Utrecht, Netherlands) (17), and vaccine strain MDV-1 CVI988 (J. van Oirschot, Department of Virology, Central Veterinary Institute, Lelystad, Netherlands) (18) were used as a template for PCR amplification. PCRs were performed with Pfu (Invitrogen), Taq (Promega), or KOD DNA polymerase (Novagen-Merck) and specific primers (Table 1) for amplification of the coding sequence of the UL49.5 genes. The sequences of the primers were based on published sequences found in the NCBI database. PCR-generated products were sequenced and inserted into the retroviral expression vectors pLZRS-IRES-GFP, behind the HCMV IE1 promoter and upstream of an internal ribosome entry site (IRES) element, followed by green fluorescent protein (GFP). SVV UL49.5 (derived from the clinical isolate Delta) (39) was amplified from pcDNA3.1 by PCR (see primers in Table 1) and cloned into pLZRS using Gateway technology (Invitrogen). ILTV UL49.5 (derived from the clinical isolate A489) was recloned from a pcDNA3.1 vector (W. Fuchs and T. C. Mettenleiter, Institute of Molecular Biology, Friedrich-Loeffler Institut, Greifswald-Insel Riems, Germany) (13, 14) into pLZRS (primers in Table 1). Information on the pLZRS vector can be obtained at www.stanford.edu/group/nolan/retroviral_systems/retsys.html.
TABLE 1.
PCR primers
| Primer | Orientationa | Sequence (5′-3′)b |
|---|---|---|
| BoHV-5 UL49.5 | F | GCCGGATCCGCTCCACGACGACCATGTCGCGC |
| BuHV-1 UL49.5 | F | GCCGGATCCGACGACCATGTCGCGCTCGCT |
| BoHV-5/BuHV-1 UL49.5 | R | GCGGAATTCCGCTCAACCCCGCCCCCGCAC |
| CvHV-1UL49.5 | F | GCCGGATCCGAGCCGAGCACCATGGCGAGG |
| CvHV-1UL49.5 | R | GCGGAATTCCGGTCAGCCCCGCCCCCGCGA |
| FeHV-1 UL49.5 | F | CGGGATCCCACCATGGATCGTTTATCC |
| FeHV-1 UL49.5 | R | GCGGAATTCTTAGTGTGGCATGC |
| SVV UL49.5 | F | GGGGACAAGTTTGTACAAAAAAGCAGGCTGAATTCACCATGGCTTCAAATTGCTCTT |
| SVV UL49.5 | R | GGGGACCACTTTGTACAAGAAAGCTGGGTCCTCGAGTTACCATGTACTACGTAAGACGGATCG |
| SVV-HA UL49.5 | R | GGGGACCACTTTGTACAAGAAAGCTGGGTCCTCGAGTTAAGCGTAGTCTGGGACGTCGTATGG |
| MDV-1 UL49.5 | F | CGGGATCCCACCATGGGACTCATG |
| MDV-1 UL49.5 | R | CCGAATTCCTTACCACTCCTCTTTAAAC |
| ILTV UL49.5 | F | CGGGATCCCACCATGAGGCTGC |
| ILTV UL49.5 | R | GGAATTCCTACCATCGAGAACTAATGAC |
F, forward; R, reverse.
Restriction sites are indicated in boldface.
The pDONR209 plasmids containing the UL49.5 constructs of MDV1 strains RB1B (clinical isolate) and CVI988 (vaccine strain) were kindly provided by J. Haas (Division of Pathway Medicine, University of Edinburgh, Edinburgh, United Kingdom). The inserts were cloned into the lentiviral vector pDEST-LV-IRES-GFP (K. Franken, Department of Immunohematology and Blood Transfusion, Leiden University Medical Center, Leiden, Netherlands) upstream of an IRES element, which was followed by eGFP using the Gateway technology. The DNA sequences of the resulting expression vectors were verified.
Cell lines and retroviruses.
The human melanoma cell line Mel JuSo (MJS), MJS BoHV-1 UL49.5-IRES-GFP (28), MJS IRES-GFP, the epithelial cell line Madin-Darby bovine kidney (MDBK) cells (American Type Culture Collection [ATCC]; commonly used to propagate BoHV-5, BuHV-1, and CvHV-1), and MDBK UL49.5-IRES-GFP (30) were maintained in RPMI 1640 medium. The feline-derived epithelial cell line CRFK (provided by R. de Groot, Department of Infectious Diseases and Immunology, Faculty of Veterinary Medicine, Utrecht University, Utrecht, Netherlands), human fibroblast cell line MRC-5 (a gift from G. M. Verjans, Rotterdam Eye Hospital, Rotterdam, Netherlands), telomerized rhesus fibroblasts (TRF; obtained from J. A. Nelson, Vaccine and Gene Therapy Institute, Oregon Health and Science University), and the avian hepatoma cell line LMH were cultured in Dulbecco modified Eagle medium (DMEM). The rhesus macaque-derived epithelial cell line LLC-MK2 (kindly provided by A. D. Hislop, School of Cancer Sciences, University of Birmingham, Birmingham, United Kingdom) were cultured in Eagle minimal essential medium supplemented with nonessential amino acids. Media were supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine (Invitrogen), 140 IU of penicillin/ml, and 140 mg of streptomycin/ml.
Retroviruses were made using the Phoenix amphotropic packaging system as described before (www.stanford.edu/group/nolan/retroviral_systems/retsys.html). The retroviruses were used to transduce target cells, after which eGFP-positive cells were selected by using a FACSAria cell sorter (Becton Dickinson). The following stable cell lines were generated: MJS expressing BoHV-5, BuHV-1, CvHV-1, FeHV-1, SVV, SVV-HA, and ILTV UL49.5; CRFK expressing FeHV-1; LLC-MK2 expressing SVV UL49.5; and LMH expressing ILTV UL49.5. The generation of recombinant retroviruses for the MDBK cell line transductions was described before (30). GP2-293 pantropic packaging cells were cotransfected with pLZRS-UL49.5-IRES-GFP and pVSV-G construct (envelope vector) to obtain retroviruses used for the establishment of MDBK stably expressing BoHV-5, BuHV-1, and CvHV-1 UL49.
Antibodies.
The following antibodies were used for flow cytometry: the anti-human MHC-I complex monoclonal antibody (MAb) W6/32 (7), anti-human MHC-II HLA-DR MAb L243 (ATCC), anti-feline MHC-I complex MAb H58A (VMRD, Inc.), anti-FeHV-1 MCA2490 (Serotec), anti-human transferrin receptor MAb CD71 (Becton Dickinson), anti-human epidermal growth factor receptor MAb Ab-5 (Calbiochem), and anti-chicken MHC-I complex MAb F21-2 (Southern Biotech).
For detection of UL49.5, rabbit polyclonal antisera were raised against a synthetic peptide (RLMGASGPNKKESRGRG) derived from the C-terminal domain of BoHV-1 UL49.5 (35). In addition, we used rabbit anti-ILTV UL49.5 (kindly provided by W. Fuchs and T. C. Mettenleiter) (14), anti-TAP1 MAb 148.3 (40), anti-TAP2 MAb 435.3 (kindly provided by P. van Endert, INSERM, U580, Université Paris Descartes, Paris, France), and rabbit anti-GFP (49). Anti-actin MAb AC-74 (Sigma-Aldrich) was used as a control.
Flow cytometry.
The surface expression levels of MHC-I and MHC-II molecules were determined by flow cytometry. The cells were stained with the indicated primary antibodies and washed with secondary goat anti-mouse allophycocyanin antibody (Leinco Technologies) or goat anti-mouse phycoerythrin antibody (Jackson Immunoresearch Laboratories) at 4°C. Stained cells were measured by using a FACSCalibur and LSR II (Becton Dickinson) and analyzed by using CellQuest (Becton Dickinson) or FlowJo (Tree Star) software. The results of one representative experiment out of two independent experiments are shown.
