Abstract
E2FBP1/hDRIL1, a DNA-binding A/T-rich interaction domain (ARID) family transcription factor, is expressed ubiquitously in human tissues and plays an essential role in maintaining the proliferation potential of passage-limited human fibroblasts by dissociating promyelocytic leukemia nuclear bodies (PML-NBs). This effect on PML-NBs is similar to that of viral immediate-early gene products, such as infected cellular protein 0 (ICP0) from human herpes simplex virus 1 (HSV-1), which also disrupts PML-NBs to override the intrinsic cellular defense. Here we report that E2FBP1 inhibits accumulation of ICP0 RNA and, at the same time, is degraded via ICP0's herpes ubiquitin ligase 2 (HUL-2) activity upon HSV-1 infection. These reciprocal regulatory roles of ICP0 and E2FBP1 are linked in an ARID-dependent fashion. Our results suggest that E2FBP1 functions as an intrinsic cellular defense factor in spite of its PML-NB dissociation function.
E2FBP1 was cloned independently by several laboratories as an enhancer for E2F1/DP1 complex-mediated transcriptional activation (65) and was shown to be a human homologue of the Drosophila development-related transcription factor dead ringer (DRI) (34). E2FBP1 is evolutionally conserved from yeast to vertebrates (24) and is a member of the DNA-binding A/T-rich interaction domain (ARID) family. ARID proteins are implicated in transcriptional regulation, chromatin remodeling, cell cycle regulation, and developmental control, including cell fate determination (72). Among ARID family members, orthologues of E2FBP1 (i.e., ARID3a) involved in development are found in mice, fruit flies, zebra fish, and nematodes (73). BRIGHT, a rodent orthologue, is a B-cell regulator of immunoglobulin heavy chain transcription (28) whose expression is restricted to B-cell lineages, and it binds A/T-rich sequences within matrix-associating regions (MARs) flanking the intronic enhancer (28). BRIGHT also contains both a nuclear localization signal (NLS) and a nuclear export signal (NES), with nucleocytoplasmic shuttling controlled by chromosome region maintenance 1 (CRM1) (33), and is known to enhance transcriptional activation in the presence of Bruton's tyrosine kinase (Btk) (58, 70) and to modulate chromatin accessibility (39). In addition to Btk, interactions with promyelocytic leukemia nuclear body (PML-NB) components Sp100 and LYSP100B modulate BRIGHT's transcriptional activity (7). A REKLES motif flanking the C terminus of the ARID is required both for homo- and heterodimer formation and for interaction with its specific DNA target (32). Human E2FBP1 shares some features with murine BRIGHT, including its target DNA sequences, interaction partners, and subcellular localization; however, it is expressed ubiquitously in a broader range of tissues (34). This difference in distribution suggests unique roles for E2FBP1 in cellular controls. In fact, E2FBP1 contributes to cellular regulatory mechanisms, including cell cycle start (65), rescue from oncogenic RasV12-induced premature senescence (56), dissociation of PML-NBs (23), transforming growth factor beta (TGF-β)-induced fibroblast growth in pulmonary fibrosis (40), and p53-mediated cell cycle arrest following DNA damage (47). Silencing of E2FBP1 expression leads to PML-NB accumulation, resulting in PML-mediated premature senescence (23). Recently, sumoylation of K398 in the ARID of E2FBP1 was shown to modulate its transcriptional activity (57).
PML-NBs, alternatively described as nuclear domain 10 (ND10), typically appear in interphase nuclei as punctate domains in close proximity to MARs. PML-NBs are composed of diverse proteins, including PML, Sp100, Daxx, Rb, p53, histone deacetylases, polymerases, and helicases, all of which dynamically change their numbers and composition during the cell cycle (2, 14). Loss of PML-NB formation as a consequence of genomic translocation t(15;17) results in leukemogenesis through interference with promyelocytic differentiation (8), and thus PML-NBs are implicated in maintenance of cellular integrity (reviewed in reference 37), whereas increases in the size and number of PML-NBs strongly suppress cell cycle progression and subsequently induce premature senescence (31, 55).
PML-NBs also play a major role against viral infection. Two major components of PML-NBs, PML and Sp100, are induced by type I and II interferons, and abrogation of PML-NBs results in increased viral titers. Moreover, diverse viruses target PML-NBs at very early stages of infection, and their components are sorted to form similar structures in the vicinity of the sites of viral replication (reviewed in references 12, 61, and 66). Among the many viral proteins targeting PML-NBs, the best studied is infected cellular protein 0 (ICP0) of herpes simplex virus 1 (HSV-1). ICP0 is an immediate-early (IE) protein of HSV-1 that exhibits multiple functions, including transcriptional activation and herpes ubiquitin ligase (HUL) activity (reviewed in references 10 and 25). These functions are regulated by posttranslational modifications, interactions with cellular and viral proteins, and its subcellular localizations and are probably required for both efficient lytic infection and reactivation from latency (26, 27, 60, 67, 75; reviewed in references 10 and 25). In infected cells, ICP0 is initially nuclear and subsequently translocates to the cytoplasm after the onset of viral DNA replication (45, 68). ICP0 in the nucleus targets PML-NBs through its RING-dependent HUL-2 activity and ubiquitylates both PML and a sumoylated form of Sp100 to disintegrate PML-NBs (3, 13, 50, 54). This function of ICP0 is important for virus replication, as ICP0-null mutant viruses, which are defective in destruction of PML-NBs, have reduced viral yields at a low multiplicity of infection (MOI) (reviewed in references 11, 48, 59, 61, 64, and 66). Moreover, a reduction of either PML or Sp100 expression in human primary foreskin fibroblasts did not affect wild-type (WT) HSV-1 replication but increased gene expression and plaque-forming efficiency of ICP0-null mutants (20). Simultaneous depletion of both PML and Sp100 resulted in a significant increase in ICP0-null mutant expression (18). Curiously, while high-level expression of transduced PML resulted in increased formation of PML-NB-like nuclear domains in Vero cells, Hep-2 cells, and telomerase-transformed human foreskin fibroblasts, it did not affect replication of WT HSV-1 (22, 44). These apparently conflicting results suggested that components of PML-NBs contribute to the intrinsic viral response. Recently, some isoforms of Sp100 were revealed to protect PML from degradation and to suppress transcription of IE genes of HSV-1, including ICP0, although details of the mechanisms remain elusive (51, 52).
In this paper, we show that E2FBP1 undergoes ICP0-induced ubiquitylation, that the RING/zinc finger element of ICP0 is required for this activity, and that E2FBP1 suppresses accumulation of ICP0 RNA. These reciprocal regulatory roles of ICP0 and E2FBP1 are linked in an ARID-dependent fashion, suggesting a role for the ARID in productive HSV-1 replication.
MATERIALS AND METHODS
Cells and viruses.
hTERT-BJ1 cells (Clontech) were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 18% 199 medium and 10% fetal bovine serum (FBS). Human fetal lung fibroblast TIG-3 cells were used at the indicated population doublings (PD). Hep-2 and Vero cells were maintained in DMEM containing 10% FBS. Hep-2-derived cells constitutively expressing hemagglutinin (HA)-tagged E2FBP1 or its ARID deletion mutant (ΔA) were established by introducing either pEF2HA-E2FBP1WT-IRESP or pEF2HA-E2FBP1ΔA-IRESP DNA (see the following section) cleaved at a unique ScaI site into plasmids at the bla gene and were selected and maintained in the presence of 3 μg/ml of puromycin. HEK293FT cells (Invitrogen) were cultured in DMEM supplemented with 10% FBS, nonessential amino acid solution (Gibco), and 1 mM sodium pyruvate. HSV-1 wild-type strain F was propagated and titrated in Vero cells. Recombinant lentiviruses were produced using ViraPower packaging mix (Invitrogen) according to the manufacturer's manual.
