Abstract
Over the past decade, a family of host proteins known as suppressors of cytokine signaling (SOCS) have emerged as frequent targets of viral exploitation. Under physiologic circumstances, SOCS proteins negatively regulate inflammatory signaling pathways by facilitating ubiquitination and proteosomal degradation of pathway machinery. Their expression is tightly regulated to prevent excessive inflammation while maintaining protective antipathogenic responses. Numerous viruses, however, have developed mechanisms to induce robust host SOCS protein expression following infection, essentially “hijacking” SOCS function to promote virus survival. To date, SOCS proteins have been shown to inhibit protective antiviral signaling pathways, allowing viruses to evade the host immune response, and to ubiquitinate viral proteins, facilitating intracellular viral trafficking and progeny virus assembly. Importantly, manipulation of SOCS proteins not only facilitates progression of the viral life cycle but also powerfully shapes the presentation of viral disease. SOCS proteins can define host susceptibility to infection, contribute to peripheral disease manifestations such as immune dysfunction and cancer, and even modify the efficacy of therapeutic interventions. Looking toward the future, it is clear that a better understanding of the role of SOCS proteins in viral diseases will be essential in our struggle to modulate and even eliminate the pathogenic effects of viruses on the host.
Viruses possess a compact genome that is only sufficient to encode the most essential viral proteins. Therefore, viruses must rely on the host cell to supply a number of additional proteins that are required for completion of the viral life cycle in order to establish a productive infection. For example, viruses may require the use of host cell surface receptors to enter a cell, DNA polymerases to replicate the viral genome, RNA polymerases to transcribe viral genes, and translational machinery to produce viral proteins, as well as various other cellular proteins to enhance intracellular trafficking of viral components and to evade immune detection. Determining the requirements for these cellular factors contributes not only to our basic knowledge of the viral life cycle but also to our understanding of viral disease. Viral dependency on a particular cellular protein can define the host range and cellular tropism, contribute to the development of virus-associated pathology, or even offer new therapeutic strategies. Considering the current obstacles to successful treatment of many viral illnesses, including human immunodeficiency virus type 1 (HIV-1) infection, identification of new targets cannot be overlooked.
The suppressors of cytokine signaling (SOCS) family has recently been identified as a group of host proteins that can be exploited for viral benefit. Members of the SOCS family are induced upon infection by a number of different viruses, including HIV-1, hepatitis C virus (HCV), hepatitis B virus (HBV), herpes simplex virus type 1 (HSV-1), respiratory syncytial virus (RSV), Ebola virus, influenza A virus, and coxsackievirus, and subsequently contribute to viral replication and pathogenesis. This review will focus on the virally exploited functions of SOCS proteins, as well as on the consequences of these functions for viral disease and therapy.
SOCS PROTEIN STRUCTURE AND FUNCTION
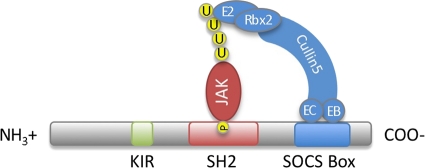
The SOCS family of proteins contains eight members, the cytokine-inducible SH2 domain-containing protein (CIS) and SOCS1 to SOCS7. Each contains a central SH2 domain, a C-terminal SOCS box, and an N-terminal domain of various lengths and compositions (Fig. 1) (78). The SH2 domain determines the target of each SOCS protein by binding specific phosphorylated tyrosine residues on its preferred substrate. Once bound, the SOCS box can interact with a complex containing Elongins B and C, Cullin5, and RING-box-2 to form an E3 ubiquitin ligase. By bringing the SH2-bound substrate into close proximity with ubiquitinating machinery, SOCS proteins facilitate the ubiquitination of target proteins, marking them for degradation via the proteosome (51). Certain SOCS proteins also harbor an additional effector domain in their N termini. SOCS1 and SOCS3 contain a kinase inhibitory region (KIR) that functions as a pseudosubstrate to inhibit kinase activity. Collectively, this multifaceted structure provides for a wide and rapidly growing range of biological effects.
FIG. 1.
SOCS protein structure. All SOCS proteins contain a central SH2 domain and a C-terminal SOCS box. The SH2 domain determines the target of each SOCS protein by binding specific phosphorylated (P) tyrosine residues on its preferred substrate (commonly JAK proteins). The SOCS box interacts with ubiquitinating machinery, including Elongin B (EB), Elongin C (EC), Cullin5, RING-box-2 (Rbx2), and an E2 ubiquitin-conjugating enzyme. By bringing the bound substrate into proximity with ubiquitinating machinery, the SOCS protein facilitates ubiquitination (U) of target proteins, thereby marking them for degradation by the proteosome. Certain SOCS proteins also contain an N-terminal kinase inhibitory region (KIR). The KIR is thought to function as a pseudosubstrate to inhibit the kinase activity of proteins that are bound by, or in close proximity to, the SOCS protein.
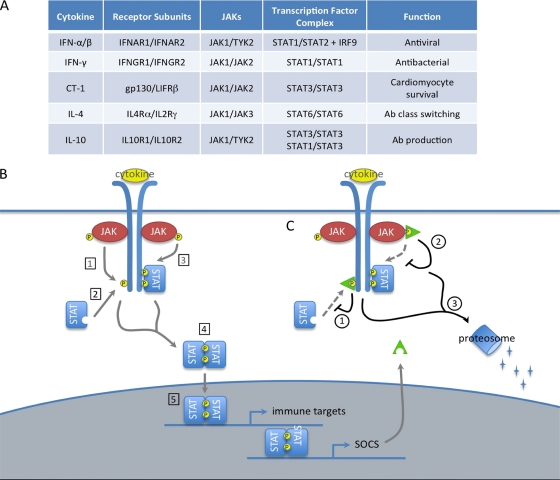
The most well-described function of SOCS proteins is the negative regulation of the JAK/STAT pathway (Fig. 2). In this pathway, cytokine stimulation of cell surface receptors activates receptor-associated tyrosine kinases known as Janus kinases (JAKs), which subsequently phosphorylate the receptor cytoplasmic domain and lead to the recruitment of signal transducers and activators of transcription (STATs). Recruited STATs are then activated by JAK phosphorylation, allowing them to dimerize and enter the nucleus to induce the transcription of target genes. SOCS proteins regulate this pathway in a family member-specific manner, by binding specific substrates within the JAK/STAT signaling receptor complex and terminating pathway activation in one of three ways: (i) SOCS box-mediated ubiquitination and degradation of bound receptor components, (ii) competition with recruited STAT proteins for shared phosphotyrosine residues, or (iii) KIR-mediated inhibition of JAK activity (78). SOCS1 and SOCS3 are thought to bind phosphotyrosine residues within the activation loop of the JAK and/or nearby residues on the receptor cytoplasmic domain and inhibit signal transduction through either their KIRs or their SOCS boxes. In contrast, CIS and SOCS2 are thought to bind phosphotyrosine residues on the cytoplasmic domain of the receptor and compete with, or sterically hinder, the binding of recruited STATs. Certain SOCS proteins have also been reported to regulate additional signal transduction pathways, although these functions have been less well described. SOCS1 can inhibit the NF-κB pathway by binding the NF-κB subunit p65 (55) or one of the upstream signaling components, MAL (36) or IRAK (14, 42), and targeting them for ubiquitination and degradation by the proteosome. SOCS1 can also induce the proteosomal degradation of ASK1 (25), an upstream activator of JNK and p38, thereby inhibiting these arms of the MAPK pathway. SOCS3 can inhibit both the NF-κB and JNK/p38 pathways by binding the upstream signaling molecule TRAF6 and preventing its association with, and activation of, TAK1 (20). Together, these SOCS protein functions limit excessive pathway activation and shape complex signaling responses.
FIG. 2.