Peptide transport assay.
Cells were permeabilized using 2.5 U of Streptolysin-O (Murex Diagnostics)/ml at 37°C for 10 min. Permeabilized cells were incubated with 4.5 μM fluorescein-conjugated synthetic peptide CVNKTERAY (the N-core glycosylation site underlined) in the presence of 10 mM ATP or 0.125 M EDTA at 37°C for 10 min. The addition of EDTA, which binds divalent cations, abrogates the ATP-hydrolyzing function of TAP. Peptide translocation was terminated by adding 1 ml of ice-cold lysis buffer (1% Triton X-100, 500 nM NaCl, 2 mM MgCl2, 50 mM Tris-HCl [pH 8.0]). After lysis for 30 min at 4°C, cells were centrifuged at 16,000 × g for 20 min at 4°C in order to obtain postnuclear lysates. Glycosylated peptides were isolated from these lysates by incubation with concanavalin A-Sepharose beads (GE Healthcare) for 2 h at 4°C. After the beads were washed, glycosylated peptides were eluted from the beads with elution buffer (500 mM mannopyranoside, 10 mM EDTA, 50 mM Tris-HCl [pH 8.0]) during a 1-h incubation step at room temperature. Fluorescence was measured by using a Mithras LB 940 multilabel reader (Berthold Technologies) or a VersaFluor fluorometer (Bio-Rad). Peptide transport is expressed as a percentage of translocation, relative to the translocation observed in control cells (set at 100%). One representative experiment out of two independent experiments is shown.
Immunoblotting.
Cells were lysed in 1% Nonidet P-40 and proteins were separated by SDS-PAGE and subsequently transferred to polyvinylidene difluoride membranes (GE Healthcare). UL49.5 proteins were separated by using 16.5%-Tricine PAGE. The blots were incubated with the indicated antibodies, followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Dako and Jackson ImmunoResearch Laboratories). Bound HRP-labeled antibodies were visualized by using ECL Plus (GE Healthcare). The results of one representative experiment out of two independent experiments are shown.
Transient expression.
MJS and LMH cells were transiently transfected by using the Amaxa Nucleofector II device according to the manufacturer's instructions. For MJS, program A-020 and solution L were used, and for LMH, program T-020 and solution T were used. At 2 days posttransfection the cells were analyzed for MHC-I expression.
Viruses and viral infections.
CRFK cells were infected with FeHV-1 (strain B927) at a multiplicity of infection (MOI) of 10 in serum-free DMEM. After 1 h of virus adsorption, DMEM containing 10% FBS was added to make the final FBS concentration 5%. At 5 or 12 h postinfection (hpi) the cells were harvested. To restrict viral gene expression to immediate-early (IE) and early (E) genes, the cells were treated with 300 μg of phosphonoacetic acid (PAA: Sigma)/ml for 2 h before addition of the virus and during infection. The Dutch BoHV-1.1 field strain Lam was used to infect MRC-5 cells at an MOI of 20. After 2 h of virus adsorption, the inoculum was removed, and the cells were incubated in the presence of complete RPMI 1640 medium for another 3 h. A bac-derived rhesus CMV (44) was used to infect TRF at an MOI of 1. The cells were harvested at 36 hpi.
For VZV infection, we used the recombinant viruses VZV pOka expressing ORF66 N-terminally tagged with eGFP (11) and VZV-GFP (34) that contains eGFP inserted between ORF65 and ORF66. Trypsinized VZV-infected MRC-5 cells were used to infect confluent monolayers of MRC-5 at a 4:1 ratio (uninfected cells to infected cells). This yielded a 100% eGFP-positive population when harvested at 72 to 88 hpi. During infection, MRC-5 cells were cultured in DMEM supplemented with 2% FBS. For SVV infections, we used the recombinant SVV delta, in which eGFP was inserted between US2 and US3 through homologous recombination (36). A confluent monolayer of TRF cells was infected in a 6:1 ratio with previously SVV-infected TRF cells. The cells were harvested 72 hpi and were 100% positive for eGFP.
In all experiments, mock-infected cells were treated under the same conditions as infected cells. The results of one representative experiment out of two independent experiments are shown.
ATP-agarose binding assay.
TAP binding to ATP-agarose was assayed as described previously (28). In brief, the cells were solubilized in 1% Nonidet P-40 or in 1% (wt/vol) digitonin, 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 150 mM NaCl, 5 mM iodoacetamide, and 1 mM AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride] to preserve the interactions between TAP1, TAP2, and UL49.5. Hydrated C-8 ATP-agarose (Fluka/Sigma) was added to the postnuclear supernatant, followed by incubation at 4°C overnight. The supernatant was separated from the ATP-agarose pellet by 5 min of centrifugation. The resulting pellet was washed three times with 0.1% (wt/vol) digitonin, 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, and 150 mM NaCl. Proteins bound to the ATP-agarose beads were eluted with 500 mM EDTA, and SDS-PAGE sample buffer was added to both the supernatant and the pellet. The samples were separated by using SDS-PAGE and analyzed by immunoblotting. The results of one representative experiment out of two independent experiments are shown.
RESULTS
TAP inhibition by herpesviruses infecting ruminants.
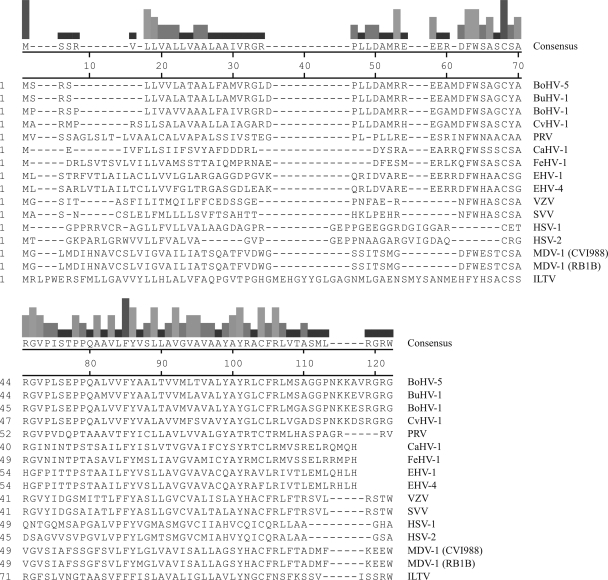
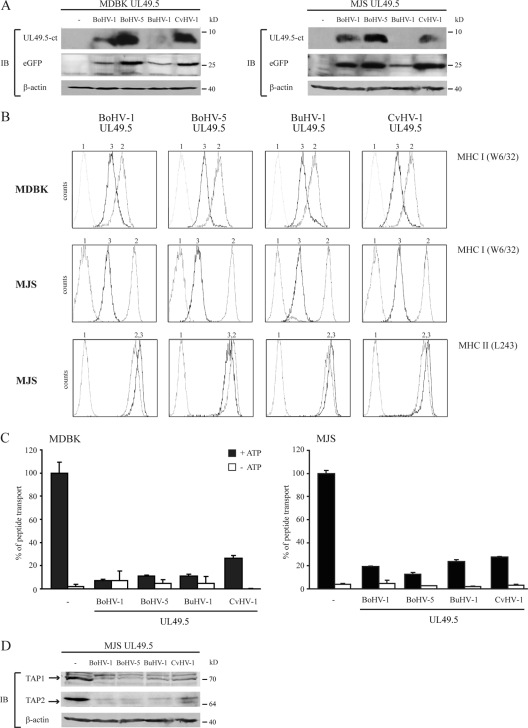
The sequences of the UL49.5 proteins encoded by BoHV-5, BuHV-1, and CvHV-1 are 79, 80, and 74% identical to the BoHV-1 UL49.5, respectively (Fig. 1 and Table 2). To determine whether the capacity to inhibit TAP is conserved for these proteins, the BoHV-5, BuHV-1, and CvHV-1 UL49.5 proteins were expressed in bovine cells (MDBK) and human (MJS) cells. Expression of the UL49.5 homologs was verified by using an antibody specific for BoHV-1 UL49.5. This antibody was capable of detecting the UL49.5 proteins of BoHV-1, BoHV-5, and CvHV-1 in MDBK and MJS cells (Fig. 2 A). BuHV-1 UL49.5 could not be detected, possibly due to differences within the C-terminal region against which the antibody was raised. In the retroviral vector used to establish the cell-lines, the UL49.5 homologs were placed upstream of an IRES that is followed by an eGFP gene. Since the two proteins are encoded by one transcript, the expression of eGFP reflects expression of the UL49.5 protein. Detection of eGFP in cells transduced with the BuHV-1 UL49.5-IRES-GFP retrovirus confirmed the expression of the corresponding transcript (Fig. 2A).
