Abstract
Pregnancy in women with diabetes is associated with a higher risk of perinatal complications. In particular, infants of diabetic mothers frequently suffer from respiratory distress syndrome (RDS), which is a leading cause of death in preterm infants and is considered to be primarily due to hyperinsulinemia in infants in response to maternal hyperglycemia. To elucidate the mechanism of how insulin signaling induces RDS, bronchoalveolar epithelium-specific Akt1 transgenic (TG) mice were generated. Akt1 overexpression in fetal lung epithelium resulted in RDS in preterm infants born by Caesarean section at embryonic day 18.5 (E18.5). The expression levels of hypoxia-inducible factor 2α (HIF-2α) and its target vascular endothelial growth factor (VEGF) were downregulated in the lung of Akt1 TG mice. Inhibition of the Akt-mammalian target of rapamycin (mTOR) signaling axis by rapamycin restored the expression of VEGF and improved the lung pathology of Akt1 TG pups. Rapamycin also attenuated the RDS phenotype in wild-type mice delivered preterm at E17.5. In cultured lung epithelial cells, insulin reduced VEGF expression and transcriptional activity of HIF-2 on VEGF promoter in an mTOR-dependent manner. Thus, aberrant activation of the Akt-mTOR pathway in lung epithelium plays a causal role in the pathogenesis of infant RDS, presumably through downregulation of HIF-2-dependent VEGF expression in the lung.
Pregnancy in women with diabetes is associated with a higher risk of perinatal complications. The estimated prevalence rate of gestational diabetes is approximately 4% and is considered to represent 90% of all cases of diabetes diagnosed during pregnancy (2). Moreover, pregnant women with type 2 diabetes are increasing in number in line with the rapid increase in the prevalence of type 2 diabetes in all age groups (9). Infants of diabetic mothers frequently suffer from macrosomia, neonatal hypoglycemia, cardiomegaly, respiratory difficulties, and other congenital anomalies (3, 5, 14, 21), which are considered to be primarily due to hyperinsulinemia in infants in response to maternal hyperglycemia. Respiratory distress syndrome (RDS) is one of the most clinically significant perinatal complications, with high morbidity and mortality (19, 22). RDS is caused by attenuated production of pulmonary surfactant, a mixture of phospholipids and surfactant-associated proteins that covers the alveolar surface and prevents alveolar collapse by reducing the surface tension of the air-water interface (12). Because pulmonary surfactant is specifically produced by type II lung epithelial cells, attenuated production of pulmonary surfactant is considered to be due to impaired differentiation and/or maturation of type II lung epithelial cells (31). Reduced expression of surfactant-associated proteins was observed in fetuses of streptozotocin-induced diabetic rats or in insulin-treated human fetal lung explants (8, 10, 11), suggesting that hyperactivation of insulin signaling in the lung plays a causal role in the pathogenesis of RDS of infants having a diabetic mother.
Phosphatidylinositol 3-kinase (PI3K) is activated by insulin and is responsible for most of the metabolic actions of insulin. PI3K also controls cell growth, differentiation, survival, and protein synthesis (28). The involvement of the PI3K pathway in RDS was suggested by a previous report showing that bronchoalveolar-specific deletion of Pten in mice led to upregulation of the PI3K pathway in the lung and resulted in RDS associated with marked hyperplasia of alveolar epithelial cells, increased numbers of bronchoalveolar stem cells (BASCs), and impaired production of surfactant proteins (SPs) (33). It was also shown that genetic ablation of hypoxia-inducible factor 2α (HIF-2α) causes RDS due to downregulation of vascular endothelial growth factor (VEGF) expression in the lung and that intratracheal VEGF administration stimulates maturation of type II lung epithelial cells (4). However, the downstream effectors of PI3K that cause RDS and the mechanistic link between the PI3K pathway and HIF-2-dependent VEGF expression in the lung have been elusive.
Using bronchoalveolar epithelium-specific Akt1 transgenic (TG) mice, we show here that aberrant activation of the Akt-mammalian target of rapamycin (mTOR) pathway in lung epithelium plays a causal role in the pathogenesis of infant RDS. We also provide evidence suggesting that sustained Akt-mTOR activation induces RDS through downregulation of HIF-2-dependent VEGF expression in the lung.
MATERIALS AND METHODS
Animals and DOX administration.
SP-C-rtTA TG mice expressing the reverse tetracycline transactivator (rtTA) protein under the control of the human surfactant protein-C promoter (pro-SP-C) on the FVB/N background were described previously (23, 29) and were purchased from the Jackson Laboratory. These mice were crossed with Tet-myrAkt1 TG mice (25) harboring a myristoylated Akt1 (myrAkt1) transgene under the control of multimerized tetO sequences maintained on a mixed background of FVB/N, C57BL/6J, and 129Sv. For myrAkt1 expression, dams were treated with doxycycline (DOX) in drinking water from embryonic day 0.5 (E0.5) at a final concentration of 0.5 mg/ml (25, 29). Respiratory rate was measured by visual inspection of the movements of thorax and abdomen for 1 min. All animal procedures were performed with the approval of the Institutional Animal Care and Use Committee of Chiba University.
Histological analysis and immunohistochemistry.
Lungs were formalin fixed without constant pressure inflation and embedded in paraffin for histological analyses. Serial sections of 4 μm were stained with hematoxylin and eosin (HE) for morphological analysis, periodic acid-Schiff (PAS) for detection of glycogen-rich cells, and Masson's trichrome (MT) for detection of fibrosis. Aerated lung area and alveolar septal thickness were measured in toluidine blue-stained sections using ImageJ software (4). Aerated lung area was measured in 10 visual fields for each animal. Septal thickness was measured at 10 points in each visual field, and 10 visual fields for each animal were used for the measurement. The size and number of saccules were measured in PAS-stained sections using ImageJ software. The size of 10 saccules was measured in each visual field, and 10 visual fields for each animal were used for the analysis. Saccular number was counted in 10 visual fields for each animal. Immunohistochemistry was performed using an avidin-biotin-horseradish peroxidase detection system with Ni-diaminobenzidine (DAB) (ABC kit; Vector Laboratories) and Nuclear Fast Red as a counterstain. The antibodies used were hemagglutinin (HA), pro-SP-C, Clara cell 10-kDa protein (CC10), aquaporin-5 (AQP5), VEGF (Santa Cruz Biotechnology), and calcitonin gene-related peptide (CGRP; BioMol).