SOCS proteins negatively regulate the JAK/STAT pathway. (A) Composition of selected JAK/STAT pathways. (B) Function of JAK/STAT pathway. Cytokine stimulation of cell surface receptors activates receptor-associated JAK proteins by phosphorylation (P). Activated JAKs phosphorylate receptor cytoplasmic domains (1), which leads to the recruitment of cytoplasmic STAT proteins (2). Recruited STATs are activated by JAK phosphorylation (3), allowing them to dimerize (4) and enter the nucleus as a transcription factor complex to induce the expression of target genes. Gene targets often include both immune effectors and SOCS proteins. (C) SOCS proteins. SOCS proteins negatively regulate this pathway by binding specific substrates within the JAK/STAT receptor complex and terminating pathway activation in one of the following ways: competition with recruited STAT proteins for shared phosphotyrosine residues (1), kinase inhibitory region (KIR)-mediated inhibition of JAK activity (2), or SOCS box-mediated ubiquitination and degradation of bound receptor components (3).
As a critical regulator of multiple signal transduction pathways, SOCS protein expression is carefully regulated in a cell type- and stimulus-specific manner (32). SOCS proteins are most classically induced by cytokine stimulation of the JAK/STAT pathway (Fig. 2B), creating a negative feedback loop to limit excessive inflammation (Fig. 2C). However, their expression can also be induced following activation of the NF-κB or MAPK pathway and in response to a variety of stimuli, including lipopolysaccharide (LPS), tumor necrosis factor alpha (TNF-α), isoproterenol, and statins. In most cases, transcriptional activation is the sole mechanism for enhancing SOCS levels, although TNF-α has been shown to promote SOCS3 mRNA stability (15). Additionally, the short half-life of SOCS proteins (typically 1 to 2 h) can be altered by phosphorylation (21) or ubiquitination (58) and by association with the serine/threonine kinase PIM1 (9, 48) or other SOCS proteins. By tightly controlling SOCS protein levels, these mechanisms provide for optimal pathway activation in a variety of cellular contexts.
Viruses, however, can independently induce SOCS proteins and exploit their functions to promote viral replication (Table 1). Enhanced SOCS expression can inhibit the normal function of JAK/STAT-regulated pathways, most notably those critical for initiating innate and adaptive antiviral immune responses. SOCS box activity can also be utilized to ubiquitinate viral proteins, thereby facilitating the intracellular trafficking of viral proteins required for progeny virus particle production. These “hijacked” host functions provide significant benefits to viral pathogens and are critical for understanding both viral replication and disease.
TABLE 1.
Viruses induce SOCS proteins and exploit their functions to promote viral replication
| Virus | SOCS protein | Functiona |
|---|---|---|
| Coxsackievirus | SOCS1 | Inhibits IFN-α, IFN-β, and CT-1 signaling |
| SOCS3 | Inhibits CT-1 signaling | |
| Ebola virus | SOCS1 | May ubiquitinate VP40 to enhance progeny virus production |
| HBV | SOCS1 | Inhibits IFN-α production |
| SOCS3 | Inhibits IFN-α production | |
| HCV | SOCS1 | Increased expression in T cells decreases T cell activation and IFN-γ production; decreased expression in B cells increases B cell activation, proliferation, and Ab production |
| SOCS3 | Inhibits IFN-α signaling | |
| HSV-1 | SOCS1 | Inhibits IFN-γ signaling |
| SOCS3 | Inhibits IFN-α and IFN-β signaling and IFN-α production | |
| HIV-1 | SOCS1 | Ubiquitinates HIV-1 Gag to enhance progeny virus production |
| SOCS1/SOCS3 | Inhibits IL-4 to prevent Ab class switching; inhibits IL-10 to prevent Ab production | |
| SOCS2 | Inhibits IFN-γ signaling | |
| SOCS3 | Inhibits IFN-β signaling | |
| Influenza virus | SOCS3 | Inhibits IFN-β signaling |
| RSV | CIS | Inhibits IFN-α signaling |
| SOCS1 | Inhibits IFN-α signaling | |
| SOCS3 | Inhibits IFN-α signaling |
Ab, antibody.
VIRALLY EXPLOITED FUNCTIONS OF SOCS PROTEINS
JAK/STAT pathway inhibition provides for viral immune evasion.
Viral infection results in a robust and multifaceted host immune response. The rapid, nonspecific innate immune response is primarily driven by the virally induced expression of the type I interferons (IFNs) alpha interferon (IFN-α) and IFN-β. Type I IFNs exert their effects by signaling through the JAK/STAT pathway to induce the transcription of multiple antiviral targets (65). Following IFN stimulation of the cell surface receptor subunits IFN-α/β receptor 1 (IFNAR1) and IFNAR2, receptor-associated JAKs (JAK1 and TYK2) recruit and phosphorylate cytoplasmic STAT1 and STAT2. The STAT1-STAT2 heterodimer associates with IFN-regulatory factor 9 (IRF9) to form IFN-stimulated gene factor 3 (ISGF3), which enters the nucleus and binds IFN-stimulated regulatory elements (ISRE) in the promoters of hundreds of antiviral IFN-stimulated genes (ISGs) to induce their expression (Fig. 2A). Some of the most well-studied antiviral ISGs include the double-stranded-RNA-dependent protein kinase (PKR), which inhibits viral protein translation, 2′,5′-oligoadenylate synthetase (OAS), which results in viral RNA cleavage, and the Mx proteins, which interfere with viral transcription. Collectively, ISGs have been shown to inhibit every stage of viral replication, from viral entry and uncoating to assembly and release, providing the host with formidable protection against viral infection.
Following the innate immune response is the slower, antigen-specific adaptive immune response. Adaptive immunity to viral infection requires the balanced effects of both cytotoxic T lymphocytes (CTLs; CD8+ T cells) and B cell-generated antibodies, the actions of which are orchestrated by CD4+ T cells (67). CTLs target virally infected cells after recognizing viral antigens presented on the cell surface in the context of major histocompatibility complex class I (MHC-I) molecules. CTLs proliferate in response to antigen recognition and CD4+ T cell help and act to eradicate viruses from these cells either through a lytic mechanism involving perforins and granzymes or through a nonlytic mechanism involving production of the antiviral cytokine IFN-γ. In contrast, B cell-generated antibodies typically target cell-free viruses. B cells that recognize virus-specific antigens proliferate in response to antigen recognition and CD4+ T cell help and generate antibodies capable of binding specific viral epitopes. These virus-specific antibodies can neutralize or promote aggregation of cell-free viruses, preventing further infection of cells, or target virally infected cells for destruction by complement- or natural killer cell-dependent mechanisms. The functions of these two arms of the adaptive immune response complement each other, and both are necessary for effective virus control.
When functioning properly, the innate and adaptive immune systems prevent productive viral infection. In response, viral evolution has favored the acquisition of a number of different immune evasion mechanisms (67). Viruses can prevent IFN production, IFN signaling, or the function of antiviral ISGs, limit the production of CTLs and their ability to recognize virally infected targets, or rapidly mutate epitopes recognized by neutralizing antibodies. Often viruses use multiple methods of immune evasion, highlighting the importance of this function for survival.
(i) Innate immunity.
The obvious potential of SOCS proteins to inhibit the innate immune response to viral infection was recognized only shortly after the initial descriptions of SOCS functions. Through the use of overexpression studies, Song and Shuai determined that SOCS1 and SOCS3, but not SOCS2, could attenuate both type I (IFN-α) and type II (IFN-γ) IFN signaling through the JAK/STAT pathway (63). They showed that exogenously expressed SOCS1 and SOCS3 were sufficient to inhibit not only IFN-induced phosphorylation and nuclear translocation of STAT1 but also the antiviral effect of IFN on vesicular stomatitis virus infection. Much later, the observation that SOCS1 knockout mice were extremely resistant to Semliki Forest virus infection provided confirmation that endogenously expressed SOCS proteins play an important role in regulating innate antiviral immunity. Hertzog and colleagues reported that SOCS1 deficiency resulted in decreased viral load and increased host survival following viral infection, which was reversed in the presence of IFNAR1 deficiency or upon treatment with IFN-α/β-neutralizing antibodies, indicating both that type I IFNs are critical to the endogenous antiviral response and that SOCS1 functions to inhibit them (16). Further studies indicated that SOCS1 exerted these effects, at least in part, by directly binding IFNAR1 to inhibit STAT1 phosphorylation and the expression of the antiviral target gene OAS. Collectively, these studies provided evidence that SOCS proteins are capable of dampening the antiviral effects of IFNs by disrupting JAK/STAT signaling within the host cell.