FIG. 1.
Alignment of the amino acid sequences of a selection of alphaherpesvirus UL49.5 proteins. The amino acid sequence alignment of UL49.5 homologs was performed by using CLUSTAL V of the MegAlign software from DNAStar. BoHV-5 UL49.5, bovine herpesvirus 5 (accession number NP_954898); BuHV-1 UL49.5, bubaline herpesvirus 1 (F. A. M. Rijsewijk, unpublished data); BoHV-1 UL49.5, bovine herpesvirus 1 (NP_045309); CvHV-1 UL49.5, cervid herpesvirus 1 (F. A. M. Rijsewijk, unpublished data); PRV/SuHV-1 UL49.5, pseudorabies virus (YP_068325); CaHV-1 UL49.5, canid herpesvirus 1 (patent EPO910406); FeHV-1 UL49.5, felid herpesvirus 1 (YP_003331529); EHV-1 UL49.5, equid herpesvirus 1 (YP_053055); EHV-4 UL49.5, equid herpesvirus 4 (NP_045227); VZV/HHV3 UL49.5, varicella-zoster virus (YP_068406); SVV UL49.5, simian varicella virus (NP_077423); HSV-1 UL49.5, herpes simplex virus 1 (NP_044652); HSV-2 UL49.5, herpes simplex virus 2 (NP_044520); MDV-1/GaHV-2 UL49.5, Marek's disease virus 1 strain CVI988 (ABF72292.1); MDV-1 strain RB1B (YP_001033979.1); ILTV/GaHV-1 UL49.5, infectious laryngotracheitis virus (YP_182341). The bars at the top of figure are proportional in height to the degree of homology of amino acid conservation among the different viral proteins.
TABLE 2.
Percentages of amino acid sequence identity between various UL49.5 homologs
| Virus | % Amino acid sequence identitya |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ILTV | MDV-1 (RBIB) | MDV-1 (CVI988) | HSV-2 | HSV-1 | SVV | VZV | EHV-4 | EHV-1 | FeHV-1 | CaHV-1 | PRV | CvHV-1 | BoHV-1 | BuHV-1 | BoHV-5 | |
| BoHV-5 | 18.9 | 22.1 | 22.1 | 23.0 | 20.9 | 26.4 | 24.1 | 31.6 | 32.6 | 28.4 | 30.1 | 30.5 | 72.6 | 78.9 | 94.7 | 100 |
| BuHV-1 | 20.0 | 23.2 | 23.2 | 20.7 | 19.8 | 26.7 | 25.3 | 31.6 | 30.5 | 28.4 | 26.7 | 29.5 | 74.7 | 80.0 | 100 | |
| BoHV-1 | 18.8 | 23.2 | 23.2 | 21.8 | 20.9 | 25.3 | 25.3 | 29.2 | 30.2 | 30.5 | 27.9 | 33.3 | 74.0 | 100 | ||
| CvHV-1 | 19.4 | 22.1 | 22.1 | 18.4 | 22.0 | 25.3 | 21.8 | 27.6 | 28.6 | 29.5 | 27.9 | 28.6 | 100 | |||
| PRV | 22.4 | 22.1 | 22.1 | 20.7 | 23.1 | 28.7 | 25.3 | 29.6 | 28.6 | 32.6 | 30.2 | 100 | ||||
| CaHV-1 | 15.1 | 26.7 | 26.7 | 14.0 | 15.1 | 26.7 | 29.1 | 46.5 | 44.2 | 53.5 | 100 | |||||
| FeHV-1 | 18.9 | 26.3 | 26.3 | 18.4 | 17.6 | 29.9 | 32.2 | 38.9 | 35.8 | 100 | ||||||
| EHV-1 | 21.0 | 23.2 | 23.2 | 18.4 | 19.8 | 33.2 | 27.6 | 88.0 | 100 | |||||||
| EHV-4 | 20.0 | 24.2 | 24.2 | 16.1 | 18.7 | 33.3 | 29.9 | 100 | ||||||||
| VZV | 23.0 | 32.2 | 32.2 | 13.8 | 11.5 | 63.2 | 100 | |||||||||
| SVV | 27.6 | 32.2 | 32.2 | 14.9 | 14.9 | 100 | ||||||||||
| HSV-1 | 17.6 | 17.6 | 17.6 | 54.0 | 100 | |||||||||||
| HSV-2 | 20.7 | 18.4 | 18.4 | 100 | ||||||||||||
| (CVI988) MDV-1 | 21.1 | 90.9 | 100 | |||||||||||||
| (RBIB) MDV-1 | 21.1 | 100 | ||||||||||||||
| ILTV | 100 | |||||||||||||||
The percentages were calculated by using the CLUSTAL V program (parameters: PAM 250) of MegAlign software from DNAStar.
FIG. 2.
The UL49.5 proteins of BoHV-5, BuHV-1, and CvHV-1 inhibit peptide transport and mediate the degradation of TAP1 and TAP2. (A) Lysates derived from control and UL49.5-expressing MDBK and MJS cells were stained for GFP and UL49.5 by SDS-PAGE and immunoblotting (IB) with specific antibodies. The β-actin signal was used as a loading control. (B) Surface expression of MHC-I (MDBK and MJS) and MHC-II (MJS) molecules was assessed by flow cytometry on untransduced cells (graph 2) and on cells expressing the UL49.5 homologs of BoHV-1, BoHV-5, BuHV-1, and CvHV-1 (graph 3) using the indicated antibodies. Graph 1, background staining in the presence of secondary antibody only. (C) Transport activity of TAP was analyzed in MDBK and MJS expressing the UL49.5 homologs. Peptide transport was evaluated in the presence of ATP (▪) or EDTA (□). (D) Steady-state levels of TAP1 and TAP2 in MJS cells were determined using specific antibodies. The β-actin signal was used as a loading control.
Analysis of MHC-I cell surface expression revealed that the BoHV-5, BuHV-1, and CvHV-1 UL49.5 proteins cause a strong downregulation on MDBK and MJS cells (Fig. 2B, upper and middle panels, respectively). The reduction was comparable to that induced by BoHV-1 UL49.5. This downregulation was specific for MHC-I, since MHC-II expression was not affected by all UL49.5 homologs (Fig. 2B, lower panels). Next, TAP function was assessed in BoHV-5, BuHV-1, and CvHV-1 UL49.5-expressing MDBK and MJS cells. Transport of the reference peptide was reduced by 70 to 90% in both human and bovine cells, indicating that the observed reduction of MHC-I results from TAP inhibition (Fig. 2C).
Previously, BoHV-1 UL49.5 was found to induce the degradation of human and bovine TAP (28, 30). To evaluate whether BoHV-5, BuHV-1, and CvHV-1 UL49.5 possess a similar capacity, TAP1 and TAP2 levels were analyzed in MJS cells expressing these proteins (Fig. 2D). TAP levels were strongly reduced in the presence of all three UL49.5 homologs tested, suggesting that UL49.5-induced degradation of the TAP complex is conserved for UL49.5 proteins of herpesviruses infecting ruminants.
