Abstract
To date, no vaccine that is safe and effective against herpes simplex virus 2 (HSV-2) disease has been licensed. In this study, we evaluated a DNA prime-formalin-inactivated-HSV-2 (FI-HSV2) boost vaccine approach in the guinea pig model of acute and recurrent HSV-2 genital disease. Five groups of guinea pigs were immunized and intravaginally challenged with HSV-2. Two groups were primed with plasmid DNAs encoding the secreted form of glycoprotein D2 (gD2t) together with two genes required for viral replication, either the helicase (UL5) and DNA polymerase (UL30) genes or the single-stranded DNA binding protein (UL29) and primase (UL52) genes. Both DNA-primed groups were boosted with FI-HSV2 formulated with monophosphoryl lipid A (MPL) and alum adjuvants. Two additional groups were primed with the empty backbone plasmid DNA (pVAX). These two groups were boosted with MPL and alum (MPL-alum) together with either formalin-inactivated mock HSV-2 (FI-Mock) or with FI-HSV2. The final group was immunized with gD2t protein in MPL-alum. After challenge, 0/9 animals in the group primed with UL5, UL30, and gD2t DNAs and all 10 animals in the mock-immunized control group (pVAX-FI-Mock) developed primary lesions. All mock controls developed recurrent lesions through day 100 postchallenge. Only 1 guinea pig in the group primed with pVAX DNA and boosted with FI-HSV2 (pVAX-FI-HSV2 group) and 2 guinea pigs in the group primed with UL5, UL30, and gD2t DNAs and boosted with FI-HSV2 (UL5, UL30, gD2t DNA-FI-HSV2 group) developed recurrent lesions. Strikingly, the UL5, UL30, gD2t DNA-FI-HSV2 group showed a 97% reduction in recurrent lesion days compared with the mock controls, had the highest reduction in days with recurrent disease, and contained the lowest mean HSV-2 DNA load in the dorsal root ganglia.
Herpes simplex virus 2 (HSV-2) infection is the primary cause of genital herpes and establishes a lifelong, latent infection that can periodically reactivate. HSV-2 is one of the most common sexually transmitted infections, with the majority of horizontal transmissions occurring during asymptomatic viral shedding (29). In the United States, the seroprevalence for HSV-2 is 16.2%, and surprisingly, 81.1% of those seropositive individuals had not been diagnosed by a health care professional, suggesting that most were asymptomatic (4). In addition to the physical pain and emotional stress caused by recurrent genital lesions, significant morbidity and mortality can result from HSV-2 infection in neonates (21) and immunocompromised hosts (12). Importantly, HSV-2 infection is significantly related to an increased risk of human immunodeficiency virus (HIV) acquisition (15, 46), which is likely due to the influx and persistence of CD4+ T cell targets in the genital skin (25, 48).
Clearly, there is a great need for a HSV-2 vaccine. Although the exact correlates of protective immunity are undefined, it appears that both cellular and humoral immune responses play a role in controlling HSV-2 infection. In humans, neutralizing antibody responses alone have not resulted in an efficacious prophylactic vaccine (6). In addition, the loss of CD4+ T cells in HIV-1-infected people correlates with HSV-2 shedding (39). In rodent models, gamma interferon (IFN-γ) has been shown to be important for T cell-mediated clearance of HSV-2 from the mucosa (30). CD4+ T cell depletion studies have also shown that these cells may be required for protection against intravaginal (i.vag.) challenge (30, 31, 38). CD8+ cytotoxic T cells have also been shown to be important for reducing HSV-2 replication and shedding. These T cells are associated with control of acute ganglion infection during primary infection (23). They accumulate at nerve endings in genital skin during reactivation (49), and their presence correlates with HSV-2 clearance (26).
Various strategies have been utilized in the attempt to make an effective HSV-2 vaccine (23). A large focus has been targeting the glycoproteins, including glycoprotein D (gD) and gB (6, 43). To date, the most promising vaccine candidate tested in clinical trials was the GlaxoSmithKline (GSK) vaccine that consisted of a secreted gD protein formulated with monophosphoryl lipid A (MPL) and alum (MPL-alum). In two clinical trials, however, this vaccine was efficacious only in HSV-1- and HSV-2-seronegative women (41). Replication-defective virus vaccines have also been successful in animal models, and their advancement to clinical trials is imminent. One potential advantage of a replication-defective virus is its ability to elicit humoral and cell-mediated immune responses similar to those of a live virus. However, for safety concerns, the introduction of multiple mutations to guarantee that this virus is replication deficient is preferred. Among the proteins required for HSV-2 replication are UL5 (DNA helicase), UL29 (single-stranded DNA binding protein), UL30 (DNA polymerase), and UL52 (DNA primase) (5, 22). A UL5 and UL29 double deletion HSV-2 mutant virus (dl5-29) has exhibited promising immunogenicity and subsequent protection in mouse (7, 9) and guinea pig (18-20) models. This replication-defective mutant virus provided protection similar to that of defective virus with a mutation only in the UL29 gene (9), which also protected both mice and guinea pigs against challenge with wild-type HSV-2 (8, 35). While the use of inactivated-virus vaccines are generally regarded as safe and are approved for poliovirus and influenza viruses, inactivated-HSV-2 vaccines tested in the past have not been as successful. Numerous studies with various preparations of inactivated HSV-2 have been performed in humans, but most lacked the necessary controls for correct data interpretation (reviewed in reference 47). However, the interpretable data gleaned from the few studies with controls emphasized the need for a durable immune response that was not elicited by inactivated virus alone (47). The use of inactivated virus combined with other vaccine platforms in a prime-boost strategy or in combination with adjuvants, such as MPL and alum, may provide such desired long-lived immunity.