It was not long before it was recognized that viruses could hijack host SOCS proteins to manipulate antiviral IFN signaling to their advantage. HCV, a small, positive-sense, single-stranded RNA (ssRNA) virus that infects hepatocytes of the liver to cause liver inflammation (hepatitis), was the first virus reported to independently induce SOCS expression. Notably, HCV was already well known for its effective suppression of the host antiviral immune response, as evidenced by its propensity for establishing a chronic, lifelong infection in approximately 80% of cases (5). But the contribution of SOCS proteins to HCV's immune evasion arsenal was not described until 2003 by Bode et al. (6). Their early studies showed that overexpression of the HCV core protein induced SOCS3 expression in HepG2 cells, which correlated with inhibition of IFN-α-induced STAT1 activation, nuclear translocation, and DNA binding (6). The authors further showed that HCV core protein was sufficient to enhance the replication of an experimental influenza virus engineered to lack its own IFN antagonist, suggesting that HCV core protein-induced SOCS3 was capable of inhibiting the antiviral type I IFN response through disruption of the JAK/STAT pathway. Later studies confirmed that SOCS3 expression was also increased in HCV-infected HepG2 cells and in the peripheral lymphocytes of patients infected with HCV compared to those of healthy controls (49). While a number of subsequent studies gave further support to the role of HCV-induced SOCS3 in viral persistence (29, 50), not all reports are consistent. Limited studies indicate that SOCS1 expression, but not SOCS3 expression, is increased in the hepatic tissues of patients with chronic HCV infection (26). Another reports that in OR6 cells and JFH1-infected Huh7.5.1 cells, SOCS3 expression inhibits HCV replication in an mTOR-dependent manner (60). While the factors contributing to these discrepancies are not yet clear, the bulk of the literature continues to suggest that enhanced expression of SOCS proteins during HCV infection dramatically impairs innate antiviral signaling, which contributes not only to the chronicity of HCV infection but also to the decreased response of patients to exogenous IFN therapy, which will be described further below.
HIV-1, a virus which infects CD4+ T cells and monocytes/macrophages, thereby causing devastating immunodeficiency of the host, has also been shown to induce SOCS proteins in order to suppress antiviral innate immunity. HIV-1 is a relatively large retrovirus with a complex immune evasion strategy, including the production of multiple viral accessory proteins (Vif, Nef, Vpu, and Vpr) designed specifically to thwart innate immunity (35). Studies performed in our lab have determined that HIV-1 Tat, a regulatory protein whose primary function is to enhance viral transcription by recruiting transcription factors to the HIV-1 promoter, also contributes to HIV-1 immune evasion by inducing SOCS3 expression (2). HIV-1 Tat-induced SOCS3 attenuated IFN-β signaling in macrophages both upstream, at the level of STAT1 and STAT2 activation, and downstream, at the level of PKR and ISG20 antiviral gene expression. In vitro, SOCS3 expression was sufficient to overcome the inhibitory effect of IFN-β and enhance HIV-1 replication in macrophages, while in vivo, SOCS3 expression in the central nervous systems (CNSs) of simian immunodeficiency virus (SIV)-infected macaques correlated with increases in CNS SIV replication and the onset of CNS disease. These studies suggest that HIV-1 Tat-induced SOCS3 disrupts protective type I IFN signaling within macrophages, allowing for enhanced viral replication and viral pathogenesis. HIV-1 Tat has also been shown to attenuate type II IFN signaling in human monocytes in a SOCS2-dependent manner (10). This finding has significant implications for protection from bacterial and parasitic infections that are common in the context of HIV-1 infection and will be described further below.
HSV-1 is a large DNA virus that chronically infects its hosts through evasion of antiviral immunity and latency (46). HSV-1 initially infects and replicates within keratinocytes of the skin and then subsequently establishes a more long-term, latent infection within adjacent neurons, where it is hidden from immune surveillance. Periodically, HSV-1 will become activated by external stimuli and productively reinfect nearby keratinocytes, resulting in skin-damaging lesions. Interestingly, SOCS proteins have been shown to contribute to HSV-1 immune evasion in multiple ways. SOCS3 is induced by HSV-1 in a number of cell lines through a mechanism that involves the viral tegument proteins UL41 and UL13 and STAT3 (76, 77). In cells which are capable of expressing SOCS3, HSV-1 is sufficient to inhibit IFN-α-induced STAT1 phosphorylation and downstream OAS production and establish a rapidly propagating infection (76). However, in cells that do not express SOCS3 in response to HSV-1, or when SOCS3 expression is inhibited with STAT3 or SOCS3 antagonists, viral replication is attenuated. This attenuation is rescued following treatment with IFN-α/β-neutralizing antibodies, indicating that HSV-1-induced SOCS3 is sufficient to inhibit antiviral type I IFN signaling, thereby enhancing viral replication (76). Notably, in addition to targeting IFN signaling, HSV-1-induced SOCS3 has also been shown to inhibit IFN-α production. During a typical antiviral response, IFN-β is initially expressed and induces the JAK/STAT-dependent production of IRF7. IRF7 then induces the expression of IFN-α to amplify the innate immune response (59). Interestingly, viruses that can induce sufficient SOCS3 also inhibit production of the IRF7 protein and downstream IFN-α transcription, while viruses deficient in the SOCS3-inducing tegument proteins UL41 and UL13 do not (77). Collectively, these studies show that HSV-1-induced SOCS3 can inhibit both the production and signal transduction of antiviral type I IFNs by disrupting the JAK/STAT pathway. In addition to type I IFNs, type II IFNs are also critical antiviral agents during HSV-1 infection that promote the establishment of latency (13). Importantly, type II IFNs are not sufficient to perform this function during the initial period of viral replication or during periodic outbreaks in keratinocytes. HSV-1 has been shown to induce the expression of SOCS1 specifically in HEL-30 keratinocytes but not in other cells such as L929 fibroblasts (18). HSV-1-induced SOCS1 expression correlates with inhibition of IFN-γ-induced STAT1 activation and enhanced viral replication in keratinocytes. However, the antiviral effect of IFN-γ on HSV-1 replication is restored in the presence of a SOCS1 antagonist, indicating that the selective expression of HSV-1-induced SOCS1 in keratinocytes inhibits the antiviral and latency-promoting effects of type II IFNs. Notably, induction of either SOCS1 or SOCS3 by HSV-1 is cell type specific. In the examples described above, the innate immune evasion provided by SOCS expression within a particular cell type is critical for defining the susceptibility of that cell to HSV-1 infection. Because the ability to evade innate immunity is required for establishing a productive viral infection, SOCS expression within a particular cell type has significant implications for the permissiveness of a wide range of cells to viral infection. This common theme will be explored in further examples below.