FeHV-1 UL49.5 inhibits TAP-mediated peptide transport.
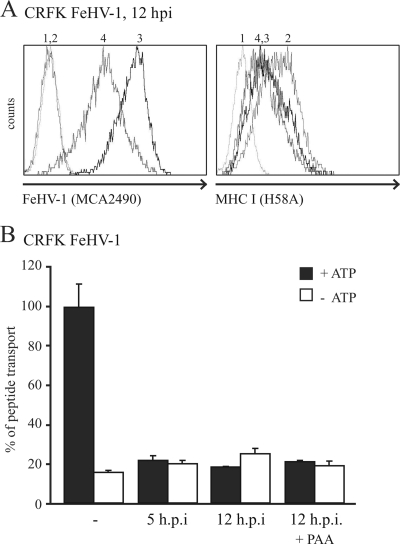
FeHV-1 has recently been found to reduce MHC-I levels on infected cells (41). To assess whether this downregulation resulted from the inhibition of peptide transport by TAP, TAP activity was analyzed in feline CRFK cells infected with FeHV-1 at 5 and 12 hpi. To evaluate the contribution of FeHV-1 late proteins to TAP inhibition, the experiment was also performed in the presence of PAA, which inhibits late viral protein synthesis. FeHV-1 infection of the cells was confirmed using an antibody recognizing several FeHV-1 glycoproteins (Fig. 3 A, left panel). As expected, MHC-I levels were downregulated on FeHV-1-infected cells (Fig. 3A, right panel) and, additionally, TAP function was strongly reduced already at 5 hpi (Fig. 3B). TAP was still inhibited after 12 h, irrespective of the presence of PAA, implying that an immediate-early or early gene is responsible for this effect, possibly UL49.5.
FIG. 3.
TAP is inhibited in FeHV-1-infected cells by an immediate-early or early protein. (A) CRFK cells were mock infected (graph 2) or infected with FeHV-1 for 12 h in the absence (graph 3) or presence (graph 4) of PAA. The expression of FeHV-1 glycoproteins and cell surface expression of MHC-I were assessed by flow cytometry using specific antibodies. Graph 1, secondary antibody only. (B) TAP activity was determined in mock- and FeHV-1-infected cells at 5 and 12 hpi (in the absence or presence of PAA). The assay was performed in the presence of ATP (▪) or EDTA (□).
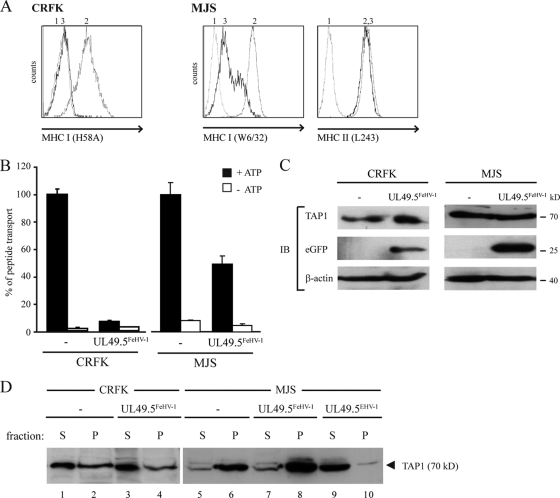
To study whether FeHV-1 UL49.5 plays a role in the observed TAP inhibition, this homolog was expressed in CRFK and MJS cells. FeHV-1 UL49.5 induced a strong downregulation of MHC-I in CRFK and a slightly weaker downregulation in MJS cells. The inhibition of MHC-I expression was specific, since MHC-II expression was not affected (Fig. 4 A). TAP function was inhibited in both FeHV-1 UL49.5-expressing cell lines, albeit less effectively in the MJS cells (Fig. 4B). In the absence of FeHV-1 UL49.5-specific antibodies, expression of UL49.5 gene was inferred from the presence of eGFP that is encoded by the same transcript (Fig. 4C). The antibody used to detect human TAP1 cross-reacted with feline TAP1 (Fig. 4C). Therefore, this antibody could be used to investigate whether FeHV-1 UL49.5 shares the capacity of UL49.5 of ruminant varicelloviruses to degrade TAP. However, in the presence of FeHV-1 UL49.5 no degradation of TAP was observed (Fig. 4C).
FIG. 4.
FeHV-1 UL49.5 downregulates MHC-I and strongly inhibits TAP-mediated peptide transport. (A) Surface expression of MHC-I (CRFK and MJS) and MHC-II (MJS) molecules was assessed by flow cytometry on untransduced cells (graph 2) and on cells expressing FeHV-1 UL49.5 (graph 3) using the indicated antibodies. Graph 1, background staining in the presence of secondary antibody only. (B) Transport activity of TAP was analyzed in CRFK and MJS expressing the FeHV-1 UL49.5. Peptide transport was evaluated in the presence of ATP (▪) or EDTA (□). (C) The steady-state levels of TAP1 and GFP in FeHV-1 UL49.5-expressing cells were determined by SDS-PAGE and immunoblotting (IB) with specific antibodies. The β-actin signal was used as a loading control. (D) Immunoblot analysis of ATP-bound (P) or unbound (S) TAP molecules found in CRFK or MJS cells expressing FeHV-1 UL49.5 or EHV-1 UL49.5 (control).
Preventing ATP-binding to TAP is a strategy often exploited by TAP inhibitors, including EHV-1 UL49.5 (30). To investigate whether FeHV-1 UL49.5 uses this strategy, the ATP-binding capacity of TAP was assessed in lysates from untransduced cells and cells stably expressing FeHV-1 or EHV-1 UL49.5. In control CRFK cells, but also in FeHV-1 UL49.5-expressing CRFK cells, TAP1 was detected in the pellet fraction, showing that TAP1 was bound to ATP (Fig. 4D, lanes 1 to 4). Correspondingly, in MJS cells expressing FeHV-1 UL49.5, TAP1 was found in the pellet fractions (Fig. 4D, lanes 5 to 8). In contrast, in the presence of EHV-1 UL49.5, very little TAP1 was found in the pellet fraction, confirming interference with ATP binding to TAP by EHV-1 UL49.5 (Fig. 3D, compare lane 9 to lane 10). These results demonstrate that ATP binding to TAP is not affected by FeHV-1 UL49.5.
Effect of SVV UL49.5 on TAP-mediated peptide transport.
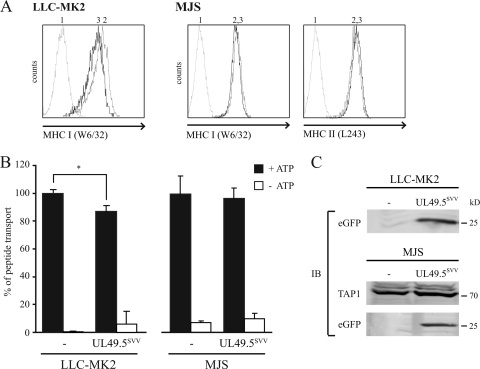
The protein sequences of VZV and SVV UL49.5 display 63% sequence identity (Fig. 1 and Table 2). Previously, we have shown that VZV UL49.5 does not interfere with TAP function (30). To investigate whether the UL49.5 protein of SVV blocks TAP, the viral protein was expressed in a rhesus macaque-derived cell line (LLC-MK2) and in MJS cells. On LLC-MK2 cells expressing SVV UL49.5, MHC-I expression was slightly reduced (Fig. 5 A). This was accompanied by a 15% reduction in peptide transport in these cells (Fig. 5B). SVV UL49.5-induced downregulation of MHC-I expression could not be detected on MJS (Fig. 5A). In accordance with this, TAP inhibition was not detectable in SVV UL49.5-expressing MJS cells (Fig. 5B). eGFP was expressed in both cell lines, indicating proper expression of the SVV UL49.5-encoding transcript (Fig. 5C). Protein steady-state levels of TAP were not affected by SVV UL49.5. Together, these data indicate that SVV UL49.5 has a slight effect on TAP function in rhesus cells.