We have previously shown that a DNA prime and inactivated virus plus adjuvant boost was protective in mice against another herpesvirus challenge, murine cytomegalovirus (MCMV) (33, 34). Using this model, we also demonstrated that the CD8+ T cell responses generated against the DNA vaccines encoding the conserved, essential MCMV genes for DNA polymerase and helicase were protective (32). Furthermore, the immunity elicited by the systemic prime-boost immunization protected mice against a mucosal challenge (33).
In this regard, we sought to apply a similar prime-boost strategy and evaluate its protective efficacy against i.vag. HSV-2 challenge. We tested this approach in the guinea pig model, which closely resembles human infection with respect to acute and recurrent genital disease. Two of the HSV-2 DNA-primed groups of guinea pigs received a plasmid DNA that expresses a truncated glycoprotein D2 (gD2t). The truncated form of gD2 lacks the transmembrane domain and is secreted (17). A previous study by Strasser et al. showed that compared to the full-length or cytosolic portion, immunization with the secreted form of gD2 produced a more balanced Th1-Th2 antibody response in mice. It also elicited the highest antibody levels in mice and guinea pigs and conferred a slightly higher level of protection against disease in guinea pigs (42). In the study presented here, guinea pigs were primed with 3 plasmid DNAs: the gD2t DNA plus two plasmids that express genes required for DNA replication (UL5 and UL30 or UL29 and UL52). Both of these groups were subsequently boosted with formalin-inactivated HSV-2 (FI-HSV2) in MPL-alum. Two additional vaccine groups were primed with plasmid backbone DNA (pVAX). One of these groups was boosted with FI-HSV-2 (as an inactivated-virus-only group), and the other group was boosted with a FI-Mock preparation (as a mock-immunized group). For comparative purposes, an additional group received vaccine consisting of a purified gD2t protein plus MPL-alum that is similar to the one administered in the GSK trials. Following i.vag. HSV-2 challenge, the protective efficacies of the vaccines were compared with respect to outcomes of acute disease and virus shedding. We also examined the animals daily over a long-term period for recurrent disease. At the end of the experiment, the levels of latent HSV-2 were measured. Taken together, the results showed that priming with UL5, UL30, and gD2t DNAs and boosting with FI-HSV-2 was highly protective against acute and recurrent HSV-2 disease in the guinea pig model.
MATERIALS AND METHODS
Cells and viruses.
Vero cells (ATCC CLL-81) were purchased from ATCC and propagated in Dulbecco's modified Eagle medium (DMEM) with low glucose (1 g of glucose per liter) supplemented with 5% (vol/vol) heat-inactivated fetal bovine serum (HI-FBS), 5% (vol/vol) heat-inactivated newborn calf serum (HI-NCS), 100 U of penicillin per ml, 100 μg streptomycin per ml, and 2 mM additional l-glutamine. 293FT cells (Invitrogen) were propagated in DMEM (4.5 g glucose per liter) supplemented with 10% (vol/vol) HI-FBS and 1× each of minimum essential medium (MEM) nonessential amino acids, penicillin, streptomycin, and additional glutamine as described above. Sera and supplements were purchased from Invitrogen Life Technologies.
HSV-2 strain G (13) was a gift from David M. Knipe and was propagated by infection of confluent Vero cell monolayers at a multiplicity of infection (MOI) of 0.1, incubation at 33°C for 72 h, and then harvest of the cell-associated virus as previously described (11). This HSV-2 stock had a titer on Vero cells of 2.08 × 108 PFU per ml and an approximate 50% lethal dose (LD50) in methyl-hydroxyprogesterone-treated BALB/c mice following intravaginal (i.vag.) infection of 5 × 103 PFU.
Formalin-inactivated HSV2 (FI-HSV2) was prepared from extracellular virus obtained following the infection of confluent Vero cell monolayers at an MOI of 0.05 to 0.1 and incubation at 33°C for 4 days. The medium was harvested and sequentially clarified by centrifugation at 500 × g for 5 min, followed by centrifugation at 4,000 × g for 10 min. The virus in the supernatant was concentrated by ultracentrifugation through a cushion of 25% (wt/vol) sucrose in Dulbecco's phosphate-buffered saline (DPBS) in an SW-27 rotor at 25,000 rpm for 1 h. Virus pellets were resuspended in DPBS on ice overnight, combined, and then sonicated in ice water in a Misonix cuphorn sonicator. The titer of resultant virus was determined on Vero cells, and the protein content was measured by a Bradford protein assay kit (Bio-Rad) using a bovine serum albumin (BSA) standard (Pierce). A 37% (wt/vol) formaldehyde stock solution was diluted 2,000-fold in DPBS, and an equal volume was added to the resuspended, sonicated virus (final formaldehyde dilution of 1:4,000 or 0.009%). After 72 h of incubation at 37°C with end-over-end mixing, equimolar sodium bisulfite was added to quench any residual formaldehyde, and 0.1 ml of the formalin-treated virus was used to infect Vero cells to confirm the absence of detectable infectivity. Vero cells were incubated for 5 days, and no cytopathic effect (CPE) was observed in any preparations. Inactivated virus was stored at −80°C in aliquots. The inactivated virus had a final titer of 3 × 108 to 6 × 108 PFU equivalents per ml, and prior to inactivation, contained ca. 1 × 106 to 2 × 106 PFU per μg of protein. A formalin-treated mock virion preparation (FI-Mock) was prepared in parallel from the medium of mock-infected Vero cells. The medium from uninfected cells was clarified and subjected to ultracentrifugation through a sucrose cushion, and the resulting pellets were sonicated and treated with formalin as described above. It should be noted that while the technique for the preparation of FI-Mock was identical to that for FI-HSV2, the FI-Mock preparation would not likely contain cellular factors present in the FI-HSV2 that are induced by virus infection, i.e., cytokines or nonvirion secretome components.