Innate immune defense mechanisms present within cardiomyocytes of the heart are not driven by IFNs alone but also by cardiotropin-1 (CT-1) (62), an interleukin-6 (IL-6) family member that signals through the JAK/STAT pathway following activation of the gp130 receptor subunit (Fig. 2A). Coxsackievirus, a positive-sense ssRNA enterovirus, has a propensity for infecting cardiomyocytes, often leading to severe inflammation of the heart tissue (myocarditis), heart damage (cardiomyopathy), and heart failure. Studies performed by Knowlton and colleagues (75) that show that coxsackievirus can induce the expression of SOCS1 and SOCS3 have implicated both of these SOCS proteins in the ability of coxsackievirus to evade antiviral immune responses within the cardiomyocyte and establish a productive infection. Early studies focused on the role of SOCS1 in this process (75). SOCS1 was found to prevent cardiomyocyte protection by IFN-β, IFN-γ, and CT-1 when overexpressed in vitro and to attenuate STAT1 and STAT3 activation, as well as to enhance viral replication, heart damage, and mortality when overexpressed in a cardiomyocyte-specific transgenic mouse in vivo. Furthermore, injection of a dominant negative SOCS1 (dnSOCS1) construct into the heart prevented virus-induced heart damage. These studies suggested that coxsackievirus-induced SOCS1 could inhibit antiviral innate immunity initiated through either the IFN or gp130 receptor, thereby enhancing replication-induced damage of cardiomyocytes. Later, studies focusing on SOCS3 provided a more complete understanding of innate immune protection in these cells (73). SOCS3 overexpression in a cardiomyocyte-specific transgenic mouse resulted in increased viral load, heart damage, and mortality following coxsackievirus infection. These results, which were similar to those of its SOCS1 counterpart, suggested that SOCS3 was also capable of dramatically inhibiting innate immunity. However, SOCS3 overexpression in vitro was only sufficient to prevent STAT3 phosphorylation and cardiomyocyte protection by CT-1 and not by IFN-β or IFN-γ, suggesting that CT-1 signaling through the gp130 receptor, rather than IFN signaling, is the critical mediator of innate immunity in cardiomyocytes. The authors further showed that CT-1 protects cardiomyocytes without suppressing viral replication, by preventing coxsackievirus-induced dystrophin cleavage and disruption of the cell membrane in a STAT3-dependent manner. These data indicate that SOCS1 and SOCS3 contribute to coxsackievirus immune evasion by attenuating JAK/STAT signaling through the gp130 receptor and that virally induced SOCS proteins are sufficient to inhibit even this alternative innate immune mechanism.
Additional viruses follow this well-established pattern and induce various SOCS proteins for the purpose of inhibiting host innate immune defenses. Influenza A virus (47, 52), RSV (24, 79), and HBV (30, 72) (Table 1) are just a few examples of viruses that benefit in this manner by exploiting SOCS protein function. It is likely that the discovery of others will soon follow.
(ii) Adaptive immunity.
SOCS-mediated inhibition of the JAK/STAT pathway can also be used by viruses to evade the adaptive immune response. As described above, antibody production by B cells is a critical component of the comprehensive immune response to viral infection. For antibodies to be fully effective, they must be capable of neutralizing viruses at portal sites of entry, including the respiratory, intestinal, and genital mucosa, in addition to the systemic circulation. To achieve this type of response, B cells must be competent to undergo class switch recombination to produce antibodies containing the multifunctional IgA, IgG, or IgE heavy chain rather than the IgM heavy chain, which has only limited potential. This switch requires T cell-dependent activation of B cells through the NF-κB and JAK/STAT pathways (66). In the context of HIV-1 infection, however, the HIV-1 accessory protein Nef is secreted by infected cells and accumulates within B cells to upregulate inhibitors of these pathways (53). Nef-induced SOCS1 and SOCS3 inhibit IL-4-induced STAT6 activation (Fig. 2A), and Nef-induced IκBα inhibits CD40L-induced NF-κB activation, effectively preventing class switch recombination in B cells. Antibody production is also dependent on JAK/STAT signaling via IL-10 activation of STAT1 and STAT3 (Fig. 2A). HIV-1 Nef-induced SOCS proteins are also sufficient to inhibit this pathway, thereby inhibiting both the quantity and efficacy of B cell-generated antibodies. In light of studies indicating that robust neutralizing-antibody responses may be responsible for the dramatically slowed disease progression found in a select group of HIV-1-infected individuals termed long-term nonprogressors (7), this role of SOCS proteins is troubling.
HCV-induced alterations in SOCS proteins also have a dramatic effect on lymphocytes of the adaptive immune system. Stimulation of CD8+ T cells with HCV core protein results in an increase in SOCS1 protein (74). This is accompanied by decreases in STAT1 activation, markers of general T cell activation, and JAK/STAT-mediated IFN-γ production, a response which greatly impedes the ability of CTLs to effectively clear viruses and promotes chronic HCV infection. In contrast, HCV core protein decreases the levels of SOCS1 in B cells (39, 74). This decrease correlates with increased STAT1 activation and markers of general B cell activation, as well as increased B cell proliferation and antibody (IgM and IgG) production. While it may seem counterintuitive for a viral protein to promote “enhancement” of an immune cell compartment, the clonal B cell proliferation and antibody production that result are relatively ineffective for viral neutralization. Furthermore, this clonal expansion of B cell populations can also lead to important complications of chronic HCV infection, which are further described below.
SOCS box-mediated ubiquitination promotes intracellular viral trafficking.
To achieve successful viral replication culminating in the production of progeny virus particles, viruses must move considerable distances within the host cell. Viruses must traverse the host cell membrane, transport their genomes to locations within the cytoplasm or nucleus for replication and transcription, and coordinate the movement of both their genome and translated viral proteins to specific locations at the plasma membrane for viral particle assembly before finally budding from the cell surface. To achieve these complex movements, viruses hijack the intrinsic transport machinery of the host cell, using the intracellular scaffold of actin microfilaments and microtubules, as well as associated kinesin and dynein motor complexes, to traffic their components throughout the cell and thereby facilitate their own propagation (41). While early studies suggested that viral components could interact directly with host transport machinery, more-recent studies have indicated that additional host proteins are required to facilitate these interactions.
Yamamoto and colleagues were the first to report that SOCS proteins play a role in promoting intracellular trafficking of viral proteins and therefore viral egress from the host cell. They showed that HIV-1 infection could induce the expression of SOCS1 in T cells, which led to enhanced HIV-1 replication and virion production in the absence of enhanced HIV-1 transcription (56). Further analysis indicated that SOCS1 achieved this effect by enhancing microtubule stability and facilitating microtubule-dependent trafficking of the HIV-1 structural protein Gag to the plasma membrane for viral particle assembly (43). SOCS1 directly binds Gag via its SH2 domain and ubiquitinates this viral substrate via its SOCS box, thereby promoting association of Gag with microtubules and enhancing its stability and trafficking. HIV-1 infection studies performed in the absence of SOCS1 yielded decreased Gag ubiquitination and Gag protein levels, as well as a decrease in subsequent particle release (43). Overexpression of SOCS3 in T cells was not sufficient to reproduce these results, indicating that the effects of SOCS1 on trafficking of viral proteins are family member-specific. Importantly, these reports describe the first SOCS-mediated enhancements of viral replication independent of immune evasion or JAK/STAT pathway inhibition.
Although HIV-1 is the only virus for which this process has been well described, recent studies with Ebola virus have suggested that this may represent a more common mechanism. Ebola virus is a negative-sense ssRNA virus that leads to the development of Ebola hemorrhagic fever. This virus disrupts both vascular integrity and effective coagulation by infecting endothelial cells and hepatocytes, respectively, resulting in the rapid leakage of blood through vessel walls and ultimately in hypovolemic shock. Glycoproteins on the Ebola virus particle surface can activate signaling downstream of Toll-like receptor 4 (TLR4), leading to the production of SOCS1 (45). Because TLR4 stimulation has previously been shown to increase Ebola virus production, and because ubiquitination of the Ebola virus structural protein VP40 has been shown to enhance virus budding, Harty and colleagues hypothesized that SOCS1 may ubiquitinate VP40 and thereby facilitate viral particle production (45). The authors cited preliminary results (not presented in the manuscript) that suggest that SOCS1 does ubiquitinate VP40, resulting in a modest increase in viral egress. Further studies will be needed to evaluate whether SOCS1-mediated ubiquitination plays a critical role in promoting intracellular trafficking and therefore viral particle production for Ebola virus or other viruses.