FIG. 5.
SVV-encoded UL49.5 does not affect TAP function. Surface expression of MHC-I (LLC-MK2 and MJS) and MHC-II (MJS) molecules was assessed by flow cytometry on untransduced cells (graph 2) and SVV UL49.5-expressing cells (graph 3) with the indicated antibodies. Graph 1, secondary antibody only. (B) Transport activity of TAP was analyzed in LLC-MK2 and MJS expressing SVV UL49.5. Peptide transport was evaluated in the presence of ATP (▪) or EDTA (□). *, A difference at P < 0.05 was considered significant. (C) The steady-state levels of TAP1 and GFP in SVV UL49.5-expressing cells were determined by SDS-PAGE and immunoblotting (IB) with specific antibodies.
VZV infection does not result in TAP inhibition.
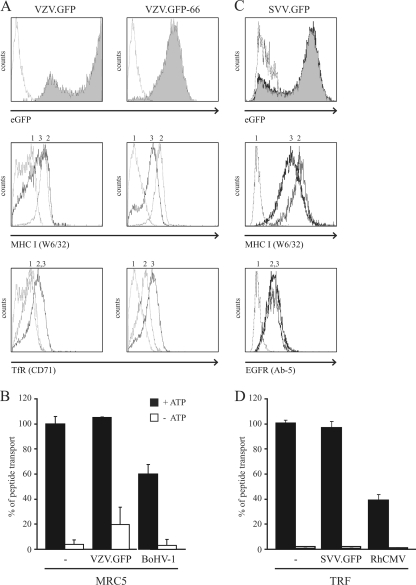
Despite the fact that VZV UL49.5 can be found in association with the TAP complex, TAP inhibition has not been observed (30). A slight reduction in TAP function was observed in the presence of SVV UL49.5 (Fig. 5B). The UL49.5 proteins of VZV and SVV might require one or more other viral gene product(s) to accomplish TAP inhibition. To investigate this possibility, TAP function was assessed in cells infected with VZV or SVV. EGFP-expressing viruses allowed us to determine the efficiency of infection by flow cytometry. For VZV, two different recombinant viruses were used. In one virus, eGFP is inserted between ORF65 and ORF66 (VZV.GFP) (34). In the second virus, eGFP is fused to the N terminus of the ORF66 protein (VZV.GFP-66) (11). MRC-5 cells were infected with VZV.GFP and VZV.GFP-66 at a ratio of 4:1 (uninfected to VZV-infected cells) for 88 h, after which the efficiency of infection was found to be nearly 100% (Fig. 6 A, upper panels). Next, cell surface levels of MHC-I and transferrin receptor (TfR) were determined on mock- and VZV-infected MRC5 cells. A moderate downregulation of MHC-I was observed with VZV-infected cells, whereas the TfR levels were upregulated (Fig. 6A, middle and lower panels). These results are in line with previous observations (8, 11). Subsequently, peptide transport was assessed in VZV.GFP-infected cells. As a positive control, MRC-5 cells were infected with BoHV-1. Infection was verified by eGFP expression (VZV) or by cell surface staining using a BoHV-1 gB-specific antibody (data not shown). TAP function was not affected in VZV.GFP-infected MRC-5 cells (Fig. 6B). In contrast, BoHV-1 infection resulted in a 40% reduction of peptide transport (Fig. 6B), which is in accordance with previous observations (29). The experiment was repeated and confirmed in MRC-5 cells infected with VZV.GFP-66 (data not shown).
FIG. 6.
VZV and SVV infection do not result in reduced TAP function. (A) MRC5 cells were either mock infected (graph 2) or infected with VZV.GFP or VZV.GFP-66 (graph 3) for 88 h and then analyzed by flow cytometry to determine the expression of eGFP and the cell surface expression of MHC-I molecules and TfR using the indicated antibodies. Graph 1, secondary antibody only. (B) MRC5 cells were mock and VZV.GFP infected for 72 h, after which peptide transport was assessed. As a control for TAP inhibition, cells were infected with BoHV-1 for 3 h at an MOI of 20. The transport activity of TAP was analyzed in the presence of ATP (▪) or EDTA (□). (C) TRF cells were mock infected (graph 2) or SVV infected (graph 3) for 72 h. eGFP expression and cell surface expression of MHC-I and EGFR were determined via flow cytometry. (D) TRF cells were mock or SVV infected, and at 72 hpi the TAP activity was determined. As a control for TAP inhibition, the TAP activity was also determined in cells that were infected with RhCMV for 36 h at an MOI of 1.
The same experiments were performed with an SVV.eGFP recombinant (with eGFP inserted into the US region) (36). Infection of telomerized rhesus fibroblasts (TRF) was monitored via eGFP expression by using flow cytometry. The infection efficiency was found to be ca. 95% at 72 hpi (Fig. 6C, upper panel). Comparing MHC-I and epidermal growth factor receptor (EGFR) cell surface expression on mock- and SVV-infected cells revealed a specific downregulation for MHC-I on SVV-infected cells (Fig. 6C, middle panel). TAP function was assessed in mock-, SVV-, and (as a positive control) rhesus CMV (RhCMV)-infected cells (Fig. 6D). Compared to mock-infected cells, peptide transport appeared to be unaffected in SVV-infected cells, whereas infection with RhCMV resulted in a 60% reduction.
These data imply that the VZV- and SVV-induced downregulation of MHC-I does not result from a block of TAP-mediated peptide transport.
UL49.5 of MDV-1 is not responsible for MHC-I downregulation by this virus.
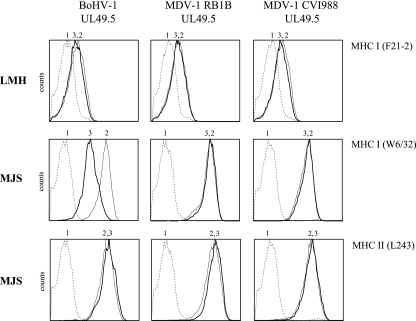
MDV-1 is known to reduce MHC-I cell surface expression levels on infected cells (18, 26). To assess whether MDV-1 UL49.5 is responsible for this phenomenon, we transiently expressed chicken hepatoma cells (LMH) and MJS cells with plasmids coding for the UL49.5 homologs of the MDV-1 strains RB1B and CVI988. BoHV-1 UL49.5 was used as a control. At 48 h posttransfection, the cell surface expression of MHC-I and, for MJS, MHC-II was analyzed. None of the UL49.5 proteins were able to reduce MHC-I cell surface expression on LMH cells (Fig. 7). In contrast, on MJS cells, BoHV-1 UL49.5 induced a reduction of MHC-I that was not observed for either MDV-1 homolog. The reduction by BoHV-1 UL49.5 was specific, since the cell surface expression of MHC-II was not affected.
FIG. 7.
The UL49.5 proteins of MDV-1 RB1B and CVI988 do not affect MHC-I surface expression. BoHV-1, MDV-1 RB1B, and MDV-1 CVI988 UL49.5 were transiently expressed in LMH and MJS cells. The cell surface expression levels of MHC-I and MHC-II molecules were assessed by flow cytometry on cells transfected with a control plasmid (graph 2) or with the UL49.5-expressing plasmids (graph 3) with specific antibodies. Graph 1, secondary antibody only.