Plasmids, synthetic oligonucleotides, and antibodies.
The coding sequences for two-tandem-repeat hemagglutinin (2HA)-tagged E2FBP1 (HA-E2FBP1) and its mutants, derived from pcDNA3 HA-E2FBP1 (23), were inserted between the XhoI and XbaI sites located downstream of the human elongation factor 1α promoter in pEF-IRESP (29). The sequences of HA-E2FBP1 and its mutants used a bovine growth hormone polyadenylation signal inserted downstream of the mouse mammary tumor virus (MMTV) 3′ long terminal repeat (LTR) promoter of pMTV-dhfr (38), and then they were substituted for the U6 short hairpin RNA (shRNA) expression unit of pLenti6-GW/U6-laminshRNA (Invitrogen) to generate a series of pLentiMMTV-2HA-E2FBP1 plasmids. For the pQE1-E2FBP1 construct, E2FBP1 was subcloned into the pQETriSystem 1 vector (Qiagen), which encodes a (His)8 tag. pDS16 (74), pCM2/7, and pCM11/93 (4, 5) are ICP0 expression plasmids for the WT and for deletion mutants lacking the RING/zinc finger motif and the C-terminal multimerization domain of ICP0, respectively. To construct a series of pGLIE0p-hRluc reporter plasmids for monitoring HSV-1 IE-0 expression, the upstream sequence of the IE-0 structural gene was amplified by PCR and subcloned between the NheI and HindIII sites of the pGL4.83 humanized Renilla reniformis luciferase (hRluc) expression plasmid, using an In-Fusion PCR cloning system. Expression plasmids employed for HA-tagged and (His)6-tagged ubiquitin (His-Ub) were pEF-IRESp-HA-Ub (6) and pCMV-His-Ub. Synthetic double-stranded small interfering RNA (siRNA) molecules for E2FBP1 and a nonsense control were designed using software provided by RNAi Co. Ltd. (Japan) and were synthesized by Japan Bio-Service Corporation and Proligo LLC (Japan). The following primary and secondary antibodies were used: ICP0 Clu 7 (42), ICP0 5H7 (Abcam), DRIL1 CBL665 (Bethyl Laboratories), ICP4 10F1 (Abcam), PML PG-M3 (SantaCruz), HA 3F10 (Roche), α-tubulin (CHI), Alexa Fluor 488-conjugated donkey anti-rat and anti-mouse IgG, Alexa Fluor 555-conjugated donkey anti-mouse and anti-rabbit IgG (Invitrogen), horseradish peroxidase (HRP)-conjugated donkey anti-mouse, anti-rabbit, and anti-rat IgG, and alkaline phosphatase (AP)-conjugated donkey anti-mouse, anti-rabbit, and anti-rat IgG (Chemicon).
Introduction of foreign DNAs and siRNAs.
Efficient DNA transformation of TIG-3 cells was achieved only within 45 PD, using Xfect reagent (Clontech). Otherwise, transformation of TIG-3 cells and HEK293FT cells was performed with either FuGene6 reagent (Roche) or Lipofectamine LTX (Invitrogen) according to the suppliers' instructions. hTERT-BJ1 cells were infected with lentiviruses expressing HA-E2FBP1 under the control of the MMTV LTR promoter, and stably transduced cell clones were isolated in the presence of 2 μg/ml blasticidin S hydrochloride. siRNA-mediated suppression of E2FBP1 was performed as previously described (23). For this experiment, TIG-3 cells at 47 PD were transformed twice with siRNAs and allowed to reach confluence. The cells were then plated on glass coverslips and transformed with pDS16, using FuGene6.
Infection.
HSV-1 infections were carried out for 30 min at room temperature, and the diluted HSV-1 stock was replaced with prewarmed (at 37°C) medium containing 10% FBS to terminate the step. The end of the infection step was taken as 0 min postinfection (mpi). Infected cells were incubated in a CO2 incubator at 37°C for the indicated times until harvest or fixation.
Immunofluorescence microscopy.
Cells grown on coverslips were fixed with phosphate-buffered saline (PBS) containing 4% paraformaldehyde and 1% FBS for 20 min at room temperature. The cells were subsequently permeabilized with 0.25% Triton X-100 for 10 min, washed with PBS three times, and then stained for 4 h with anti-DRIL1 for E2FBP1 at a dilution of 1:2,000 and with 5H7 for ICP0 at a dilution of 1:20,000 at room temperature. Cells treated with primary antibodies were washed with PBS and then stained for 2 h with a secondary antibody solution containing 250 ng/ml of DAPI (4′,6-diamidino-2-phenylindole) and a 1:2,000-diluted mix of anti-rabbit IgG and anti-mouse IgG conjugated with Alexa 488 and Alexa 555, respectively. After being washed with PBS, specimens were mounted on glass slides with ProLong Gold antifade reagent (Molecular Probes) and subjected to fluorescence microscopy using an FV1000 laser scanning confocal microscope system with Fluoview software, version 1.6 (Olympus, Japan).
Extract preparation, Ni-NTA pulldown, and immunoprecipitation.
In most instances, cells were lysed in high-salt buffer (HSB; 300 mM NaCl, 50 mM HEPES-sodium, pH 7.0, 1 mM EDTA, 0.1% NP-40, 1 mM Na3VO4) containing protease inhibitors [PI mix; 2 μM MG115, 2 μM MG132, 1 mM Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK), 400 μM 4-amidinophenylmethanesulfonyl fluoride hydrochloride, and 400 μM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride]. Cellular debris was removed by centrifugation at 15,000 × g, and protein concentrations of cleared lysates were measured using a bicinchoninic acid protein assay kit (Sigma-Aldrich). For Ni-nitrilotriacetic acid (Ni-NTA) pulldown, cells were disrupted with 6 M guanidine hydrochloride solution containing 20 mM imidazole (GHI), or cells suspended in 150 μl of PBS were lysed with 900 μl of GHI. Lysates were sonicated and mixed with 40 μl of 50% Ni-NTA agarose beads (Qiagen) equilibrated with GHI to collect His-tagged proteins. Following 1 h of gentle rocking, beads were washed three times with 1 ml GHI, followed by two washes with PBS containing 0.25% Tween 20 (PBST). After removal of the buffer, Ni-NTA agarose beads were boiled with 50 μl of 2× Laemmli sample buffer (120 mM Tris-HCl, pH 6.8, 250 mM dithiothreitol [DTT], 3% SDS, 20% glycerol, 0.02% bromphenol blue) (36), and 25-μl aliquots were analyzed by SDS-PAGE. For immunoprecipitation, cells were treated with 10 μM (each) proteasome inhibitors MG115 and MG132 for 15 min and then lysed with medium-salt buffer (MSB; 250 mM NaCl, 50 mM HEPES-sodium, pH 7.0, 1 mM EDTA, 0.1% NP-40, 1 mM Na3VO4) containing PI mix without TLCK, and 2 mg of each cell lysate was incubated with 0.5 μg of primary antibody and 5 μl of 50% protein A Sepharose-FF beads (Pharmacia-GE Healthcare) for 2 h. The beads were washed four times with MSB, and antigen was released by boiling with 15 μl of 2× Laemmli sample buffer for 5 min and then subjected to immunoblotting.
Immunoblotting.
