Abstract
Fowlpox virus (FWPV) is a double-stranded DNA virus long used as a live-attenuated vaccine against poultry diseases, but more recent interest has focused on its use as a mammalian vaccine vector. Here, in a mouse model system using FWPV encoding the nominal target antigen chicken ovalbumin (OVA) (FWPVOVA), we describe for the first time some of the fundamental processes by which FWPV engages both the innate and adaptive immune systems. We show that Toll-like receptor 7 (TLR7) and TLR9 are important for type I interferon secretion by dendritic cells, while TLR9 is solely required for proinflammatory cytokine secretion. Despite this functional role for TLR7 and TLR9 in vitro, only the adapter protein myeloid differentiation primary response gene 88 (MyD88) was shown to be essential for the formation of adaptive immunity to FWPVOVA in vivo. The dependence on MyD88 was confined only to the T-cell compartment and was not related to its contribution to TLR signaling, dendritic cell maturation, or the capture and presentation of FWPV-derived OVA antigen. We demonstrate that this is not by means of mediating T-cell-dependent interleukin-1 (IL-1) signaling, but rather, we suggest that MyD88 functions to support T-cell-specific IL-18 receptor signaling, which in turn is essential for the formation of adaptive immunity to FWPV-encoded OVA.
Like other poxviruses, fowlpox virus (FWPV) is a double-stranded DNA (dsDNA) virus. Recombinant FWPVs (rFWPVs) are being developed clinically as vaccine vectors because of their impeccable safety profile and large cloning capacity (enabling the expression of multiple heterologous genes) as well as their ability to induce cell-mediated immunity (15; reviewed in reference 4). Recombinant FWPV-vectored vaccines have delivered promising preclinical and clinical results, particularly when used as part of homologous or heterologous prime-boost immunization regimens (18, 21, 25, 27, 28, 40, 55, 58, 62). Most recently, in humans, and following antiretroviral drug therapy withdrawal, homologous prime-boost vaccination with rFWPV encoding human immunodeficiency virus (HIV) Gag-Pol and human gamma interferon (hIFN-γ) was associated with a partial control of HIV replication in a subset of individuals (22). Furthermore, priming with recombinant canarypox virus (a closely related avipox virus), encoding multiple full-length HIV proteins and defined human cytotoxic T-cell epitopes, followed by a recombinant HIV gp120 protein boost resulted in a reduced risk of HIV infection (56). Despite these encouraging indications that recombinant avipox viruses can contribute to therapeutic and protective vaccine-induced responses in humans, the potency and durability of these responses may be further enhanced. However, only a thorough understanding of the interaction between virally vectored vaccines and the host immune system is likely to produce the desired improvements.
Productive adaptive immunity to the antigens encoded by dsDNA viral vaccine vectors depends on the integration of the innate immune signals that are initiated by the pathogen recognition receptor (PRR) ligation of conserved pathogen-associated molecular patterns (20). The PRRs represent a diverse family of well-defined receptors, including the Toll-like receptors (TLRs), Nod-like receptors (NLRs), Rig-like receptors (RLRs), and C-type lectin receptors (CLRs), in addition to an increasing number of cytosolic receptors, some of which have specificity for nucleic acids of dsDNA viral origin (reviewed in reference 35). The TLRs represent the best-studied PRRs, sensing dsDNA virus-derived DNA, RNA, and protein. For instance, TLR9 recognizes viral DNA that contains unmethylated CpG motifs (31); TLR3 binds to dsRNA, which presumably results from bidirectional viral gene transcription (2); and TLR2 interacts with viral core and envelope proteins (12, 65, 76). Although TLR7 recognizes single-stranded RNA (ssRNA), like TLR9, it also participates in the innate recognition of the dsDNA virus murine cytomegalovirus (MCMV) (77).
Upon ligation, TLRs signal through two adaptor proteins, MyD88, which is responsible for signaling downstream of TLR1, TLR2, and TLR4 to TLR12, and TRIF, which operates exclusively downstream of TLR3 and contributes (with MyD88) to TLR4 signaling (32). In addition to its role as a signaling adapter molecule for TLRs, MyD88 also has a role in interleukin-1 (IL-1) receptor (IL-1R) and IL-18R signal transduction and, hence, is relevant to the important immunological functions of IL-1β and IL-18 (16).
The engagement of PRRs induces the secretion of type I IFNs and proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), IL-12, and IL-6. In addition, the proinflammatory cytokines pro-IL-1β and pro-IL-18 generally require further processing by caspase-1, which is itself controlled by PRR-regulated inflammasome assembly, to produce biologically active cytokines (61); however, recently reported evidence suggests that pro-IL-1β can also be cleaved by granzyme B (53). Subsequently, these soluble mediators orchestrate antiviral effector responses, which include the recruitment of effector cells such as macrophages and neutrophils and the activation of the specific dendritic cell (DC) subsets required to drive productive T-cell responses (70). One such subset, the plasmacytoid dendritic cell (pDC), is increasingly implicated in antiviral immunity (63). Indeed, we have recently shown that pDCs are essential for mediating adaptive immunity to an FWPV-encoded antigen (15). This activity most likely results from the ability of pDCs to either directly present or cross-present antigens rather than from their ability to secrete copious amounts of type I IFNs (43). In this study, we further our understanding of the way in which the innate arm of the mammalian immune system recognizes rFWPV as well as provide new detail on the PRRs involved and their relevance to the formation of innate and adaptive immune responses. We find that immunization with rFWPV stimulates the secretion of type I IFNs in a TLR7- and TLR9-dependent fashion, which, as expected, relies on MyD88 signaling. However, only those mice deficient in MyD88, but not those doubly deficient in TLR7 and TLR9, displayed impaired adaptive immune responses to an FWPV-encoded antigen. These findings indicate that the TLR-mediated innate recognition of rFWPV is not required for the development of adaptive immune responses to an FWPV-encoded antigen and that the dependence on MyD88 for adaptive immunity to rFWPV is confined to the T-cell compartment as a result of its ability to transduce essential IL-18-mediated signaling in T cells.
MATERIALS AND METHODS
Mice and viruses.
Strains included TLR2-deficient (TLR2−/−) mice (65) on a BALB/c genetic background and TLR3-deficient (TLR3−/−) (2), TLR7-deficient (TLR7−/−) (30), TLR9-deficient (TLR9−/−) (31), MyD88-deficient (MyD88−/−) (1), TRIF-deficient (TRIF−/−) (73), MyD88/TRIF doubly deficient (MyD88−/−/TRIF−/−), TLR7/TLR9 doubly deficient (TLR7−/−/TLR9−/−), and IL-1 receptor-deficient (IL-1R−/−) mice (39), all on a C57BL/6 (B6) background. TLR3−/−, TLR9−/−, MyD88−/−, TRIF−/−, and MyD88−/−/TRIF−/− mice were kindly provided by Gabrielle Belz (Walter and Eliza Hall Institute of Medical Research) and Gayle Davey (University of Melbourne). All experiments utilized 6- to 12-week-old mice, with all procedures approved and conducted in accordance with institutional ethical guidelines.
Fowlpox virus mild strain 3 (6) was used either in its wild-type form (FWPVWT) or as a recombinant version encoding the full-length ovalbumin (OVA) gene under the control of a fowlpox bidirectional promoter element with early/late and late functions (FWPVOVA) (38). Both FWPVWT and FWPVOVA were routinely propagated and titrated on chicken embryonic fibroblast (CEF) cells as previously described (15). UV-inactivated FWPVWT (FWPVUV) was produced by the exposure of FWPVOVA to a UV lamp within a type II biohazard cabinet for 1 h at room temperature (RT), which resulted in the virus being unable to undergo RNA transcription and protein translation, as confirmed by measurements of OVA mRNA transcripts and protein secretion (data not shown).
Generation of dendritic cell cultures.
Bone marrow (BM) was harvested from the femur and tibia of experimental mice by flushing with complete RPMI medium supplemented with 10% fetal calf serum (FCS) (CM). Following red blood cell (RBC) lysis, BM cells were washed thoroughly and seeded at 1 × 106 cells/ml in CM supplemented with 100 ng/ml recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems) or at 2 × 106 cells/ml in CM supplemented with 200 ng/ml human Flt-3 ligand (FL) (kindly provided by Amgen Inc.) in 1-ml aliquots in 24-well plates unless otherwise stated. Cells were cultured for 8 days prior to infection or stimulation, with a 50% medium change daily from days 4 to 8 for GM-CSF cultures and on day 4 for FL cultures. The majority of GM-CSF-expanded DCs took on a conventional DC (cDC) phenotype, while FL-expanded DCs consisted of both cDCs and pDCs as previously described (7, 24, 43) and confirmed by flow cytometric analysis of B220, CD11b, and CD11c expressions (data not shown).
