Abstract
Enterovirus 71 (EV71) is the major causative agent of hand, foot, and mouth disease (HFMD) in young children and has been consistently associated with the most severe complications of the disease, including central nervous system inflammation and pulmonary edema. Increasing frequency and amplitude of EV71 outbreaks have raised awareness and concerns worldwide. Previous reports proposed that overwhelming virus replication combined with the induction of massive proinflammatory cytokines is responsible for the pathogenicity of EV71. Specifically, elevated interleukin-6 (IL-6) levels were observed consistently in patients and strongly correlated with disease severity. In this study, we show in the neonate mouse model that sustained high levels of IL-6 produced upon EV71 infection lead to severe tissue damage and eventually death of the animals. Administration of anti-IL-6 neutralizing antibodies after the onset of the clinical symptoms successfully improved the survival rates and clinical scores of the infected hosts. Compared to untreated infected controls, anti-IL-6-treated mice displayed reduced tissue damage, absence of splenic atrophy, and increased immune cell activation. In addition, markedly elevated systemic levels of IL-10 were measured in the protected animals. Furthermore, there was no significant difference in virus titers between anti-IL-6-treated mice and untreated mice, indicating that the anti-IL-6 antibody-mediated protection is independent of the virus load. Our findings thus demonstrate that IL-6 plays a major role in EV71-induced immunopathogenesis. As there is still neither vaccine nor treatment available against EV71, anti-IL-6 antibody treatment represents a potential therapeutic approach to providing protection from the most severe complications of the disease.
Enterovirus 71 (EV71) is the major etiological agent of hand, foot, and mouth disease (HMFD). It is a small, nonenveloped virus with a positive-stranded RNA genome size of about 7.4 kb. Taxonomically, EV71 belongs to the Enterovirus genus of the Picornaviridae family. Since its first description in the United States in 1969 (41), EV71 has been associated with several epidemics of HFMD, severe neurological disease, and other complications in Australia, Europe, Asia, and the United States (11, 15, 31). In recent years, multiple reports of large-scale HFMD outbreaks in Singapore, India, Thailand, Hong Kong, Malaysia, and Brunei have been received (38). In 2009, the disease caused 155 deaths in China alone, where health authorities reported 436,221 cases between 1 March and 31 May (55). Strikingly, EV71-mediated HFMD infections reported in 2009 in Malaysia and Brunei increased tremendously compared to levels for the same period the year before (55). As there is no effective vaccine or antiviral treatment available on the market, EV71 infections have increasingly become a public health concern in developed and developing countries.
EV71 infection affects mainly infants and young children, is transmitted via the oral-fecal route, and results usually in a mild and self-limiting disease characterized by herpangina, sore throat, and fever. However, EV71 infection may occasionally lead to severe complications such as aseptic meningitis, brain stem encephalitis, and acute flaccid paralysis, a polio-like syndrome (4, 16, 27, 28, 56). Autopsies of EV71-associated deaths revealed various contributing factors, including extensive neuronal degeneration, severe central nervous system (CNS) inflammation, and pulmonary congestion with hemorrhage (pulmonary edema [PE]) (5, 6). A number of animal models have been developed to detail the pathogenesis of EV71 infection (7, 33, 36, 54). However, the majority of the research has been devoted to understanding the neurotropism and neuropathogenesis of EV71, whereas the immunopathogenesis aspect of the viral infection has remained largely unknown. As with many acute viral infections, the role of viral versus immunopathological events in EV71 pathogenesis has been discussed; it was proposed that overwhelming virus replication combined with the induction of massive proinflammatory cytokines is responsible for the pathogenicity of EV71 (24, 25, 52). Indeed, high levels of interleukin-1β (IL-1β), IL-6, IL-10, IL-13, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) in the serum and cerebral spinal fluid (CSF) from EV71-infected patients have been consistently reported (24, 51, 52). In particular, CSF levels of IL-1β, IL-6, and TNF-α were found significantly elevated in patients with PE and encephalitis, demonstrating a strong correlation between proinflammatory cytokine production and clinical severity in EV71 infections (25, 54). Furthermore, administration of intravenous immunoglobulin (IVIG), a critical treatment upon diagnosis of neuro-dysregulation in EV71-infected patients, could effectively reduce the level of proinflammatory cytokines such as IL-6 and IL-8 during the early phase of EV71-associated autonomic nervous system (ANS) dysregulation and prevent further progression to PE (50). IVIG contains natural anticytokine antibodies such as antibodies against IL-1, IL-6, and interferons that modulate the cytokine cascade (1). As there were no significant changes in neutralizing antibody (Ab) titers against EV71 before and after the administration of IVIG in the recovered patients, the changes in concentrations of pro- and anti-inflammatory cytokines, as well as of soluble cytokine receptor and receptor antagonists, are likely responsible for the therapeutic effect of IVIG. Collectively, these observations support the idea that proinflammatory cytokines, especially IL-6, seem to play a prominent role in the overwhelming disease process induced upon EV71 infection.
In this study, we assessed the role of IL-6 during EV71 infection in the neonatal mouse model (9, 40, 46). Our results indicate that EV71 infection leads to the production of sustained high levels of IL-6 in the animals, which correlated with severe organ damage. Furthermore, the administration of anti-IL-6 neutralizing antibodies after disease symptoms started to manifest significantly improved the survival rate and prevented clinical symptoms and disease severity from progressing further.
MATERIALS AND METHODS
Virus and growth conditions.
EV71 strain 5865/SIN/00009 (accession number AF316321), designated strain 41, was propagated in rhabdomyosarcoma (RD) cells at 37°C in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) with 100 U/ml penicillin and 10 μg/ml streptomycin (Invitrogen). Once the cells displayed cytopathic effect (CPE), they were harvested, and cellular debris was removed by centrifugation at 10,000 × g for 30 min. The virus was purified by precipitation with 7% polyethylene glycol 8000 (PEG 8000) (Sigma-Aldrich, St. Louis, MO) and then subjected to centrifugation on a 30% sucrose cushion at 25,000 × g for 4 h. The 50% tissue culture infective dose (TCID50) was determined in RD cells using the Reed and Muench formula (39).
Animals and treatments.
Inbred BALB/c mice were obtained from the Centre for Animal Resources (CARE) of the National University of Singapore. All institutional guidelines for animal care and use were strictly followed throughout the experiments. One-day-old mice were infected with a lethal dose (104 TCID50) of EV71 strain 41 in 100 μl via the intraperitoneal (i.p.) route. At the time of viral challenge (day 0), or at 3 or 6 days postinfection, mice were injected i.p. with purified neutralizing monoclonal rat anti-mouse IL-6 IgG monoclonal antibody (anti-IL-6 MAb) (BD PharMingen, San Jose, CA) at 1.33 mg/kg per mouse. Suckling mice from the control group were injected with 1.33 mg/kg/mouse of purified rat IgG1 (isotype control) (BD PharMingen). All suckling mice were monitored daily upon infection for occurrence of clinical symptoms and mortality up to day 25 postinfection as the experimental endpoint.