Proteins separated by SDS-PAGE were transferred to Immobilon P polyvinylidene difluoride membranes (Millipore), and membranes were probed with a primary antibody suspended in PBS containing 0.2% I-Block blocking reagent (Tropix) for 2 h at room temperature, washed with PBST, and incubated with an appropriate secondary antibody conjugated with HRP or AP for 1 h. The membranes were subsequently washed with PBST, exposed to enhanced chemiluminescence (ECL) reagent (GE Healthcare Bioscience) or CDP-Star chemiluminescence substrate (Millipore), and detected following exposure to X-ray film or with a Chemidoc chemiluminescence/fluorescence imaging instrument with Quantity One software, version 4.6.2 (Bio-Rad).
RT-qPCR analysis of transiently transformed and infected cells.
TIG-3 cells (1.8 × 106 cells/dish) at 43 to 45 PD seeded in a 100-mm dish were transformed with 30 μg/dish of pEF-IRESP, pEF2HA-E2FBP1WT-IRESP, and pEF2HA-E2FBP1ΔA-IRESP DNAs, using Xfect (Clontech). On the third day after seeding, cells were infected with HSV-1 at the stated MOI, and subsequently, infected cells were collected at various times postinfection, washed with ice-cold PBS, and suspended in 400 μl of PBS; aliquots of the suspension (200 μl) were then subjected to either genomic DNA or total RNA extraction. A mixture of genomic and viral DNAs (genomic/viral DNA) was extracted with a NucleoSpin Blood kit (Macherey-Nagel) according to the manufacturer's instructions. Yields of genomic DNA mixtures were measured with a Nanodrop 1000 spectrophotometer (Thermo Fisher) and ranged from 18 to 250 μg. Total RNA extraction was carried out with a High Pure RNA isolation kit (Roche Applied Science). RNA yields were measured with a Nanodrop 1000 spectrophotometer and ranged from 13.5 to 23 μg. The resulting RNAs were converted to cDNAs with a Transcriptor High Fidelity cDNA synthesis kit. Real-time quantitative PCR (RT-qPCR) was performed on a LightCycler 480 instrument (Roche Applied Science) with either 20 ng of genomic/viral DNA or 200 ng of cDNA, using a LightCycler 480 Probes master reagent kit (Roche Applied Science) equipped with specific hydrolysis probes. The PCR program consisted of the following steps: primary denaturation at 95°C for 5 min; 45 PCR cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 1 s; and termination at 50°C for 30 s. The sequences of primers and combined hydrolysis probes shown in Table 1 were designed with ProbeFinder online software, versions 2.44 and 2.45, provided by the Roche Applied Science Assay Design Center. The genes for RNase P RNA component H1 (RPPH1; GenBank accession number NR_002312) and 18S rRNA (GenBank accession number X03205) were employed as references for normalizing copy numbers of genomic/viral DNA (43) and expressed transcripts (53), respectively, and data were processed both by LCS480 software, version 1.5.0.39, and manually by comparative threshold cycle (CT) numbers as described previously (43, 63). Copy numbers of HSV-1 DNA were calculated as a half of the ICP0 gene number detected in the genomic/viral DNA mixture. Copy numbers of transformed plasmids in the genomic/viral DNA were calculated based on the internal ribosome entry site/Cap-independent translation enhancer (IRES) sequence from the encephalomyocarditis virus polyprotein gene present in pEF-x-IRESP plasmids. ICP0 RNA levels from infected HSV-1 were first normalized to 18S rRNA levels and then to the number of HSV-1 genomes (53). Every experiment was done in triplicate and repeated at least twice.
TABLE 1.
Primers and combined hydrolysis probes designed for quantitative PCRa
| Target | GenBank accession no. | Primer direction | Sequence | Probe |
|---|---|---|---|---|
| E2FBP1 | NM_005224 | Forward | GCACTCCAGCAGAACTTCCT | UPL 44 |
| Reverse | AGAGTCCTGGCGGCTTTC | GCTGCCCA | ||
| ICP0 | X04614 | Forward | AGCCCCGTCTCGAACAGT | UPL 56 |
| Reverse | ACCACCATGACGACGACTC | TGCTGTCC | ||
| IRES | M81861 | Forward | TGGCTCTCCTCAAGCGTATT | UPL 41 |
| Reverse | CCCATACAATGGGGTACCTTC | GGCTGAAG | ||
| 18S rRNA | X03205 | Forward | CGATTGGATGGTTTAGTGAGG | UPL 81 |
| Reverse | AGTTCGACCGTCTTCTCAGC | GGCCCTGG | ||
| RPPH1 | NR_002312 | Forward | CCGGAGCTTGGAACAGACT | UPL 30 |
| Reverse | GTAGTCTGAATTGGGTTATGAGGTC | GGCTGAGG |
UPL, universal probe library probe provided by Roche Applied Science; IRES, cap-independent translation enhancer sequence from encephalomyocarditis virus polyprotein gene (nucleotides 335 to 834) subcloned into pEF-x-IRESP; RPPH1, RNase P RNA component H1.
Luciferase assay.
To observe the activity of the IE-0 gene promoter, TIG-3, hTERT-BJ1, and Hep-2 cells were plated in 24-well culture dishes and transformed with a DNA mixture containing pGLIE0p-hRluc, pcycD1Pr-luc(−30) (carrying the Photinus pyralis luciferase [luc] gene linked with a 31-bp fragment of rat cyclin D1 upstream sequence [GenBank accession number AF148946] without any obvious regulatory motifs), and pEF2HA-E2FBP1-IRESP (expression plasmid for wild type or ARID deletion mutant of E2FBP1). Eighteen hours after transformation, cells were washed with ice-cold PBS and lysed with 100 μl of passive lysis buffer. Dual-luciferase assays were then performed with a dual-luciferase reporter assay system (Promega) according to the manufacturer's manual. All assays were done in triplicate and repeated.
RESULTS
E2FBP1 interacts and colocalizes with ICP0.
Immunoprecipitation was used to ask if E2FBP1 and ICP0 interacted. HEK293FT cells were cotransformed with an ICP0 expression plasmid (pDS16) and an E2FBP1 expression plasmid (HA-E2FBP1), and cell lysates were subjected to both immunoblotting and immunoprecipitation (Fig. 1 A). Proteins precipitated with anti-HA antibody included ICP0. This result suggested a possible interaction between ICP0 and E2FBP1. The reciprocal immunoprecipitation with anti-ICP0 antibody was not successful, as E2FBP1 binds with protein A-Sepharose beads under nondenaturing conditions. The amount of ICP0 detected was less than 1% of the input from the whole-cell lysate (WCL). These data suggest that the E2FBP1 and ICP0 interaction is either weak or unstable.
FIG. 1.
Interaction and colocalization of E2FBP1 and ICP0 in HEK293FT and TIG-3 cells. (A) Interaction between E2FBP1 and ICP0 was detected by immunoprecipitation of cell lysates. HEK293FT cells were transformed with pDS16 (ICP0), pcDNA3-2HA-E2FBP1 (HA-E2FBP1), and pcDNA3 (empty vector) and cultivated for 18 h. Cell were treated with 10 μM (each) proteasome inhibitors MG115 and MG132 for 15 min, and lysates were prepared and subjected to immunoblotting and immunoprecipitation. The numbers on top of the lanes indicate the relative amounts of the plasmids. Lanes 1 to 3 received 20 μg of cell lysate, whereas lanes 4 to 6 contained coprecipitated materials obtained from 2 mg of cell lysates incubated with either anti-ICP0 (5H7) or anti-HA (3F10) antibody. WCL, whole-cell lysate; IB, immunoblotting; IP, immunoprecipitation. (B) Endogenous E2FBP1 and ICP0 expressed from transformed plasmid DNA colocalized in PML-NB-like nuclear subdomains. TIG-3 cells treated with or without E2FBP1 siRNA (siE2FBP1) or nonsense control siRNA (siControl) were subsequently transformed with pDS16. After 2 days, cells were stained with anti-ICP0 (5H7) (green), anti-DRIL1 (red), and DAPI (gray), and images were captured by confocal microscopy.