Assessment of cell viability.
Cells (1 × 105) were resuspended in annexin binding buffer (100 μl; BD Biosciences) and stained with fluorescein isothiocyanate (FITC)-annexin V (5 μl) and propidium iodide (2 μl) at RT in the dark (15 min). Following incubation, annexin binding buffer was added (400 μl), and samples were analyzed immediately with a flow cytometer. Dead cells were identified as those staining positive for annexin V and propidium iodide.
ELISA analysis of in vitro cytokine and OVA production.
Expanded DC cultures from knockout and control mice were infected for 24 h at a multiplicity of infection (MOI) of 1 (unless otherwise indicated) with FWPVWT (for cytokine analysis) or FWPVOVA (for analysis of OVA production), after which cell-free supernatants were harvested and stored at −20°C until enzyme-linked immunosorbent assay (ELISA) analysis was performed. For analyses of cytokines, matched antibody pairs and standards were used with sandwich ELISA protocols as previously described (43). Ovalbumin secretion was assessed by use of polyclonal goat anti-OVA capture antibody (ICN) and biotinylated rabbit anti-OVA detection antibody (Rockland), followed by streptavidin-horseradish peroxidase (HRP) (Rockland) and substrate detection (Fast OPD; Sigma-Aldrich) performed with OVA protein standards (grade V; Sigma-Aldrich) with a standard sandwich ELISA protocol.
Western blotting for NF-κB activation.
FL-expanded DCs cultured in 6-well plates (10 × 106 cells/5 ml) were infected with FWPVWT (MOI of 1) or a diluent control (phosphate-buffered saline [PBS]) for the times indicated in Fig. 3d. Cells were subsequently harvested, with lysates being subjected to polyacrylamide gel electrophoresis, before proteins were transferred onto polyvinylidene difluoride (PVDF) membranes for the assessment of IκBα phosphorylation as previously described (43).
Direct chromium release CTL assay.
For cytolytic T-lymphocyte (CTL) assays, mice were immunized with FWPVOVA (1 × 107 PFU) via intraperitoneal (i.p.) injection, and splenocyte suspensions were prepared at the peak of the cell-mediated response, 5 days postimmunization. Following RBC lysis, cells were incubated in vitro to remove the adherent cell population, with the nonadherent cells subsequently being assessed for potential OVA-specific cytolytic activity starting at a 200:1 effector-to-target cell ratio according to previously described methods (43). The percent specific lysis was determined by using the following equation: [(experimental 51Cr release − spontaneous 51Cr release)/(maximum 51Cr release − spontaneous 51Cr release)] ×100. The percent spontaneous lysis was <10% in all assays. Nonspecific lysis was routinely <5% and was subtracted from specific lysis results.
T-helper-cell [3H]thymidine incorporation assay.
Samples of total splenocyte suspensions obtained as described above were resuspended in CM and seeded (2 × 105 cells/ml) with various concentrations of OVA protein (6.25 to 400 μg/ml) in flat-bottom 96-well plates. Three days later, cells were pulsed with [3H]thymidine (1 μCi/well), and 18 h later, cells were harvested onto glass fiber filters (Packard) for measurements of [3H]thymidine incorporation (TopCount NXT β-scintillation counter; Perkin-Elmer).
Anti-FWPV ELISA.
Mouse serum samples serially diluted 1:40 to 1:320 were assessed for native FWPV binding abilities with a standard ELISA protocol using plates coated with FWPVWT as previously described (43).
Maturation status of DC subsets following infection with FWPV.
FL-expanded DCs were prepared in 12-well plates (4 × 106 cells/well) as described above and subsequently infected with FWPVWT (MOI of 1) or stimulated with PBS (uninfected) or CpG 2216 (1 μg/ml) for 24 h. Cells were subsequently harvested and stained for the expression of the surface markers B220, CD11b, and either CD40, CD80, or CD86 for flow cytometric analysis as previously described (43). Plasmacytoid DCs were gated as B220+/CD11blo, and cDCs were gated as B220−/CD11bint/hi.
In vitro OT-I mouse proliferation assay.
Splenocytes were prepared from FWPVOVA-immunized B6 or MyD88−/− mice 24 h postinfection by cutting the dissected spleens into small pieces and digesting them in 2% FCS-CM supplemented with collagenase (2 mg/ml) and DNase I (20 μg/ml) for 30 min at 37°C with gentle shaking. EDTA (0.1 M; pH 7.22) was added for the final 5 min of incubation. Cells were subsequently filtered (70-μm pore size), and CD11c+ DCs were purified by MACS separation using anti-CD11c (N418) microbeads (Miltenyi Biotec) according to the manufacturer's instructions. Purified DCs were subsequently incubated alone or with 4 × 104 lymph node (LN) cells from T-cell receptor-transgenic OT-I mice at various ratios (5:1 to 0.625:1) in U-bottom 96-well plates for 48 h prior to the addition of 1 μCi [3H]thymidine. After a further 18 h, cells were harvested, and the amount of incorporated [3H]thymidine was counted (TopCount NXT; Perkin-Elmer).
CFSE labeling of OT-I LN cells.
Single-cell suspensions prepared from the major LNs of OT-I mice were fluorescently labeled with 5 (and 6-)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) as previously described (43).
Adoptive transfer presentation assay.
For analyses of T-cell proliferation in vivo, 107 CFSE-labeled congenic (CD45.1+) OT-I LN cells were adoptively transferred into recipient B6 or MyD88−/− mice following the footpad administration of FWPVOVA and PBS, with the resultant proliferation (determined by CFSE dilution) being analyzed by flow cytometry 3 days later, as previously described (43).
Kinetic analysis of antigen-specific CD8+ T-cell expansion in vivo.
B6 or MyD88−/− mice were administered FWPVOVA (1 × 107 PFU i.p.), and peripheral blood was sampled daily for endogenous OVA-specific CD8+ T-cell expansion. Blood (20 μl) obtained from the tail vein of gently heated mice was mixed in an equal volume of heparin (Sigma) and stained with a phycoerythrin (PE)-conjugated H-2Kb-restricted SIINFEKL tetramer (1/500) and PE-Cy5-anti-CD8 (1/100; BD Pharmingen) on ice for 30 min. Cells were subsequently washed, and RBCs were lysed, washed further, fixed, and stored at 4°C until flow cytometric analysis (FACSCalibur; BD). Tetramers were synthesized in the Australian Cancer Research Foundation (ACRF) Biomolecular Resource Facility at the John Curtin School of Medical Research, using the BirA enzyme synthesized as previously described (52).
T-cell adoptive transfer.
For each experiment, two B6 mice were euthanized, and splenocyte suspensions were prepared. Cells were washed in fluorescence-activated cell sorter (FACS) buffer (1% FCS, 1× PBS) and subsequently stained with FITC-anti-CD3, PE-anti-CD8, and PE-Cy5-anti-CD4 for 30 min on ice. Cells were washed three times, passed through a 70-μm-pore-size filter, and sorted into CD4+/CD3+ or CD8+/CD3+ donor T-cell populations by using a high-speed cell sorter (FACSaria; Becton Dickinson) (Detmold Imaging Centre, SA Pathology). The equivalent number of cells from one B6 mouse (either CD4+/CD3+ or CD8+/CD3+) was used for adoptive transfer into MyD88−/− recipients. Twenty-four hours following donor cell transfer, mice were immunized with FWPVOVA, and CTL assays were performed 5 days later, as described above.
IL-18 depletion in vivo.
B6 mice were depleted of circulating IL-18 following the i.p. administration of 15 μg IL-18 binding protein (IL-18BP) (122-BP; R&D Systems) at the same time as FWPVOVA immunization (1 × 107 PFU i.p.). The effect of IL-18 depletion on the cell-mediated immune response was examined 5 days later with CTL assays.
Statistical analysis.
Statistical comparisons were performed by using GraphPad Prism v5 software. Analysis of variance (ANOVA) was used to deduce significant differences among groups, with a Bonferroni posttest comparison used to report P values.
RESULTS
Infection of DCs with FWPV results in cytokine secretion in a dose-dependent manner.