Cytokine quantification.
Blood was collected from mice of various groups at the time points indicated in the figure legends and centrifuged for 10 min at 800 × g at 4°C. The sera were then frozen and stored at −80°C until further examination. Brain, limb muscle, intestine, spleen, and lungs were harvested from sacrificed animals at indicated time points, weighed, and homogenized in 500 μl of 1× phosphate-buffered saline (PBS) immediately. The homogenates were centrifuged at 13,000 × g for 10 min at 4°C, and the supernatant was collected and stored frozen at −80°C until further analysis. The levels of cytokine were measured using a mouse IL-6 enzyme-linked immunosorbent assay (ELISA) set and IL-10 ELISA set (eBioscience, San Diego, CA) according to the manufacturer's guidelines.
Histology.
Mice were euthanized, and the tissues were harvested and immediately fixed in a fixative decalcifier, Formica-4 (Decal Chemical Corporation, Tallman, NY), for 72 h at room temperature. Fixed tissues were paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E).
Virus quantification.
Intestines from mice were aseptically harvested in DMEM and homogenized for total RNA extraction using an RNeasy extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The extracted RNA was then analyzed for the presence of EV71 using a real-time hybridization probe and reverse transcription-PCR (RT-PCR) for the detection of EV71 RNA as described previously (45). Each assay was carried out in triplicates.
Flow cytometry analysis.
Spleens were harvested, and total splenocyte suspensions were prepared and stained with the following antibodies: Pacific blue-conjugated anti-CD4 (RM4-5; eBioscience), fluorescein isothiocyanate (FITC)-conjugated anti-CD8α (53-7.7; eBioscience), Cy-5-conjugated anti-CD19 (6D5; Beckman Coulter, Brea, CA), phycoerythrin-conjugated anti-CD69 (H1.2F3; eBioscience), and allophycocyanin (APC)-conjugated anti-CD138 (281-2; BD Biosciences) for 45 min on ice. The stained cells were then analyzed by a Cyan Flow Cytometer using Summit software (Beckman Coulter).
Statistics.
All statistical analysis was done with GraphPad Prism, version 5.0 (GraphPad Software, San Diego, CA), for Mac. Kaplan-Meier survival curves were analyzed by a log rank test. Clinical disease score curves were analyzed by a Wilcoxon test. Other experiments were analyzed by a two-tailed Student's t test, for which a P value of <0.05 was considered significant.
RESULTS
Systemic and local levels of IL-6 are elevated in EV71-infected mice.
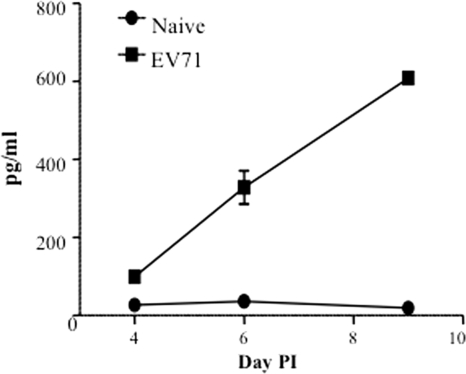
One-day-old suckling BALB/c mice were infected with a lethal dose (104 TCID50) of EV71 strain 41, a lab-passaged strain that originates from a fatal human case (43). As described previously by us (9), the infected pups displayed a range of clinical symptoms over the course of infection including hunched back, reduced motility, hind limb paralysis, and eventually death at day 12 postinfection. Furthermore, the systemic levels of IL-6 were monitored over the course of infection and were compared to age-matched noninfected animals. Significantly higher levels of serum IL-6 were measured in the EV71-infected mice and gradually increased over time with a peak observed at day 9 postinfection (Fig. 1).
FIG. 1.
Systemic IL-6 level in EV71-infected mouse neonates. Groups of 1-day-old suckling mice were challenged with EV71 strain 41 at a lethal dose of 104 TCID50 per mouse. At days 4, 6, and 9 postinfection (PI), four mice per group per time point were sacrificed, and individual serum samples were collected for IL-6 quantification by ELISA. The results are expressed as the mean ± standard error of the mean of the four serum samples.
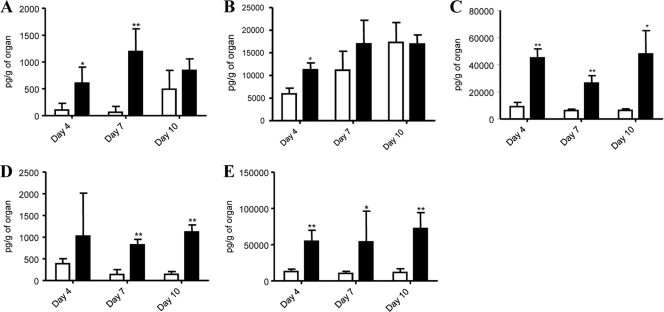
In addition, local IL-6 production in the brain, muscle, intestines, spleen, and lungs from EV71-infected mice was analyzed. Consistent with the increased IL-6 production measured in the serum, IL-6 levels were significantly higher in the various organ homogenates prepared from EV71-infected animals than in those from noninfected controls (Fig. 2). These results thus indicate that a single intraperitoneal (i.p.) inoculation with EV71 stimulates the local and systemic inflammatory response, as shown by increased tissue and serum levels of IL-6.
FIG. 2.
IL-6 production in the brain, muscle, intestines, spleen, and lungs from EV71-infected mouse neonates. Groups of 1-day-old suckling mice were challenged with EV71 strain 41 at a lethal dose of 104 TCID50 per mouse. At days 4, 7, and 10 postinfection, four mice per group per time point were sacrificed, and various organs were harvested and homogenized. The levels of IL-6 in the brain (A), muscle (B), intestine (C), spleen (D), and lung (E) homogenates from naïve (open bar) and EV71-infected (black bar) animals were quantified individually by ELISA. The results are expressed as the means ± standard error of the means. *, P < 0.05; **, P < 0.01 (in relation to naïve group).
Anti-IL-6 antibody treatment protects mice from lethal EV71 infection.
Previous literature indicates that high levels of serum and CSF IL-6 correlate with disease severity in EV71 patients (24, 51) and that they play an important role in the development of the meningitis-associated inflammatory reaction (54). We thus examined whether IL-6 blockade may improve the disease outcome in the mouse model of EV71 infection.