For preparation of HSV-2 virion for an enzyme-linked immunosorbent assay (ELISA), HSV-2 was propagated in Vero cells as described above. On day 4 postinfection (p.i.), the cells were released into the medium from T-175 flasks by shaking, pelleted at 500 × g, and washed once with DPBS. The cells were resuspended in 5 ml of sterile DPBS containing 150 μg/ml dextran sulfate (average molecular mass of 9,000 to 20,000; Sigma) per T-175 flask. The cells were resuspended gently to prevent cell lysis and then mixed by inverting the tubes 20 times. The cells were pelleted at 500 × g, and the supernatant was clarified by centrifugation at 2,000 × g for 10 min. Sterile NaCl was slowly added to the supernatant while mixing to increase the NaCl concentration from 150 to 650 mM, and the virus was pelleted through a sucrose cushion as described above. Virus pellets were resuspended in DPBS on ice overnight, sonicated, sampled for infectious virus by plaque assay on Vero cells, and assayed for protein as described above. Sterile glycerol in DPBS was added to a final concentration of 15% (vol/vol) before the aliquots were frozen at −80°C. The infectivity of the frozen virus was found to be maintained after an additional freeze-thaw cycle.
For use in virus neutralization assays, HSV-2 was propagated as described above except that serum-free medium was used. The extracellular medium was collected and clarified twice. The virus in the supernatant was pelleted, resuspended in DPBS, sonicated, and stored in 15% (vol/vol) glycerol at −80°C as described above.
Plasmid construction and expression.
HSV-2 open reading frames (ORFs) from the virion DNA of strain G were amplified by PCR and cloned into a modified pVAX1 vector (Invitrogen). Expression from the pVAX1 plasmid vector is driven by the strong, constitutive human cytomegalovirus (HCMV) major immediate-early (IE) promoter/enhancer. This vector was modified by QuikChange mutagenesis to modify the multicloning site to contain a unique HincII site followed by a FLAG tag-coding sequence and 3 in-frame stop codons. The final vector, designated pVAX1.2 and abbreviated below as pVAX, was the basis for the cloning and expression of carboxy-terminal FLAG-tagged HSV-2 ORFs UL5 (helicase), UL29 (single-stranded DNA binding protein), UL30 (DNA polymerase), UL52 (helicase subunit), and gD2t (the extracellular portion of gD2 consisting of amino acids [aa] 1 to 327).
Plasmid DNA used for injection was purified by Qiagen Endo-Free Mega or Giga anion-exchange columns. DNAs were resuspended at a final concentration of 2 to 3 mg per ml in endotoxin-free Tris-HCl (pH 8) and stored at −20°C in aliquots. For each preparation, the expression of full-length ORF-FLAG fusion proteins was confirmed by Lipofectamine 2000 (Invitrogen)-mediated transient transfection into 293FT cells followed by Western blotting with anti-FLAG mouse monoclonal antibody (clone M2; Sigma). Immediately prior to injection, DNAs were thawed and diluted in endotoxin-free Tris-buffered saline (TBS), pH 8.
Preparation of the gD2 subunit vaccine.
Truncated gD2 subunit vaccine was purified from the medium of transiently transfected 293FT cells. The medium from pc3Δneo-gD2t-transfected 293FT cells was collected 4 days posttransfection and clarified. The carboxy-terminally FLAG-tagged gD2 protein in the supernatant was purified by immunoaffinity purification using anti-FLAG M2 resin (Sigma) and elution with 3×FLAG peptide (Sigma). Fractions containing gD2 protein were mixed and stored in aliquots at −80°C. Purity and gD2 protein concentration were determined by SDS-PAGE and Coomassie blue staining (GelCode Blue; Pierce), with bovine serum albumin standards run on the same gel for quantification purposes. Using Image-J software (NIH), a standard curve of BSA concentrations was generated by optical density, and the gD2 protein concentrations were interpolated. The yield was approximately 5 μg of gD2 protein per ml of medium.
Immunization of guinea pigs.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (36a). All use of vertebrate animals was approved by the Institutional Animal Care and Use Committee, University of California, San Diego. Specific-pathogen-free female Hartley guinea pigs were purchased from Charles River Laboratories when the animals were 5 weeks old and housed in microisolator cages. The guinea pigs were 12 weeks old when they were first immunized, and each prime-boost immunization group contained 10 guinea pigs.
For DNA priming of guinea pigs, the hindquarters of each guinea pig were shaved to ca. 3 to 4 cm from the caudal end. A total of 100 μl of DNA in endotoxin-free saline was injected intradermally (i.d.) at 3 separate locations. Groups received either (i) 200 μg of pVAX, (ii) a cocktail of 66.7 μg (each) UL5, UL30, and gD2t DNAs, or (iii) a cocktail of 66.7 μg (each) UL29, UL52, and gD2t DNAs. Three immunizations were given over 5 weeks.
For FI-HSV2 and gD2 vaccination, monophosphoryl lipid A (MPL) from Salmonella minnesota Re 595 was purchased from Sigma, resuspended at a concentration of 1 mg per ml of 0.5% (vol/vol) triethanolamine, emulsified by sonication as previously described (2), and stored at 4°C until use. FI-HSV2 or purified gD2 subunit stocks were thawed and diluted in DPBS, and then MPL was added. Alum adjuvant (Alhydrogel; Accurate Chemical & Scientific, Westbury, NY) was added dropwise while mixing and then additionally mixed for 1 h at room temperature before injection.