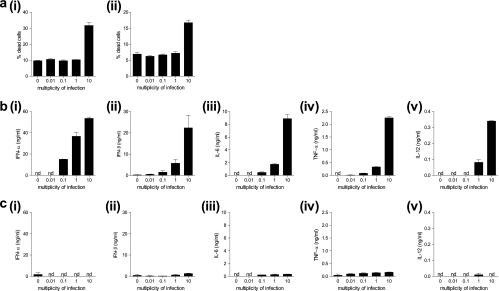
To examine the innate immune response to infection with FWPV, bone marrow (BM)-derived DCs (BMDCs) were generated ex vivo using FL or GM-CSF and infected for 24 h with FWPV ranging in dose from an MOI of 0.01 to an MOI of 10. After infection, these cells were analyzed for viability and the production of type I IFNs and proinflammatory cytokines. The results demonstrated that cells infected with FWPV at the highest MOI tested (MOI of 10) were significantly less viable (∼32% dead FL-DCs and 17% GM-CSF DCs) (Fig. 1ai and ii) than those infected at the other three MOIs, which were not significantly different from the mock-infected control (∼9 to 10% FL-DCs and ∼7 to 8% GM-CSF DCs) (Fig. 1ai and ii). In regard to cytokine secretion, the results indicated that FL-expanded DCs infected with FWPV at an MOI of 10 generated the highest level of all cytokines tested (IFN-α, 54 ng/ml; IFN-β, 22.3 ng/ml; IL-6, 8.8 ng/ml; TNF-α, 2.3 ng/ml; IL-12, 0.34 ng/ml) (Fig. 1b), while the GM-CSF-expanded cultures failed to generate the majority of cytokines except for TNF-α, where a small amount was measured (Fig. 1c). Cells infected at an MOI of 1 still reliably demonstrated cytokine secretion; however, this level was substantially lower than that observed for the cells infected at an MOI of 10 (IFN-α, 37 ng/ml; IFN-β, 5.7 ng/ml; IL-6, 1.8 ng/ml; TNF-α, 0.33 ng/ml; IL-12, 0.08 ng/ml). The two lower MOIs tested (0.01 and 0.1) yielded variable results, and in most cases, an MOI of 0.01 failed to result in detectable levels of cytokine secretion (Fig. 1b).
FIG. 1.
Infection at an MOI of 1 elicits the optimal innate immune response to FWPV. (a) FL-DCs (i) or GM-CSF DCs (ii) were infected with FWPVWT (MOI of 0.01, 0.1, 1, or 10) or PBS (MOI of 0) for 24 h, and supernatants were harvested and analyzed for cell viability via annexin V and propidium iodide costaining and are expressed as a percentage of dead cells within each culture. (b and c) FL-DCs (b) and GM-CSF DCs (c) were infected as described above (a) except that the production of IFN-α (i), IFN-β (ii), IL-6 (iii), TNF-α (iv), and IL-12 (v) was assessed via ELISA. Results are representative of data from two separate experiments with cells from separate mice and represent the means ± standard errors of the means (SEM). nd, not determined.
Infection with FWPV induces type I IFN secretion in a MyD88-dependent and TLR7- and TLR9-mediated fashion, which is not dependent on de novo viral gene expression.
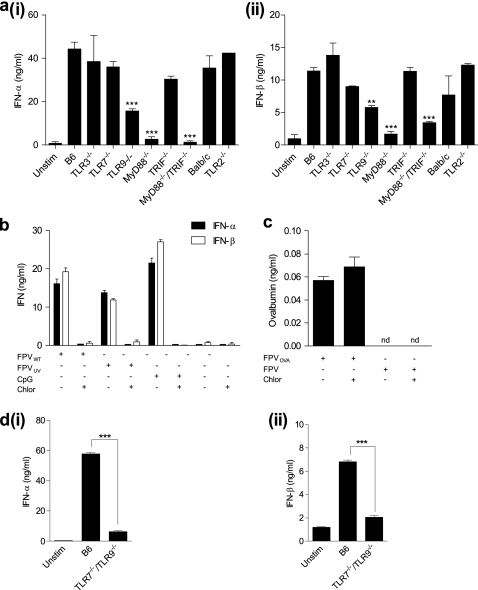
Toll-like receptors 2, 3, 7, and 9 and the adapter molecules MyD88 and TRIF have all been implicated in the induction of innate and adaptive immunity to different dsDNA viruses, including poxviruses (12, 37, 76, 77). To assess the role of these various TLR signaling components in FWPV-mediated immune responses, BMDC cultures prepared from TLR and TLR adaptor molecule knockout or control mice were infected with FWPVWT, and culture supernatants were analyzed 24 h later for the production of IFN-α and IFN-β. Results from normal B6 DC cultures indicated strong IFN-α (Fig. 2ai) and IFN-β (Fig. 2aii) responses (∼44 ng/ml and ∼11 ng/ml, respectively), which were abrogated only in MyD88−/− and MyD88−/−/TRIF−/− DC cultures. In contrast, TRIF-deficient cultures did not show any significant defect in type I IFN secretion. This suggested that type I IFN production following FWPV infection of DC cultures depends only on the TLR adapter molecule MyD88. Interestingly, only a partial reduction (∼2-fold) in type I IFN secretion was observed for TLR9−/− DCs, even though it was anticipated that TLR9-deficient cells would not be capable of responding to a DNA virus. This suggested that another MyD88-restricted viral recognition pathway was important for type I IFN secretion.
FIG. 2.
Infection with FWPV induces type I IFN secretion in a MyD88-dependent and TLR7- and TLR9-mediated fashion and does not depend on de novo viral gene expression. (a) FL-expanded DCs from TLR and TLR adapter molecule knockout mice and relevant controls were infected with FWPVWT (MOI of 1) or PBS diluent (unstimulated [unstim]) for 24 h. Culture supernatants were subsequently analyzed for IFN-α (i) and IFN-β (ii) via ELISA. (b) FL-expanded DCs obtained from B6 mice were either treated with chloroquine (10 μM for 2 h) or not treated prior to the addition of FWPVWT (MOI of 1), FWPVUV (MOI of 1), CpG 2216 (10 μg/ml), or PBS (unstim). Culture supernatants were analyzed for IFN-α and IFN-β production 24 h later. (c) Same as above (b) except that DCs were infected with FWPVOVA or FWPVWT after treatment with chloroquine or control diluent, and culture supernatants were analyzed for OVA production via ELISA. (d) FL-expanded DCs obtained from TLR7/TLR9 double-knockout or B6 mice were infected with FWPVWT (MOI of 1) or PBS (unstim), with culture supernatants being analyzed for IFN-α (i) and IFN-β (ii) production via ELISA. Data are representative of at least two independent experiments each containing cells derived from two mice per group and represent the means ± SEM. **, P < 0.01; ***, P < 0.001.
To identify the subcellular location of the putative secondary sensing mechanism, we determined whether type I IFN secretion relied on endosomal acidification. The pH-dependent endosomal pathway is important for the entry of many viruses, which rely on the acidic environment to uncoat and replicate (60, 67). Furthermore, the acidic endosomal environment is critically required for TLR7-, TLR9-, and, depending on the cell type, TLR3-mediated sensing of pathogens (14, 29, 47). As we wished to determine whether the live nature of the virus was required for recognition, it was important to confirm our ability to inactivate FWPV prior to infection. For this experiment, we used a recombinant FWPV vector encoding the secreted form of the chicken ovalbumin protein (FWPVOVA). FL-expanded DCs were either left unstimulated or infected with live or UV-inactivated FWPVOVA, and expression levels of OVA mRNA and protein were determined. The results indicated that UV inactivation inhibited OVA mRNA and protein expression, with levels not significantly different from those of the uninfected controls, as opposed to live infection, which yielded high levels of both OVA mRNA and protein (data not shown). Therefore, using this same protocol, FL-expanded DCs were left untreated or were pretreated with chloroquine, an inhibitor of endosomal acidification, prior to stimulation with FWPVWT, FWPVUV, or the control TLR9 ligand CpG, which requires endosomal acidification to signal. Following the addition of the stimulus, supernatants were collected and analyzed for IFN-α and IFN-β secretion (Fig. 2b). As expected, DC cultures that had been stimulated with CpG secreted a substantial amount of both IFN-α (∼22 ng/ml) and IFN-β (∼27 ng/ml) in quantities not dissimilar to those observed after FWPVWT infection (∼16 and ∼19 ng/ml, respectively) or UV-inactivated FWPV (FWPVUV) infection (13.7 ng/ml IFN-α and 11.8 ng/ml IFN-β). However, chloroquine treatment completely inhibited the induction of type I IFN in all cultures (Fig. 2b).
As poxviruses use both pH-dependent and neutral-pH pathways to infect target cells (9, 66-68), it was necessary to confirm whether chloroquine treatment simply inhibited pH-dependent viral entry into these cells or whether the lack of type I IFN production was due to an inhibition of endosomal TLR signaling. To explore these possibilities, we measured levels of FWPV-derived OVA expression in supernatants from chloroquine-treated and untreated FWPVOVA-infected DC cultures. The viral inoculum contains only negligible amounts of preformed OVA, and OVA synthesis depends on FPWV coopting the translation machinery located in the cytoplasm (data not shown). Thus, the presence of similar concentrations of OVA in the supernatants of chloroquine-treated and untreated cells (Fig. 2c) indicated that despite the inhibition of endosomal acidification, FWPV was still able to gain entry into the cell cytoplasm and express the transgene, presumably via the neutral-pH pathway. A concurrent analysis of type I IFN concentrations in these culture supernatants confirmed our previous findings, demonstrating a complete inhibition of type I IFN production following chloroquine treatment (data not shown).