Fluorescence imaging.
A BZ-9000 (Keyence) microscope was used for fluorescence imaging. Paraffin-embedded lung sections were incubated with isolectin B4-fluorescein isothiocyanate (FITC) conjugate (Sigma) to detect endothelial cells and with wheat germ agglutinin-tetramethyl rhodamine isothiocyanate (TRITC) conjugate (Sigma) to counterstain the cell membrane. Vascular cell counts with three replications were performed with BZ application software (Keyence), and the median value was used for each sample.
Western blot analysis.
Total protein lysate was extracted from lung tissue, and SDS-PAGE was performed as described previously (26). The antibodies used were HA, total Akt1, total S6 kinase (S6K), SP-A, pro-SP-C, AQP5, CC10, VEGF, glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology), phospho-Akt (Ser473), phospho-S6K1 (Thr389), phospho-S6 (Ser235/236), total S6, phospho-glycogen synthase kinase 3β (phospho-GSK3β) (Ser9), phospho-FOXO3a (318/321), total FOXO3a (Cell Signaling Technology), SP-B, SP-D (Chemicon/Millipore), and HIF-2α (Novus Biologicals). Densitometric analysis was performed using ImageJ.
Mouse model of preterm infants.
Pups were collected by Caesarean section at E18.5 for Akt1 TG mice and at E17.5 for wild-type ICR mice (4, 20). The umbilical cord was cut, amniotic fluid and membranes were removed from the mouth and nose, and body temperature was kept at 37°C.
Administration of rapamycin.
Rapamycin (LC Laboratories) was prepared in the solvent containing 0.2% sodium carboxymethylcellulose and 0.25% polysorbate-80 in water (24, 25). Rapamycin (1 mg/kg of body weight/day) or vehicle was administered subcutaneously to dams.
Reporter gene assays.
Luciferase assays were performed essentially as previously described (18). The plasmids used were VEGF reporter plasmid (pGL2hVEGF) and the expression vectors for HIF-1α (phHIF-1α), HIF-2α (phEP-1), and ARNT (phARNT). A549 cells were transfected with the plasmids indicated in the Fig. 8 legend and treated with 250 μM CoCl2 to mimic hypoxic condition.
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM). Statistical significance between two groups was determined with a Student's t test or χ2 test. Probability values of <0.05 were considered to be statistically significant.
RESULTS
Generation of bronchoalveolar epithelium-specific Akt1 TG mice.
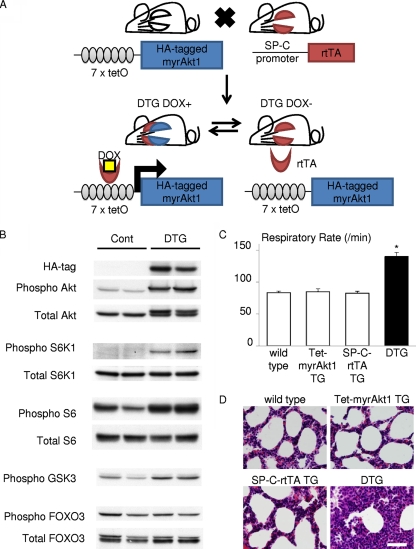
Two lines of TG mice (Tet-myrAkt1 and SP-C-rtTA) were used to generate bronchoalveolar epithelium-specific Akt1 TG mice (Fig. 1A). The Tet-myrAkt1 TG line harbors an active form of the Akt1 transgene (myrAkt1) under the control of multimerized tetracycline operator (tetO) sequences (25), and the SP-C-rtTA TG line expresses the reverse tetracycline transactivator (rtTA) in respiratory epithelial cells driven by human surfactant protein-C (SP-C) promoter (23, 29). DOX enables rtTA binding to tetO sequences by inducing its conformational change. Therefore, DOX treatment of double-TG (DTG) mice harboring both transgenes induces transcription of the myrAkt1 gene in lung epithelial cells (Fig. 1A). Mating of SP-C-rtTA mice with Tet-myrAkt1 mice resulted in the generation of mice with four different genotypes (wild type, Tet-myrAkt1 single TG, SP-C-rtTA single TG, and DTG) at the expected frequencies. Western blot analysis of lung lysates harvested at postnatal day 0 (P0) revealed that the transgene product detected by anti-HA blotting was observed in the lung of DTG mice treated with DOX (Fig. 1B). The induced expression of myrAkt1 was associated with a marked increase in phosphorylation levels of several downstream effectors such as S6K1, S6, and glycogen synthase kinase-3 (GSK3), but not FOXO3 (Fig. 1B). Because it was reported that treatment of SP-C-rtTA mice with DOX may exert toxic effects on alveolar epithelial cells (20), the phenotype of DOX-treated SP-C-rtTA single-TG mice was carefully compared with littermates of other genotypes (wild type, Tet-myrAkt1 single TG, and DTG). However, although there was an obvious lung phenotype in DTG mice (described in detail below), no apparent abnormality was observed in mice with other genotypes under our experimental conditions (Fig. 1C and D). We therefore used DOX-treated single-TG littermates as controls in this study.
FIG. 1.
Generation of bronchoalveolar epithelium-specific Akt1 TG mice. (A) Schematic illustration of binary TG system. (B) Western blot analysis of the whole-lung lysate from control and DTG mice at P0. (C) Respiratory rate at P0. DOX-treated SP-C-rtTA TG mice do not show respiratory distress. *, P < 0.05 versus DOX-treated wild-type, Tet-myrAkt1 TG mice, and SP-C-rtTA TG mice (n = 7, 5, 6, and 6 mice, respectively, from 3 dams). All animals were treated with DOX in the drinking water. (D) HE staining of the lung sections. Scale bar, 50 μm.