Six weeks after the last DNA injection, guinea pigs received the first of two boosts. Each of the DNA-primed groups was subcutaneously (s.c.) boosted in the middle of their back with 2 × 107 PFU equivalents of FI-HSV2 (12.2 μg protein), 12.5 μg MPL, and Alhydrogel corresponding to 305 μg Al(OH)3 and 108 μg Al (according to the manufacturer's titration). We found that by using these amounts of FI-HSV2, MPL, and Alhydrogel and adsorption conditions, all detectable gD2 protein from the virion (>95% of input) remained bound to the Alhydrogel (data not shown). For a group immunized with FI-HSV2 alone, half of the pVAX-primed guinea pigs were boosted with FI-HSV2 as described above. As a vehicle-only negative-control group, the other half of the pVAX-primed guinea pigs received a volume of FI-Mock equal to that of FI-HSV2 above together with MPL and Alhydrogel as described above. Finally, a group immunized with the gD2 subunit (gD2 subunit group) (that was previously naïve) received 5 μg of purified gD2 protein together with 12.5 μg MPL and Alhydrogel corresponding to 125 μg Al(OH)3 and 44.4 μg Al. Guinea pigs were given an identical boost 4 weeks later, similar to the two-dose schedule carried out in the GlaxoSmithKline (GSK) trials.
Blood samples were collected from the saphenous vein for IgG and neutralizing antibody determination on the weeks shown below in Fig. 1. Sera were prepared and stored at −20°C until assay.
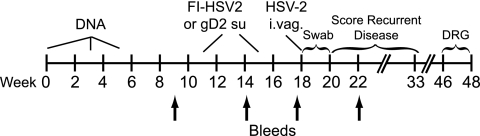
FIG. 1.
Immunization scheme and timeline. The vaccine components for each immunization group are shown in Table 1. Groups receiving a DNA prime and FI-HSV2 (or FI-Mock) boost (groups 1 to 4) were i.d. immunized between weeks 0 and 5 and then s.c. boosted on weeks 11 and 15. The gD2 subunit control group (gD2 su) received s.c. injections concurrently with groups receiving FI-HSV2 boosts. Each vaccine group consisted of 10 guinea pigs. The HSV-2 challenge (6 × 105 PFU) was given intravaginally (i.vag.) at week 18. The acute disease phase was defined as up to 14 days postchallenge (weeks 18 to 20), and the recurrent phase was defined as days 15 to 100 postchallenge (to week 33). Guinea pigs were sacrificed, and the dorsal root ganglia (DRG) were harvested over the last 2 weeks of the experiment. The arrows show the weeks of test bleeds for antibody determination.
Intravaginal virus challenge, disease scoring, and quantification of viral shedding.
Three weeks after the final boost, ketamine-xylazine-anesthetized guinea pigs were challenged intravaginally by micropipette-based instillation of 25 μl of sterile DPBS containing 1% (vol/vol) HI-NCS and 0.1% (wt/vol) glucose (DNG) and 6.0 × 105 PFU of HSV-2. Before the guinea pigs were given anesthesia, the individual guinea pig cages were each assigned a new identifying cage card labeled only with a random number (http://www.random.org/sequences/). Thus, the personnel (C.S.M. and M.S.L.) were able to perform the disease scoring and vaginal swab collection in a blinded manner.
The guinea pigs were scored daily for 100 days for the severity of vaginal disease using a previously described (40) scoring system of 0 (no lesions), 1 (erythema only), 2 (a single vesicle or a few vesicles), 3 (large or fused vesicles), or 4 (ulcerated lesions). In addition, a score of 0.5 was given for slight erythema, swelling, or nonlesion papules or patches. In addition, a score of 5 was given for death due to HSV-2 disease. To confirm vaginal viral replication and measure shedding, vaginal swab samples were collected on days 1, 3, 5, 7, 9, and 12 postchallenge using 15-cm polyester-tipped swabs (catalog no. 25-806-1PD; Puritan Medical Products, Guilford, ME) that were premoistened with DNG. The swabs were stored in 1 ml of DNG at −80°C until titration by plaque assay on Vero cell monolayers.
For plaque assays, the swabs were quickly thawed in a 37°C water bath and vortex mixed at full speed for 30 s prior to serial dilution in DNG. Confluent monolayers of Vero cells in 24-well plates were infected for 1 h at 37°C on an orbit shaker set at 50 to 60 rpm with an infection volume of 0.25 ml of DNG per well. After adsorption, the inocula were removed, and the monolayers were overlaid with 0.5 ml of DMEM with 1% (vol/vol) HI-NCS and 0.35% (wt/vol) sterile agarose. After a 2-day incubation at 37°C and 7% CO2, formalin in DPBS was added to a final formalin concentration of 4%. The monolayers were fixed at 25°C for at least 3 h prior to aspiration of the agarose overlay-formalin and staining with crystal violet. The assay limit of sensitivity was 10 PFU per swab. Assays yielding no plaques were assigned a titer of 5 PFU per swab for graphing and statistical analysis.
Antibody quantification. (i) HSV-2 virion-specific IgG ELISA.
Each well of a 96-well Maxisorp plate (Nunc) was coated with 0.5 μg of either dextran sulfate-released HSV-2 virion or sonicate from mock-infected Vero cells. It is noteworthy that the HSV-2 virion that was released from the surfaces of infected Vero cells by dextran sulfate was found to result in both lower background and higher signal in the ELISA compared with ELISA using purified extracellular HSV-2 (data not shown). The plates were incubated overnight at 4°C and then treated with 200 mJ/cm2 of UV light in a Stratalinker 1800 (Stratagene) to inactivate residual virus infectivity. The plates were washed with TBS four times and blocked for at least 1 h at room temperature with 2.5% (wt/vol) casein (Hammarsten grade; USB) in TBS. All room temperature incubations were performed on a platform orbit shaker at 200 rpm. Sera from individual immunized guinea pigs were initially diluted 1:40 and then serially diluted 4-fold in 1.25% (wt/vol) casein and 50% (vol/vol) HI-NCS in TBS. The plates were washed four times with TBS, and serum dilutions were added to the plates. After a 1-h incubation at room temperature, the plates were washed six times with TBS, and then goat anti-guinea pig IgG (whole molecule)-alkaline phosphatase conjugate (Sigma) diluted 1:2,500 in 2.5% casein in TBS was added to each well. The plates were incubated at room temperature for 1 h and then washed six times with TBS. SigmaFast p-nitrophenyl phosphate (disodium salt) substrate (Sigma) was added to each well and incubated for another hour prior to detecting the absorbance at 405 nm (A405) on a Spectramax plate reader (Molecular Devices). The endpoint titer was defined as the highest reciprocal dilution of serum at which the A405 in the virion-coated well was greater than or equal to twice the A405 of the same serum dilution in the well coated with mock-infected Vero cell lysate.