EFFECTS OF SOCS EXPRESSION ON VIRAL DISEASE
Susceptibility to viral infection.
When the host innate immune response is fully functional, a virus cannot establish a productive infection. Therefore, to achieve successful infection in a particular host or cell type, viruses must be able to at least partially circumvent the IFN response in that environment. Differences in the ability of a given virus to overcome this response in various hosts or cell types can contribute to differences in susceptibility to viral infection. Two of the most current examples of this phenomenon involve HIV-1. While HIV-1 readily infects human T cells and monocytes/macrophages, it is not capable of infecting cells of the rhesus macaque. Macaque infection is prevented by TRIM5α, an IFN-induced antiviral protein present in the macaque cell cytoplasm that recognizes a motif in the HIV-1 capsid and interferes with proper uncoating (68). Human cells contain a slightly different version of TRIM5α that is not capable of inhibiting HIV-1, whereas SIV is less sensitive to the macaque TRIM protein, thereby allowing each retrovirus to infect its preferred host without innate immune interference. The efficacy of apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G), another IFN-inducible antiviral protein, can determine the cell type specificity of HIV-1 infection (11). APOBEC3G can inhibit HIV-1 replication in three ways: (i) by inhibiting reverse transcription, (ii) by inducing G-to-A hypermutation, and (iii) by inhibiting integration. However, the ability of APOBEC3G to perform these functions is highly dependent on its assembly state in a particular cell, with its arrangement in low-molecular-mass (LMM) ribonucleoprotein complexes being permissive for full inhibitory function and its arrangement in high-molecular-mass (HMM) complexes being nonpermissive. Consequently, resting CD4+ T cells in the peripheral blood that contain LMM complexes cannot be productively infected, while the same cells in lymphoid tissues containing HMM complexes are readily infected by HIV-1 (12, 31). Similarly, LMM complexes are converted to HMM complexes during monocyte differentiation to macrophages, which parallels an increase in HIV-1 infectivity (12). Clearly the ability of a virus to overcome host IFN responses is a critical predictor of permissiveness to infection.
In this regard, the cell type specificity of SOCS expression can determine susceptibility to infection. RSV, a negative-sense ssRNA virus, is an interesting example of this phenomenon. RSV causes a severe respiratory tract infection in its host, targeting both pulmonary epithelial cells and macrophages. However, differences in the ability of RSV to productively infect cells of the monocyte/macrophage lineage have been tied to SOCS expression. While RSV can induce the expression of SOCS1, SOCS3, and CIS and establish a successful infection in macrophages (phorbol myristate acetate [PMA]-differentiated U937 cells), it is not capable of SOCS expression or infection in monocytes (undifferentiated U937 cells) (79). A subsequent report showed that knockdown of any of these SOCS proteins in cells susceptible to infection resulted in an increase in STAT1/2 phosphorylation and expression of the IFN-induced antiviral target gene OAS, as well as a correlating decrease in RSV replication (24). These studies suggest that SOCS expression contributes to the differences in permissiveness to RSV infection that accompany the cellular differentiation state of macrophages.
HIV-1-induced SOCS expression has also been shown to play a role in permitting infection. Wahl and colleagues observed that tonsil mucosal associated lymphoid tissues are much more susceptible to HIV-1 infection than peripheral blood mononuclear cells (PBMCs), in part due to the increased levels of SOCS1 and SOCS3 present in these tissues (40). Increased SOCS expression in tonsil tissues, both constitutively and in response to HIV-1 infection, correlated with decreased STAT1 activation in response to IFN-α and a decreased TH1 response in the presence of IFN-γ. These studies suggest that SOCS expression may contribute to determining susceptibility to HIV-1 infection by inhibiting both the innate and adaptive immune responses to infection. In addition, studies from our lab show that HIV-1 Tat-induced SOCS3 expression is more selective for cells of macrophage lineage than for other CNS cells examined, such as astrocytes and neurons (2). The mechanism behind this specificity requires further study but may involve differential surface receptor expression or pathway activation between cell types. It is intriguing to note, however, that the cell types in which Tat is able to induce SOCS3 and thereby overcome the innate antiviral response are synonymous with those able to support a productive infection of HIV-1 within the brain (i.e., macrophages and microglia). Although further studies are required in this area, it is enticing to speculate that Tat-induced SOCS3 may contribute to determining permissiveness for HIV-1 infection in the brain.
Virus-associated diseases.
SOCS proteins are induced by viruses to promote the successful completion of their own life cycles. However, aberrant SOCS expression can also powerfully shape the overall presentation of viral disease by contributing to the peripheral manifestations of viral infection. For example, while the direct result of HIV-1 replication is CD4+ T cell death, the most well-recognized manifestation of HIV-1 disease may be infection with opportunistic pathogens such as mycobacteria, protozoa, and fungi. These pathogens are cleared in the immunocompetent host primarily by T cell-mediated IFN-γ signaling. While decreased T cell numbers in the HIV-1-infected host certainly hinder proper clearance, it has been shown that even exogenously supplied IFN-γ is not fully effective (4), suggesting that nonproductive signaling is also at fault. SOCS1 and SOCS3 expression during Mycobacterium avium infection has been shown to correlate with decreased STAT1 activation and failed clearance following IFN-γ treatment (69). It seems likely that HIV-1-induced SOCS1 and SOCS3 proteins function similarly during coinfection to provide a safe haven for M. avium by inhibiting IFN-γ signaling. Furthermore, HIV-1 Tat-induced SOCS2 has also been reported to inhibit IFN-γ signaling in monocytes (10). Cheng et al. recently showed that HIV-1 Tat is sufficient to dampen both STAT1 activation and downstream target gene expression in response to IFN-γ but that this protective immune response is restored following knockdown of SOCS2 (10). Although the authors of this study did not evaluate the effect of Tat-induced SOCS2 on pathogen clearance, these data suggest that multiple HIV-1-induced SOCS proteins may promote secondary infections by inhibiting the antipathogenic effects of IFN-γ.
Patients with chronic HCV infection experience multiple associated diseases, several of which have been linked to SOCS protein expression. A propensity toward glucose intolerance and type II diabetes mellitus has been documented (37, 38) and is thought to result from reduced expression of insulin receptor substrates 1 (IRS1) and IRS2 during chronic HCV disease (27). White and colleagues have shown that overexpressed SOCS1 and SOCS3 are capable of binding both IRS proteins and targeting them for ubiquitination and proteosomal degradation in a SOCS box-dependent manner (54). Furthermore, liver-specific overexpression of SOCS1 in a mouse model in vivo also reduced IRS1 and IRS2 protein levels and subsequently led to insulin resistance (54). A later report provided evidence that overexpression of the HCV core protein, either in vitro or in vivo, could promote a similar decrease in IRS1 and IRS2 expression but only following the induction of SOCS3 (27). These data suggest that HCV-induced SOCS3 can target critical IRS proteins for proteosomal degradation, thereby contributing to the glucose intolerance characteristic of HCV infection. Recent studies demonstrate that HBV-induced SOCS3 can also mediate the ubiquitination of IRS1 and thereby inhibit insulin signaling (28). Although limited, these results suggest that SOCS-induced dysfunction of insulin signaling may be a broader phenomenon.
While most reports have focused on HCV-induced increases in SOCS protein expression, several have shown that HCV-related decreases in SOCS proteins within specific cell types or tissue regions may also be associated with disease. A major extrahepatic manifestation of HCV infection affecting 30 to 50% of patients is mixed cryoglobulinemia, which is caused by rampant overproduction of monoclonal or polyclonal IgM and IgG antibodies by B cells (8). Another associated disease, known as non-Hodgkin lymphoma, results directly from aberrant B cell proliferation (70). As previously mentioned, SOCS1 is decreased in B cells taken from HCV-infected patients (39) or those incubated with HCV core protein in vitro (74). Decreased SOCS1 expression correlates with increased STAT1 activation and with increases in both B cell proliferation and IgM/IgG production. Therefore, HCV-induced decreases in SOCS1 expression in B cells may promote B cell dysregulation and clonal expansion, leading to the lymphoproliferative disorders characteristic of HCV infection.