Akt activation in lung epithelial cells in utero results in transient tachypnea associated with delayed maturation of the lung.
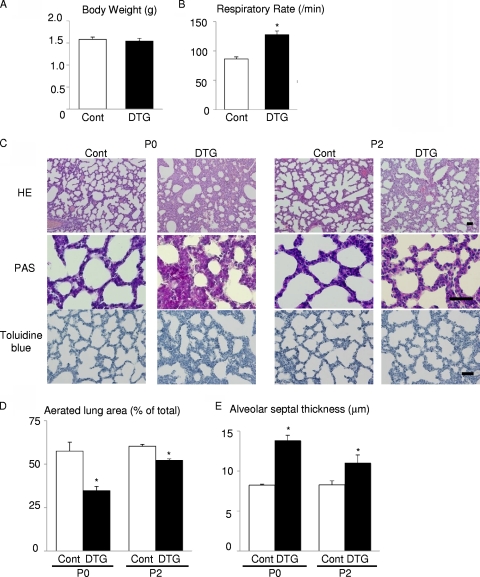
Activation of Akt signaling in the lung was achieved by DOX treatment of dams starting at embryonic day 0.5 (E0.5). When analyzed at P0, DTG pups were viable and exhibited no cyanosis or growth retardation (Fig. 2A). Postnatal growth of DTG animals was also comparable to that of control mice. However, DTG mice at P0 exhibited significant tachypnea (Fig. 2B) and various histological abnormalities such as markedly reduced aerated space, increased atelectasis, bronchiolar hyperplasia, impaired thinning of the alveolar septa, and abundant PAS-positive glycogen stores (Fig. 2C to E). Since PAS-positive glycogen is normally converted to surfactant phospholipids in mature epithelial cells, these observations suggest that the differentiation of lung epithelial cells is impaired by aberrant activation of Akt signaling. Of note, these histological findings were improved spontaneously at P2 (Fig. 2C to E). Although mild elastic fiber deposition was observed at P2, no such pathology was evident at the age of 12 weeks (data not shown). Thus, Akt activation in lung epithelium induces transient respiratory difficulties associated with lung maturational defects, which improves spontaneously after birth.
FIG. 2.
Akt activation in lung epithelium induces tachypnea and delayed maturation of the lung. (A) Body weight at P0 of control (Cont; n = 6) and DTG (n = 4) mice from 2 dams. (B) Respiratory rate at P0 of control (n = 14) and DTG (n = 8) mice from 4 dams. (C) Histological analysis. HE, PAS, and toluidine blue staining of lung sections at P0 and P2. Scale bar, 50 mm. (D) Aerated lung area in control and DTG mice at P0 and P2. *, P < 0.05 versus control. (E) Thickness of alveolar septum in control and DTG mice at P0 and P2. *, P < 0.05 versus control. For experiments shown in panels D and E, n = 3 from a single dam under all conditions.
Akt activation in lung epithelial cells in utero results in increased numbers of CC10/SP-C double-positive cells and defective maturation of lung epithelial cells.
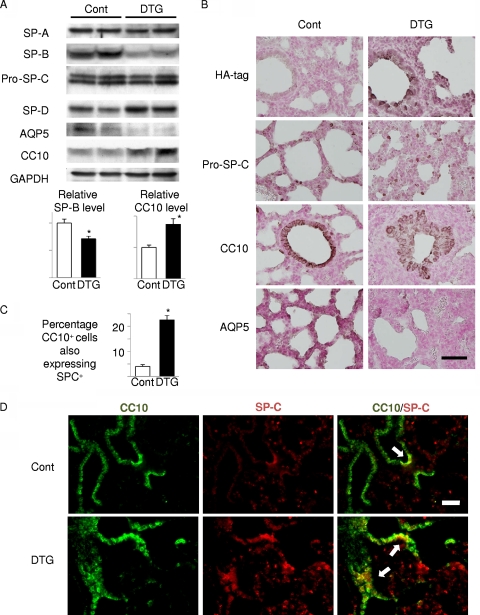
In order to further assess the extent of epithelial differentiation, we performed Western bolt analysis of surfactant proteins (SP-A to SP-D) and immunohistochemistry of marker proteins: SP-C for type II alveolar epithelial cells, Clara cell 10-kDa protein (CC10) for Clara cells, aquaporin-5 (AQP5) for type I alveolar epithelial cells, and calcitonin gene-related peptide (CGRP) for neuroendocrine cells. Western blot analysis of surfactant proteins revealed downregulation of SP-B and upregulation of SP-D, whereas the expression levels of SP-A and SP-C were not altered (Fig. 3A). Western blot analysis and immunohistochemistry also revealed increased expression of CC10 and reduced expression of AQP5 in the lung of DTG mice (Fig. 3A and B). CGRP expression was not altered between control and DTG mice (date not shown). Previous studies characterized CC10/SP-C double-positive cells as BASCs that reside at the bronchoalveolar duct junction and differentiate to both Clara cells and type II alveolar epithelial cells; the latter further differentiate to type I alveolar epithelial cells (15). Double immunostaining revealed that CC10/SP-C double-positive cells were increased in number in the lung of DTG mice (Fig. 3C and D). Collectively, these findings suggest that aberrant activation of Akt signaling in lung epithelial cells results in increased numbers of CC10/SP-C double-positive cells and impaired differentiation of alveolar epithelial cells.
FIG. 3.
Akt activation in lung epithelium results in defective maturation of lung epithelial cells and expansion of CC10/SP-C double-positive cells. (A) Western blot analysis of surfactant proteins (SP-A, SP-B, pro-SP-C, and SP-D), CC10 (a marker of Clara cells), and AQP5 (a marker of type I alveolar cells). Lower panels show densitometric analysis. *, P < 0.05 versus control (n = 4 mice from 2 dams for both controls and DTG). (B) Immunohistochemical analysis of HA tag (Akt1 transgene), SP-C, CC10, and AQP5. SP-C, CC10, and AQP5 were detected in cuboidal type II alveolar cells, Clara cells, and flat type I alveolar cells, respectively. Scale bar, 50 μm. (C and D). Double immunostaining of CC10 and SP-C. CC10/SP-C double-positive cells are indicated by arrows. Scale bar, 100 μm. *, P < 0.05 versus control (n = 5 control and n = 3 DTG mice from 3 dams).