Colocalization of endogenous E2FBP1 and ICP0 was studied by confocal microscopy. TIG-3 cells transformed with siRNA against E2FBP1 (siE2FBP1) or with a nonsense control (siControl) were further transformed with the ICP0 expression plasmid pDS16. In mock-treated and siControl-treated cells (Fig. 1B, left and right panels, respectively), E2FBP1 was spread ubiquitously in the nucleoplasm in the absence of ICP0, as previously reported (23). In these cells, ICP0 was found along with endogenous E2FBP1 in subnuclear foci that were reminiscent of enlarged PML-NBs (yellow-green signals observed in both left and right panels). Colocalization of ICP0 and endogenous E2FBP1 in nuclear foci was observed in 100% of transformed cells (n = 40). In contrast, when cells were pretreated with siE2FBP1, ICP0 was localized diffusely throughout the nucleoplasm (Fig. 1B, middle panels). A diffuse nuclear distribution of ICP0 was also observed in 100% of cells that expressed undetectable levels of E2FBP1 (n = 16). These data reveal that interaction of E2FBP1 and ICP0 in vivo affects ICP0's nuclear distribution.
ICP0 accelerates polyubiquitylation of E2FBP1.
We next asked if an interaction between E2FBP1 and ICP0 could be detected in HSV-1-infected cells. Reciprocal immunoprecipitations with anti-HA or anti-ICP0 were not successful (data not shown). It is possible that other HSV immediate-early proteins further weaken this interaction, making it difficult to detect by immunoprecipitation. Because ICP0 is a biheaded ubiquitin ligase (E3), we tested whether E2FBP1 was polyubiquitylated during the early phase of HSV-1 infection. HEK293FT cells were transformed with HA-E2FBP1 and His-Ub expression plasmids and subsequently infected with HSV-1 at an MOI of 10. Cell lysates were prepared at various times postinfection and subjected to immunoblotting and Ni-agarose (Ni-NTA) pulldown to check expression levels or to collect His-tagged ubiquitylated proteins (Fig. 2 A). E2FBP1 was polyubiquitylated in uninfected cells, and the polyubiquitylation level gradually increased after infection (lanes 7 to 9). ICP0 abundance increased after 80 mpi (lanes 2 and 3), suggesting to us that it was a potential E3 enzyme for E2FBP1.
FIG. 2.
ICP0 induces polyubiquitylation of E2FBP1. (A) The level of polyubiquitylated E2FBP1 increased during the immediate-early phase of HSV-1 infection. HEK293FT cells expressing HA-E2FBP1 and His-Ub were infected with HSV-1 at an MOI of 10. Cell lysates were prepared at the indicated times postinfection. The left and middle panels show expression levels of ICP0 (lanes 1 to 3) and HA-E2FBP1 (lanes 4 to 6). The right panel shows polyubiquitylated forms of HA-E2FBP1 (lanes 7 to 9) collected from cell lysates. (B) Polyubiquitylation of E2FBP1 was enhanced by expression of ICP0. The relative ratios of plasmids transformed into HEK293FT cells are shown at the top of the figure. Cell lysates were prepared after 20 h of transformation and subjected to immunoblotting (lanes 1 to 6) or Ni-NTA pulldown (lanes 7 to 12). The positions of ICP0 and nonubiquitylated endogenous or His-tagged E2FBP1 are indicated on the left. mpi, minutes postinfection.
This supposition was verified by immunoblotting and Ni-NTA pulldown of cell lysates prepared from HEK293FT cells transformed with expression plasmids for ICP0, E2FBP1-His, and HA-Ub. These experiments confirmed that E2FBP1 is polyubiquitylated in the absence of ICP0 (Fig. 2B, lanes 8 and 11). However, expression of ICP0 induced greater levels of polyubiquitylated E2FBP1, seen as slower-migrating species of E2FBP1-His (Fig. 2B, lanes 9 and 12). Thus, E2FBP1 was further ubiquitylated in the presence of ICP0 during the immediate-early phase of HSV-1 infection. Interaction of ICP0 and E2FBP1 is most probably a transient enzyme-substrate interaction, and the polyubiquitylation process may lead to degradation of the targeted substrate, which could explain the inefficient recovery of E2FBP1-ICP0 complexes from infected cell lysates.
ICP0 mediates polyubiquitylation of E2FBP1 through its RING/HUL-2 domain.
To examine whether ICP0 E3 activity mediates polyubiquitylation of E2FBP1, expression plasmids for ICP0 mutants lacking either the entire RING/HUL-2 domain (ΔRING) or the C-terminal half of the HUL-1 domain (ΔC) (Fig. 3 A) were transformed with HA-E2FBP1 and His-Ub expression plasmids. Immunoblotting and Ni-NTA pulldown assays revealed that highly polyubiquitylated, slowly migrating E2FBP1 accumulated in the presence of WT and ΔC ICP0 proteins (Fig. 3B, lanes 2, 4, 6, and 8). In contrast, ΔRING ICP0 did not enhance levels of highly polyubiquitylated E2FBP1 (lanes 3 and 7). These data revealed that ICP0 polyubiquitylated E2FBP1 through its RING/HUL-2 domain.
FIG. 3.
ICP0 mediates polyubiquitylation of E2FBP1 through its RING/HUL-2 domain. (A) Schematic representation of ICP0 and its deletion mutants. (B) ICP0 lacking the RING/HUL-2 domain fails to induce E2FBP1 polyubiquitylation. Expression plasmids were transformed into HEK293FT cells at the ratios indicated at the top of the figure. Cell lysates were prepared after 20 h of transformation and subjected to immunoblotting (lanes 1 to 4) (top, ICP0; bottom, HA-E2FBP1) and Ni-NTA pulldown followed by immunoblotting (lanes 5 to 8) (HA-E2FBP1). Positions for nonubiquitylated and ubiquitylated HA-E2FBP1 and ICP0 mutant proteins are shown to the left of the figure.
The ARID of E2FBP1 is targeted for polyubiquitylation by ICP0.
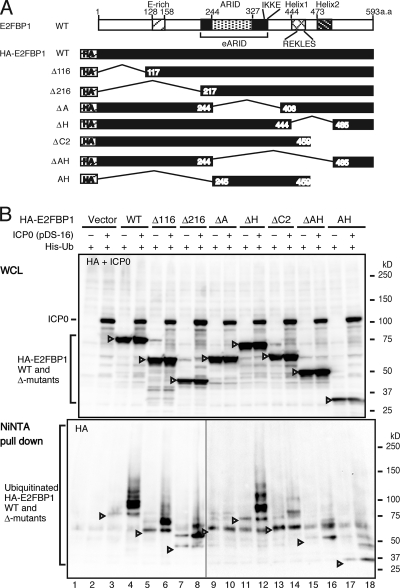
We next asked which region of E2FBP1 was targeted for polyubiquitylation by ICP0. Extracts from HEK293FT cells transformed with the plasmid combinations shown in Fig. 4 B were subjected to immunoblotting and Ni-NTA pulldown assays. All E2FBP1 deletion mutants were polyubiquitylated, regardless of whether ICP0 was present (Fig. 4B, lanes 3, 5, 7, 9, 11, 13, 15, and 17). Therefore, the endogenous ubiquitin ligase(s) targets multiple residues in E2FBP1, spanning the entire molecule. Ectopic expression of ICP0 resulted in greater levels of polyubiquitylated E2FBP1 and its mutants, except for the ΔA mutant (Fig. 4B, lanes 4, 6, 8, 12, 14, 16, and 18). Therefore, most target sites (i.e., lysine residues) for ICP0-mediated ubiquitylation reside in the ARID (compare lanes 9 and 10). Among these residues, Lys398 and Lys399, present in the Ile-Lys-Lys-Glu (IKKE) motif, are known targets for sumoylation (57). Importantly, ICP0 significantly increased polyubiquitylation of the ΔAH protein (Fig. 4B, compare lanes 15 and 16), and thus a Lys residue(s) residing outside the ARID and the helix-loop-helix (HLH) domain might be polyubiquitylated by ICP0. Because the Arg-Glu-Lys-Leu-Glu-Ser (REKLES) motif residing in the HLH domain is required for dimerization of ARID3 members (32), the discrepancy between the ICP0-mediated polyubiquitylation statuses of ΔA and ΔAH proteins may be explained by an additional target site(s) besides those in the ARID. The target Lys residues residing outside the ARID might be concealed after REKLES-mediated homodimerization of E2FBP1. The high level of ICP0-dependent polyubiquitylation of the ΔH protein (Fig. 4B, compare lanes 11 and 12) could result from polyubiquitylation of Lys residues residing both inside and outside the ARID.