Patients with chronic HCV infection also display a propensity toward hepatocellular carcinoma (HCC) (33). Interestingly, while HCV infection has been shown in a number of studies to increase SOCS1 and SOCS3 expression in hepatocytes to promote viral replication, HCV-induced decreases in these SOCS proteins tend to correlate with hepatocarcinogenesis. A report by Yoshimura and colleagues provides some resolution to this conflict by establishing that SOCS3 levels are increased in the livers of patients with HCV infection compared to those in normal controls but that within HCV-infected livers, hepatocytes in regional areas of HCC contain drastically reduced levels of SOCS3 compared to those in non-HCC areas (44). The lower levels of SOCS3 in HCC liver regions correlate with increased STAT3 activation, which may lead to enhanced expression of antiapoptotic molecules such as Bcl-2 and Bcl-XL and promote cancer development. Further studies will be required to determine the cause of differential regulation of SOCS proteins by HCV in different regions of the liver.
Therapeutic potential.
Because the exploitation of host SOCS protein functions provides such a potent viral advantage, these proteins also possess considerable therapeutic potential. One of the most straightforward therapeutic applications of SOCS proteins has been shown in the context of chronic HCV infection. The most common treatment for HCV infection is the exogenous administration of IFN-α (17, 19), given to augment the endogenous antiviral host response. However, a significant proportion of patients, particularly those with genotype 1 HCV infection, do not respond to therapy (17, 19). Multiple studies have shown that HCV-induced SOCS3 expression, which is known to inhibit endogenous host IFN responses, can also be used as an independent predictor of therapeutic response to IFN-α treatment (29, 49, 50). SOCS3 expression is elevated in nonresponders compared to that in responders in both peripheral blood mononuclear cells (49) and hepatic tissue (29). These data also provide evidence that differences in HCV-induced SOCS3 expression may be dependent on both viral and host factors. Patients with genotype 1 HCV infection tend to have higher levels of SOCS3 expression, providing a rationale for their propensity toward a lack of therapeutic response (29, 49). In addition, patients with a particular SOCS3 genotype (−4874 AA) also express SOCS3 at elevated levels and consequently have a poorer response to therapy (50). Not all studies agree, however, as one report suggests that elevated levels of SOCS1 but not SOCS3 in the livers of HCV-infected patients provide a better prediction of response to viral therapy (26). Regardless, analyzing the levels of SOCS protein expression in the HCV-infected patient may allow for early prediction of therapeutic response and therefore more appropriate treatment decisions. This correlation may also provide the information necessary for developing a more effective treatment in the future.
One therapeutic strategy to consider would be suppression of SOCS protein levels or function during viral infection. The effectiveness of this approach in dampening viral replication has been explored in a small number of studies. A dnSOCS1 construct containing a point mutation in the KIR (F59D), previously shown to cause destabilization of both SOCS1 and SOCS3 (23), was evaluated in the treatment of coxsackievirus infection (75). In an in vitro reporter assay, dnSOCS1 enhanced heart-protective CT-1-induced STAT3 activity and overcame STAT3 inhibition in response to SOCS1 overexpression in cardiomyocytes. In addition, it was able to sustain STAT1 and STAT3 phosphorylation in response to the innate antiviral agents IFN-γ and CT-1, respectively. In vivo, injection of dnSOCS1 into the hearts of coxsackievirus-infected mice attenuated both virus replication and cardiomyocyte damage. These studies suggest that dnSOCS1, by reducing levels of coxsackievirus-induced SOCS1 and SOCS3, allows endogenous antiviral JAK/STAT signaling to protect the heart from viral infection. Another SOCS1 antagonist, a peptide mimic of the phosphorylated JAK2 activation loop (pJAK2), exhibits even broader antiviral activity. By binding the KIR of SOCS1 and preventing its function, pJAK2 has been shown to reverse the inhibition of SOCS1 overexpression on IL-6-induced STAT3 phosphorylation and to enhance reporter assay activity at the promoter of antiviral target genes in response to IFN-γ (71). Keratinocytes, which are highly susceptible to HSV-1 infection due to virus-induced SOCS1 expression, are protected from HSV-1-induced death following pretreatment with pJAK2 (18). Treatment with pJAK2 alone enhances keratinocyte survival only modestly, while cotreatment with IFN-γ results in complete protection. These data indicate that pJAK2 is sufficient to attenuate SOCS1 function, thereby allowing either endogenous or exogenous JAK/STAT signaling pathways to exert their antiviral effects, and to promote keratinocyte survival. In a similar manner, pretreatment of mice with pJAK2 also provides protection against lethal vaccinia and encephalomyocarditis virus infections (1). While these examples are limited and preliminary, they suggest that manipulation of SOCS proteins is possible during viral infection and may provide a potent mechanism for inhibiting viral replication.
Enhanced expression of SOCS proteins during viral infection may actually be favorable for one therapeutic strategy. Oncolytic HSVs (oHSVs) are robust anticancer therapeutics engineered to infect and replicate within tumor cells, thereby reducing tumor burden through direct cell lysis. A recent study has shown that the ability of the oHSV vector G207 to replicate within target cells and thereby have a therapeutic effect is dependent on its induction of SOCS1 (34). oHSV induced SOCS1 and inhibited STAT1 activation in cells permissive for replication but not in cells where replication was poor. SOCS1 knockdown in oHSV-permissive cells resulted in a >10-fold decrease in viral replication. Therefore, oHSV-induced expression of SOCS1 is critical for the susceptibility of cells to viral replication and, consequently, to therapeutic effect. Exogenous overexpression of SOCS1 by therapeutic viral vectors has been proposed in order to inhibit the host innate immune response (57). This type of strategy may enhance the range and efficacy of viral vectors in the future.
Manipulation of SOCS proteins may even be used to prevent viral infection entirely. Currently utilized vaccine strategies have been unable to produce a meaningful immune response to vaccination with HIV-1 antigens (3). One obstacle is that endogenous SOCS1 expression in host dendritic cells (DCs) inhibits effective antigen presentation to T and B cells, thereby limiting immune activation (22, 61). In a series of experiments comparing mice immunized with either normal or SOCS1-depleted DCs carrying the HIV-1 envelope protein gp120, Chen and colleagues showed that DC function is enhanced in the absence of SOCS1, allowing for more robust cellular and humoral responses to HIV-1 antigens (64). Immunization with SOCS1-depleted DCs resulted in more-robust CTL activity against gp120-pulsed target cells, along with increased numbers of IFN-γ- and perforin-positive CD8+ T cells. Gp120-specific CD4+ T cells were also present in greater numbers in vivo and exhibited increased proliferation and increased production of both TH1- and TH2-polarizing cytokines following interaction with gp120-pulsed DCs ex vivo. The HIV-1-specific humoral response was also enhanced. The number of gp120-specific IgG-producing B cells was increased, as were markers of their activation state and their production of gp120-specific antibodies. In addition, mice immunized with SOCS1-depleted DCs generated a much longer lasting immune response, exhibiting drastically superior gp120-specific CTL and antibody responses 6 months after immunization compared to those of mice immunized with SOCS1-containing DCs. Together, these data indicate that SOCS1 expression in DCs critically regulates anti-HIV immunity and that an enhanced but balanced cellular and humoral response can be generated against HIV-1 antigens in the absence of SOCS1. In light of these results, the authors further examined whether the administration of SOCS1 small interfering RNA (siRNA) expressor DNA (pSuper-SOCS1-siRNA) could enhance the potency of a coadministered HIV-1 DNA vaccine. Coadministration of SOCS1 siRNA increased gp120-specific antibody titers as well as CTL and CD4+ T cell responses, suggesting that limiting SOCS1 expression may be an effective adjunct to current vaccine development strategies.