Akt activation in lung epithelial cells in utero results in RDS in preterm infants.
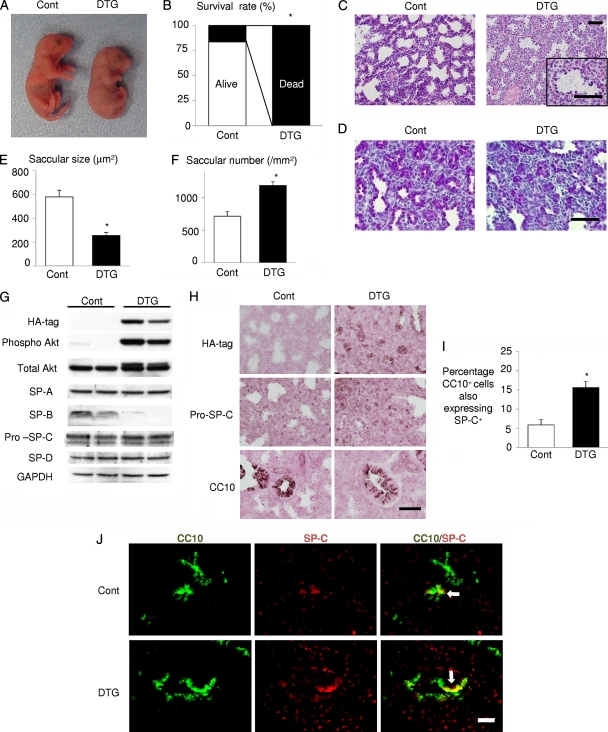
Since RDS is more frequent in preterm infants than in full-term infants, we examined preterm infants born by Caesarean section at E18.5. DTG mice born at E18.5 showed cyanosis (Fig. 4A) associated with an irregular respiratory pattern, and all DTG mice died by 2 h after birth (Fig. 4B). The airway spaces of DTG lungs were filled with amorphous, proteinaceous material that was not observed in control lungs (Fig. 4C). PAS staining revealed that the size and the density of saccules in DTG lung were smaller and higher than those of controls, respectively (Fig. 4D to F). Western blot analysis of surfactant proteins revealed downregulation of SP-B, whereas the expression levels of SP-A, -C, and -D were not altered (Fig. 4G). Immunohistochemistry also revealed expansion of CC10/SP-C double-positive cells in the lung of DTG mice (Fig. 4H to J). AQP5 expression was barely detectable at this stage (data not shown). These results collectively suggest that Akt activation in lung epithelial cells in utero results in RDS and perinatal lethality in preterm infants.
FIG. 4.
Akt activation in lung epithelium results in RDS and lethality in preterm infants. (A) Gross appearance of infants. (B) Survival rate by 2 h after delivery. *, P < 0.05 (n = 6 control and n = 5 DTG mice from 2 dams). (C) HE staining of the lung sections of infants delivered by Caesarean section at E18.5. Scale bar, 50 μm. (D) PAS staining of the lung sections of infants delivered by Caesarean section at E18.5. Scale bar, 50 μm. (E) Saccular size at E18.5. *, P < 0.05 versus control. (F) Saccular number at E18.5. *, P < 0.05 versus control. For the experiments shown in panels E and F, n = 3 control and n = 4 DTG mice from 2 dams. (G) Western blot analysis of surfactant proteins (SP-A, SP-B, pro-SP-C, and SP-D). (H) Immunohistochemistry of HA tag (Akt1 transgene), pro-SP-C, and CC10 at E18.5. Scale bar, 50 μm. (I and J) Double immunostaining of CC10 and SP-C. CC10/SP-C double-positive cells are indicated by arrows. Scale bar, 100 μm. For both panels, n = 4 (Cont) and n = 3 (DTG) from 2 dams.
Rapamycin improves respiratory distress and lung maturational defects induced by Akt1 overexpression in lung epithelium.
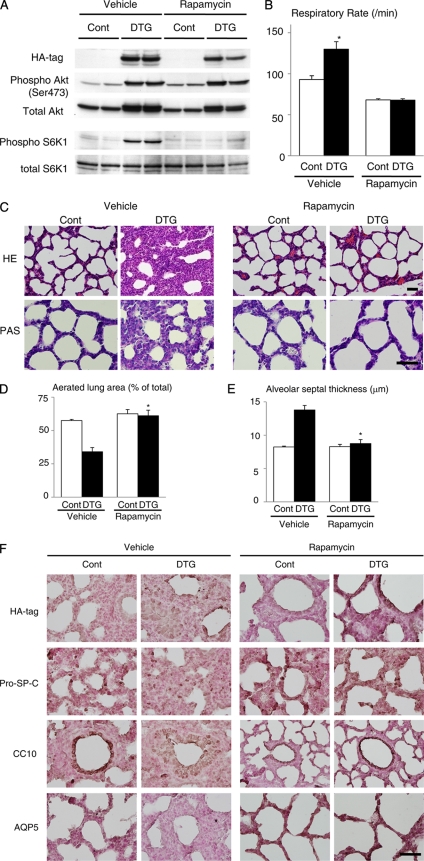
To test whether the Akt-mTOR pathway mediates lung pathology in DTG mice, rapamycin was administered to dams for 3 days (at E17.5, E18.5, and E19.5). Downregulation of mTOR signaling by rapamycin was confirmed by reduced phosphorylation levels of S6K1 (Fig. 5A). Examination of infants at P0 demonstrated that tachypnea observed in vehicle-treated DTG mice was improved by rapamycin treatment (Fig. 5B). Hematoxylin and eosin (HE) staining revealed that abnormal morphology of lung alveoli in DTG mice was reversed by rapamycin treatment (Fig. 5C, upper panel), and PAS staining demonstrated a reduced number of PAS-positive glycogen stores following rapamycin treatment (Fig. 5C, lower panel). By quantitative analysis of toluidine blue-stained sections, a decrease in aerated space and an increase in alveolar septal thickness observed in DTG mice were rescued by rapamycin treatment (Fig. 5D and E). Impaired differentiation of lung epithelial cells in DTG mice was also improved by rapamycin treatment, as evidenced by reduced expression of CC10, increased expression of AQP5, and a normal expression pattern of SP-C (Fig. 5F). These results suggest that mTOR is critically involved in respiratory distress and lung maturational defects induced by Akt1 overexpression in lung epithelium.