One-day-old suckling BALB/c mice were lethally infected with EV71 strain 41 and were injected i.p. 3 or 6 days later with a monoclonal anti-IL-6 IgG neutralizing antibody at 1.33 mg/kg of body weight. A control group was injected with an irrelevant IgG antibody (isotype Ab). An additional control group of noninfected pups was i.p. injected with anti-IL-6 neutralizing antibody.
In a first set of experiments, mice from all groups were sacrificed at day 9 postinfection, and their systemic levels of IL-6 were measured individually. The results showed that a single dose of anti-IL-6 antibody given at day 3 or day 6 post-EV71 infection significantly reduced the serum IL-6 concentration to a level comparable to that measured in the uninfected mice, whereas high IL-6 levels were detected in nontreated infected mice and in EV71-infected mice injected with an irrelevant antibody (Table 1).
TABLE 1.
Systemic IL-6 levels in EV71-infected mice either untreated or treated with anti-IL-6 neutralizing antibodies postinfectiona
| Animal treatment group | Mean IL-6 (pg/ml [SEM]) |
|---|---|
| Naïve | 19.61 (4.72) |
| Anti-IL-6 only | 68.80 (21.04) |
| EV71 challenge + anti-IL-6 (day 3) | 51.23 (31.62) |
| EV71 challenge + anti-IL-6 (day 6) | 7.51 (1.88) |
| EV71 challenge + isotype Ab | 682.17 (36.74) |
| EV71 challenge | 668.78 (59.34) |
Groups (n = 4) of 1-day-old suckling mice were lethally challenged with EV71 strain 41 at a lethal dose of 104 TCID50 per mouse. Two groups received neutralizing anti-IL-6 antibodies at 1.33 mg/per kg of body weight on day 3 and day 6 postinfection. At day 9 postinfection, individual serum samples were collected for IL-6 quantification. Serum samples were diluted 1:25.
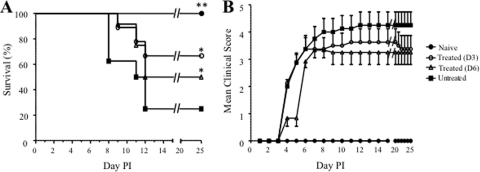
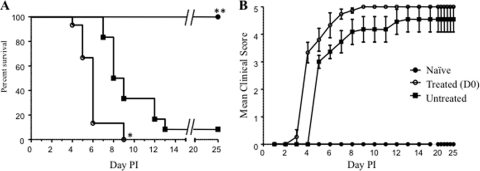
In a second set of experiments, survival of the mice from each of the groups described above was monitored over 25 days post-EV71 infection. Anti-IL-6 treatment resulted in significantly improved survival of the infected pups when the treatment was administered at day 3 or day 6 postinfection, with a 67% and 50% survival rate, respectively, whereas the survival rate of the untreated infected mice was 25% (Fig. 3 A). Infected mice treated with an irrelevant IgG antibody displayed a survival rate similar to that of the infected nontreated group (data not shown).
FIG. 3.
Survival rate and clinical score of EV71-infected mouse neonates either untreated or treated with anti-IL-6 antibodies postinfection (PI). Groups of 1-day-old mice were lethally infected with EV71 strain 41 and treated with anti-IL-6 antibodies at 1.33 mg/kg of body weight at 3 days (D3; n = 9) or 6 days (D6; n = 12) post-EV71 challenge. The control groups are either uninfected animals (Naïve; n = 6) or untreated EV71-infected animals (Untreated; n = 8). The survival rates and clinical scores were determined for each mouse group based on daily observation of the animals. Clinical scores were defined as follows: 0, healthy; 1, ruffled hair, hunchbacked; 2, reduced mobility; 3, limb weakness; 4, limb paralysis; and 5, death. *, P < 0.05; **, P < 0.01 (in relation to the untreated group).
To further evaluate the protective potential of the anti-IL-6 antibody treatment, the mean clinical score was determined for each mouse group upon daily observation and recording of relevant clinical manifestations. Mice from the untreated infected group (Fig. 3B) or from the group treated with an irrelevant antibody (data not shown) typically displayed reduced motility at day 4. The disease severity then developed progressively to limb weakness at day 5 and hind limb paralysis from day 8 postinfection onwards; the majority of the animals died by day 12 postinfection. The anti-IL-6 antibody-treated mice exhibited symptoms similar to those of the untreated controls during the early course of infection (before the antibody treatment was administered), i.e., hunched back, reduced motility, and limb weakness (Fig. 3B). However, as soon as 24 h after antibody treatment, further manifestations of clinical symptoms stopped, and the mice eventually recovered their full motion capabilities.
Collectively, these data demonstrate that treatment up to at least 6 days postinfection with anti-IL-6 neutralizing antibodies significantly protects neonatal mice against EV71 infection, improving the survival rate and preventing the most severe clinical symptoms from manifesting.
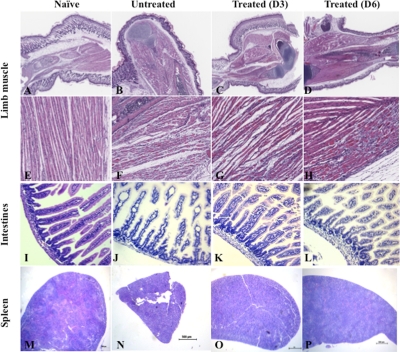
Anti-IL-6 antibody treatment prevents tissue damage in EV71-infected mouse neonates.
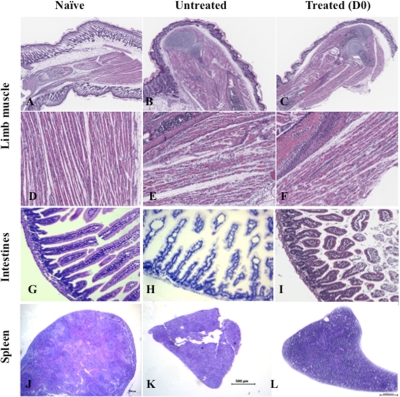
We along with others have previously reported severe tissue damage in the mouse model of EV71 infection (9, 46, 53). To further assess the protective effect of an anti-IL-6 neutralizing antibody treatment, central and peripheral nervous systems, brains, limb muscles, intestines, and spleens from uninfected mice and treated and untreated infected animals were harvested at day 10 postinfection, and a comparative histopathology analysis was performed. No marked lesions and/or obvious signs of inflammation in the brain and central or peripheral nervous tissue were observed from the infected animals (data not shown). In contrast, massive muscle damage characterized by the presence of foci of myositis and myonecrosis associated with neutrophilic infiltration was observed in the limb musculature from EV71-infected mice (Fig. 4 B and F). Moreover, no apparent tissue damage was noticed in limb muscle from the anti-IL-6-treated mice (Fig. 4C, D, G, and H). However, compared to noninfected animals, the limb musculature of the anti-IL-6-treated mice displayed slight myofiber regeneration with nuclear rowing, suggesting signs of recovery from the virus infection (Fig. 4G and H).