FIG. 4.
The ARID of E2FBP1 is polyubiquitylated in response to expression of ICP0. (A) Schematic representation of HA-E2FBP1 and its deletion mutants. (B) Polyubiquitylation of all E2FBP1 mutants but the ΔA mutant was induced by ICP0. Expression plasmids for the E2FBP1 deletion mutants shown in panel A, for His-Ub, and for ICP0 were transformed into HEK293FT cells as indicated. Cell lysates were prepared and subjected to immunoblotting (upper panel) (HA-E2FBP1 and ICP0) and Ni-NTA pulldown followed by immunoblotting (lower panel) (HA-E2FBP1). The levels of various E2FBP1 proteins and ICP0 are shown in the top panel. ICP0 and nonubiquitylated and ubiquitylated HA-E2FBP1 proteins are indicated. The bottom panel shows the levels of ubiquitylated E2FBP1 proteins.
The decrease in E2FBP1 levels after infection with HSV-1 requires the ARID.
As shown in Fig. 5 A, both endogenous and exogenous E2FBP1 levels in Hep-2 cells were decreased within 130 mpi in response to infection with HSV-1. In contrast, the abundance of ΔA HA-E2FBP1, lacking the ARID, was unaffected by HSV-1 infection (Fig. 5A, bottom panels). These effects were enhanced in hTERT-BJ1 cells at 120 mpi (Fig. 5B, upper panels). These immunofluorescence analyses support the biochemical data shown in Fig. 4B and suggest that E2FBP1 is degraded by ICP0 through polyubiquitylation within the ARID.
FIG. 5.
Accumulation of E2FBP1 or ICP0 in HSV-1-infected cells is affected by the level of the other. (A) Hep-2 cells were transformed with a control (vector) expression plasmid or with expression plasmids for wild-type (WT) or ΔA HA-E2FBP1. The cells were then grown on glass coverslips and infected with HSV-1 at an MOI of 10. After infection, cells were further cultivated for the indicated times and then fixed and stained with anti-E2FBP1 (DRIL1; red), anti-ICP0 (5H7; green), and DAPI (gray). (B) hTERT-BJ1 cells were infected with recombinant lentiviruses encoding either HA-E2FBP1 or its ΔA mutant driven by an MMTV LTR promoter. The cells were then grown on glass coverslips, treated with 2 μM dexamethasone (Dex) to induce expression of HA-E2FBP1 for 18 h, infected with HSV-1 at an MOI of 5 in the presence of Dex, and maintained in the presence of Dex until fixation at 120 mpi. Cells were stained with anti-HA (red), anti-ICP0 (green) (left panels), and DAPI (gray). (C) Colocalization of E2FBP1 proteins and ICP0 in HSV-1-infected hTERT-BJ1-derived cells. hTERT-BJ1 cells were infected with recombinant lentiviruses encoding the indicated mutants of HA-E2FBP1 driven by an MMTV LTR promoter. The cells were then infected with HSV-1 and treated as described above. Cells were stained at 120 mpi with anti-HA (red), anti-ICP0 (green), and DAPI (gray).
E2FBP1's C terminus is required for interaction with ICP0.
Together with the results shown in Fig. 1B, the colocalization of ΔA E2FBP1 with ICP0 (Fig. 5A and B) suggested that these proteins interact in the cell nucleus. Accordingly, confocal microscopy was used to ask which domain of E2FBP1 was required for interaction with ICP0 (Fig. 5C). The proteins encoded by all deletion mutants of HA-E2FBP1, except for the ΔC2 mutant, showed a high degree of nuclear colocalization; in contrast, colocalization of the ΔC2 protein with ICP0 was rarely observed. These results suggested that E2FBP1's C terminus (i.e., amino acids 485 to 593) is likely required for interaction with ICP0.
E2FBP1 represses ICP0 expression at the level of transcription.
Because E2FBP1 was originally reported to be a transcription factor (65), it was conceivable that decreased ICP0 resulted from E2FBP1-repressed transcription from the IE-0 gene. High expression levels of ΔA E2FBP1 and ICP0 (Fig. 5A and B) were probably a consequence of deletion of a polyubiquitylation target region in E2FBP1 and the loss of its function as a transcriptional repressor. HEK293FT cells ectopically expressing HA-E2FBP1 were infected with HSV-1 at various MOIs, and cell lysates were examined for ICP0 levels by immunoblotting at 120 mpi (Fig. 6 A). Accumulation of ICP0 was detected in cells with ectopic E2FBP1 expression only after infection at an MOI of 1 (Fig. 6A, lane 10). This level of ICP0 was equivalent to what was seen in control cells at an MOI of 0.1 (Fig. 6A, lane 4). Moreover, in the absence of ectopic expression of E2FBP1, infection at an MOI of 1 resulted in a substantially higher level of ICP0 (Fig. 6A, lane 5). Thus, accumulation of ICP0 was repressed in response to ectopic expression of E2FBP1.
FIG. 6.
Expression of endogenous E2FBP1 affects ICP0 accumulation, and vice versa, during HSV-1 infection. (A) Accumulation of ICP0 protein during HSV-1 infection was repressed by ectopic expression of E2FBP1 in HEK293FT cells. HEK293FT cells were transformed with either pcDNA3 (empty vector) or pcDNA3-2HA-E2FBP1 and infected with HSV-1 at the indicated MOIs. Cell lysates were prepared at 120 mpi and subjected to immunoblotting with anti-ICP0 (upper panel) and anti-HA (lower panel). (B) Ectopic expression of ICP0 is repressed by ectopic expression of E2FBP1. HEK293FT cells were transformed with the indicated expression plasmids, and cell lysates were prepared after 120 mpi and subjected to immunoblotting. Expression of ICP0 is shown in the upper panel, and that of E2FBP1 is shown in the lower panel. Numbers at the top of the figure indicate relative amounts of expression plasmids. endo-E2FBP1, endogenous E2FBP1.
To examine whether E2FBP1-repressed ICP0 expression occurred in the absence of other HSV-1-derived factors, extracts from HEK293FT cells cotransformed with an ICP0 expression plasmid (pDS16) together with an HA-E2FBP1 expression plasmid were subjected to immunoblotting. There was a significant dose-dependent reduction of ICP0 levels (Fig. 6B). The unexpectedly increased level of endogenous E2FBP1 (Fig. 6B, lanes 2 and 3) may have been a consequence of dilution of ICP0's E3 activity. These results led us to posit that E2FBP1 represses accumulation of ICP0 by decreasing transcription of its RNA.