FIG. 5.
Rapamycin improves respiratory distress and lung maturational defects induced by Akt1 overexpression in lung epithelium. (A) Western blot analysis of Akt and S6K1 in the lung. (B) Respiratory rate of vehicle- or rapamycin-treated pups at P0. For vehicle experiments, n = 7 for both control and DTG mice from 3 dams; for rapamycin experiments, n = 14 (control) and n = 11 (DTG) mice from 4 dams. (C) Histological analysis. HE and PAS staining of lung sections at P0. Scale bar, 50 μm. (D) Aerated lung area in vehicle- or rapamycin-treated pups at P0. *, P < 0.05 versus vehicle-treated DTG mice. (E) Thickness of alveolar septum in vehicle- or rapamycin-treated pups at P0. *, P < 0.05 versus vehicle-treated DTG mice. For experiments shown in panels D and E, n = 3 control and n =3 DTG vehicle-treated mice from 3 dams, and n = 8 control and n = 7 DTG rapamycin-treated mice from 4 dams. (F) Immunohistochemistry of HA tag, pro-SP-C, CC10, and AQP5 at P0. Scale bar, 50 μm.
Rapamycin improves respiratory distress induced by preterm delivery.
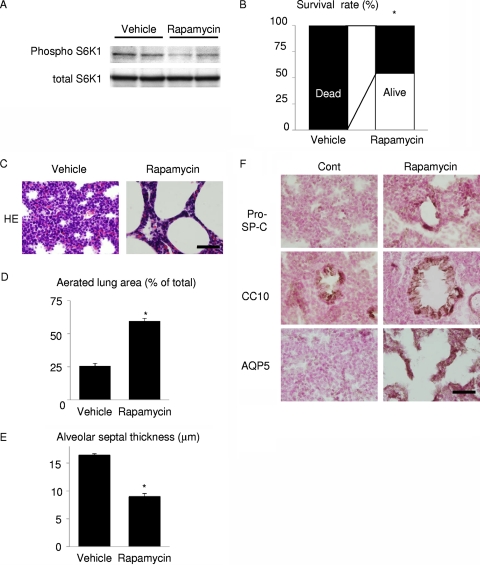
The above-mentioned results suggest the possibility that mTOR activation mediates RDS in wild-type infants delivered preterm. To test this hypothesis, pregnant wild-type mice were treated with vehicle or rapamycin at E15.5 and E16.5, and pups were collected at E17.5 by Caesarean section. Inhibition of mTOR in the lung of rapamycin-treated pups was confirmed by reduced phosphorylation levels of S6K1 (Fig. 6A). All pups delivered from vehicle-treated dams died within 1 h after birth, whereas more than 50% of pups delivered from rapamycin-treated dams survived in this time frame (Fig. 6B). Histological examination revealed that rapamycin treatment promoted lung maturation, as demonstrated by increased aerated lung area, decreased alveolar septal wall thickness, a normal expression pattern of SP-C, and increased expression of AQP5 (Fig. 6C to F). Thus, downregulation of mTOR signaling has a therapeutic potential for RDS induced by preterm delivery in wild-type mice.
FIG. 6.
Rapamycin improves respiratory distress and lung maturational defects induced by preterm delivery. (A) Western blot analysis of S6K1 in the lung. (B) Survival rate of wild-type pups 1 h after Caesarean section at E17.5. *, P < 0.05 versus vehicle-treated group. In the vehicle-treated group, n = 15 from a single dam. In the rapamycin group, n = 24 from 2 dams. (C) HE staining at 1 h after delivery. Scale bar, 50 μm. (D) Aerated lung area in vehicle- or rapamycin-treated pups. *, P < 0.05 versus vehicle-treated group. In the vehicle-treated group, n = 3 from a single dam; in the rapamycin group, n = 4 from 2 dams. (E) Thickness of alveolar septum in vehicle- or rapamycin-treated pups. *, P < 0.05 versus vehicle-treated group. In the vehicle-treated group, n = 3 from a single dam; in the rapamycin group, n = 4 from 2 dams. (F) Immunohistochemistry of pro-SP-C, CC10, and AQP5. Scale bar, 50 μm.
Activation of the Akt-mTOR pathway attenuates HIF-2-dependent VEGF expression in lung epithelial cells.