(ii) gD2-specific IgG ELISA.
The gD2-specific IgG ELISA was performed as described above for the HSV-2 virion-specific IgG ELISA, except that the wells on the plates were coated with 50 ng of gD2 protein purified from the medium of transfected 293FT cells as described above. Individual guinea pig sera were initially diluted 1:20 and then serially diluted 4-fold in TBS containing 2.5% casein. For the gD2 protein ELISA, the endpoint titer was defined as the highest reciprocal dilution of sera at which the A405 was greater than or equal to twice the average of naïve guinea pig sera against gD2 protein at the same dilution. To address the possibility that a significant percentage of the antibodies elicited by the FLAG-tagged gD2 protein were specific for the FLAG tag rather than the gD2 subunit, the sera from the gD2 protein-vaccinated group that were collected at the peak level of gD2-specific IgG (week 18) were pooled and tested in a FLAG-specific ELISA. The wells on the plates were coated with 1 μg of 3×FLAG peptide (Sigma) or 50 ng of the FLAG-tagged gD2 protein per well. A mouse anti-FLAG monoclonal antibody (clone M2; Sigma) confirmed that the 3×FLAG peptide efficiently bound to the plate and that the FLAG tag on the plate-bound gD2 protein was accessible to antibody binding (data not shown). The pooled sera from the animals immunized with the gD2 subunit showed only a background level of binding in the FLAG peptide-coated wells (titer of 320) and high-level binding to the FLAG-tagged gD2-coated wells (titer of 81,920 to 327,680) (data not shown).
(iii) Neutralizing antibody assay.
Individual guinea pig sera were thawed and mixed, and 2-fold serial dilutions were made in DNG. To 50 μl of each dilution was added 50 μl of DNG containing ca. 50 PFU of HSV-2 that was purified from serum-free medium from infected Vero cells. With the addition of virus, the final reciprocal serum dilutions were 2-fold dilutions ranging from 20 to 2,560. The serum-virus mixtures were incubated at 37°C for 1 h before they were added to confluent Vero cells in wells on 24-well plates. The cells were infected, incubated, fixed, and stained, and the plaques were counted as described above for the plaque assay. For the determination of input virus for each assay day, DNG was used instead of serum in the assay, and the geometric mean of 16 replicate wells of input virus was calculated. The neutralization titer of each serum was determined by Fit spline/LOWESS interpolation for the reciprocal serum dilution that neutralized 50% of the input virus for the corresponding assay (Prism 5.0b for Macintosh; GraphPad Software, Inc.). To normalize the neutralization activities for each assay day, each assay contained the same internal controls consisting of 3 sera collected from a guinea pig 6, 9, and 13 weeks following i.vag. infection with 105.25 PFU of HSV-2 as well as a purified, pooled human IgG preparation (without sodium azide; Innovative Research, Novi, MI). The assay-to-assay variability of each serum was approximately 2-fold. Neutralization titers below the limit of sensitivity were assigned a value of 10 for graphing purposes.
Quantification of HSV-2 DNA in the DRG.
At the conclusion of the disease scoring period (see Fig. 1), the guinea pigs were sacrificed, and the lumbosacral dorsal root ganglia (DRG) (L3 through S1 or S2) from individual guinea pigs were harvested bilaterally. They were snap-frozen on dry ice in a DNA-free tube and stored at −80°C until DNA extraction. The DRG from each animal were collected into a single tube, and care was taken to prevent animal-to-animal cross-contamination of DNA by either autoclaving/baking of instruments or treatment with 10% (vol/vol) bleach in double-distilled water (ddH2O), followed by thorough rinsing with ddH2O. The DRG DNA from each guinea pig was extracted using the DNeasy blood and tissue kit (Qiagen) according to the manufacturer's recommendations of overnight proteinase K digestion and RNase addition, except that double volumes of all reagents, except AE elution buffer (Qiagen), were used. DNA concentrations were determined by A260 measurement (NanoDrop 2000; Thermo Scientific), and both the quality of DNA and the absence of residual RNA were determined by agarose gel electrophoresis and ethidium bromide staining. For a control for the remote, yet unknown possibility that HSV-2 DNA derived from the FI-HSV2 immunization could locate to and be maintained in the DRG of immunized guinea pigs, 3 naïve guinea pigs were subcutaneously immunized with FI-HSV2 as described above, and 6 weeks after the last immunization, the DRG were harvested and DNA was extracted as described above.