In addition, baculoviruses were used to express BoHV-1 UL49.5 and MDV-1 CVI988 UL49.5 in MJS cells. Although BoHV-1 UL49.5-expressing cells displayed evident MHC-I downregulation, a reduction of MHC-I cell surface levels could not be detected in cells with MDV-1 UL49.5 (see Fig. S1A in the supplemental material). Also, TAP function was not affected by MDV-1 UL49.5, whereas BoHV-1 UL49.5 reduced peptide transport by 40% in the same assay (see Fig. S1B in the supplemental material).
These two independent experiments indicate that MDV-1 UL49.5 does not affect the transport of peptides via TAP.
TAP function is not affected by ILTV UL49.5.
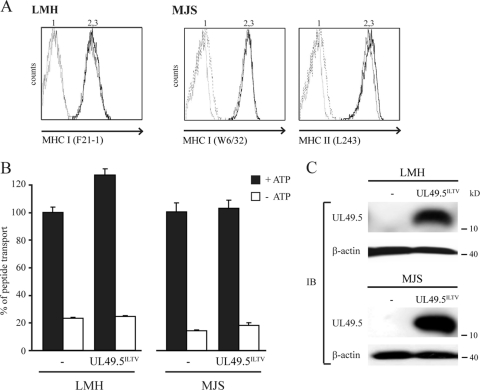
To investigate whether ILTV UL49.5 interferes with peptide transport, the protein was expressed in LMH or MJS cells by retroviral transduction. Analysis of MHC-I cell surface levels on UL49.5-expressing cells did not reveal ILTV UL49.5-induced downregulation (Fig. 8 A). In accordance with this, reduced TAP function was not detected in ILTV UL49.5-expressing cells (Fig. 8B). Proper expression of ILTV UL49.5 was demonstrated using a specific antibody (Fig. 8C). Together, these results indicate that ILTV UL49.5 does not function as a TAP inhibitor.
FIG. 8.
ILTV-encoded UL49.5 does not affect TAP function. (A) Surface expression of MHC-I (LMH and MJS) and MHC-II (MJS) molecules was assessed by flow cytometry on untransduced cells (graph 2) and ILTV UL49.5-expressing cells (graph 3) with the indicated antibodies. Graph 1, secondary antibody only. (B) Transport activity of TAP was analyzed in LMH and MJS expressing ILTV UL49.5. Peptide transport was evaluated in the presence of ATP (▪) or EDTA (□). (C) The steady-state levels of UL49.5 (MJS and LMH) and TAP1 (MJS) were determined by using SDS-PAGE and immunoblotting (IB) with the indicated antibodies. The β-actin signal was used as a loading control.
DISCUSSION
Herpesviruses employ many strategies to avoid elimination by the host immune system (20). A strategy often exploited by these viruses is the inhibition of TAP-mediated peptide transport, illustrating the key role of this process for immunity against these viruses. UL49.5 proteins of varicelloviruses have been identified as a new class of TAP inhibitors that now includes the UL49.5 homologs encoded by BoHV-1, BoHV-5, BuHV-1, CvHV-1, PRV, EHV-1, EHV-4, and FeHV-1 (28, 30; the present study). SVV UL49.5 was found to have a minor effect on TAP-mediated peptide transport, whereas VZV UL49.5 did not block TAP at all, despite its observed interaction with the complex (30). No TAP inhibition was found after infection with VZV or SVV, suggesting that no other viral proteins contribute to UL49.5-mediated TAP inhibition or exhibit this capacity themselves. Expression of the UL49.5 proteins encoded by alphaherpesviruses belonging to the genera Mardivirus (MDV-1) and Iltovirus (ILTV) did not result in reduced MHC-I expression or inhibition of TAP function, suggesting that TAP inhibition is a property unique to UL49.5 proteins encoded by varicelloviruses.
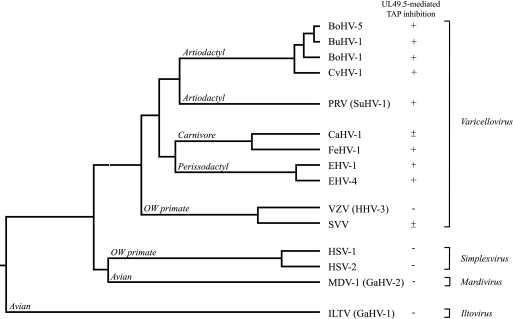
Within the genus Varicellovirus, clusters of closely related herpesviruses can be identified (Fig. 9). Varicelloviruses infecting ruminants, including BoHV-1, BoHV-5, BuHV-1, and CvHV-1, evolved in close proximity (46) and share a high degree of sequence identity (see Table 2; the homologies between BoHV-1 and BoHV-5, BuHV-1 and CvHV-1 are 79, 80, and 74%, respectively). Here, we show that the UL49.5 proteins of these viruses affect TAP to the same extent and, most probably, via the same mechanism. BoHV-1 UL49.5 renders the transporter in a translocation-incompetent state and induces the degradation of both TAP subunits. Correspondingly, the UL49.5 proteins of BoHV-5, BuHV-1, and CvHV-1 also induce the degradation of TAP. Thus, the strategy to inhibit TAP seems conserved within the cluster of varicelloviruses of ruminants.
FIG. 9.
Phylogenetic tree of a selection of alphaherpesviruses based on gB sequences. The tree has been constructed by using the CLUSTAL W (slow/accurate, Gonnet) method of the alignment program MegAlign 500 of the sequence analysis software of DNAStar, Inc. Genus and species nomenclature were previously described by A. Davison et al. (10). BoHV-5 gB, bovine herpesvirus 5 (AAD46112.2); BuHV-1 gB, bubaline herpesvirus 1 (AAL88794.1); BoHV-1 gB, bovine herpesvirus 1 (P12640.1); CvHV-1 gB, cervid herpesvirus 1 (AAD46115.2); PRV/SuHV-1 gB, pseudorabies virus (ACT78489.1); CaHV-1 gB, canid herpesvirus 1 (AAK51052.1); FeHV-1 gB, felid herpesvirus 1 (YP_003331552.2); EHV-1 gB, equid herpesvirus 1 (YP_053078.1); EHV-4 gB, equid herpesvirus 4 (P17472.1); VZV/HHV3 gB, varicella-zoster virus (AAP32845.1); SVV gB, simian varicella virus (NC_002686.2); HSV-1 gB, herpes simplex virus 1 (ABM66851.1); HSV-2 gB, herpes simplex virus 2 (AAA60540.1); MDV-1/GaHV-2 gB, Marek's disease virus 1 (BAA02866); ILTV/GaHV-1 gB, infectious laryngotracheitis virus (CAA39573.1). OW, Old World.
As displayed in Fig. 1, regions of homology between all UL49.5 proteins are limited and scattered throughout the sequence. This makes it difficult to locate domains or individual amino acid residues that might be responsible for TAP inhibition. Thus far, the only exception is the UL49.5-mediated degradation of TAP, which appears to be related to defined amino acid residues within the cytoplasmic tail of the UL49.5 protein (28). Preliminary data suggest that the conserved RGRG sequence at the C termini of BoHV-1, BoHV-5, BuHV-1, and CvHV-1 UL49.5 is involved in the degradation process (M.C. Verweij, A. D. Lipińska, J. Koch [Georg-Speyer-Haus, Frankfurt, Germany], and E. J. H. J. Wiertz, unpublished data).
CaHV-1 and FeHV-1, both infecting carnivores, are genetically and serologically related (16). As depicted in Fig. 9, these viruses are thought to have a common ancestor. The UL49.5 proteins of these viruses share 54% sequence identity (Table 2). Earlier, we showed that CaHV-1 UL49.5 has a minor effect on TAP function in canine cells (30). Surprisingly, in the present study, FeHV-1 UL49.5 was identified as a potent inhibitor of feline TAP and, to a lesser extent, of human TAP. FeHV-1 and CaHV-1 are related to EHV-1 and EHV-4, herpesviruses infecting odd-toed ungulates or Perissodactyla (Fig. 9). The UL49.5 homologs encoded by these viruses were previously shown to strongly inhibit TAP (30). Hence, in this cluster of related varicelloviruses, CaHV-1 appears to be the odd one out, coding for a UL49.5 protein that only moderately affects TAP. This UL49.5 homolog might have lost the capacity to strongly inhibit TAP.