These results suggested that the TLR(s) responsible for sensing FWPV and driving type I IFN expression was located within the endosomal compartment and that signaling within this compartment depended on endosomal acidification. As TLR7 and TLR9 are the only TLRs located within the endosome that signal through MyD88, and evidence suggests that TLR7 can comediate the sensing of MCMV with TLR9 (77), we speculated that TLR7 may also contribute to sensing FWPV infection, despite the fact it showed no obvious role in driving FWPV-mediated type I IFN production in isolation. We therefore infected FL-expanded TLR7−/−/TLR9−/− and B6 DCs with FWPVWT and measured type I IFN production. Compared to control B6 DCs, TLR7 and TLR9 doubly deficient DC cultures exhibited a profound reduction in levels of IFN-α (Fig. 2di) and IFN-β (Fig. 2dii) secretion, which indicated that endosomal TLR7 and TLR9 both contribute to driving type I IFN responses to FWPV via MyD88, although it appeared that TLR9 was the primary sensing molecule involved.
FWPV-mediated proinflammatory cytokine production is dependent on TLR9 and MyD88 signaling.
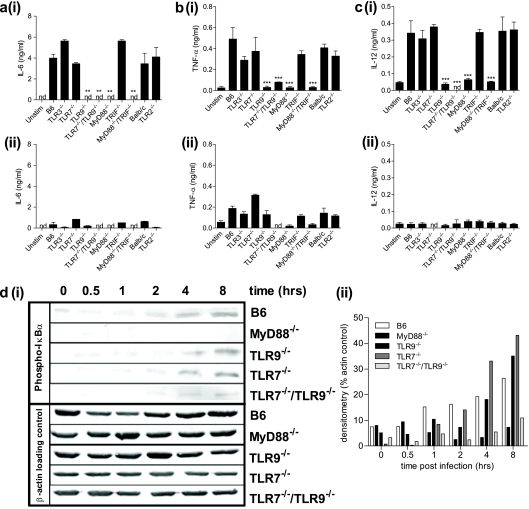
Since proinflammatory cytokine secretion from different populations of BM-derived cells has been shown to be important for determining the outcome of antiviral immune responses (50), different DC subpopulations from TLR and TLR adaptor molecule knockout mice were relatively enriched for pDCs and cDCs by culturing BM cells in FL or GM-CSF, respectively (43). After infection with FWPVWT or a diluent control, culture supernatants were analyzed for the presence of IL-6, TNF-α, and IL-12. The results confirmed previously reported findings (43) that the majority of these proinflammatory cytokines were produced in FL-expanded DC cultures (Fig. 3a to ci), with GM-CSF-expanded DCs producing only minor amounts of TNF-α (Fig. 3bii) and only background levels of IL-6 (Fig. 3aii) and IL-12 (Fig. 3cii). FWPV-infected and FL-expanded DCs taken from mice deficient in either TLR9, MyD88, MyD88 and TRIF, or TLR7 and TLR9 failed to secrete levels of IL-6 (Fig. 3ai), TNF-α (Fig. 3bi), and IL-12 (Fig. 3ci) above those observed for uninfected cultures. GM-CSF-differentiated DCs displayed a similar trend of TNF-α secretory responses in FL-expanded DCs albeit at much reduced levels (Fig. 3bii). Together, these results indicated that the majority of FWPV-induced proinflammatory cytokine production was instigated by the TLR9-mediated recognition of FWPV in FL-expanded DCs.
FIG. 3.
FWPV-mediated proinflammatory cytokine production is dependent on TLR9 and MyD88 signaling. (a to c) FL-expanded (panels i) or GM-CSF-expanded (panels ii) DCs obtained from TLR and TLR adapter molecule knockout mice and relevant wild-type controls were infected with FWPVWT (MOI of 1) or PBS (unstim). Culture supernatants were analyzed for IL-6 (a), TNF-α (b), and IL-12 (c) secretion 24 h later via ELISA. (d) FL-expanded DCs cultured in 6-well plates (5 ml) were infected with FWPVWT for 30 min, 1 h, 2 h, 4 h, or 8 h or mock infected with PBS (0 h) before lysis. (i) Cell lysates were analyzed for NF-κB activation as indicated by the presence of phosphorylated IκBα, with β-actin as a loading control. (ii) A densitometric analysis of each band was performed by using ImageJ software (NIH), and data are depicted as a percentage of the density of the actin loading control. All data are representative of results from at least 2 independent experiments each containing cells obtained from separate mice and, in the case of data in a to c, represent the means ± SEM. **, P < 0.01; ***, P < 0.001.
The roles of TLR7, TLR9, and MyD88 in sensing FWPV were confirmed by analyzing the upstream regulator of proinflammatory cytokine secretion, NF-κB. FL-expanded DCs from control B6, TLR7−/−, TLR9−/−, TLR7−/−/TLR9−/−, and MyD88−/− mice were infected with FWPVWT at various time points prior to cell harvest and lysis. Western blot analyses were performed on the resultant lysates to detect phosphorylated IκBα (phospho-IκBα) as an indirect measure of NF-κB activation (Fig. 3di). Quantification of the density of the resulting phospho-IκBα bands as a percentage of the corresponding actin control clearly indicated that the level of IκBα phosphorylation in MyD88−/− and TLR7−/−/TLR9−/− cells was reduced to approximately one-third of that seen in control B6 cells at 8 h postinfection (7.2% and 10.9% versus 26.4% for control cells). These levels were not significantly different from those observed for the corresponding cells immediately before FWPV infection (8.1% and 3.25%). Interestingly, DC cultures from mice deficient in TLR7 or TLR9 in isolation did not have defective NF-κB activation after 8 h of FWPV infection (43% and 35%, respectively), thus indicating a level of redundancy in TLR7 and TLR9 signaling for NF-κB activation (Fig. 3dii).
Adaptive immune responses to FWPV-encoded OVA partially depend on MyD88 expression.
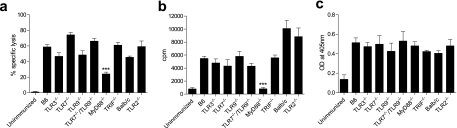
We next sought to determine whether TLR7, TLR9, or MyD88, implicated here in the innate recognition of FWPV, or any of the other TLRs or TLR adaptor molecules, played a role in the development of an adaptive immune response to FWPV. Mice deficient in the various TLR and TLR adaptor molecules were immunized with FWPVOVA and assessed for their abilities to generate CTL responses, CD4+ T-helper recall proliferative responses, and anti-FWPV IgG antibody production (Fig. 4). The results indicated that only mice deficient in MyD88 displayed a significant reduction in primary CTL responses to OVA-expressing targets compared to control B6 mice (24.2% versus 58.7% specific lysis) (Fig. 4a). In contrast, all other deficient mice produced CTL responses that were not significantly different from those exhibited by the relevant wild-type controls. Similarly, only MyD88 appeared to contribute to an anti-OVA T-helper-cell recall proliferative response, with results indicating a complete abrogation of responses in the cells obtained from MyD88-deficient immunized mice compared to the B6 controls (965.7 cpm versus 5,153.9 cpm) (Fig. 4b). All other TLR and TLR adaptor molecule mice had T-helper recall responses comparable to those of the appropriate control animals. Interestingly, when IgG antibody responses to FWPV in the various knockout mice were examined, no difference was observed between the different experimental groups at all serum dilutions tested (Fig. 4c). In summary, these results indicated that only MyD88 made a detectable contribution to the induction of adaptive immunity to FWPV and was important only for CTL and T-helper proliferative responses but not antibody-mediated immunity.
FIG. 4.
Adaptive immune responses to FWPV-encoded OVA are partially dependent on MyD88 expression. Mice deficient in various TLR and TLR adaptor molecules and relevant controls were immunized with FWPVOVA (107 PFU) or PBS. (a) Five days later, splenocytes were harvested and assessed for cytolytic activity in an 18-h CTL assay against specific or nonspecific 51Cr-labeled EL-4 target cells, with results shown as the percent specific lysis obtained at a 200:1 effector-to-target cell ratio. (b) Whole-splenocyte preparations were analyzed for their T-helper-cell proliferative capacities in a [3H]thymidine incorporation assay. Results represent counts per minute (cpm) of wells containing 100 μg/ml OVA protein. (c) Collected sera were analyzed for the presence of anti-FWPV IgG antibodies via ELISA. Results are presented as raw optical density readings obtained at a representative 1:40 serum dilution. Results are representative of data from between 5 and 10 mice per group and represent the means ± SEM. ***, P < 0.001.