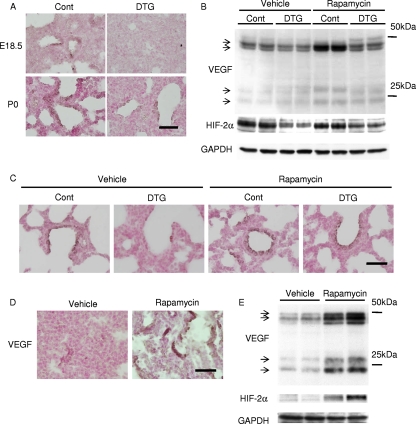
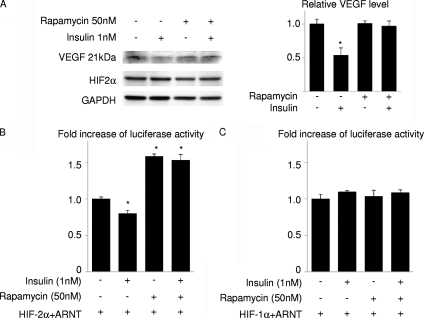
To investigate the mechanism by which Akt-mTOR signaling affects lung maturation, we examined the expression of VEGF, an angiogenic growth factor that is produced in the distal airway during late gestation and regulates coordinated development of alveolar epithelium and capillaries (4). Immunohistochemistry and Western blot analysis revealed that the expression levels of VEGF were downregulated in DTG mice both at E18.5 and P0 and were restored by rapamycin treatment (Fig. 7A to C). Since the expression of VEGF in the lung has been reported to depend on HIF-2 activity (4), we next examined the expression of HIF-2α protein in the lung. Western blot analysis revealed that the HIF-2α protein amount was downregulated in the lung of DTG mice (Fig. 7B). The expression levels of VEGF and HIF-2α were also examined in vehicle- or rapamycin-treated wild-type pups delivered at E17.5. Immunohistochemistry and Western blot analysis demonstrated the upregulation of VEGF and HIF-2α expression by rapamycin in wild-type mice delivered preterm (Fig. 7D and E). It was therefore concluded that activation of the Akt-mTOR pathway attenuates the expressions of VEGF and HIF-2α in the lung. However, it was also noted that the expression levels of HIF-2α do not necessarily correlate with those of VEGF because rapamycin treatment highly upregulated VEGF expression in control animals without altering HIF-2α expression levels (Fig. 7B, compare the vehicle control group and the rapamycin control group). We therefore examined whether Akt-mTOR signaling regulates the transcriptional activity of HIF-2. In cultured A549 lung epithelial cells, insulin induced downregulation of VEGF expression, which was reversed by rapamycin treatment (Fig. 8A). Luciferase assays using VEGF-luc as a reporter gene revealed that insulin attenuated transcriptional activity of HIF-2 on VEGF promoter, which was reversed by rapamycin treatment (Fig. 8B), while both insulin and rapamycin had minimal effects on HIF-1 transcriptional activity (Fig. 8C). These results suggest that activation of Akt-mTOR signaling attenuates HIF-2-dependent VEGF expression with respect to both the amount of HIF-2α protein and HIF-2 transcriptional activity.
FIG. 7.
Akt-mTOR pathway downregulates VEGF expression in the lung. (A) Immunostaining of VEGF at E18.5 and P0 in Akt1 TG mice. Scale bar, 50 μm. (B) Western blot analysis of VEGF and HIF-2α at P0 in Akt1 TG mice. (C) Immunostaining of VEGF at P0 in Akt1 TG mice. Scale bar, 50 μm. (D) Immunostaining of VEGF at E17.5 in wild-type mice. Scale bar, 50 μm. (E) Western blot analysis of VEGF and HIF-2α at E17.5 in wild-type mice.
FIG. 8.
Insulin attenuates VEGF expression and HIF-2 transcriptional activity on VEGF promoter in an mTOR-dependent manner in lung epithelial cells. (A) Western blot analysis of VEGF and HIF-2α in A549 cells treated with insulin and/or rapamycin for 48 h. The right panel shows the densitometric analysis. *, P < 0.05 versus the rapamycin (−)/insulin (−) group (n = 3 for each group). (B and C) Luciferase assays in A549 cells. A549 cells were transfected with a VEGF-luc reporter and expression vectors for HIF-1α, HIF-2α, and ARNT and treated with insulin and/or rapamycin. All experiments were performed in the presence of CoCl2 to mimic hypoxic conditions. *, P < 0.05 versus the rapamycin (−)/insulin (−) group.
Activation of the Akt-mTOR pathway reduces alveolar capillary density.
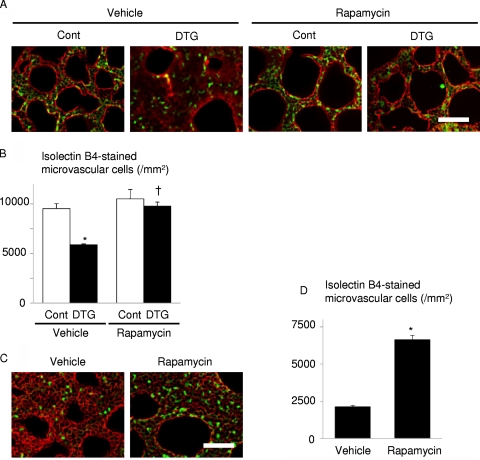
To test whether Akt-mTOR-mediated downregulation of VEGF is associated with attenuated alveolar angiogenesis, vascular morphometry was performed in DTG and control mice at P0. Isolectin B4 staining revealed that alveolar capillary density was significantly reduced in DTG mice compared to that in control mice, and this reduction was rescued by rapamycin treatment (Fig. 9A and B). We also found that alveolar capillary density in wild-type mice delivered preterm at E17.5 was increased by rapamycin treatment (Fig. 9C and D). Thus, alveolar capillary density is closely linked to the level of VEGF expression in the lung. These results are consistent with our hypothesis that Akt-mTOR signaling promotes RDS through downregulation of VEGF.
FIG. 9.
Alveolar capillary bed formation is impaired by Akt1 overexpression in lung epithelium, which is improved by rapamycin administration to the dams. (A) Histological analysis of vehicle- or rapamycin-treated pups at P0. Sections were stained with isolectin B4-FITC conjugate (green) to detect endothelial cells and with wheat germ agglutinin-TRITC conjugate (red) for membrane staining. Scale bar, 50 μm. (B) Alveolar capillary density. *, P < 0.05 versus vehicle-treated control mice; †, P < 0.05 versus vehicle-treated DTG mice. In the vehicle-treated group, n = 4 control and n = 3 DTG mice from 2 dams; in the rapamycin group, n = 4 control and n = 4 DTG mice from 2 dams. (C) Histological analysis of vehicle- or rapamycin-treated wild-type pups born by Caesarean section at E17.5. Scale bar, 50 μm. (D) Alveolar capillary density. *, P < 0.05 versus vehicle-treated mice (n = 4 vehicle-treated mice from a single dam, and n = 4 rapamycin-treated mice from 2 dams).