FIG. 4.
Histological examination of the muscles, intestines, and spleen from EV71-infected mice either untreated or treated with anti-IL-6 antibodies postinfection. EV71 infection and anti-IL-6 antibody treatment were performed as described in the legend of Fig. 3. At day 10 postinfection, three mice per group were euthanized. Paraffin sections of the limb muscle, intestines, and spleen were stained by hematoxylin and eosin. The specimens shown are representatives of three mice in each group with similar histologies. Observations were made at magnifications of ×2.5 (A to D and M to P) and ×20 (E to H and I to L).
Histological analysis of the intestines from untreated EV71-infected mice revealed dilation of the villi as well as mild villous blunting with associated crypt hyperplasia, suggesting a substantial degree of tissue damage in the intestines (Fig. 4J). In contrast, no apparent tissue damage was noticed in intestines from anti-IL-6-treated mice (Fig. 4K and L). However, compared to noninfected animals, slight thickening of the intestinal epithelium could be observed in the anti-IL-6-treated group, indicating some degree of mucosal inflammation.
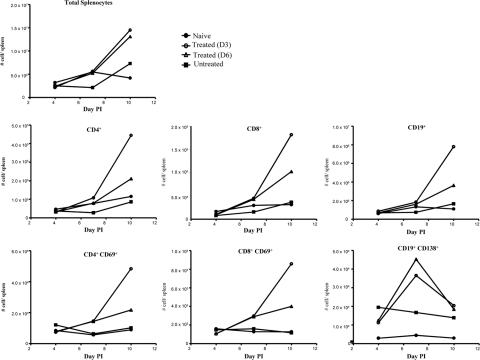
The spleens harvested from untreated infected mice were strikingly smaller in size than the spleens from naïve or anti-IL-6-treated groups (Fig. 4M to P). In addition, signs of immune cell activation and proliferation were observed in the spleens from the anti-IL-6-treated mice, as evidenced by the enlargement of the white pulp compared to the red pulp (Fig. 4O and P). Fluorescence-activated cell sorting (FACS) analysis further revealed a reduced number of total splenocytes in EV71-infected animals which likely accounted for the splenic atrophy visually observed in this animal group, whereas anti-IL-6 treatment resulted in a marked increase of the total number of splenocytes over time (Fig. 5). Such an increase was reflected by an increase of various immune cell populations, including CD4+ and CD8+ T cells and B cells, as well as their activated counterparts, further supporting the histological observations (Fig. 5).
FIG. 5.
Spleen cell composition in EV71-infected mice either untreated or treated with anti-IL-6 antibodies postinfection (PI). EV71 infection and anti-IL-6 antibody treatment were performed as described in the legend of Fig. 3. At days 4, 7, and 10 postinfection, four mice per group and per time point were euthanized, and their spleens were harvested and pooled. Total splenocytes were counted or stained with monoclonal antibodies specific to CD4, CD8, CD19, CD69, and CD138 and analyzed by flow cytometry. The data show the absolute numbers of total splenocytes and the respective cellular subsets as indicated.
Altogether, these data demonstrate that treatment with anti-IL-6 neutralizing antibodies prevents EV71-induced organ (muscle and intestine) damage and spleen atrophy and results in strong immune cell activation.
Anti-IL-6 antibody treatment did not affect the viral load.
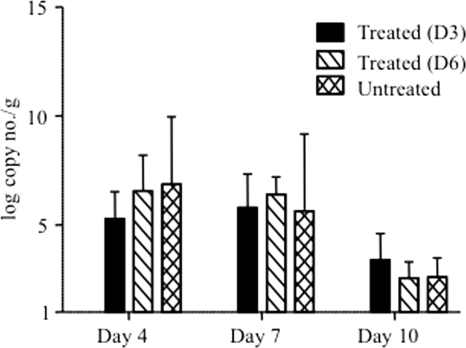
To further characterize the anti-IL-6 antibody-mediated protection against EV71, the viral load was determined by real-time PCR as described previously (45) in the anti-IL-6-treated mice and compared to the load in untreated infected controls. While PCR amplification was obtained from blood, intestines, and brain samples (data not shown), the intestines represent the only tissue from which viral RNA could be consistently and reproducibly extracted and quantified. No significant differences in the intestinal viral loads were found between treated and untreated groups over the course of infection, demonstrating that the protective effect of neutralizing anti-IL-6 antibodies does not result in reduction of the viral load (Fig. 6). Importantly, this observation further supports the idea that EV71-induced clinical manifestations and disease outcome are not a direct consequence of the cytopathic effect of the virus but, rather, result from an exacerbated and uncontrolled inflammation reaction, which leads to the host tissue damage.
FIG. 6.
Viral load in the intestines of EV71-infected mice either untreated or treated with anti-IL-6 antibodies postinfection. EV71 infection and anti-IL-6 antibody treatment were performed at day 3 or 6 postinfection as described in the legend of Fig. 3. Four animals per group and per time point were sacrificed, and their intestines were harvested and homogenized immediately. The viral load was determined by real-time PCR and is expressed as the mean value of the number of viral genome copies/g of tissue. The data represent the means ± standard error of the means of the four homogenates for each group.
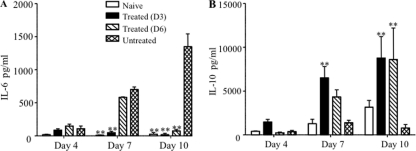
Anti-IL-6 antibody treatment increased serum IL-10 production.
Our data support the notion that anti-IL-6 neutralizing antibodies protect against EV71 infection via the reduction of the systemic and local inflammation reaction, thereby leading to limited tissue damage and reduced disease severity. To further confirm this hypothesis, the levels of both IL-6 and IL-10 were determined over the course of infection in treated and nontreated infected animals, and the ratio of IL-10 to IL-6, as a reflection of the inflammatory status of the animals, was derived. Upon treatment with anti-IL-6 antibodies, the serum concentration of IL-6 was brought back to basal levels while the serum concentration of IL-10 increased and was significantly higher than that measured in the infected untreated animals (Fig. 7). These data translated into IL-10/IL-6 ratios that were significantly higher in mice treated with anti-IL-6 antibodies than in uninfected mice (Table 2).