TaqMan real-time quantitative PCR (Applied Biosystems, Inc.) primers and probe were designed based on the sequence of the glycoprotein G (gG) ORF of HSV-2 strain HG52 (GenBank accession no. Z86099.2) as follows: primer gGsen (sen stands for sense) (CAA GCT CCC GCT AAG GAC AT, primer gGant (ant stands for antisense) (GGT GGT GCT GAT GAT AAA GAG G), and probe gG (6-carboxyfluorescein [6-FAM]-TGG TTC CTA ACG GCC TCC CCT GC-Black Hole Quencher-1). The primers and probe were obtained from Integrated DNA Technologies, San Diego, CA. PCR mixtures consisted of 50 μl of 1× TaqMan universal master mix, 0.3 μM (each) primer, 0.25 μM probe, and 250 ng of DRG DNA in 10 μl of AE elution buffer (Qiagen) per reaction mixture. Replicate reactions for the standard curve amplified 10 μl of AE buffer containing 250 ng of DRG DNA from naïve guinea pigs together with 5 to 104 copies of HSV-2 strain G DNA that was extracted from dextran sulfate-released HSV-2 by the DNeasy kit as described above. Control HSV-2 DNA was initially quantified by A260 and validated by agarose gel electrophoresis as described above. The amount of residual Vero DNA in the HSV-2 DNA preparation was quantified by real-time PCR using Vero cell-specific primers (based on the glyceraldehyde-3-phosphate dehydrogenase [GAPDH] sequence of Macaca mulatta) and SYBR green-based reactions (data not shown). Vero cell sequences were found to comprise approximately 7% of the control HSV-2 DNA preparation, and the copy number calculation for the control DNA was adjusted accordingly. To ensure that the mass and integrity of each DRG DNA was uniform and that each preparation could be amplified, the cellular DNA was also quantified using TaqMan primers and probe specific for guinea pig β-actin (GenBank accession no. AF508792.1). The β-actin primers and probes were as follows: sense primer (ACG GAG CGT GGC TAC AGT TT), antisense primer (TCC TTG ATG TCA CGC ACA ATT T), and probe (6-FAM-ACC ACC ACG GCC GAG CGG-Black Hole Quencher-1).
To determine the amount of HSV-2 DNA in the DRG, 3 independent reaction mixtures containing all of the DRG DNAs, standard curve replicates, and no-template control reactions were performed on different days. Loading orders for the wells on the 96-well plates for each reaction mixture were randomized to avoid bias or well-to-well influence. The mean DNA load for each guinea pig was calculated from the 3 reactions and expressed as HSV-2 DNA copy number per 250 ng of DRG DNA.
Statistical analyses.
The rates of the development of acute and recurrent lesions were compared by Fisher's exact test. The numbers of recurrent lesion episodes, recurrent lesion days, recurrent disease days, cumulative acute disease scores, levels of HSV-2 shedding, and the amounts of HSV-2 DNA in the DRG were analyzed first by Kruskal-Wallis nonparametric one-way analysis of variance. If statistical significance was achieved, Dunn's multiple-comparison test was subsequently performed on all pairs of vaccine groups (FI-Mock versus each vaccine and each vaccine versus the others). All P values were two tailed, and a P of <0.05 was considered significant. Analyses were performed using Prism 5.0b for Macintosh.
RESULTS
Immunization of guinea pigs.
We previously found that DNA immunization with plasmids expressing either the helicase (M105) or DNA polymerase (M54) genes of murine cytomegalovirus (MCMV) elicited CD8+ T cell responses and provided suppression of virus replication in the spleen after sublethal systemic MCMV challenge (32). Later studies showed protection following immunization with the single-stranded DNA binding protein gene (M57) (data not shown). To test whether DNA immunization using plasmids encoding conserved, essential genes of HSV-2 could augment the responses elicited by FI-HSV2, we cloned the HSV-2 homologs of the above genes, UL5, UL30, and UL29, respectively, as well as the UL52 helicase-primase subunit gene. Because vaccination with gD2-MPL-alum has shown some clinical success and gD2t DNA immunization has shown efficacy in guinea pigs, we included gD2t DNA with the conserved, essential genes to test their combined efficacy against acute and recurrent HSV-2 disease. Table 1 shows the combination of DNA plasmids used to prime each group of guinea pigs in the vaccine study; one group received UL5, UL30, and gD2t DNAs (group 3), and another group received UL29, UL52, and gD2t DNAs (group 4). To assess any protective contribution of the DNAs in the prime-boost strategy, a guinea pig group was primed with empty vector (pVAX) and boosted with FI-HSV2 (group 2). While our DNA prime-inactivated-virus boost vaccine strategy against MCMV included only alum adjuvant with the inactivated MCMV, we included MPL-alum with the inactivated HSV-2 because the GSK HSV-2 gD-MPL-alum subunit vaccine had shown some clinical efficacy (41). The mock-immunized control group was primed with pVAX and boosted with FI-Mock. FI-Mock is a formalin-inactivated preparation from the clarified medium of Vero cells that were mock infected at the time of FI-HSV2 preparation (group 1). Finally, for a positive-control group for protection and for comparative purposes, a group of guinea pigs was immunized with purified gD2 subunit together with MPL and alum (group 5). This is analogous to the vaccine that has shown partial protection in HSV-1- and HSV-2-seronegative women.
TABLE 1.
DNA prime and boost treatments for the five groups of guinea pigs in this studya
| Group | DNA primeb | Boost (with MPL-alum)c |
|---|---|---|
| 1 | pVAX | FI-Mock |
| 2 | pVAX | FI-HSV2 |
| 3 | UL5, UL30, and gD2t DNAs | FI-HSV2 |
| 4 | UL29, UL52, and gD2t DNAs | FI-HSV2 |
| 5 | None | gD2 subunit |
DNA prime and boost treatments explained in detail in Materials and Methods.
DNA was delivered by needle i.d.
Protein-based (“Boost”) vaccines were delivered s.c.
As described in detail in Materials and Methods and using the timeline in Fig. 1, 10 guinea pigs per prime-boost group were intradermally (i.d.) immunized with DNA three times over 5 weeks and then subcutaneously (s.c.) boosted with FI-HSV2 or FI-Mock 6 and 10 weeks after the last DNA immunization. The guinea pigs in the group immunized with gD2 subunit (gD2 subunit group) were left naïve until they received gD2 protein immunizations at the same time as the other groups were given FI-HSV2 or FI-Mock boosts.
Antibody responses in the immunized guinea pigs.