DISCUSSION
In this study we have demonstrated that activation of Akt signaling in lung epithelial cells during embryogenesis results in transient respiratory difficulties in full-term infants and in RDS in preterm infants. These respiratory defects were associated with bronchiolar hyperplasia, expansion of CC10/SP-C double-positive cells, and impaired maturation of lung epithelial cells. We also found that Akt-mTOR signaling is critically involved in the pathogenesis of RDS because rapamycin treatment improved respiratory distress and lung maturational defects induced by Akt activation or preterm delivery. Mechanistically, Akt-mTOR signaling attenuates both the protein amounts and transcriptional activity of HIF-2, leading to downregulation of HIF-2-dependent expression of VEGF, an angiogenic growth factor that is required for maturation of alveolar epithelial cells. These observations suggest the possibility that aberrant activation of Akt-mTOR signaling or preterm delivery before the appropriate downregulation of this signaling axis plays a causal role in RDS through downregulation of HIF-2-dependent VEGF expression and that the mTOR-HIF-2 pathway may be a novel therapeutic target for infant RDS.
RDS is frequently observed in infants of diabetic mothers, and hyperactivation of insulin signaling in response to maternal hyperglycemia has been proposed to play a pathogenic role (19, 22). It was also previously shown that alveolar epithelium-specific deletion of Pten results in RDS, with approximately 90% of neonates dying within 2 h after birth (33). This phenotype of conditional Pten deletion is more severe than that of alveolar epithelium-specific Akt1 transgenic mice, suggesting the possibility that PI3K-dependent but Akt-independent pathways are also implicated in the occurrence of RDS. However, because another line of lung epithelium-specific Pten knockout mice generated by a similar method exhibit a very mild phenotype (i.e., lack of neonatal lethality, normal postnatal development, and mild bronchiolar hyperplasia) (7), the variation of phenotypes among different animal models may be due in part to the differences in the genetic background of the animals. Furthermore, rapamycin treatment of dams significantly improved the phenotype of alveolar epithelium-specific Akt1 transgenic mice. Taken together, these observations suggest that activation of the PI3K-Akt-mTOR pathway in lung epithelial cells in utero plays a causal role in the pathogenesis of RDS in infants with diabetic mothers.
Lung development in mice is histologically divided into four phases: the pseudoglandular stage (E9.5 to 16.5), canalicular stage (E16.5 to 17.5), terminal sac stage (E17.5 to P5), and alveolar stage (P5 to P30) (30). Differentiation of type I and type II epithelial cells and high levels of Pten expression in respiratory epithelium occur at the terminal sac stage (17), which is consistent with the idea that downregulation of the PI3K-Akt-mTOR pathway is required for epithelial differentiation. In humans, lung development is relatively advanced compared with that of mice, and the terminal sac stage corresponds to human preterm infants between 26 and 36 weeks of gestation, when a high complication rate of RDS is observed. Thus, inhibition of the PI3K-Akt-mTOR pathway at specific time points during a later stage of embryogenesis appears to be critical for normal lung development and maturation, and aberrant activation of Akt-mTOR signaling or preterm delivery before this signaling pathway is appropriately downregulated may result in the occurrence of RDS. The observation that rapamycin was effective for RDS also suggests that downregulation of mTOR signaling is sufficient to induce maturation of lung epithelial cells. Previous studies also implicated GATA6-Wnt/β-catenin signaling and calcineurin/nuclear factor of activated T cells (NFAT) signaling in lung maturation (6, 34). How these two signaling pathways and the PI3K-Akt-mTOR pathway coordinately regulate normal lung development remains to be investigated. It should also be noted that VEGF-induced angiogenesis is enhanced in the presence of rapamycin, which is inconsistent with the observation that VEGF induces endothelial cell proliferation via the Akt-mTOR pathway (32). This may be in part explained by the proangiogenic effects of the rapamycin-insensitive downstream effectors of Akt such as glycogen synthase kinase-3 and the FOXO family of transcription factors (1, 16).
Epithelial-endothelial interactions during lung development are critical in establishing a functional blood-gas interface, and normal lung function depends on the coordinated development of alveolar epithelium and capillaries, which is primarily regulated by VEGF (27). It was previously shown that deletion of HIF-2α in mice causes RDS due to downregulation of HIF-2-dependent VEGF expression in the lung (4). The similarity of the phenotypes between lung epithelium-specific Akt1 TG mice and HIF-2α-deficient mice prompted us to investigate the mechanistic link between mTOR and HIF-2. Previous studies showed that mTOR enhances the transcriptional activity of HIF-1 (13) and that VEGF expression in the heart is induced by activation of the Akt-mTOR pathway in TG mice in which the Akt1 transgene is inducible in the heart by doxycycline treatment (25), suggesting that mTOR promotes HIF-1-dependent VEGF expression. However, VEGF expression in the lung was downregulated by Akt1 overexpression and restored by rapamycin treatment. Consistently, HIF-2α expression levels were downregulated in the lung of DTG mice, and reporter gene assays in cultured lung epithelial cells revealed that insulin attenuates the transcriptional activity of HIF-2 on VEGF promoter in an mTOR-dependent manner. These results suggest the possibility that HIF-2 and VEGF are situated downstream of mTOR in the pathogenesis of RDS and that activation of the Akt-mTOR pathway in the lung leads to RDS through the downregulation of HIF-2-dependent VEGF expression. How mTOR differentially regulates the transcriptional activity of HIF-1 and HIF-2 awaits further investigation.
There are several limitations in this study. First, because the experiments to test whether overexpression of HIF-2 or VEGF in lung epithelium rescues the RDS phenotype of Akt1 TG mice were not performed, the direct link between the Akt-mTOR pathway and the HIF-2-VEGF pathway remains to be determined. Second, although it is highly possible that hyperactivation of insulin signaling plays a causal role in RDS (8, 10, 11), the present study does not provide definitive evidence showing that insulin signaling is, indeed, activated in lung epithelium of infants having a diabetic mother. It is also possible that other factors that activate the PI3K-Akt-mTOR pathway (e.g., insulin-like growth factors) may be involved in the pathogenesis of RDS. Third, the TG mouse system used in this study may activate Akt in the lung to a supra-physiological level that is not comparable to the level of Akt activation observed in infants of diabetic mothers.