Previously, it has been shown that EHV-1 UL49.5 arrests the transporter in a translocation-incompetent state that is incompatible with ATP binding (30). In the presence of FeHV-1 UL49.5, TAP was still capable of binding ATP. Thus, the mechanism through which FeHV-1 UL49.5 inhibits TAP differs from that utilized by the related EHV-1 and EHV-4 UL49.5 proteins. Similar to FeHV-1 UL49.5, BoHV-1 and PRV UL49.5 do not interfere with ATP binding to TAP (30). Presumably, differences in the capacity to inhibit ATP binding result from slightly different conformational changes in TAP that are induced by these proteins.
VZV and SVV belong to the evolutionary cluster of varicelloviruses that infect Old World primates (Fig. 9). Despite an observed interaction between TAP and VZV UL49.5, this protein does not inhibit TAP (30). SVV UL49.5 moderately affects peptide transport in rhesus macaque cells. Possibly, the UL49.5 proteins of these viruses have lost the capacity to (strongly) interfere with TAP function or, alternatively, have never (fully) acquired this property. This somewhat unique situation among varicelloviruses might be related to the evolutionary position of these viruses, which seem to have separated from the other varicelloviruses at a relatively early stage (Fig. 9).
Cells infected with VZV display reduced expression of MHC-I at the cell surface (2, 8, 11). The VZV ORF66-encoded serine-threonine protein kinase has been shown to contribute to the observed downregulation (2, 11), but additional proteins appear to be involved (11). We did not observe reduced TAP function in VZV-infected cells, indicating that VZV does not code for a TAP inhibitor. This finding is rather surprising, since most of the alphaherpesviruses encode a TAP inhibitor (20). The isolated overexpression of the SVV UL49.5 protein resulted in a slightly reduced TAP activity; however, this inhibition was not observed in SVV-infected cells. Therefore, it is unlikely that the MHC-I downregulation observed in SVV-infected cells is caused by inhibition of TAP by UL49.5 or other SVV gene products.
All MDV serotypes, including MDV-1, MDV-2, and Meleagrid herpesvirus 1 or Turkey herpesvirus (HVT), reduce MHC-I expression on infected cells (26). Highly virulent strains of MDV-1, such as RB1B, cause a stronger MHC-I downregulation than less virulent strains or vaccine strains such as CVI988 (18). Nevertheless, downregulation of MHC-I was not detectable in chicken and human cells expressing the UL49.5 proteins of RB1B and CVI988. In addition, MDV-1 CVI988 UL49.5 did not detectably affect TAP-mediated peptide transport in human cells. The UL49.5 protein of ILTV did not reduce TAP-mediated peptide transport either. ILTV and the recently identified psittacid herpesvirus 1 (PsHV-1) are the only known iltoviruses (38). These viruses have been classified as members of the Alphaherpesvirinae (10) but are distantly related to all other members (Fig. 9). Similarly, MDV-1 branched away from the varicelloviruses and simplexviruses relatively early in evolution (Fig. 9). Given their distant relatedness, ILTV and MDV-1 probably evolved genes other than UL49.5 to circumvent MHC-I-mediated antigen presentation.
In conclusion, TAP-inhibiting UL49.5 proteins have been identified uniquely in varicelloviruses, which now includes the viruses BoHV-1, BoHV-5, BuHV-1, CvHV-1, PRV, EHV-1, EHV-4, and FeHV-1. These UL49.5 homologs appear to have acquired a function in immune evasion, in addition to their common function as a molecular chaperone for gM that seems to be conserved among all herpesviruses. Possibly, this dual function relies on a hitherto-unidentified structural similarity between TAP and gM.
Despite the conserved function of the TAP-inhibiting UL49.5 proteins, their sequence identity appears to be strikingly low. For example, the identity between the strong TAP inhibitors BoHV-1 and EHV-1 UL49.5 is only 30%. In contrast, the non-TAP-inhibiting mardivirus MDV-1 UL49.5 shares 25% sequence identity with BoHV-1 UL49.5. The sequence identity between CaHV-1 and FeHV-1 UL49.5 is 54%, but the latter protein inhibits TAP much more strongly than the former. FeHV-1 UL49.5 displays a functional resemblance to the BoHV-1, EHV-1, and PRV proteins, with which the sequence similarity is only ca. 30%. Thus, the homology between UL49.5 proteins is not indicative of the capacity to inhibit TAP and, as suggested earlier, the different clades of TAP-inhibiting homologs may have unique sequences that are mediating this function.
The UL49.5 homologs of the simplexviruses HSV-1 and HSV-2, the mardivirus MDV-1, the iltovirus ILTV, the betaherpesvirus HCMV, and the gammaherpesvirus EHV have no effect on TAP function (28). However, within all three subfamilies other potent TAP inhibitors have been identified (e.g., ICP47 in HSV-1 and -2, US6 in HCMV, and BNLF2a in EBV), making TAP inhibition by herpesviruses a striking example of functional convergent evolution.
Supplementary Material
Acknowledgments
This study was supported by a grant from The Macropa Foundation, Leiden, Netherlands (M.C.V.), the START program of the Foundation for Polish Science (A.D.L.), the intramural research program of the National Institute of Allergy and Infectious Diseases (J.I.C.), grants NS064022 and EY08098 of the National Institute of Health (P.R.K.), grant R01 AG037042-01 from the National Institutes of Health (I.M.), and NWO Vidi grant 917.76.330 from the Netherlands Scientific Organization (M.E.R.).
We thank Femke Walraven-Berkhoff, Edwin Quinten, Guido de Roo, and Menno van der Hoorn for their technical support.