FIG. 7.
Serum IL-6 and IL-10 levels in EV71-infected mice either untreated or treated with anti-IL-6 antibodies postinfection. EV71 infection and anti-IL-6 antibody treatment at day 3 or 6 postinfection were performed as described in the legend of Fig. 3. Four animals per group were sacrificed at the indicated time points, and individual serum samples were collected for IL-6 and IL-10 quantification. Serum samples were diluted at 1:25. The data are expressed as the means ± standard error of the means of the four serum samples per group and per time point. **, P < 0.01, in relation to untreated group.
TABLE 2.
IL-10/IL-6 ratios in EV71-infected mice either untreated or treated with anti-IL-6 antibodies postinfectiona
| Day postinfection | Mean IL-10/IL-6 ratio by treatment group (SEM) |
|||
|---|---|---|---|---|
| Naïive | Treated (day 3) | Treated (day 6) | Untreated | |
| 4 | 23.65 (5.83) | 18.40 (3.27) | 1.90 (0.87) | 5.73 (2.74) |
| 7 | 103.76 (50.68) | 436.42 (225.38) | 7.42 (1.37) | 2.00 (0.47) |
| 10 | 50.57 (9.88) | 311.22 (15.37) | 121.28 (32.16) | 0.82 (0.56) |
EV71 infection and anti-IL-6 antibody treatment were performed as described in the legend of Fig. 3. At the indicated time points postinfection, four mice per group per time point were sacrificed, and their blood was collected to determine the IL-6 and IL-10 serum concentrations.
In conclusion, these data confirm that treatment with anti-IL-6 neutralizing antibodies given at day 3 or 6 postinfection changes drastically the pro- versus anti-inflammatory balance of the immune response, thereby counteracting the strong inflammation induced upon EV71 infection.
Anti-IL-6 treatment at the time of infection is detrimental to the mice.
Previous literature reported that IL-6 knockout (KO) mice were more susceptible to EV71 infection than their parental counterparts, suggesting a protective role for IL-6 during EV71 infection (26). In contrast, we found that anti-IL-6 antibody treatment resulted in better survival and clinical outcome, thus suggesting instead that IL-6 is detrimental to the host. These apparent contradictory observations may indicate that while IL-6 is beneficial and necessary to the host at an early stage of the infection to trigger the antiviral innate and adaptive immunity, thereby limiting viral replication, sustained elevated levels of this proinflammatory mediator over the course of infection are harmful and are responsible for the immunopathological aspect of the disease. To test this hypothesis, mice were cotreated with anti-IL-6 antibodies at the time of EV71 infection (day 0). Survival rates and clinical scores indicated that the treated animals died significantly faster than the untreated infected mice (Fig. 8), thus supporting previous observations with IL-6 KO mice (26). Consistently, mice cotreated with anti-IL-6 antibodies at the time of infection displayed severe tissue (limb muscle) damage, with myonecrosis even more pronounced than in the untreated infected animals, and spleen atrophy (Fig. 9). FACS analysis further revealed that the antibody treatment at the time of infection resulted in reduced total numbers of splenocytes and reduced production of various immune cell populations, including CD4+ and CD8+ T cells and B cells, as well as their activated counterparts (see Fig. S1 in the supplemental material). The reduced numbers were either comparable to or even lower than those obtained with the nontreated infected animals.
FIG. 8.
Survival rate and clinical score of EV71-infected neonatal mice either untreated or cotreated with anti-IL-6 antibodies. Groups of 1-day-old mice were lethally infected with EV71 strain 41 and cotreated with anti-IL-6 antibodies (1.33 mg/kg of body weight/mouse) (Treated D0; n = 15) at the time of infection. The control groups are either uninfected animals (Naïve; n = 6) or infected animals (Untreated; n = 10). The survival rates and clinical scores were determined for each mouse group based on daily observation of the animals. Clinical scores were defined as follows: 0, healthy; 1, ruffled hair, hunchbacked; 2, reduced mobility; 3, limb weakness; 4, limb paralysis; and 5, death. *, P < 0.05; **, P < 0.01 (in relation to the untreated infected group).
FIG. 9.
Histological examination of the limb muscle, intestines, and spleen from EV71-infected mice either untreated or cotreated with anti-IL-6 antibodies. EV71 infection and anti-IL-6 antibody cotreatment were performed as described in the legend of Fig. 8. At day 7 postinfection, three mice per group were euthanized. Paraffin sections of the limb muscle, intestines, and spleen were stained by hematoxylin and eosin. The specimens shown are representative of three mice per group with similar histology. Observations were made at magnifications of ×2.5 (A to C and J to L) and ×20 (D to F and G to I).
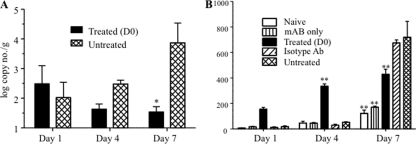
However, unexpectedly, either the intestinal viral loads were comparable (days 1 and 4 postinfection) in the treated and untreated groups, or they were significantly lower (day 7 postinfection) in the treated group than in the untreated animals (Fig. 10 A), in contrast to a previous study that reported higher viral loads in IL-6 KO mice (26). Furthermore, systemic levels of IL-6 in the anti-IL-6-treated animals were higher than the levels measured in the untreated mice (Fig. 10B). Altogether, these data and observations indicated that the administration of anti-IL-6 antibodies at the time of infection paradoxically triggered increased systemic levels of IL-6, which resulted in lower viral loads but severe immunopathology with a worse disease outcome.
FIG. 10.
Viral load and systemic IL-6 levels in EV71-infected mice either untreated or cotreated with anti-IL-6 antibodies. EV71 infection and anti-IL-6 antibody cotreatment were performed as described in the legend of Fig. 8. Four animals per group per time point were sacrificed for blood collection and intestine harvest. (A) The viral load in the intestine homogenates was determined by real-time PCR and expressed as the mean value of the number of viral genome copies/g of tissue. The data represent the means ± standard error of the means of the four intestine homogenates per group per time point. *, P < 0.05 in relation to the untreated infected group. (B) Systemic IL-6 concentration was measured by ELISA from serum samples diluted 1:25. The data represent the means ± standard error of the means of the four serum samples per group and per time point. **, P < 001 in relation to the untreated infected group.