In summary, the present study demonstrates a crucial role for the Akt-mTOR pathway in the pathogenesis of infant RDS and suggests that the mTOR-HIF-2 signaling axis is a novel therapeutic target for this disease state.
Acknowledgments
We thank S. L. McKnight for phEP-1, A. Ochiai and G. Ishii for A549 cells, and E. Fujita, R. Kobayashi, and Y. Ishiyama for technical assistance.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology to I.K.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print on 28 December 2010.
REFERENCES
- 1.Abid, M. R., et al. 2004. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24:294-300. [DOI] [PubMed] [Google Scholar]
- 2.Bentley-Lewis, R., S. Levkoff, A. Stuebe, and E. W. Seely. 2008. Gestational diabetes mellitus: postpartum opportunities for the diagnosis and prevention of type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 4:552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clausen, T. D., et al. 2005. Poor pregnancy outcome in women with type 2 diabetes. Diabetes Care 28:323-328. [DOI] [PubMed] [Google Scholar]
- 4.Compernolle, V., et al. 2002. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat. Med. 8:702-710. [DOI] [PubMed] [Google Scholar]
- 5.Crowther, C. A., et al. 2005. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 352:2477-2486. [DOI] [PubMed] [Google Scholar]
- 6.Dave, V., et al. 2006. Calcineurin/Nfat signaling is required for perinatal lung maturation and function. J. Clin. Invest. 116:2597-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dave, V., et al. 2008. Conditional deletion of Pten causes bronchiolar hyperplasia. Am. J. Respir. Cell Mol. Biol. 38:337-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekowski, S. A., and J. M. Snyder. 1992. Insulin regulation of messenger ribonucleic acid for the surfactant-associated proteins in human fetal lung in vitro. Endocrinology 131:669-676. [DOI] [PubMed] [Google Scholar]
- 9.Feig, D. S., and V. A. Palda. 2002. Type 2 diabetes in pregnancy: a growing concern. Lancet 359:1690-1692. [DOI] [PubMed] [Google Scholar]
- 10.Guttentag, S. H., D. S. Phelps, W. Stenzel, J. B. Warshaw, and J. Floros. 1992. Surfactant protein A expression is delayed in fetuses of streptozotocin-treated rats. Am. J. Physiol. 262:L489-L494. [DOI] [PubMed] [Google Scholar]
- 11.Guttentag, S. H., D. S. Phelps, J. B. Warshaw, and J. Floros. 1992. Delayed hydrophobic surfactant protein (SP-B, SP-C) expression in fetuses of streptozotocin-treated rats. Am. J. Respir. Cell Mol. Biol. 7:190-197. [DOI] [PubMed] [Google Scholar]
- 12.Hallman, M., V. Glumoff, and M. Ramet. 2001. Surfactant in respiratory distress syndrome and lung injury. Comp. Biochem. Physiol. Part A 129:287-294. [DOI] [PubMed] [Google Scholar]
- 13.Hudson, C. C., et al. 2002. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22:7004-7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen, D. M., et al. 2004. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care 27:2819-2823. [DOI] [PubMed] [Google Scholar]
- 15.Kim, C. F., et al. 2005. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121:823-835. [DOI] [PubMed] [Google Scholar]
- 16.Kim, H. S., et al. 2002. Regulation of angiogenesis by glycogen synthase kinase-3β. J. Biol. Chem. 277:41888-41896. [DOI] [PubMed] [Google Scholar]
- 17.Luukko, K., A. Ylikorkala, M. Tiainen, and T. P. Makela. 1999. Expression of LKB1 and PTEN tumor suppressor genes during mouse embryonic development. Mech. Dev. 83:187-190. [DOI] [PubMed] [Google Scholar]
- 18.Maemura, K., et al. 1999. Generation of a dominant-negative mutant of endothelial PAS domain protein 1 by deletion of a potent C-terminal transactivation domain. J. Biol. Chem. 274:31565-31570. [DOI] [PubMed] [Google Scholar]
- 19.Montan, S., and S. Arulkumaran. 2006. Neonatal respiratory distress syndrome. Lancet 367:1878-1879. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto, M., and R. Kopan. 2009. rtTA toxicity limits the usefulness of the SP-C-rtTA transgenic mouse. Dev. Biol. 325:171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nold, J. L., and M. K. Georgieff. 2004. Infants of diabetic mothers. Pediatr. Clin. North Am. 51:619-637. [DOI] [PubMed] [Google Scholar]
- 22.Northway, W. H., Jr., R. C. Rosan, and D. Y. Porter. 1967. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 276:357-368. [DOI] [PubMed] [Google Scholar]
- 23.Perl, A. K., S. E. Wert, A. Nagy, C. G. Lobe, and J. A. Whitsett. 2002. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl. Acad. Sci. U. S. A. 99:10482-10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shioi, T., et al. 2003. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 107:1664-1670. [DOI] [PubMed] [Google Scholar]
- 25.Shiojima, I., et al. 2005. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Invest. 115:2108-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiojima, I., et al. 2002. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J. Biol. Chem. 277:37670-37677. [DOI] [PubMed] [Google Scholar]
- 27.Stenmark, K. R., and S. H. Abman. 2005. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu. Rev. Physiol. 67:623-661. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi, C. M., B. Emanuelli, and C. R. Kahn. 2006. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7:85-96. [DOI] [PubMed] [Google Scholar]
- 29.Tichelaar, J. W., W. Lu, and J. A. Whitsett. 2000. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 275:11858-11864. [DOI] [PubMed] [Google Scholar]
- 30.Warburton, D., et al. 2000. The molecular basis of lung morphogenesis. Mech. Dev. 92:55-81. [DOI] [PubMed] [Google Scholar]
- 31.Whitsett, J. A., and T. E. Weaver. 2002. Hydrophobic surfactant proteins in lung function and disease. N. Engl. J. Med. 347:2141-2148. [DOI] [PubMed] [Google Scholar]
- 32.Xue, Q., et al. 2009. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler. Thromb. Vasc. Biol. 29:1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanagi, S., et al. 2007. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J. Clin. Invest. 117:2929-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, Y., et al. 2008. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat. Genet. 40:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]