Dendritic cells from MyD88-deficient mice display no defect in maturation or antigen processing or presentation following infection with FWPVOVA.
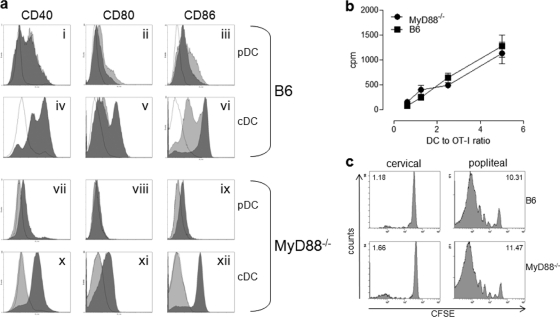
In an effort to determine a mechanism by which MyD88−/− mice lack the ability to generate a robust adaptive cellular immune response to FWPV-encoded OVA, DCs from MyD88−/− mice were analyzed for their ability to undergo maturation, a key step enabling the efficient licensing and activation of antigen-specific T cells (45). In this experiment, MyD88−/− and control B6 FL-expanded DCs were stimulated in culture for 24 h with either PBS, FWPVWT, or CpG and then analyzed for an upregulation of the classical DC activation markers CD40, CD80, and CD86 in both the pDC and cDC populations (Fig. 5a). FWPVWT-infected pDCs from B6 mice displayed a prominent biphasic upregulation of CD40 (Fig. 5ai) and a complete shift in CD80 (Fig. 5aii) and CD86 (Fig. 5aiii) expressions over uninfected controls. Conventional DCs from the same cultures showed an enhanced biphasic expression of both CD40 (Fig. 5aiv) and CD80 (Fig. 5av), with a strong upregulation of CD86 being apparent (Fig. 5avi). Similar expression pattern profiles were noticeable following the CpG stimulation of all activation markers except for CD86 in cDCs, where biphasic upregulation was observed (Fig. 5avi). In MyD88−/− cultures, low-level upregulations of CD40 (Fig. 5avii), CD80 (Fig. 5aviii), and CD86 (Fig. 5aix) were observed for the pDC subpopulation, although the upregulation of CD40 and CD80 was reduced compared to that of B6 pDC cultures. In contrast, MyD88−/− cDCs exhibited a strong, complete upregulation of CD40 (Fig. 5ax) and CD80 (Fig. 5axi) expression levels compared to B6 cDC cultures, while the CD86 (Fig. 5axii) expression level increased to the same extent. As expected, CpG stimulation failed to induce any increase in activation marker expression levels in either MyD88−/− DC subpopulation, as it relies exclusively on TLR9, and therefore MyD88, for effective signal transduction. Together, these results indicate no major differences in DC maturation between control B6 and MyD88−/− cultures, which suggests that ineffective DC licensing is not the reason why these mice fail to generate an optimal adaptive immune response to FWPV-encoded OVA.
FIG. 5.
Dendritic cells from MyD88-deficient mice display no defect in maturation, antigen processing, or antigen presentation following infection with FWPVOVA. (a) FL-expanded B6 or MyD88−/− DC cultures were incubated with FWPVWT (MOI of 1), CpG 2216 (1 μg/ml), or PBS in 12-well plates for 24 h. Recovered cells were stained with anti-B220, anti-CD11b, and either anti-CD40, anti-CD80, or anti-CD86 for flow cytometric analysis. Plasmacytoid DCs were gated as B220+/CD11blo, and cDCs were gated as B220−/CD11bint/hi, with CD40 (i, iv, vii, and x), CD80 (ii, v, viii, and xi), and CD86 (iii, vi, ix, and xii) expression levels presented as histograms. Unfilled dotted line, diluent control; light-gray filled histogram, CpG stimulation; dark-gray filled histogram, FWPVWT infection. (b) Control B6 or MyD88−/− mice were immunized with 107 PFU FWPVOVA (i.p.). Twenty-four hours later, purified CD11c+ splenic DCs were serially diluted and incubated in culture with 4 × 104 OT-I T cells. Two days later, cells were pulsed with [3H]thymidine, and 18 h later, thymidine incorporation was measured and is represented as cpm values. (c) Control B6 or MyD88−/− mice were immunized in the right footpad with 5 × 106 PFU FWPVOVA and received 107 CFSE-labeled OT-I cells the same day. Three days later, draining and nondraining LNs were harvested and individually assessed for OT-I proliferation via flow cytometric analysis of CFSE dilution, with proliferative index (PI) values shown to indicate the extent of proliferation. Results are representative of data from two experiments performed in triplicate (a); three separate experiments, depicted as the means ± SEM (b); or five replicate mice per group (c).
To determine whether DCs from MyD88−/− mice possess any defect in antigen-processing and antigen presentation functions, we sought to compare the abilities of MyD88−/− and B6 mice to capture FWPV-derived antigen in vivo and present it to antigen-specific T cells ex vivo. Mice were immunized with FWPVOVA, and 24 h later, splenocytes were harvested and dendritic cells were purified and incubated at various concentrations with antigen-naïve and OVA-specific OT-I T cells. T-cell proliferation was assessed 2 days later by measurements of tritiated thymidine incorporation. The results indicated no difference in the abilities of B6 and MyD88−/− DCs to capture and present the antigen to cognate T cells (Fig. 5b).
Finally, antigen presentations in MyD88−/− and B6 mice were compared in vivo by using an adoptive transfer model in which CFSE-labeled OT-I T cells were used to report the extent of virally derived OVA presentation after FWPVOVA immunization. Mice were immunized with FWPVOVA in one footpad and received adoptively transferred OT-I T cells immediately afterwards. Three days later, draining (popliteal) LNs and control nondraining (cervical) LNs were harvested and analyzed for the extent of OT-I proliferation by measuring CFSE dye dilution. The results indicated that B6 and MyD88−/− mice displayed an equally high level of OT-I T-cell proliferation within the draining popliteal LNs (proliferative index [PI], 10.31 and 11.47, respectively), with no significant proliferation evident within nondraining LNs (PI, 1.18 and 1.66, respectively) (Fig. 5c). Taken together, these results indicated that MyD88-deficient DCs are as competent as wild-type DCs in the antigen-processing and -presenting steps required to prime cognate T cells.
T-cell responses to FWPV-encoded OVA are intrinsically regulated by MyD88 expression.
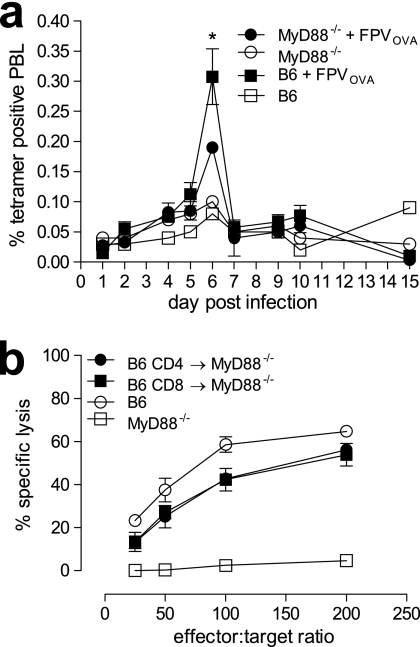
As there appeared to be no significant defect in DC immune function in MyD88−/− mice compared to the B6 controls, we next sought to determine whether T cells from these mice were functionally impaired, thus providing a possible explanation for the observed reduction in proliferative and CTL responses to FWPV-encoded OVA. First, endogenous OVA-specific CD8+ T-cell expansion in MyD88−/− and B6 mice was monitored for 15 days after immunization with FWPVOVA. The results indicated that the peak CD8+ T-cell response to OVA occurred at day 6 postinfection in both groups of mice, with expansion being enhanced in B6 mice (0.31% peripheral blood lymphocytes [PBL]) compared to MyD88−/− mice (0.19% PBL) (Fig. 6a).
FIG. 6.