DISCUSSION
IL-6 is a pleiotropic cytokine with a variety of biological activities. It plays a key role in both pro- and anti-inflammatory responses and has been observed in the context of infection, inflammation, and autoimmunity (17, 19). Typically, IL-6 is induced following pathogen stimulation as part of the innate inflammatory response, and it functions as the main inducer of acute-phase response and T- and B-cell stimulation (18). As such, IL-6 is an essential participant in the control of microbial infection in which it serves as a major signal that recruits and activates cells involved in inflammation and the induction of adaptive immunity for pathogen clearance (20).
On the other hand, there is increasing evidence that high levels of IL-6 can overwhelm immune defenses and cause severe autoimmune and chronic inflammatory proliferative disease (16). In mice, IL-6 was reported to be pathogenic in bacterial sepsis and meningitis as well as during influenza A virus infection (14, 21, 30, 44). A number of clinical studies have suggested that IL-6 overproduction could be responsible for severe forms of EV71 brainstem encephalitis (24, 25, 50, 52, 54). Treatment with IVIG resulted in a rapid decrease in IL-6 levels in both serum and the CNS, without a significant change in anti-EV71 antibody titers (50). These observations highlight the possibility that the EV71-induced systemic inflammatory response is a result of IL-6 overproduction that may contribute to the severe EV71-associated complications such as meningitis and pulmonary edema. Here, we demonstrate the detrimental effect of high and sustained systemic levels of IL-6 induced upon EV71 infection in the neonatal mouse model and the possibility to improve the disease outcome via the administration of anti-IL-6 neutralizing antibodies.
The lack of a suitable animal model of EV71 infection has resulted in limited knowledge in EV71 pathogenesis and has greatly hampered the development of effective prophylactic and therapeutic treatments. Experimental infection has been reported in cynomolgus monkeys, but their use is limited due to ethical and economic concerns (13, 33). Furthermore, only 10% of infected monkeys develop clinical symptoms, which reflects human clinical disease but which makes such a model impractical at the experimental level. As for mice, adult immunocompetent mice are not permissive to EV71 infection, and a mouse-adapted strain of EV71 has been obtained by serial passages of the virus in the mouse brain; although it was more virulent compared to its parental counterpart, this mouse-adapted EV71 strain displayed a relative infectivity limited to 14-day-old or younger mice (36). In addition, adapting the virus through serial passages in mouse brain has resulted in the selection of a highly neurotropic virus strain, whereas viruses from the Picornaviridae family are known to exhibit diverse tissue tropisms (35, 49). Alternatively, we (9) along with others (40, 46) have reported that 1-day-old neonatal mice are susceptible to infection with non-mouse-adapted EV71 strains. In this neonate mouse model of EV71 infection, the infected pups typically display a range of clinical symptoms over the course of infection, including hunched back, reduced motility, hind limb paralysis, and eventually death at day 12 postinfection (9, 40). The present work provides further histopathological insights on EV71 infection in the neonate mouse model. Whereas no sign of tissue damage was observed in the brain and central and peripheral nervous systems, a high level of inflammation and severe tissue damage were apparent in the limb muscle from the infected mice, which is likely to be responsible for the hind limb paralysis observed and which suggests a myotropism of the virus in this animal model.
In addition, mild tissue damage in the intestines and splenic atrophy correlating with a reduced number of total splenocytes were noticed in the infected animals. A decrease in various immune cell populations including CD4+ and CD8+ T cells and B cells was measured. Consistent with this observation, a previous study reported that whereas the white blood cell counts increased in EV71-infected patients, the amounts of NK cells and CD4+ and CD8+ T lymphocytes decreased dramatically during the first 2 days of hospitalization, and this correlated with the occurrence of pulmonary edema (51).
Furthermore, in agreement with observations made in patients, EV71-infected neonatal mice displayed elevated levels of systemic and local IL-6 throughout the course of infection, which increased with disease progression.
To address the functional significance of increased IL-6 levels after the onset of clinical symptoms, IL-6 neutralization was initiated 3 days or 6 days post-EV71 infection of neonatal mice. Such treatment successfully improved the survival rate and clinical score of the infected animals, demonstrating the detrimental effects of the high levels of endogenous IL-6 produced upon EV71 infection. In addition to reduced mortality and an improved clinical score, the anti-IL-6-treated mice displayed reduced myonecrosis, absence of splenic atrophy, and strong immune cell activation. Such attenuation of disease severity was not due to a better ability to control virus replication as there was no significant difference in the intestinal virus titers between anti-IL-6-treated and untreated infected animals. Hence, our data indicate that the high levels of IL-6 produced during EV71 infection play a major role in EV71-induced immunopathogenesis.
Our finding appears to contradict two recent studies which reported that IL-6-deficient mice infected with EV71 (26) or coxsackievirus B3 (37), a close relative to EV71 that belongs to the same genus, displayed increased disease severity compared to their immunocompetent counterparts, and administration of recombinant IL-6 early postinfection was sufficient to decrease the chronic disease pathology (37). These studies thus supported a protective role for IL-6 during EV71 infection. Such a protective effect was also described in a model of encephalomyocarditis virus (EMCV) infection in which administration of recombinant IL-6 postinfection was beneficial to the host (18). In contrast, EMCV infection of mice constitutively overexpressing IL-6 led to increased disease severity (47). Altogether, these studies and the present work suggest that IL-6 production is beneficial to the host early postinfection to trigger the antiviral host response through attraction of various immune cells; however, sustained high levels of IL-6 may cause tissue damage and immunopathology. To address this hypothesis, anti-IL-6 treatment was administered at the time of EV71 infection (day 0). Such treatment worsened the disease outcome, with mice dying even earlier than the untreated infected controls. This observation thus supported previous data obtained in IL-6 KO mice (26). However, the intestinal viral load from the anti-IL-6-treated animals was significantly lower than that in the untreated mice, which suggested that the treated mice did not die because of overwhelming viral replication, as reported in the IL-6 KO mice (26). Furthermore, we found that the systemic levels of IL-6 upon administration of anti-IL-6 antibodies were strikingly high compared to levels in the infected untreated animals. Such a phenomenon has been previously reported and was attributed to the deregulation of IL-6 production and/or to the reduced clearance of the antibody-antigen complexes circulating in the serum although no experimental evidence has ever been provided to support or refute these hypotheses (10, 48). The fact that we did not observe such a phenomenon when the antibodies were administered at day 3 or 6 postinfection would support the first scenario although one would have to assume that regulation of IL-6 production at day 0 of infection differs from that at day 3 or 6 postinfection. Histological analysis in the anti-IL-6-treated animals revealed severe myonecrosis and splenic atrophy, thus further supporting the immunopathological origin of the animals' death.