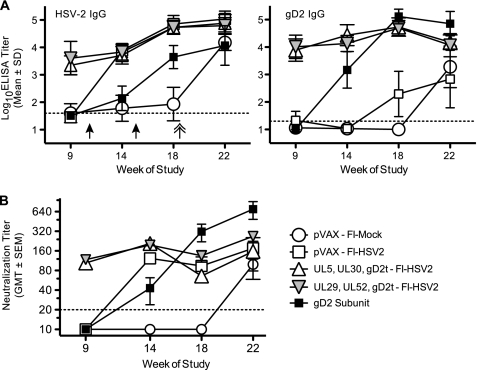
On the weeks of the experiment shown in Fig. 1, blood samples were collected from each guinea pig for virus-specific antibody determinations. HSV-2-specific IgG levels were measured in sera from individual guinea pigs by ELISAs using wells coated with whole, purified HSV-2. Both groups that received a DNA prime containing gD2t DNA had high antibody titers (mean log10 titers of 3.3 or 3.6) against HSV-2 virion (Fig. 2A). The first boost with FI-HSV2 increased HSV-2-specific titers approximately 2-fold, and the second boost further increased titers to a level that was 13- to 24-fold higher than their respective preboost levels. Similar titers after the boost were obtained in the group that was primed with the pVAX plasmid (empty vector) and given the FI-HSV2 boost (pVAX-FI-HSV2 group). In contrast, HSV-2 virion-specific titers in the group boosted with gD2 subunit were 50-fold and 14-fold lower than the FI-HSV2-immunized groups following the first and second immunizations, respectively. Prior to challenge, the background in the group of guinea pigs primed with pVAX and boosted with FI-Mock (pVAX-FI-Mock group) increased slightly. This was likely due to the increasing antibody responses to the Vero cell and/or serum proteins that were present in both the FI-Mock immunogen and HSV-2 virion coating the wells of the ELISA plates. Three weeks after i.vag. HSV-2 challenge, all 7 of the mock-immunized guinea pigs (pVAX-FI-Mock) that survived the challenge seroconverted to HSV-2 with an average log10 reciprocal titer of 4.2. Postchallenge titers in the FI-HSV2-immunized groups showed only modest increases over their respective prechallenge levels.
FIG. 2.
Virus-specific antibody levels in immunized guinea pigs. (A) Endpoint reciprocal IgG titers measured by an ELISA against either purified, dextran sulfate-released HSV-2 virion or purified, truncated gD2 protein. For reference, the weeks of immunization with FI-HSV2, FI-Mock, or gD2 subunit are shown by black arrows, and the week of i.vag. HSV-2 challenge is shown by a double-headed arrow. The symbols show the group means of the log10 endpoint IgG titers as described in Materials and Methods, and the error bars depict the standard deviations (n = 10 for all groups.) Dashed lines represent the assay limit of sensitivity, and individual titers below the assay limit were assigned a value of one-half the assay limit for calculation and graphing purposes. (B) Virus neutralizing antibody titers were measured against HSV-2 that was purified from the serum-free medium of infected Vero cells. Symbols show the geometric mean titers (GMT) of the groups, and error bars represent the standard errors (SEM). The assay limit of sensitivity and treatment of below-limit values are as described for panel A.
Taken together, we found that both groups primed with HSV-2 DNAs gave high levels of HSV-2 virion-specific IgG that were further increased by 2 boosts with FI-HSV2. Guinea pigs that were immunized only with FI-HSV2 had titers equal to those that received the HSV-2 plasmid DNA priming. However, the titer in the group immunized with gD2 subunit was consistently lower than the titers in the groups immunized with FI-HSV2.
Because gD2 is a major target of virus neutralizing antibodies, the gD2-specific IgG level in each serum sample was analyzed by an ELISA. The wells on the plates were coated with truncated gD2 protein purified from the medium of transfected 293FT cells. Priming with either of the gD2t DNA-containing cocktails elicited high gD2-specific IgG log10 titers of 3.9 to 4 (Fig. 2A). After 2 boosts with FI-HSV2, these titers were increased approximately 5-fold. In the pVAX-primed group, a gD2-specific IgG response was observed only after two boosts with FI-HSV2. Notably, this level was 2.5 log units lower than in the DNA prime-FI-HSV2 boost groups. In contrast, gD2 subunit immunization elicited the strongest gD2-specific IgG responses. After 2 immunizations, the ELISA titers (mean of 5.1) were 15-fold higher than those following 3 doses of either gD2t DNA-containing cocktail (week 18 titers of gD2 subunit groups versus week 9 titers of the gD2t DNA-primed groups). The titers in the gD2 subunit group were still 2-fold higher than those in the DNA-primed groups after they were boosted with FI-HSV2. Following challenge, all of the surviving pVAX-FI-Mock-immunized guinea pigs developed gD2-specific IgG responses that were similar to those in the pVAX-FI-HSV2 group after challenge.
Taken together, immunization with gD2t DNA in either DNA cocktail resulted in higher gD2-specific IgG levels than 2 immunizations with FI-HSV2. Subsequent boosts of the gD2t DNA-primed guinea pigs with FI-HSV2 resulted in only a slight increase in gD2-specific antibody levels. Two immunizations with gD2 subunit provided the best prechallenge gD2-specific IgG levels, with average levels over 2.5 log units higher than those in the animals immunized with pVAX-FI-HSV2. Note that because we used the FLAG-tagged gD2 subunit as the immunogen in the gD2 subunit group and as the ELISA antigen, we were concerned that the ELISA titers measured in this group represented both the contributions of gD2-specific IgG and FLAG-specific IgG. As described in Materials and Methods, we determined that the vast majority of IgG detected in the gD2 subunit-immunized guinea pigs was specific for gD2 epitopes and not the FLAG tag.