T-cell responses to FWPV-encoded OVA are intrinsically regulated by MyD88 expression. (a) B6 or MyD88−/− mice were immunized with FWPVOVA (107 PFU i.p.), and blood was harvested from the tail vein on the days shown. Heparin-admixed blood was stained with tetramer and anti-CD8 antibodies and analyzed via flow cytometry for the assessment of OVA-specific CD8+ T-cell proliferation. Results are expressed as the mean percentages ± SEM of tetramer-positive peripheral blood lymphocytes (PBL) and represent data from five mice per group except for controls, which show data for one mouse per group. (b) B6 or MyD88−/− mice which had received purified B6 CD3+/CD4+ or CD3+/CD8+ T cells 1 day prior were immunized with 107 PFU FWPVOVA (i.p.). Splenocytes were harvested 5 days later and assayed directly in an 18-h CTL assay against specific and nonspecific 51Cr-labeled EL-4 target cells. Results indicate percent specific lysis, and data are representative of four separate experiments and represent the means ± SEM. *, P < 0.05.
To confirm that the impaired immune responses observed for MyD88−/− mice were due to the absence of MyD88 signaling specifically within the T-cell compartment, we asked whether the adoptive transfer of MyD88-competent T cells to a MyD88-deficient host could restore the ability of these mice to generate a robust CTL and T-helper proliferative response to FWPV-encoded OVA. Here, purified donor B6 naïve CD4+ or CD8+ T cells were adoptively transferred into MyD88−/− recipient mice such that each recipient received the per-mouse equivalent of a naïve B6 T-cell repertoire. The following day, reconstituted mice were immunized with FWPVOVA, and 5 days later, CTL (Fig. 6b) responses were assessed. The results indicated that MyD88−/− mice that received CD4+ or CD8+ T cells were able to achieve target cell killings of 56% and 54%, respectively, at the highest effector-to-target cell ratio. This level was slightly lower than the 60% observed for normal B6 mice but significantly higher than the 5% observed for the MyD88−/− controls (Fig. 6b). This indicated that the provision of MyD88-competent CD4+ or CD8+ T cells to MyD88-deficient mice was sufficient to restore CTL responses to FWPV-encoded OVA. Overall, these results support our hypothesis that the T-cell expression of MyD88 is critical for the formation of adaptive immune responses to FWPVOVA.
Interleukin-18, but not IL-1R signaling, is necessary for the induction of cellular adaptive immune responses to FWPVOVA immunization.
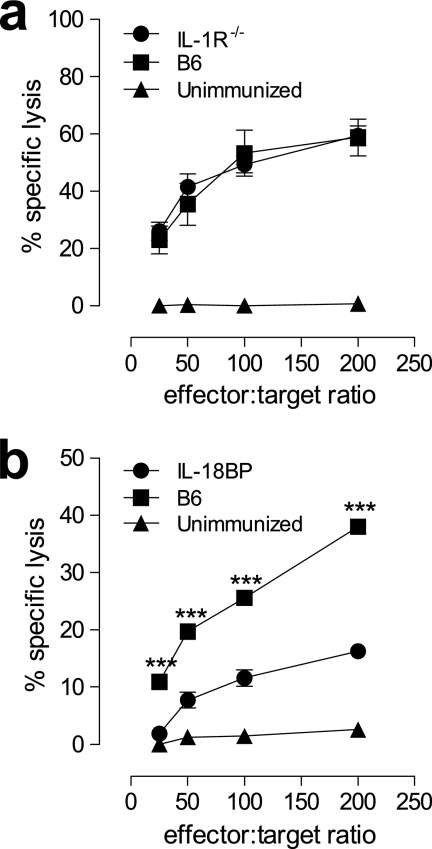
Given that we have provided evidence that TLR signaling is unlikely to contribute to the adaptive immune response to FWPV-encoded OVA and that the adaptive immune response depended on the T-cell expression of MyD88, other biological functions of the adapter molecule MyD88 clearly warranted further investigation. To this end, we examined the role that MyD88 had in IL-1 and IL-18 receptor signaling. To assess this, IL-1R−/− mice, as well as B6 mice rendered IL-18 deficient by the administration of high-affinity IL-18 binding protein (IL-18BP) in vivo, were immunized with FWPVOVA, and CTL responses were examined 5 days later (Fig. 7). The results showed no significant differences in CTL killing between IL-1R−/− and control B6 mice (58% and 60%, respectively, at a 200:1 effector-to-target cell ratio) (Fig. 7a). However, the in vivo neutralization of IL-18 significantly inhibited CTL responses, from the 38% killing (200:1 ratio) observed for untreated control B6 mice to 16% killing in IL-18BP-treated mice (Fig. 7b). These results indicated that IL-18 plays an essential role, through MyD88, in mediating the induction of T-cell effector responses to FWPV-derived antigens.
FIG. 7.
Interleukin-18, but not IL-1R signaling, is necessary for the induction of cellular adaptive immune responses to FWPVOVA immunization in vivo. (a) Control B6 or IL-1R−/− mice were immunized with FWPVOVA (107 PFU) or PBS. Splenocytes were harvested 5 days later and assayed directly for cytolytic activity in an 18-h CTL assay against specific and nonspecific 51Cr-labeled EL-4 target cells. (b) Same as above (a) except that B6 mice were either left untreated or treated with IL-18BP (15 μg/mouse i.p.) at the same time as they were immunized with FWPVOVA (107 PFU) or PBS. Data are expressed as means ± SEM and represent data from two experiments each containing 3 mice per group. ***, P < 0.001.
DISCUSSION
We have previously shown that adaptive immune responses to FWPV depend on the presence of pDCs (15), which upon further investigation appears to be at least partially attributable to their antigen presentation capability (43). Here, we sought to extend these findings by examining the mechanisms by which FWPV is recognized by the innate arm of the immune system and how these events are integrated to produce adaptive immune responses to FWPV-encoded OVA. We found that the ligation of either TLR7 or TLR9 by components of FWPV, together with downstream signaling mediated by MyD88, was necessary to instigate type I IFN responses, while TLR9 sensing in isolation appeared to be responsible for triggering proinflammatory cytokine secretions.
Given that pDCs are essential for the formation of an adaptive immune response to FWPV-encoded OVA (15), it was not entirely unexpected to find that TLR7 and TLR9 comediated the innate recognition of FWPV in vitro. Toll-like receptor 9 is overexpressed in pDCs (49), and it is recognized for its role in the innate recognition of other dsDNA viruses, including MCMV, herpes simplex virus (HSV), and adenovirus type 5 (Ad5) (37, 44, 64). Toll-like receptor 7, on the other hand, has a less restricted expression profile, but nonetheless, it is expressed at a high level in pDCs (17). Despite previously reported evidence of an innate immune recognition of other dsDNA viruses via TLR3 (64, 69) and TLR2 (12, 76), we found no role for these TLRs in generating anti-FWPV responses.
In investigating the role of the cytosolic sensing of FWPV in type I IFN responses, we found that the neutral-pH pathway (9) for the entry of FWPV was fully functional, as similar levels of viral transgene expression were detected in FL-expanded DCs regardless of chloroquine treatment (60). Despite FWPV entry into cells, the blocking of endosomal acidification inhibited FWPV signaling through TLR7 and TLR9 and prevented type I IFN secretion, which suggests that the low-pH endocytic environment (48, 67) is required for these processes. Moreover, our results suggest that the TLR-independent cytosolic recognition of FWPV-derived nucleic acids, for example, by the recently described STING- and TBK1-dependent pathway (34), does not contribute to FWPV-mediated type I IFN responses.
We investigated proinflammatory cytokine secretion by BM-derived DCs after FWPV infection and, as previously described (43), found that cultures of FL-expanded DCs (enriched for pDCs and cDCs) produced significantly more IL-6, TNF-α, and IL-12 than did cultures of GM-CSF-expanded DCs (enriched for cDCs). Unlike type I IFN production, which could be initiated via TLR7 or TLR9 signaling, the secretion of these cytokines depended exclusively on TLR9 signaling. Differential effects of TLR signaling have also been observed after MCMV infection (77). In seeking a mechanism for these differential effects, NF-κB signaling downstream of TLR activation was analyzed. Obvious NF-κB activation was lacking in the FWPV-infected and FL-expanded DCs that were derived from mice deficient in MyD88 and significantly inhibited in cells deficient in both TLR7 and TLR9, whereas NF-κB activation was normal in the DCs from mice singly deficient in TLR7 or TLR9. Therefore, defective NF-κB activation did not explain the absence of proinflammatory cytokine secretion by FWPV-infected TLR9−/− cultures and indicated that NF-κB activation could occur through either TLR7 or TLR9 signaling. Thus, although all TLR signaling pathways converge on NF-κB (8, 33), signaling pathways that diverge downstream of NF-κB may explain how a host generates pathogen-specific effector immune responses after the pathogen-mediated ligation of different TLRs.