IL-10 is a major anti-inflammatory cytokine as it interferes with many signaling pathways that result in proinflammatory cytokine and chemokine production and major histocompatibility complex class II (MHC-II) expression (32). IL-10 is produced by various immune cell types including subsets of activated dendritic cells, macrophages, activated regulatory T cells, B cells, and subsets of natural killer cells following stimulation of Toll-like receptor (TLR) ligands (8). Production of proinflammatory cytokines and IL-10 is initiated following the same stimuli in a cell-intrinsic manner, and this acts to regulate the inflammatory immune response (12). Therefore, the balance between pro- and anti-inflammatory cytokines is critical, and determining the ratio between major pro- and anti-inflammatory cytokines helps in the evaluation of the inflammatory status of an infected individual. The ratios of IL-10 to IL-6 were thus determined over the course of infection in untreated mice and mice treated with anti-IL-6 neutralizing antibodies. Anti-IL-6 treatment resulted in dramatically increased IL-10/IL-6 ratios, reflecting the upregulation of IL-10 production in the treated animals. As such, upregulation of IL-10 likely helped constrain the inflammatory reactions and subsequent tissue damage, as previously shown during infection with HCV and HIV (3, 29, 34).
In conclusion, we showed that in the neonate mouse model of EV71 infection, sustained high levels of IL-6 are detrimental to the host by causing organ (limb muscle, in particular) damage and spleen atrophy and that neutralization of IL-6 production after the onset of the clinical symptoms significantly improved the survival rate and clinical score, suggesting that anti-IL-6 neutralizing antibodies represent an interesting therapeutic approach to limiting disease progression and improving disease outcome in children infected with EV71. However, given the immaturity of the adaptive immune system in mouse neonates (2, 22, 23, 42), the actual role of IL-6 in EV71 pathogenesis in humans may not be as critical as it is in the neonate mouse model. It would thus be important and necessary to evaluate the role of this proinflammatory mediator and assess the effect of IL-6 neutralization in an immunologically mature animal model when/if it becomes available.
Supplementary Material
Acknowledgments
We are grateful to Vincent Chow (NUS, Department of Microbiology) for his kind gift of EV71 strain 41. We also thank George Yip (NUS, Department of Anatomy) for his help in analyzing some of the histology data.
This work was supported by the National Medical Research Council, Republic of Singapore (IRG/1135/2007).
Footnotes
Published ahead of print on 12 January 2011.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abe, Y., A. Horiuchi, M. Miyake, and S. Kimura. 1994. Anti-cytokine nature of natural human immunoglobulin: one possible mechanism of the clinical effect of intravenous immunoglobulin therapy. Immunol. Rev. 139:5-19. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. 4:553-564. [DOI] [PubMed] [Google Scholar]
- 3.Brockman, M. A., et al. 2009. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114:346-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, L. G., et al. 2000. Deaths of children during an outbreak of hand, foot and mouth disease in Sarawak, Malaysia, clinical and pathological characteristics of the disease. Clin. Infect. Dis. 31:678-683. [DOI] [PubMed] [Google Scholar]
- 5.Chang, L. Y., C. Y. Huang, and T. Y. Lin. 1998. Fulminant neurogenic pulmonary oedema with hand, foot and mouth disease. Lancet 352:367-368. [DOI] [PubMed] [Google Scholar]
- 6.Chang, L. Y., et al. 1999. Clinical features and risk factors of pulmonary oedema after enterovirus 71-related hand, foot and mouth disease. Lancet 354:1682-1686. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y. C., et al. 2004. A murine oral enterovirus 71 infection model with central nervous system involvement. J. Gen. Virol. 85:69-77. [DOI] [PubMed] [Google Scholar]
- 8.Couper, K. N., D. G. Blount, and E. M. Riley. 2008. IL-10: the master regulator of immunity to infection. J. Immunol. 180:5771-5777. [DOI] [PubMed] [Google Scholar]
- 9.Foo, D. G. W., et al. 2007. Passive protection against lethal enterovirus 71 infection in newborn mice by neutralizing antibodies elicited by a synthetic peptide. Microbes Infect. 9:1299-1306. [DOI] [PubMed] [Google Scholar]
- 10.Gijbels, K., S. Brocke, J. S. Abrams, and L. Steinman. 1995. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol. Med. 1:795-805. [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert, G. L., et al. 1988. Outbreak of enterovirus 71 infection in Victoria, Australia, with a high incidence of neurologic involvement. Pediatr. Infect. Dis. J. 7:484-488. [DOI] [PubMed] [Google Scholar]
- 12.Girndt, M., and H. Kohler. 2003. Interleukin-10 (IL-10): an update on its relevance for cardiovascular risk. Nephrol. Dial. Transplant. 18:1976-1979. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto, I., and A. Hagiwara. 1982. Pathogenicity of a poliomyelitis-like disease in monkeys infected orally with enterovirus 71: a model for human infection. Neuropathol. Appl. Neurobiol. 8:149-156. [DOI] [PubMed] [Google Scholar]
- 14.Heremans, H., C. Dillen, W. Put, V. J. Damme, and A. Billiau. 1992. Protective effect of anti-interleukin (IL)-6 antibody against endotoxin associated with paradoxically increased IL-6 levels. Eur. J. Immunol. 22:2395-2401. [DOI] [PubMed] [Google Scholar]
- 15.Herrero, L. J., et al. 2003. Molecular epidemiology of enterovirus 71 in peninsular Malaysia, 1997-2000. Arch. Virol. 148:1369-1385. [DOI] [PubMed] [Google Scholar]
- 16.Huang, C. C., et al. 1999. Neurological complications in children with enterovirus 71 infection. N. Engl. J. Med. 341:936-942. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara, K., and T. Hirano. 2002. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 13:357-368. [DOI] [PubMed] [Google Scholar]
- 18.Kanda, T., et al. 1996. Modification of viral myocarditis in mice by interleukin-6. Circ. Res. 78:848-856. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto, T. 2006. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res. Ther. 8:2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopf, M., et al. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339-342. [DOI] [PubMed] [Google Scholar]
- 21.Kosai, K., et al. 2008. Gabexate mesilate suppresses influenza pneumonia in mice through inhibition of cytokines. J. Int. Med. Res. 36:322-328. [DOI] [PubMed] [Google Scholar]