Footnotes
Published ahead of print on 15 December 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abele, R., and R. Tampe. 1999. Function of the transport complex TAP in cellular immune recognition. Biochim. Biophys. Acta 1461:405-419. [DOI] [PubMed] [Google Scholar]
- 2.Abendroth, A., I. Lin, B. Slobedman, H. Ploegh, and A. M. Arvin. 2001. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J. Virol. 75:4878-4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, K., et al. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. [DOI] [PubMed] [Google Scholar]
- 4.Ahn, K., et al. 1996. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 15:3247-3255. [PMC free article] [PubMed] [Google Scholar]
- 5.Aisenbrey, C., et al. 2006. Structure and dynamics of membrane-associated ICP47, a viral inhibitor of the MHC I antigen-processing machinery. J. Biol. Chem. 281:30365-30372. [DOI] [PubMed] [Google Scholar]
- 6.Amills, M., V. Ramiya, J. Norimine, and H. A. Lewin. 1998. The major histocompatibility complex of ruminants. Rev. Sci. Technol. 17:108-120. [DOI] [PubMed] [Google Scholar]
- 7.Barnstable, C. J., et al. 1978. Production of monoclonal antibodies to group A erythrocytes, HLA, and other human cell surface antigens-new tools for genetic analysis. Cell 14:9-20. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, J. I. 1998. Infection of cells with varicella-zoster virus downregulates surface expression of class I major histocompatibility complex antigens. J. Infect. Dis. 177:1390-1393. [DOI] [PubMed] [Google Scholar]
- 9.D'Arce, R. C., et al. 2002. Restriction endonuclease and monoclonal antibody analysis of Brazilian isolates of bovine herpesviruses types 1 and 5. Vet. Microbiol. 88:315-324. [DOI] [PubMed] [Google Scholar]
- 10.Davison, A. J., et al. 2009. The order Herpesvirales. Arch. Virol. 154:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisfeld, A. J., M. B. Yee, A. Erazo, A. Abendroth, and P. R. Kinchington. 2007. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J. Virol. 81:9034-9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruh, K., et al. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415-418. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs, W., and T. C. Mettenleiter. 1996. DNA sequence and transcriptional analysis of the UL1 to UL5 gene cluster of infectious laryngotracheitis virus. J. Gen. Virol. 77:2221-2229. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, W., and T. C. Mettenleiter. 2005. The nonessential UL49.5 gene of infectious laryngotracheitis virus encodes an O-glycosylated protein which forms a complex with the non-glycosylated UL10 gene product. Virus Res. 112:108-114. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Borges, C. N., B. Phanavanh, and M. D. Crew. 2006. Characterization of porcine TAP genes: alternative splicing of TAP1. Immunogenetics 58:374-382. [DOI] [PubMed] [Google Scholar]
- 16.Gaskell, R., and K. Willoughby. 1999. Herpesviruses of carnivores. Vet. Microbiol. 69:73-88. [DOI] [PubMed] [Google Scholar]
- 17.Gaskell, R. M., and R. C. Povey. 1979. The dose response of cats to experimental infection with feline viral rhinotracheitis virus. J. Comp. Pathol. 89:179-191. [DOI] [PubMed] [Google Scholar]
- 18.Gimeno, I. M., et al. 2001. Marek's disease virus infection in the brain: virus replication, cellular infiltration, and major histocompatibility complex antigen expression. Vet. Pathol. 38:491-503. [DOI] [PubMed] [Google Scholar]
- 19.Halenius, A., et al. 2006. Physical and functional interactions of the cytomegalovirus US6 glycoprotein with the transporter associated with antigen processing. J. Biol. Chem. 281:5383-5390. [DOI] [PubMed] [Google Scholar]
- 20.Hansen, T. H., and M. Bouvier. 2009. MHC class I antigen presentation: learning from viral evasion strategies. Nat. Rev. Immunol. 9:503-513. [DOI] [PubMed] [Google Scholar]
- 21.Hengel, H., et al. 1997. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity 6:623-632. [DOI] [PubMed] [Google Scholar]
- 22.Hewitt, E. W., S. S. Gupta, and P. J. Lehner. 2001. The human cytomegalovirus gene product US6 inhibits ATP binding by TAP. EMBO J. 20:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill, A., et al. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 24.Hislop, A. D., et al. 2007. A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J. Exp. Med. 204:1863-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horst, D., et al. 2009. Specific targeting of the EBV lytic phase protein BNLF2a to the transporter associated with antigen processing results in impairment of HLA class I-restricted antigen presentation. J. Immunol. 182:2313-2324. [DOI] [PubMed] [Google Scholar]
- 26.Hunt, H. D., et al. 2001. Marek's disease virus downregulates surface expression of MHC (B complex) class I (BF) glycoproteins during active but not latent infection of chicken cells. Virology 282:198-205. [DOI] [PubMed] [Google Scholar]
- 27.Klupp, B. G., R. Nixdorf, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein M inhibits membrane fusion. J. Virol. 74:6760-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koppers-Lalic, D., et al. 2005. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc. Natl. Acad. Sci. U. S. A. 102:5144-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koppers-Lalic, D., et al. 2003. Bovine herpesvirus 1 interferes with TAP-dependent peptide transport and intracellular trafficking of MHC class I molecules in human cells. Arch. Virol. 148:2023-2037. [DOI] [PubMed] [Google Scholar]
- 30.Koppers-Lalic, D., et al. 2008. Varicellovirus UL49.5 proteins differentially affect the function of the transporter associated with antigen processing, TAP. PLoS Pathog. 4:e1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehner, P. J., J. T. Karttunen, G. W. Wilkinson, and P. Cresswell. 1997. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl. Acad. Sci. U. S. A. 94:6904-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehner, P. J., and J. Trowsdale. 1998. Antigen presentation: coming out gracefully. Curr. Biol. 8:R605-R608. [DOI] [PubMed] [Google Scholar]
- 33.Levy, A. M., I. Davidson, S. C. Burgess, and H. E. Dan. 2003. Major histocompatibility complex class I is downregulated in Marek's disease virus infected chicken embryo fibroblasts and corrected by chicken interferon. Comp. Immunol. Microbiol. Infect. Dis. 26:189-198. [DOI] [PubMed] [Google Scholar]
- 34.Li, Q., M. A. Ali, and J. I. Cohen. 2006. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell 127:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipinska, A. D., et al. 2006. Bovine herpesvirus 1 UL49.5 protein inhibits the transporter associated with antigen processing despite complex formation with glycoprotein M. J. Virol. 80:5822-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahalingam, R., et al. 1998. Infectious simian varicella virus expressing the green fluorescent protein. J. Neurovirol. 4:438-444. [DOI] [PubMed] [Google Scholar]
- 37.McCluskey, J., J. Rossjohn, and A. W. Purcell. 2004. TAP genes and immunity. Curr. Opin. Immunol. 16:651-659. [DOI] [PubMed] [Google Scholar]
- 38.McGeoch, D. J., F. J. Rixon, and A. J. Davison. 2006. Topics in herpesvirus genomics and evolution. Virus Res. 117:90-104. [DOI] [PubMed] [Google Scholar]
- 39.Messaoudi, I., et al. 2009. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella-zoster virus infection in humans. PLoS Pathog. 5:e1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer, T. H., P. M. van Endert, S. Uebel, B. Ehring, and R. Tampe. 1994. Functional expression and purification of the ABC transporter complex associated with antigen processing (TAP) in insect cells. FEBS Lett. 351:443-447. [DOI] [PubMed] [Google Scholar]
- 41.Montagnaro, S., et al. 2009. Feline herpesvirus-1 downregulates MHC class I expression in an homologous cell system. J. Cell Biochem. 106:179-185. [DOI] [PubMed] [Google Scholar]
- 42.Ohta, Y., et al. 2003. Two highly divergent ancient allelic lineages of the transporter associated with antigen processing (TAP) gene in Xenopus: further evidence for co-evolution among MHC class I region genes. Eur. J. Immunol. 33:3017-3027. [DOI] [PubMed] [Google Scholar]
- 43.Osterrieder, N., et al. 1996. The equine herpesvirus 1 glycoprotein gp21/22a, the herpes simplex virus type 1 gM homolog, is involved in virus penetration and cell-to-cell spread of virions. J. Virol. 70:4110-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers, C. J., and K. Fruh. 2008. Signal peptide-dependent inhibition of MHC class I heavy chain translation by rhesus cytomegalovirus. PLoS Pathog. 4:e1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.St George, T. D., and M. Philpott. 1972. Isolation of infectious bovine rhinotracheitis virus from the prepuce of water buffalo bulls in Australia. Aust. Vet. J. 48:126. [DOI] [PubMed] [Google Scholar]
- 46.Thiry, J., et al. 2006. Ruminant alphaherpesviruses related to bovine herpesvirus 1. Vet. Res. 37:169-190. [DOI] [PubMed] [Google Scholar]
- 47.Tomazin, R., et al. 1996. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 15:3256-3266. [PMC free article] [PubMed] [Google Scholar]
- 48.Tomazin, R., et al. 1998. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J. Virol. 72:2560-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Born, E., C. C. Posthuma, K. Knoops, and E. J. Snijder. 2007. An infectious recombinant equine arteritis virus expressing green fluorescent protein from its replicase gene. J. Gen. Virol. 88:1196-1205. [DOI] [PubMed] [Google Scholar]
- 50.Wu, S. X., X. P. Zhu, and G. J. Letchworth. 1998. Bovine herpesvirus 1 glycoprotein M forms a disulfide-linked heterodimer with the U(L)49.5 protein. J. Virol. 72:3029-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.