Our in vivo studies of FWPV-infected TLR and TLR adaptor knockout mice indicated that only MyD88-deficient mice had impaired T-cell immune responses to the FWPV-encoded antigen, a finding recently reported for adaptive immune responses to the replication-incompetent Ad5 vector (41). Despite the demonstrated importance of TLR7 and TLR9 signaling to the recognition of FWPV by pDCs in vitro, our results indicate that the requirements for the specific T-cell recognition of FWPV-derived antigens are met irrespective of the status of TLR7 and TLR9 signaling in vivo. This result would not be entirely unexpected for the intact animal, because the presumed major target of FWPV infection, pDCs, will interact with many other types of immune cells in vivo, including cDCs, which, with appropriate costimulation and other cytokines, still induce an adaptive immune response to the FWPV-vectored antigen.
Consequently, we hypothesized that the importance of MyD88 to FWPV immunity resided in its role in IL-1R and IL-18R signaling rather than as a TLR adaptor protein. In support of this hypothesis, and as was demonstrated previously for vaccinia virus (VACV) (75), we found that MyD88-deficient DCs matured in vitro in response to FWPV infection and successfully captured and presented FWPV-derived antigens to cognate T cells in vivo. Here, we discovered that the efferent rather than the afferent limb of the immune response to FWPV immunity was impaired in MyD88-deficient mice. For example, in response to FWPVOVA immunization, MyD88-deficient tetramer-positive T cells expanded less than did wild-type tetramer-positive T cells. Furthermore, the provision of MyD88-competent T cells to MyD88-deficient mice before FWPVOVA immunization restored antigen-specific CTL immune responses.
Although we found that adaptive immunity to FWPV-encoded OVA depended on the T-cell expression of MyD88, the role of T-cell signaling mediated by the TLR or IL-1R family (1, 46) was uncertain. However, antigen-specific cellular immune responses were normal in FWPVOVA-immunized IL-1R-deficient mice. On the other hand, the depletion of IL-18 in FWPVOVA-immunized wild-type mice significantly impaired antigen-specific CTL responses, which provided strong evidence for a critical T-cell-specific role for this cytokine. Interleukin-18 has been shown to make a pivotal contribution to cellular immunity against murine influenza virus (13) and ectromelia virus (mouse poxvirus) in conjunction with IL-12 (71), but a role for MyD88 signaling in the T-cell response to IL-18 has not been formally evaluated. After HSV-1 challenge, pDCs produce IL-18, which is implicated in NK cell activation and IFN-γ production (3). Although we do not believe that NK cells are involved in adaptive immune responses to FWPV-encoded antigens (data not shown for in vivo NK cell depletion experiments), the idea that pDCs are a primary source of IL-18 fits well with our previously reported findings showing that pDCs are crucial for productive adaptive immune responses to FWPVOVA (15, 43). Pro-IL-18 is expressed constitutively (26) and is stored preformed in the DC cytoplasm (23). The processing of pro-IL-18 and the release of the active IL-18 cytokine are generally considered to depend on inflammasome assembly (61), although alternative mechanisms were suggested by previously reported in vitro studies (53). Consequently, we hypothesize that following immunization, FWPV directly infects pDCs and drives inflammasome assembly by the cytosolic recognition of FWPVOVA-derived dsDNA, putatively via AIM2 (57), which in turn triggers the release of active IL-18, thus facilitating T-cell adaptive immune responses to FWPV-encoded antigens. It has been postulated that IL-18 signaling within T cells subsequently drives the T-cell-intrinsic secretion of IFN-γ, which would explain why IFN-γ secretion is actively targeted by immunomodulatory genes of many members of the poxvirus family (51, 54, 59). Furthermore, we propose that this immune response occurs even in the absence of the TLR-meditated innate immune recognition of rFWPV, because FWPV infection enables inflammasome activation and the release of preformed pro-IL-18 from pDCs and results in MyD88-dependent signaling through the T cell's IL-18R (61). However, since the clearly demonstrated role for IL-18 and MyD88 does not account entirely for the ability of the murine immune system to form an adaptive immune response to FWPV, alternative mechanisms may be responsible.
If the results of our planned experiments support the hypothesis of FWPV-initiated activation of the inflammasome complex with consequent pro-IL-18 processing (61), then inflammasome activation may also initiate a cell death cascade that is inherently inflammatory in nature and which itself can result in an inflammation-induced immune response. These forms of cell death, pyroptosis or pyronecrosis (11, 72), can result in the “spilling out” of cellular contents upon death. The released components include many that ordinarily would be protected from the extracellular environment, some of which are recognized as danger-associated molecular patterns (DAMP) by the host immune system (19, 72). One of these molecules, the high-mobility-group box 1 (HMGB1) protein, is a potent DAMP which can induce potent proinflammatory cytokine secretion and the recruitment of immune cells and can drive the maturation of important immune cells, such as DCs, upon its release into the extracellular environment (5, 42). It is therefore possible that HMGB1, or a molecule of a similar nature, could provide the necessary signals to drive an adaptive immune response to FWPV in the absence of MyD88, albeit a severely impaired response (10). This would depend on the ability of FWPV-derived components to ligate an inflammasome-forming NOD-like receptor, for example, AIM2, which can sense DNA (57), and, in the case of pyroptosis, concurrently activate caspase-1 (36). It is interesting that HMGB1 is also capable of binding DNA within the cytosol and can participate in amplifying the innate immune responses to DNA ligands, including those derived from viruses (74). Thus, the roles of inflammasome-associated molecules and DAMPs such as HMGB1 remain the focus of ongoing investigations.
In summary, immunization with rFWPV results in the production of type I IFNs in a TLR7-, TLR9-, and MyD88-dependent fashion, while proinflammatory cytokine secretion depends solely on TLR9. Despite the importance of TLR7, TLR9, and MyD88 in innate immunity to rFWPV, only a MyD88 deficiency impairs adaptive immune responses to FWPV-encoded antigens. Here, the T-cell expression of MyD88 is critical, presumably because of its essential role in the IL-18 signaling cascade within T cells. A better understanding of the mechanisms involved in the innate immune sensing of FWPV and the subsequent development of adaptive immune responses to FWPV-encoded antigens, as described here, will guide the rational design of strategies aimed at improving the quantity and quality of immune responses elicited by this safe and versatile viral vaccine vector.
Acknowledgments
The work was supported in part by funding from Australian Research Council Linkage Project grant LP05618109 (J.D.H. and M.P.B.) and NHMRC program grant PG453556 (S.A.R.). E.L.L. was supported by the Cancer Council of South Australia through the provision of a Ph.D. stipend. Material support for this study was provided by Virax Holdings Ltd. and Regeneron Ltd.