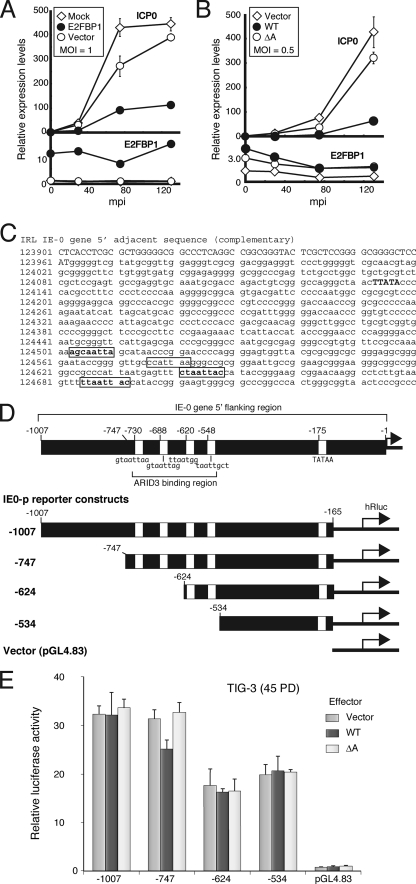
To examine the molecular basis of E2FBP1-mediated repression of ICP0, TIG-3 cells at 43 PD were transformed with HA-E2FBP1 DNA and then infected with HSV-1 at an MOI of 1. TIG-3 cells transformed with empty vector and nontransformed TIG-3 cells were infected as controls. Samples were collected at 0, 30, 75, and 130 mpi and then subjected to RT-qPCR analyses. ICP0 RNA was detected readily at 30 mpi and was increased in both nontransformed (mock) and empty vector control cells (Fig. 7 A). In contrast, ICP0 RNA accumulation was significantly lowered in cells expressing E2FBP1.
FIG. 7.
The E2FBP1 ARID is required for repression of accumulation of ICP0 transcripts. (A) E2FBP1 decreases accumulation of transcripts encoding ICP0 from HSV-1. TIG-3 cells at 43 PD were transformed with either a plasmid expressing E2FBP1 or empty vector, incubated for 44 h, and then infected with HSV-1 at an MOI of 1 for 30 min. Total cellular RNA was collected at the indicated times postinfection and subjected to RT-qPCR analyses. The upper chart represents the relative accumulation of ICP0 RNA, and the lower chart shows the relative accumulation of E2FBP1 RNA. Nontransformed TIG-3 cells (mock) served as a control for transformation. (B) E2FBP1 requires its ARID to repress accumulation of ICP0 RNA. TIG-3 cells at 45 PD were transformed with empty vector or plasmid expressing either wild-type (WT) or ΔA E2FBP1, incubated for 44 h, and then infected with HSV-1 at an MOI of 0.5 for 30 min. Total cellular RNA was then collected at the indicated times postinfection and subjected to RT-qPCR analyses. (C) Potential ARID3-binding motifs in the IE-0 promoter. The IRL region of HSV-1 strain F (GenBank accession no. GU734771) was searched for ARID3-binding consensus motifs. The DNA sequence constituting the promoter for IE-0 is shown, and boxes identify ARID3 consensus and consensus-like motifs. (D) Schematic representation of ARID3 consensus motifs residing in the IE-0 promoter and its truncated sequences linked upstream of the hRluc gene in reporter constructs. (E) Relative expression levels of hRluc activity in the presence or absence of wild-type or ΔA mutant E2FBP1 expressed from reporter constructs.
Because ICP0 levels were unaffected after infection of cells expressing ΔA E2FBP1, ICP0 RNA levels were compared in cells expressing wild-type and ΔA HA-E2FBP1 after infection with HSV-1 at an MOI of 0.5. RT-qPCR analysis revealed that WT E2FBP1 repressed ICP0 RNA levels to a similar extent to that in the previous experiment, whereas ICP0 RNA levels increased in cells expressing ΔA E2FBP1 (Fig. 7B). Sequence analysis of the HSV-1 F strain genome revealed the presence of multiple ARID3 consensus and consensus-like motifs, including 5′-GTAATTAA/G-3′ and 5′-TAATTGCT-3′ motifs upstream of the IE-0 gene (Fig. 7C). These results strongly suggest that E2FBP1 represses expression of ICP0 as a result of transcriptional repression. To examine this hypothesis, we subcloned various lengths of wild-type HSV-1 IE-0 promoter sequence 5′ of a humanized Renilla luciferase (hRluc) coding region in pGL4.83 (Fig. 7D) and performed dual-luciferase assays. Ratios of hRluc to luc were calculated and aligned by comparison with pGL4.83 activity expressed in the absence of ectopic E2FBP1 expression (Fig. 7E). As expected, expression from the −747 fragment retaining four ARID3 consensus motifs was suppressed by wild-type E2FBP1, while it was unaffected by the ΔA mutant. Deletion of two upstream consensus motifs abrogated the effect of E2FBP1, although the relative promoter activity of this construct was decreased even in the absence of ectopic E2FBP1. Moreover, the −1007 fusion construct also diminished the suppressive effect of E2FBP1, revealing how complicated the regulation of the IE-0 promoter is and that it is controlled not only by ARID proteins but also by other host proteins. The precise mechanism of E2FBP1-mediated repression remains to be elucidated.
DISCUSSION
We report here that ICP0 depletes E2FBP1 as its HUL-2 substrate, by ubiquitin-mediated degradation, and that a major polyubiquitylation target region is the ARID of E2FBP1. Contemporaneously with this event, E2FBP1 represses accumulation of ICP0 transcripts in an ARID-dependent manner. As a result of these interactions, E2FBP1 is degraded, RNA encoding ICP0 is modulated, and PML-NBs are dissociated (Fig. 8). These interactions between E2FBP1 and ICP0 suggest that E2FBP1 contributes to the cellular defense response against establishment of HSV-1 infection and that ICP0 works as the first wave of attack to repel the host response by degrading this defense factor. Intrinsic cellular defense is initiated by proteins interacting with PML-NBs (reviewed in references 11, 12, 41, 61, 66, and 71). However, the relationship between the replication machinery of HSV-1 and the role of PML-NBs that impinge on viral replication has not been sorted out fully. A highly debated issue is the significance of ICP0 disruption of PML-NBs during viral infection. It has been reported that a lack of PML-NB disruption as a consequence of infection with ICP0-deficient HSV-1 interferes with replication of HSV-1 in limited-passage human fibroblasts. However, other reports revealed that high-level ectopic expression of PML in Vero cells, Hep-2 cells, and telomerase-transformed immortalized human foreskin fibroblasts did not affect viral replication, although the virus accumulated in the PML-NB-like nuclear domains (22, 44). The key factor(s) contributing to intrinsic cellular defense is therefore implied to be associated with PML-NBs (1, 9, 17-19, 21, 46). This factor(s) is likely to be targeted by ICP0 during the immediate-early phase of infection. Alternatively, it may associate directly with HSV-1 genomes to suppress their transcription and replication (15, 16). While attempting to elucidate the molecular bases for this intrinsic cellular defense mechanism, we showed that E2FBP1 interacts with PML-NBs to dissociate them. In the absence of E2FBP1, passage-limited human fibroblasts lost their proliferation potential, resulting in premature senescence accompanied by ectopic accumulation of PML-NBs (23). Recently, human Daxx and its partner, α-thalassemia/mental retardation syndrome X-linked (ATRX), were investigated as candidates for such DNA-associating suppressive factors by use of an RNA interference (RNAi)-mediated knockdown method, and they were revealed to contribute to intrinsic cellular defense (46). Both of these PML-NB-associating proteins are involved in the chromatin-remodeling complex and exhibit transcription-repressing activities (41, 49, 61, 62).
FIG. 8.