CONCLUSIONS
Virus-induced SOCS proteins represent a powerful tool for virus survival. They can mediate inhibition of the JAK/STAT pathway, allowing viruses to evade the host immune response, or viral protein ubiquitination, facilitating protein trafficking and progeny virus assembly. Virally enhanced expression of SOCS proteins also has a profound role in determining the presentation of viral disease, due to its peripheral effects on the host organism. Understanding the role of SOCS proteins in the context of viral diseases will provide us not only with a better understanding of the complexities of virus-host interactions but also potentially with new therapeutic targets.
Acknowledgments
This work was funded in part by Public Health Service grants NS-57-563 and NS-50665 from NINDS, a grant from the National Multiple Sclerosis Society (RG-3892-A-12), and a Collaborative MS Research Center Award from the NMSS (CA 1059-A-13). L.N.A. is supported by the UAB Medical Scientist Training Program and by Public Health Service grants T32-AI-07493 and F30-NS-65600.
Footnotes
Published ahead of print on 17 November 2010.
REFERENCES
- 1.Ahmed, C. M., et al. 2010. Enhancement of antiviral immunity by small molecule antagonist of suppressor of cytokine signaling. J. Immunol. 185:1103-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhtar, L. N., et al. 2010. Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. J. Immunol. 185:2393-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal, G. P., A. Malaspina, and J. Flores. 2010. Future paths for HIV vaccine research: exploiting results from recent clinical trials and current scientific advances. Curr. Opin. Mol. Ther. 12:39-46. [PubMed] [Google Scholar]
- 4.Biggs, B. A., M. Hewish, S. Kent, K. Hayes, and S. M. Crowe. 1995. HIV-1 infection of human macrophages impairs phagocytosis and killing of Toxoplasma gondii. J. Immunol. 154:6132-6139. [PubMed] [Google Scholar]
- 5.Bode, J. G., E. D. Brenndorfer, and D. Haussinger. 2007. Subversion of innate host antiviral strategies by the hepatitis C virus. Arch. Biochem. Biophys. 462:254-265. [DOI] [PubMed] [Google Scholar]
- 6.Bode, J. G., et al. 2003. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 17:488-490. [DOI] [PubMed] [Google Scholar]
- 7.Cecilia, D., C. Kleeberger, A. Munoz, J. V. Giorgi, and S. Zolla-Pazner. 1999. A longitudinal study of neutralizing antibodies and disease progression in HIV-1-infected subjects. J. Infect. Dis. 179:1365-1374. [DOI] [PubMed] [Google Scholar]
- 8.Charles, E. D., and L. B. Dustin. 2009. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 76:818-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, X. P., et al. 2002. Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc. Natl. Acad. Sci. U. S. A. 99:2175-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, S. M., et al. 2009. HIV-1 transactivator protein induction of suppressor of cytokine signaling-2 contributes to dysregulation of IFNγ signaling. Blood 113:5192-5201. [DOI] [PubMed] [Google Scholar]
- 11.Chiu, Y. L., and W. C. Greene. 2008. The APOBEC3 cytidine deaminases: an innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu. Rev. Immunol. 26:317-353. [DOI] [PubMed] [Google Scholar]
- 12.Chiu, Y. L., et al. 2005. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature 435:108-114. [DOI] [PubMed] [Google Scholar]
- 13.Decman, V., P. R. Kinchington, S. A. Harvey, and R. L. Hendricks. 2005. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 79:10339-10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimitriou, I. D., et al. 2008. Putting out the fire: coordinated suppression of the innate and adaptive immune systems by SOCS1 and SOCS3 proteins. Immunol. Rev. 224:265-283. [DOI] [PubMed] [Google Scholar]
- 15.Ehlting, C., et al. 2007. Regulation of suppressor of cytokine signaling 3 (SOCS3) mRNA stability by TNF-alpha involves activation of the MKK6/p38MAPK/MK2 cascade. J. Immunol. 178:2813-2826. [DOI] [PubMed] [Google Scholar]
- 16.Fenner, J. E., et al. 2006. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat. Immunol. 7:33-39. [DOI] [PubMed] [Google Scholar]
- 17.Forde, K. A., and K. R. Reddy. 2009. Hepatitis C virus infection and immunomodulatory therapies. Clin. Liver Dis. 13:391-401. [DOI] [PubMed] [Google Scholar]
- 18.Frey, K. G., et al. 2009. HSV-1-induced SOCS-1 expression in keratinocytes: use of a SOCS-1 antagonist to block a novel mechanism of viral immune evasion. J. Immunol. 183:1253-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried, M. W., et al. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 20.Frobøse, H., et al. 2006. Suppressor of cytokine signaling-3 inhibits interleukin-1 signaling by targeting the TRAF-6/TAK1 complex. Mol. Endocrinol. 20:1587-1596. [DOI] [PubMed] [Google Scholar]
- 21.Haan, S., et al. 2003. Tyrosine phosphorylation disrupts elongin interaction and accelerates SOCS3 degradation. J. Biol. Chem. 278:31972-31979. [DOI] [PubMed] [Google Scholar]
- 22.Hanada, T., et al. 2005. Induction of hyper Th1 cell-type immune responses by dendritic cells lacking the suppressor of cytokine signaling-1 gene. J. Immunol. 174:4325-4332. [DOI] [PubMed] [Google Scholar]
- 23.Hanada, T., et al. 2001. A mutant form of JAB/SOCS1 augments the cytokine-induced JAK/STAT pathway by accelerating degradation of wild-type JAB/CIS family proteins through the SOCS-box. J. Biol. Chem. 276:40746-40754. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto, K., et al. 2009. RSV replication is attenuated by counteracting expression of the suppressor of cytokine signaling (SOCS) molecules. Virology 391:162-170. [DOI] [PubMed] [Google Scholar]
- 25.He, Y., W. Zhang, R. Zhang, H. Zhang, and W. Min. 2006. SOCS1 inhibits tumor necrosis factor-induced activation of ASK1-JNK inflammatory signaling by mediating ASK1 degradation. J. Biol. Chem. 281:5559-5566. [DOI] [PubMed] [Google Scholar]
- 26.Imanaka, K., et al. 2005. Enhanced expression of suppressor of cytokine signalling-1 in the liver of chronic hepatitis C: possible involvement in resistance to interferon therapy. J. Viral Hepat. 12:130-138. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi, T., et al. 2004. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am. J. Pathol. 165:1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, K., K. H. Kim, and J. Cheong. 2010. Hepatitis B virus X protein impairs hepatic insulin signaling through degradation of IRS1 and induction of SOCS3. PLoS One 5:e8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, K. A., et al. 2009. Hepatic SOCS3 expression is strongly associated with non-response to therapy and race in HCV and HCV/HIV infection. J. Hepatol. 50:705-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koeberlein, B., et al. 2010. Hepatitis B virus overexpresses suppressor of cytokine signaling-3 (SOCS3) thereby contributing to severity of inflammation in the liver. Virus Res. 148:51-59. [DOI] [PubMed] [Google Scholar]
- 31.Kreisberg, J. F., W. Yonemoto, and W. C. Greene. 2006. Endogenous factors enhance HIV infection of tissue naive CD4 T cells by stimulating high molecular mass APOBEC3G complex formation. J. Exp. Med. 203:865-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo, M., T. Hanada, and A. Yoshimura. 2003. Suppressors of cytokine signaling and immunity. Nat. Immunol. 4:1169-1176. [DOI] [PubMed] [Google Scholar]
- 33.Levrero, M. 2006. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene 25:3834-3847. [DOI] [PubMed] [Google Scholar]
- 34.Mahller, Y. Y., et al. 2008. Molecular analysis of human cancer cells infected by an oncolytic HSV-1 reveals multiple upregulated cellular genes and a role for SOCS1 in virus replication. Cancer Gene Ther. 15:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malim, M. H., and M. Emerman. 2008. HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe 3:388-398. [DOI] [PubMed] [Google Scholar]