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1.Adachi, O., et al. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Barr, D. P., et al. 2007. A role for plasmacytoid dendritic cells in the rapid IL-18-dependent activation of NK cells following HSV-1 infection. Eur. J. Immunol. 37:1334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beukema, E. L., M. P. Brown, and J. D. Hayball. 2006. The potential role of fowlpox virus in rational vaccine design. Expert Rev. Vaccines 5:565-577. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi, M. E., and A. A. Manfredi. 2007. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 220:35-46. [DOI] [PubMed] [Google Scholar]
- 6.Boyle, D., A. Pye, and B. Coupar. 1996. Comparison of field and vaccine strains of Australian fowlpox viruses. Arch. Virol. 142:737-748. [DOI] [PubMed] [Google Scholar]
- 7.Brasel, K., T. De Smedt, J. L. Smith, and C. R. Maliszewski. 2000. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 96:3029-3039. [PubMed] [Google Scholar]
- 8.Carmody, R. J., and Y. H. Chen. 2007. Nuclear factor-kappaB: activation and regulation during Toll-like receptor signaling. Cell. Mol. Immunol. 4:31-41. [PubMed] [Google Scholar]
- 9.Carter, G. C., M. Law, M. Hollinshead, and G. L. Smith. 2005. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J. Gen. Virol. 86:1279-1290. [DOI] [PubMed] [Google Scholar]
- 10.Castiglioni, A., V. Canti, P. Rovere-Querini, and A. A. Manfredi. 2011. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res. 343:189-199. [DOI] [PubMed] [Google Scholar]
- 11.Cookson, B. T., and M. A. Brennan. 2001. Pro-inflammatory programmed cell death. Trends Microbiol. 9:113-114. [DOI] [PubMed] [Google Scholar]
- 12.Delaloye, J., et al. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 5:e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Denton, A. E., P. C. Doherty, S. J. Turner, and N. L. La Gruta. 2007. IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur. J. Immunol. 37:368-375. [DOI] [PubMed] [Google Scholar]
- 14.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 15.Diener, K. R., et al. 2008. Recombinant fowlpox virus elicits transient cytotoxic T cell responses due to suboptimal innate recognition and recruitment of T cell help. Vaccine 26:3566-3573. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello, C. A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519-550. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, A. D., et al. 2003. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33:827-833. [DOI] [PubMed] [Google Scholar]
- 18.Emery, S., et al. 2007. Influence of IFNgamma co-expression on the safety and antiviral efficacy of recombinant fowlpox virus HIV therapeutic vaccines following interruption of antiretroviral therapy. Hum. Vaccin. 3:260-267. [DOI] [PubMed] [Google Scholar]
- 19.Franchi, L., T. Eigenbrod, R. Munoz-Planillo, and G. Nunez. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10:241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser, C. K., K. R. Diener, M. P. Brown, and J. D. Hayball. 2007. Improving vaccines by incorporating immunological coadjuvants. Expert Rev. Vaccines 6:559-578. [DOI] [PubMed] [Google Scholar]
- 21.Fraser, C. K., et al. 2010. Induction of both cellular and humoral immunity following a rational prime-boost immunization regimen that incorporates recombinant ovine atadenovirus and fowlpox virus. Clin. Vaccine Immunol. 17:1679-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French, M. A., et al. 2010. Vaccine-induced IgG2 anti-HIV p24 is associated with control of HIV in patients with a ‘high affinity’ FcγRIIa genotype. AIDS 24:1983-1990. [DOI] [PubMed] [Google Scholar]
- 23.Gardella, S., C. Andrei, A. Poggi, M. R. Zocchi, and A. Rubartelli. 2000. Control of interleukin-18 secretion by dendritic cells: role of calcium influxes. FEBS Lett. 481:245-248. [DOI] [PubMed] [Google Scholar]
- 24.Gilliet, M., et al. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenough, T. C., et al. 2008. Safety and immunogenicity of recombinant poxvirus HIV-1 vaccines in young adults on highly active antiretroviral therapy. Vaccine 26:6883-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu, Y., et al. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275:206-209. [DOI] [PubMed] [Google Scholar]
- 27.Gulley, J. L., et al. 2009. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol. Immunother. 59:663-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulley, J. L., et al. 2008. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin. Cancer Res. 14:3060-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hacker, H., et al. 1998. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 17:6230-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmi, H., et al. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 31.Hemmi, H., et al. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 32.Hoebe, K., et al. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743-748. [DOI] [PubMed] [Google Scholar]
- 33.Huang, Q., et al. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870-875. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa, H., Z. Ma, and G. N. Barber. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawai, T., and S. Akira. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373-384. [DOI] [PubMed] [Google Scholar]
- 36.Kepp, O., L. Galluzzi, L. Zitvogel, and G. Kroemer. 2010. Pyroptosis—a cell death modality of its kind? Eur. J. Immunol. 40:627-630. [DOI] [PubMed] [Google Scholar]
- 37.Krug, A., et al. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21:107-119. [DOI] [PubMed] [Google Scholar]
- 38.Kumar, S., and D. B. Boyle. 1990. A poxvirus bidirectional promoter element with early/late and late functions. Virology 179:151-158. [DOI] [PubMed] [Google Scholar]
- 39.Labow, M., et al. 1997. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J. Immunol. 159:2452-2461. [PubMed] [Google Scholar]
- 40.Lechleider, R. J., et al. 2008. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin. Cancer Res. 14:5284-5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsay, R. W., et al. 2010. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J. Immunol. 185:1513-1521. [DOI] [PubMed] [Google Scholar]
- 42.Lotze, M. T., and K. J. Tracey. 2005. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5:331-342. [DOI] [PubMed] [Google Scholar]
- 43.Lousberg, E. L., C. K. Fraser, M. G. Tovey, K. R. Diener, and J. D. Hayball. 2010. Type I interferons mediate the innate cytokine response to recombinant fowlpox virus but not the induction of plasmacytoid-dependent adaptive immunity. J. Virol. 84:6549-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macagno, A., G. Napolitani, A. Lanzavecchia, and F. Sallusto. 2007. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 28:227-233. [DOI] [PubMed] [Google Scholar]
- 46.Mannel, D. N., S. B. Mizel, T. Diamantstein, and W. Falk. 1985. Induction of interleukin 2 responsiveness in thymocytes by synergistic action of interleukin 1 and interleukin 2. J. Immunol. 134:3108-3110. [PubMed] [Google Scholar]
- 47.Matsumoto, M., and T. Seya. 2008. TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 60:805-812. [DOI] [PubMed] [Google Scholar]
- 48.Mercer, J., and A. Helenius. 2008. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320:531-535. [DOI] [PubMed] [Google Scholar]
- 49.Naik, S. H., et al. 2005. Generation of splenic CD8+ and CD8− dendritic cell equivalents in fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 174:6592-6597. [DOI] [PubMed] [Google Scholar]
- 50.Netea, M. G., J. W. M. Van der Meer, R. P. Sutmuller, G. J. Adema, and B.-J. Kullberg. 2005. From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrob. Agents Chemother. 49:3991-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuara, A. A., et al. 2008. Structure and mechanism of IFN-gamma antagonism by an orthopoxvirus IFN-gamma-binding protein. Proc. Natl. Acad. Sci. U. S. A. 105:1861-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Callaghan, C., et al. 1999. BirA enzyme: production and application in the study of membrane receptor-ligand interactions by site-specific biotinylation. Anal. Biochem. 266:9-15. [DOI] [PubMed] [Google Scholar]
- 53.Omoto, Y., et al. 2010. Granzyme B is a novel interleukin-18 converting enzyme. J. Dermatol. Sci. 59:129-135. [DOI] [PubMed] [Google Scholar]
- 54.Puehler, F., et al. 2003. An interferon-gamma-binding protein of novel structure encoded by the fowlpox virus. J. Biol. Chem. 278:6905-6911. [DOI] [PubMed] [Google Scholar]
- 55.Radaelli, A., E. Pozzi, S. Pacchioni, C. Zanotto, and C. de Giuli Morghen. 2010. Fowlpox virus recombinants expressing HPV-16 E6 and E7 oncogenes for the therapy of cervical carcinoma elicit humoral and cell-mediated responses in rabbits. J. Transl. Med. 8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rerks-Ngarm, S., et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209-2220. [DOI] [PubMed] [Google Scholar]
- 57.Roberts, T. L., et al. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323:1057-1060. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg, S. A., et al. 2003. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. Clin. Cancer Res. 9:2973-2980. [PMC free article] [PubMed] [Google Scholar]
- 59.Sakala, I. G., et al. 2007. Poxvirus-encoded gamma interferon binding protein dampens the host immune response to infection. J. Virol. 81:3346-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savarino, A., J. R. Boelaert, A. Cassone, G. Majori, and R. Cauda. 2003. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 3:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sims, J. E., and D. E. Smith. 2010. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10:89-102. [DOI] [PubMed] [Google Scholar]
- 62.Smith, F. O., J. A. Klapper, J. R. Wunderlich, S. A. Rosenberg, and M. E. Dudley. 2009. Impact of a recombinant fowlpox vaccine on the efficacy of adoptive cell therapy with tumor infiltrating lymphocytes in a patient with metastatic melanoma. J. Immunother. 32:870-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swiecki, M., and M. Colonna. 2010. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 234:142-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabeta, K., et al. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. U. S. A. 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takeuchi, O., et al. 1999. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 66.Townsley, A. C., and B. Moss. 2007. Two distinct low-pH steps promote entry of vaccinia virus. J. Virol. 81:8613-8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Townsley, A. C., A. S. Weisberg, T. R. Wagenaar, and B. Moss. 2006. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J. Virol. 80:8899-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1998. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79:877-887. [DOI] [PubMed] [Google Scholar]
- 69.Vercammen, E., J. Staal, and R. Beyaert. 2008. Sensing of viral infection and activation of innate immunity by Toll-like receptor 3. Clin. Microbiol. Rev. 21:13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villadangos, J. A., and P. Schnorrer. 2007. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 7:543-555. [DOI] [PubMed] [Google Scholar]
- 71.Wang, Y., G. Chaudhri, R. J. Jackson, and G. Karupiah. 2009. IL-12p40 and IL-18 play pivotal roles in orchestrating the cell-mediated immune response to a poxvirus infection. J. Immunol. 183:3324-3331. [DOI] [PubMed] [Google Scholar]
- 72.Willingham, S. B., et al. 2007. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2:147-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamamoto, M., et al. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 74.Yanai, H., et al. 2009. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462:99-103. [DOI] [PubMed] [Google Scholar]
- 75.Zhao, Y., et al. 2009. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J. Immunol. 182:6278-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu, J., J. Martinez, X. Huang, and Y. Yang. 2007. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 109:619-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zucchini, N., et al. 2008. Overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J. Immunol. 180:5799-5803. [DOI] [PubMed] [Google Scholar]