Schematic representation of possible interactions between E2FBP1 and ICP0 in infected cell nuclei. ICP0 targets E2FBP1 as a HUL-2 substrate to deplete it via the ubiquitin pathway. Contemporaneously with this event, E2FBP1 targets the IE-0 promoter to repress transcription of ICP0 RNA. Target Lys residues in E2FBP1 reside both inside and outside the ARID. The latter sites may be occluded from ubiquitylation as a consequence of REKLES-mediated dimerization. Interactions of PML-NBs with both E2FBP1 and ICP0 are also illustrated. K, Lys residues available for ICP0-mediated ubiquitylation; Ub, ubiquitin moiety.
Our results identify the ARID of E2FBP1 as another target of ICP0-mediated polyubiquitylation (Fig. 4 and 5). Since the ARID is a highly conserved domain within the ARID protein family (reviewed in references 35, 69, 72, and 73), other family members are potential host targets for ICP0-mediated degradation. ARID proteins are involved in multiple cellular processes to maintain chromosomal integrity, including chromatin remodeling, DNA repair, and transcriptional controls. Therefore, our results may provide insight into host-virus interactions, specifically into how other ARID family members interact with HSV-1 and its gene products. Of possible relevance is a report that the ARID5B transcription factor Mrf-2 suppresses the human cytomegalovirus enhancer (30).
Finally, the ability of ICP0 to suppress the host cell cycle during a productive infection may also be connected to the depletion of E2FBP1. E2FBP1 activates the E2F1/DP1 complex to enhance transcription levels of target genes that are important for S-phase entry (65). Therefore, depletion of E2FBP1 should result in a delay in the G1/S transition.
Acknowledgments
We thank Naoki Inoue at the National Institute of Infectious Diseases of Japan for WT HSV-1 strain F and Ikuo Morita, Miki Yokoyama, Podyma-Inoue Katarzyna-Anna, Ichiro Nakagawa, and Kenji Yamato at TMDU for discussions.
This study was supported by grants from the NIH Public Health Service (AI024021 to S.J.S. and CA127378-01A1 to A.M.I.).
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1.Boutell, C., et al. 2008. Herpes simplex virus type 1 ICP0 phosphorylation mutants impair the E3 ubiquitin ligase activity of ICP0 in a cell type-dependent manner. J. Virol. 82:10647-10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand, P., T. Lenser, and P. Hemmerich. 2010. Assembly dynamics of PML nuclear bodies in living cells. PMC Biophys. 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chelbi-Alix, M. K., and H. de Thé. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 4.Chen, J., C. Panagiotidis, and S. Silverstein. 1992. Multimerization of ICP0, a herpes simplex virus immediate-early protein. J. Virol. 66:5598-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J., and S. Silverstein. 1992. Herpes simplex viruses with mutations in the gene encoding ICP0 are defective in gene expression. J. Virol. 66:2916-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng, L., et al. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351-361. [DOI] [PubMed] [Google Scholar]
- 7.Dent, A. L., et al. 1996. LYSP100-associated nuclear domains (LANDs): description of a new class of subnuclear structures and their relationship to PML nuclear bodies. Blood 88:1423-1426. [PubMed] [Google Scholar]
- 8.de The, H., et al. 1991. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell 66:675-684. [DOI] [PubMed] [Google Scholar]
- 9.Everett, R. D. 2010. Depletion of CoREST does not improve the replication of ICP0 null mutant herpes simplex virus type 1. J. Virol. 84:3695-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 11.Everett, R. D. 2006. Interactions between DNA viruses, ND10 and the DNA damage response. Cell. Microbiol. 8:365-374. [DOI] [PubMed] [Google Scholar]
- 12.Everett, R. D., and M. K. Chelbi-Alix. 2007. PML and PML nuclear bodies: implications in antiviral defence. Biochimie 89:819-830. [DOI] [PubMed] [Google Scholar]
- 13.Everett, R. D., et al. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett, R. D., P. Lomonte, T. Sternsdorf, R. van Driel, and A. Orr. 1999. Cell cycle regulation of PML modification and ND10 composition. J. Cell Sci. 112:4581-4588. [DOI] [PubMed] [Google Scholar]
- 15.Everett, R. D., and J. Murray. 2005. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 79:5078-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett, R. D., J. Murray, A. Orr, and C. M. Preston. 2007. Herpes simplex virus type 1 genomes are associated with ND10 nuclear substructures in quiescently infected human fibroblasts. J. Virol. 81:10991-11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett, R. D., and A. Orr. 2009. Herpes simplex virus type 1 regulatory protein ICP0 aids infection in cells with a preinduced interferon response but does not impede interferon-induced gene induction. J. Virol. 83:4978-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett, R. D., C. Parada, P. Gripon, H. Sirma, and A. Orr. 2008. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 82:2661-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everett, R. D., M. L. Parsy, and A. Orr. 2009. Analysis of the functions of herpes simplex virus type 1 regulatory protein ICP0 that are critical for lytic infection and derepression of quiescent viral genomes. J. Virol. 83:4963-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett, R. D., et al. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 80:7995-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett, R. D., D. F. Young, R. E. Randall, and A. Orr. 2008. STAT-1- and IRF-3-dependent pathways are not essential for repression of ICP0-null mutant herpes simplex virus type 1 in human fibroblasts. J. Virol. 82:8871-8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everett, R. D., and A. Zafiropoulos. 2004. Visualization by live-cell microscopy of disruption of ND10 during herpes simplex virus type 1 infection. J. Virol. 78:11411-11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuyo, Y., K. Mogi, Y. Tsunematsu, and T. Nakajima. 2004. E2FBP1/hDril1 modulates cell growth through downregulation of promyelocytic leukemia bodies. Cell Death Differ. 11:747-759. [DOI] [PubMed] [Google Scholar]
- 24.Gregory, S. L., R. D. Kortschak, B. Kalionis, and R. Saint. 1996. Characterization of the dead ringer gene identifies a novel, highly conserved family of sequence-specific DNA-binding proteins. Mol. Cell. Biol. 16:792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagglund, R., and B. Roizman. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 78:2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halford, W. P., and P. A. Schaffer. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 75:3240-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris, R. A., R. D. Everett, X. X. Zhu, S. Silverstein, and C. M. Preston. 1989. Herpes simplex virus type 1 immediate-early protein Vmw110 reactivates latent herpes simplex virus type 2 in an in vitro latency system. J. Virol. 63:3513-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrscher, R. F., et al. 1995. The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: a B cell-specific trans-activator that describes a new DNA-binding protein family. Genes Dev. 9:3067-3082. [DOI] [PubMed] [Google Scholar]
- 29.Hobbs, S., S. Jitrapakdee, and J. C. Wallace. 1998. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem. Biophys. Res. Commun. 252:368-372. [DOI] [PubMed] [Google Scholar]
- 30.Huang, T. H., et al. 1996. Repression by a differentiation-specific factor of the human cytomegalovirus enhancer. Nucleic Acids Res. 24:1695-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, W. Q., and N. Ringertz. 1997. Altered distribution of the promyelocytic leukemia-associated protein is associated with cellular senescence. Cell Growth Differ. 8:513-522. [PubMed] [Google Scholar]
- 32.Kim, D., L. Probst, C. Das, and P. W. Tucker. 2007. REKLES is an ARID3-restricted multifunctional domain. J. Biol. Chem. 282:15768-15777. [DOI] [PubMed] [Google Scholar]