- 36.Mansell, A., et al. 2006. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7:148-155. [DOI] [PubMed] [Google Scholar]
- 37.Mehta, S. H., et al. 2003. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 38:50-56. [DOI] [PubMed] [Google Scholar]
- 38.Mehta, S. H., et al. 2000. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann. Intern. Med. 133:592-599. [DOI] [PubMed] [Google Scholar]
- 39.Moorman, J., et al. 2009. Abnormal B-cell activation associated with TALL-1 over-expression and SOCS-1 suppression during chronic hepatitis C virus infection. Immunology 128:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moutsopoulos, N. M., et al. 2006. Regulation of the tonsil cytokine milieu favors HIV susceptibility. J. Leukoc. Biol. 80:1145-1155. [DOI] [PubMed] [Google Scholar]
- 41.Naghavi, M. H., and S. P. Goff. 2007. Retroviral proteins that interact with the host cell cytoskeleton. Curr. Opin. Immunol. 19:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa, R., et al. 2002. SOCS-1 participates in negative regulation of LPS responses. Immunity 17:677-687. [DOI] [PubMed] [Google Scholar]
- 43.Nishi, M., et al. 2009. Requirement for microtubule integrity in the SOCS1-mediated intracellular dynamics of HIV-1 Gag. FEBS Lett. 583:1243-1250. [DOI] [PubMed] [Google Scholar]
- 44.Ogata, H., et al. 2006. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology 131:179-193. [DOI] [PubMed] [Google Scholar]
- 45.Okumura, A., P. M. Pitha, A. Yoshimura, and R. N. Harty. 2010. Interaction between Ebola virus glycoprotein and host Toll-like receptor 4 leads to induction of proinflammatory cytokines and SOCS1. J. Virol. 84:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paladino, P., and K. L. Mossman. 2009. Mechanisms employed by herpes simplex virus 1 to inhibit the interferon response. J. Interferon Cytokine Res. 29:599-607. [DOI] [PubMed] [Google Scholar]
- 47.Pauli, E. K., et al. 2008. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 4:e1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peltola, K. J., et al. 2004. Pim-1 kinase inhibits STAT5-dependent transcription via its interactions with SOCS1 and SOCS3. Blood 103:3744-3750. [DOI] [PubMed] [Google Scholar]
- 49.Persico, M., et al. 2007. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: insulin resistance and response to antiviral therapy. Hepatology 46:1009-1015. [DOI] [PubMed] [Google Scholar]
- 50.Persico, M., et al. 2008. Elevated expression and polymorphisms of SOCS3 influence patient response to antiviral therapy in chronic hepatitis C. Gut 57:507-515. [DOI] [PubMed] [Google Scholar]
- 51.Piessevaux, J., D. Lavens, F. Peelman, and J. Tavernier. 2008. The many faces of the SOCS box. Cytokine Growth Factor Rev. 19:371-381. [DOI] [PubMed] [Google Scholar]
- 52.Pothlichet, J., M. Chignard, and M. Si-Tahar. 2008. Cutting edge: innate immune response triggered by influenza A virus is negatively regulated by SOCS1 and SOCS3 through a RIG-I/IFNAR1-dependent pathway. J. Immunol. 180:2034-2038. [DOI] [PubMed] [Google Scholar]
- 53.Qiao, X., et al. 2006. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat. Immunol. 7:302-310. [DOI] [PubMed] [Google Scholar]
- 54.Rui, L., M. Yuan, D. Frantz, S. Shoelson, and M. F. White. 2002. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 277:42394-42398. [DOI] [PubMed] [Google Scholar]
- 55.Ryo, A., et al. 2003. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 12:1413-1426. [DOI] [PubMed] [Google Scholar]
- 56.Ryo, A., et al. 2008. SOCS1 is an inducible host factor during HIV-1 infection and regulates the intracellular trafficking and stability of HIV-1 Gag. Proc. Natl. Acad. Sci. U. S. A. 105:294-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakurai, H., et al. 2008. Adenoviral expression of suppressor of cytokine signaling-1 reduces adenovirus vector-induced innate immune responses. J. Immunol. 180:4931-4938. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki, A., et al. 2003. The N-terminal truncated isoform of SOCS3 translated from an alternative initiation AUG codon under stress conditions is stable due to the lack of a major ubiquitination site, Lys-6. J. Biol. Chem. 278:2432-2436. [DOI] [PubMed] [Google Scholar]
- 59.Sato, M., et al. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 60.Shao, R. X., et al. 2010. Suppressor of cytokine signaling 3 suppresses hepatitis C virus replication in an mTOR-dependent manner. J. Virol. 84:6060-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen, L., K. Evel-Kabler, R. Strube, and S. Y. Chen. 2004. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat. Biotechnol. 22:1546-1553. [DOI] [PubMed] [Google Scholar]
- 62.Sheng, Z., D. Pennica, W. I. Wood, and K. R. Chien. 1996. Cardiotrophin-1 displays early expression in the murine heart tube and promotes cardiac myocyte survival. Development 122:419-428. [DOI] [PubMed] [Google Scholar]
- 63.Song, M. M., and K. Shuai. 1998. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J. Biol. Chem. 273:35056-35062. [DOI] [PubMed] [Google Scholar]
- 64.Song, X. T., et al. 2006. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 3:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 66.Stavnezer, J., J. E. Guikema, and C. E. Schrader. 2008. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 26:261-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strauss, J. H., and E. G. Strauss. 2002. Host defense against viral infection and viral counter defenses, p. 303-346. In J. H. Strauss and E. G. Strauss (ed.), Viruses and human disease. Academic Press, San Diego, CA.
- 68.Stremlau, M., et al. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848-853. [DOI] [PubMed] [Google Scholar]
- 69.Vázquez, N., T. Greenwell-Wild, S. Rekka, J. M. Orenstein, and S. M. Wahl. 2006. Mycobacterium avium-induced SOCS contributes to resistance to IFN-gamma-mediated mycobactericidal activity in human macrophages. J. Leukoc. Biol. 80:1136-1144. [DOI] [PubMed] [Google Scholar]
- 70.Viswanatha, D. S., and A. Dogan. 2007. Hepatitis C virus and lymphoma. J. Clin. Pathol. 60:1378-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waiboci, L. W., et al. 2007. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J. Immunol. 178:5058-5068. [DOI] [PubMed] [Google Scholar]
- 72.Xu, Y., et al. 2009. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol. Immunol. 46:2640-2646. [DOI] [PubMed] [Google Scholar]
- 73.Yajima, T., et al. 2006. Innate defense mechanism against virus infection within the cardiac myocyte requiring gp130-STAT3 signaling. Circulation 114:2364-2373. [DOI] [PubMed] [Google Scholar]
- 74.Yao, Z. Q., D. Prayther, C. Trabue, Z. P. Dong, and J. Moorman. 2008. Differential regulation of SOCS-1 signalling in B and T lymphocytes by hepatitis C virus core protein. Immunology 125:197-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yasukawa, H., et al. 2003. The suppressor of cytokine signaling-1 (SOCS1) is a novel therapeutic target for enterovirus-induced cardiac injury. J. Clin. Invest. 111:469-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yokota, S., N. Yokosawa, T. Okabayashi, T. Suzutani, and N. Fujii. 2005. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virology 338:173-181. [DOI] [PubMed] [Google Scholar]
- 77.Yokota, S., et al. 2004. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J. Virol. 78:6282-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshimura, A., T. Naka, and M. Kubo. 2007. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7:454-465. [DOI] [PubMed] [Google Scholar]
- 79.Zhao, D. C., T. Yan, L. Li, S. You, and C. Zhang. 2007. Respiratory syncytial virus inhibits interferon-alpha-inducible signaling in macrophage-like U937 cells. J. Infect. 54:393-398. [DOI] [PubMed] [Google Scholar]