- 22.Lambert, P. H., M. Liu, and C. A. Siegrist. 2005. Can. successful vaccines teach us how to induce efficient protective immune responses? Nat. Med. 211:S54-S62. [DOI] [PubMed] [Google Scholar]
- 23.Levy, O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. 7:379-390. [DOI] [PubMed] [Google Scholar]
- 24.Lin, T. Y., et al. 2002. Different proinflammatory reactions in fatal and non-fatal enterovirus 71 infections: implications for early recognition and therapy. Acta Paediatr. 91:632-635. [DOI] [PubMed] [Google Scholar]
- 25.Lin, T. Y., S. H. Hsia, Y. C. Huang, C. T. Wu, and L. Y. Chang. 2003. Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clin. Infect. Dis. 36:269-274. [DOI] [PubMed] [Google Scholar]
- 26.Lin, Y. W., S. W. Wang, Y. Y. Tung, and S. H. Chen. 2009. Enterovirus 71 infection of human dendritic cells. Exp. Biol. Med. (Maywood) 234:1166-1173. [DOI] [PubMed] [Google Scholar]
- 27.Liu, C. C., H. W. Tseng, S. M. Wang, J. R. Wang, and I. J. Su. 2000. An outbreak of enterovirus 71 infection in Taiwan, 1998: epidemiologic and clinical manifestations. J. Clin. Virol. 17:23-30. [DOI] [PubMed] [Google Scholar]
- 28.Lum, L. C., et al. 1998. Fatal enterovirus 71 encephalomyelitis. J. Pediatr. 133:795-798. [DOI] [PubMed] [Google Scholar]
- 29.Mangia, A., et al. 2004. IL-10 haplotypes as possible predictors of spontaneous clearance of HCV infection. Cytokine 25:103-109. [DOI] [PubMed] [Google Scholar]
- 30.Marby, D., G. R. Lockhart, R. Raymond, and J. G. Linakis. 2001. Anti-interleukin-6 antibodies attenuate inflammation in a rat meningitis model. Acad. Emerg. Med. 8:946-949. [DOI] [PubMed] [Google Scholar]
- 31.McMinn, P. C., et al. 2001. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J. Virol. 75:7732-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore, K. W., D. W. Malefyt, R. R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 33.Nagata, N., et al. 2004. Differential localization of neurons susceptible to enterovirus 71 and poliovirus type 1 in the central nervous system of cynomolgus monkeys after intravenous inoculation. J. Gen. Virol. 85:2981-2989. [DOI] [PubMed] [Google Scholar]
- 34.Naicker, D. D., et al. 2009. Interleukin-10 promoter polymorphisms influence HIV-1 susceptibility and primary HIV-1 pathogenesis. J. Infect. Dis. 200:448-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathanson, N. 2008. The pathogenesis of poliomyelitis: what we don't know. Adv. Virus Res. 71:1-50. [DOI] [PubMed] [Google Scholar]
- 36.Ong, K. C., et al. 2008. Pathologic characterization of a murine model of human enterovirus 71 encephalomyelitis. J. Neuropathol. Exp. Neurol. 67:532-542. [DOI] [PubMed] [Google Scholar]
- 37.Poffenberger, M. C., et al. 2009. Lack of IL-6 during Coxsackievirus Infection heightens the early immune response resulting in increased severity of chronic autoimmune myocarditis. PLoS One 4:e6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Program for Monitoring Emerging Diseases. 2001. Enterovirus 71. International Society for Infectious Diseases, Brookline, MA. http://www.promedmail.org.
- 39.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:4931-4937. [Google Scholar]
- 40.Sasaki, O., T. Karaki, and J. Imanishi. 1986. Protective effect of interferon on infections with hand, foot, and mouth disease virus in newborn mice. J. Infect. Dis. 153:498-502. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt, N. J., E. H. Lennette, and H. H. Ho. 1974. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 129:304-309. [DOI] [PubMed] [Google Scholar]
- 42.Siegrist, C. A. 2001. Neonatal and early life vaccinology. Vaccine 19:3331-3346. [DOI] [PubMed] [Google Scholar]
- 43.Singh, S., C. L. Poh, and V. T. Chow. 2002. Complete sequence analyses of enterovirus 71 strains from fatal and non-fatal cases of the hand, foot and mouth disease outbreak in Singapore (2000). Microbiol. Immunol. 46:801-808. [DOI] [PubMed] [Google Scholar]
- 44.Starnes, H. F. J., et al. 1990. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-challenge in mice. J. Immunol. 145:4185-4191. [PubMed] [Google Scholar]
- 45.Tan, E. L., et al. 2006. Specific detection of enterovirus 71 directly from clinical specimens using real-time RT-PCR hybridization probe assay. Mol. Cell Probes 20:135-140. [DOI] [PubMed] [Google Scholar]
- 46.Tan, E. L., T. M. C. Tan, V. T. K. Chow, and C. L. Poh. 2007. Inhibition of enterovirus 71 in virus-infected mice by RNA interference. Mol. Ther. 15:1931-1938. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka, T., et al. 2001. Overexpression of interleukin-6 aggravates viral myocarditis: impaired increase in tumor necrosis factor-alpha. J. Mol. Cell Cardiol. 33:1627-1635. [DOI] [PubMed] [Google Scholar]
- 48.van Zaanen, H. C., et al. 1996. Endogenous interleukin 6 production in multiple myeloma patients treated with chimeric monoclonal anti-IL-6 antibodies indicates the existence of a positive feed-back loop. J. Clin. Invest. 98:1441-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, S. M., T. Ho, C. Shen, and C. C. Liu. 2008. Enterovirus 71, one virus and many stories. Pediatr. Neonatol. 49:113-115. [DOI] [PubMed] [Google Scholar]
- 50.Wang, S. M., et al. 2006. Modulation of cytokine production by intravenous immunoglobulin in patients with enterovirus 71-associated brainstem encephalitis. J. Clin. Virol. 37:47-52. [DOI] [PubMed] [Google Scholar]
- 51.Wang, S. M., et al. 2003. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J. Infect. Dis. 188:564-570. [DOI] [PubMed] [Google Scholar]
- 52.Wang, S. M., et al. 2007. Cerebrospinal fluid and cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin. Microbiol. Infect. 13:677-682. [DOI] [PubMed] [Google Scholar]
- 53.Wang, Y., et al. 2004. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J. Virol. 78:7916-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weng, K. F., L. L. Chen, P. N. Huang, and S. R. Shih. 2010. Neural pathogenesis of enterovirus 71 infection. Microbes Infect. 12:505-510. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization. 2009. Hand, foot and mouth disease: a rising menace in Asia. World Health Organization for the Western Pacific, Manila, Philippines. http://www.wpro.who.int/media_centre/news/news_20090713.htm.
- 56.Yan, J. J., J. R. Wang, C. C. Liu, H. B. Yang, and I. J. Su. 2000. An outbreak of enterovirus 71 in Taiwan 1998—a comprehensive pathological, virological and molecular study on a case of fulminant encephalitis. J. Clin. Virol. 17:13-22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.