- 33.Kim, D., and P. W. Tucker. 2006. A regulated nucleocytoplasmic shuttle contributes to Bright's function as a transcriptional activator of immunoglobulin genes. Mol. Cell. Biol. 26:2187-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kortschak, R. D., et al. 1998. The human dead ringer/bright homolog, DRIL1: cDNA cloning, gene structure, and mapping to D19S886, a marker on 19p13.3 that is strictly linked to the Peutz-Jeghers syndrome. Genomics 51:288-292. [DOI] [PubMed] [Google Scholar]
- 35.Kortschak, R. D., P. W. Tucker, and R. Saint. 2000. ARID proteins come in from the desert. Trends Biochem. Sci. 25:294-299. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 37.Lallemand-Breitenbach, V., and H. de The. 2010. PML nuclear bodies. Cold Spring Harb. Perspect. Biol. 2:a000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, F., R. Mulligan, P. Berg, and G. Ringold. 1981. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature 294:228-232. [DOI] [PubMed] [Google Scholar]
- 39.Lin, D., et al. 2007. Bright/ARID3A contributes to chromatin accessibility of the immunoglobulin heavy chain enhancer. Mol. Cancer 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin, L., et al. 2008. Cross talk between Id1 and its interactive protein Dril1 mediate fibroblast responses to transforming growth factor-beta in pulmonary fibrosis. Am. J. Pathol. 173:337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsay, C. R., V. M. Morozov, and A. M. Ishov. 2008. PML NBs (ND10) and Daxx: from nuclear structure to protein function. Front. Biosci. 13:7132-7142. [DOI] [PubMed] [Google Scholar]
- 42.Lium, E. K., C. A. Panagiotidis, X. Wen, and S. Silverstein. 1996. Repression of the alpha0 gene by ICP4 during a productive herpes simplex virus infection. J. Virol. 70:3488-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 44.Lopez, P., R. J. Jacob, and B. Roizman. 2002. Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins. J. Virol. 76:9355-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez, P., C. Van Sant, and B. Roizman. 2001. Requirements for the nuclear-cytoplasmic translocation of infected-cell protein 0 of herpes simplex virus 1. J. Virol. 75:3832-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lukashchuk, V., and R. D. Everett. 2010. Regulation of ICP0-null mutant herpes simplex virus type 1 infection by ND10 components ATRX and hDaxx. J. Virol. 84:4026-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma, K., et al. 2003. E2FBP1/DRIL1, an AT-rich interaction domain-family transcription factor, is regulated by p53. Mol. Cancer Res. 1:438-444. [PubMed] [Google Scholar]
- 48.Maul, G. G. 1998. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays 20:660-667. [DOI] [PubMed] [Google Scholar]
- 49.Maul, G. G., D. Negorev, P. Bell, and A. M. Ishov. 2000. Review: properties and assembly mechanisms of ND10, PML bodies, or PODs. J. Struct. Biol. 129:278-287. [DOI] [PubMed] [Google Scholar]
- 50.Muller, S., and A. Dejean. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73:5137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Negorev, D. G., O. V. Vladimirova, A. Ivanov, F. Rauscher III, and G. G. Maul. 2006. Differential role of Sp100 isoforms in interferon-mediated repression of herpes simplex virus type 1 immediate-early protein expression. J. Virol. 80:8019-8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Negorev, D. G., O. V. Vladimirova, and G. G. Maul. 2009. Differential functions of interferon-upregulated Sp100 isoforms: herpes simplex virus type 1 promoter-based immediate-early gene suppression and PML protection from ICP0-mediated degradation. J. Virol. 83:5168-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nystrom, K., et al. 2004. Real time PCR for monitoring regulation of host gene expression in herpes simplex virus type 1-infected human diploid cells. J. Virol. Methods 118:83-94. [DOI] [PubMed] [Google Scholar]
- 54.Parkinson, J., and R. D. Everett. 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74:10006-10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearson, M., et al. 2000. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406:207-210. [DOI] [PubMed] [Google Scholar]
- 56.Peeper, D. S., et al. 2002. A functional screen identifies hDRIL1 as an oncogene that rescues RAS-induced senescence. Nat. Cell Biol. 4:148-153. [DOI] [PubMed] [Google Scholar]
- 57.Prieur, A., K. Nacerddine, M. van Lohuizen, and D. S. Peeper. 2009. SUMOylation of DRIL1 directs its transcriptional activity towards leukocyte lineage-specific genes. PLoS One 4:e5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajaiya, J., M. Hatfield, J. C. Nixon, D. J. Rawlings, and C. F. Webb. 2005. Bruton's tyrosine kinase regulates immunoglobulin promoter activation in association with the transcription factor Bright. Mol. Cell. Biol. 25:2073-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roizman, B. 1999. HSV gene functions: what have we learned that could be generally applicable to its near and distant cousins? Acta Virol. 43:75-80. [PubMed] [Google Scholar]
- 60.Russell, J., N. D. Stow, E. C. Stow, and C. M. Preston. 1987. Herpes simplex virus genes involved in latency in vitro. J. Gen. Virol. 68:3009-3018. [DOI] [PubMed] [Google Scholar]
- 61.Saffert, R. T., and R. F. Kalejta. 2008. Promyelocytic leukemia-nuclear body proteins: herpesvirus enemies, accomplices, or both? Future Virol. 3:265-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salomoni, P., and A. F. Khelifi. 2006. Daxx: death or survival protein? Trends Cell Biol. 16:97-104. [DOI] [PubMed] [Google Scholar]
- 63.Schmittgen, T. D., and K. J. Livak. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101-1108. [DOI] [PubMed] [Google Scholar]
- 64.Sternsdorf, T., T. Grotzinger, K. Jensen, and H. Will. 1997. Nuclear dots: actors on many stages. Immunobiology 198:307-331. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki, M., et al. 1998. A novel E2F binding protein with Myc-type HLH motif stimulates E2F-dependent transcription by forming a heterodimer. Oncogene 17:853-865. [DOI] [PubMed] [Google Scholar]
- 66.Tavalai, N., and T. Stamminger. 2008. New insights into the role of the subnuclear structure ND10 for viral infection. Biochim. Biophys. Acta 1783:2207-2221. [DOI] [PubMed] [Google Scholar]
- 67.Thompson, R. L., and N. M. Sawtell. 2006. Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J. Virol. 80:10919-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Sant, C., P. Lopez, S. J. Advani, and B. Roizman. 2001. Role of cyclin D3 in the biology of herpes simplex virus 1 ICP0. J. Virol. 75:1888-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Webb, C. F. 2001. The transcription factor, Bright, and immunoglobulin heavy chain expression. Immunol. Res. 24:149-161. [DOI] [PubMed] [Google Scholar]
- 70.Webb, C. F., et al. 2000. The transcription factor Bright associates with Bruton's tyrosine kinase, the defective protein in immunodeficiency disease. J. Immunol. 165:6956-6965. [DOI] [PubMed] [Google Scholar]
- 71.Wileman, T. 2007. Aggresomes and pericentriolar sites of virus assembly: cellular defense or viral design? Annu. Rev. Microbiol. 61:149-167. [DOI] [PubMed] [Google Scholar]
- 72.Wilsker, D., A. Patsialou, P. B. Dallas, and E. Moran. 2002. ARID proteins: a diverse family of DNA binding proteins implicated in the control of cell growth, differentiation, and development. Cell Growth Differ. 13:95-106. [PubMed] [Google Scholar]
- 73.Wilsker, D., et al. 2005. Nomenclature of the ARID family of DNA-binding proteins. Genomics 86:242-251. [DOI] [PubMed] [Google Scholar]
- 74.Zhu, X. X., J. X. Chen, and S. Silverstein. 1991. Isolation and characterization of a functional cDNA encoding ICP0 from herpes simplex virus type 1. J. Virol. 65:957-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu, X. X., J. X. Chen, C. S. Young, and S. Silverstein. 1990. Reactivation of latent herpes simplex virus by adenovirus recombinants encoding mutant IE-0 gene products. J. Virol. 64:4489-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]