The levels of virus neutralizing antibody were measured by plaque reduction assay (Fig. 2B). Overall, neutralizing antibody levels were found to mirror the ELISA IgG levels against the HSV-2 virion, with some exceptions. Immunization with either of the HSV-2 DNA cocktails resulted in geometric mean titers (GMTs) of approximately 100 to 120 in the neutralization assay (measured at week 9). Following an FI-HSV2 boost, these GMTs increased to between 200 and 210 (week 14). However, the group given only the FI-HSV2 boost (pVAX-FI-HSV2) had a GMT of 120 (week 14), which was approximately 2-fold lower than the titers in the primed groups. While one immunization with gD2 subunit elicited neutralization titers (GMT of 43) that were 3- to 4-fold lower than in the FI-HSV2 groups, the second immunization with gD2 subunit resulted in the highest observed levels of neutralizing antibodies both pre- and postchallenge (GMT of 317). Prechallenge (week 18) levels in the gD2 subunit group were 2- to 5-fold higher than the FI-HSV2 groups. Neutralizing antibody titers in the FI-HSV2 groups were similar after the first and second boosts compared with the approximate 2-fold assay-to-assay variability that was measured using internal control sera.
Protection against acute vaginal HSV-2 disease.
Three weeks after the last immunization, guinea pigs were anesthetized and intravaginally challenged with HSV-2. The anogenital region of each guinea pig was scored daily by blinded observers for 100 days postchallenge. Days 1 to 14 were defined as the acute disease phase, and days 15 to 100 were defined as the recurrent phase. The outcomes of disease and lesion development as well as deaths are summarized in Table 2. We found that the highly virulent challenge dose of virus (6 × 105 PFU) resulted in overwhelming disease followed by death of 3 of the 10 pVAX-FI-Mock-immunized guinea pigs between days 11 and 14 postchallenge. An additional guinea pig in this group developed a severe, macerating disease by day 9 postchallenge that resulted in enough sequelae to preclude the unequivocal determination of recurrent lesion development. Therefore, recurrent-phase disease scores from this animal were omitted from the study. Finally, another guinea pig in this group died on day 34 postchallenge. This guinea pig accumulated 7 acute-phase and 5 recurrent-phase lesion days through the first 20 days of the recurrent phase, and disease scores from this animal were included in both acute and recurrent disease analyses. In summary, 9 of the 10 pVAX-FI-Mock-immunized guinea pigs developed acute lesions, with the remaining guinea pig developing swelling, fecal retention and impaction, and anogenital hair loss.
TABLE 2.
Measures of disease and lesion development in groups of guinea pigs given different HSV-2 vaccines
| Vaccination group | Lesion ratea |
Mean no. of recurrent lesion episodesb | Reduction in the no. of recurrent lesion days (%)c | |
|---|---|---|---|---|
| Acute phase | Recurrent phase | |||
| pVAX-FI-Mock | 9/10 | 6/6d | 3.67 | |
| pVAX-FI-HSV2 | 1/10 | 1/10 | 0.70f | 79f |
| UL5, UL30, gD2t DNA-FI-HSV2 | 0/9e | 2/9 | 0.22f | 97f |
| UL29, UL52, gD2t DNA-FI-HSV2 | 1/10 | 4/10 | 0.90g | 81g |
| gD2 subunit | 1/10 | 3/10 | 0.80g | 82g |
The lesion rate is the number of guinea pigs that developed at least one lesion/total number of guinea pigs during the acute phase (days 0 to 14 postchallenge) or recurrent phase (days 15 to 100 postchallenge). The values for all vaccine groups were significantly different (P ≤ 0.001) from the value for the control group (pVAX-FI-Mock) by Fisher's exact test.
A lesion episode is defined as one or more consecutive days with a disease score of 2 or above.
Reduction in the cumulative number of recurrent lesion days per guinea pig compared to the number for the pVAX-FI-Mock group.
Three animals died before the recurrent phase; 1 survivor failed to resolve primary disease and was omitted.
One animal did not recover from anesthesia used during challenge.
Significantly different (P < 0.05) from the value for the pVAX-FI-Mock group by Kruskal-Wallis analysis plus Dunn's multiple-comparison test.
Significantly different (P < 0.01) from the value for the pVAX-FI-Mock group by Kruskal-Wallis analysis plus Dunn's multiple-comparison test.
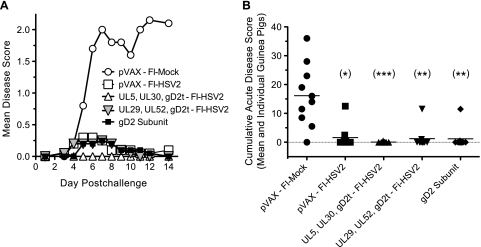
In contrast to the severe disease and deaths in this control group, none of the 9 guinea pigs in the group primed or immunized with UL5, UL30, and gD2t DNAs and boosted with FI-HSV2 (UL5, UL30, gD2t DNA-FI-HSV2 group) developed lesions during the acute phase, with only 1 guinea pig showing slight erythema (score of 0.5) on day 3 only. There were only 9 guinea pigs to observe in this group due to the failure of 1 to regain consciousness after receiving the prechallenge anesthetic cocktail that contained ketamine from a lot that has since been recalled. One of 10 guinea pigs in each of the other immunization groups developed lesions lasting 4 or 5 days. In addition, 1 to 3 animals in each of these groups showed erythema on only 1 to 3 days. Each of the vaccines significantly reduced the rates of acute lesion development (P = 0.0001 for the UL5, UL30, gD2t DNA-FI-HSV2 group and P = 0.001 for the other groups relative to mock-immunized controls by Fisher's exact test). No significant differences were observed between the protective vaccines. The time course of the mean disease score of each group is shown in Fig. 3A.
FIG. 3.
Protection against acute HSV-2 disease. Guinea pigs were i.vag. challenged with HSV-2 and then scored daily by blinded observers over the 14-day acute disease period using the disease scoring scale as described in Materials and Methods. This scale represents the standard scale from 0 to 4, except that a score of 0.5 was given for slight erythema, swelling, or papules/patches and a score of 5 was given to animals that died of HSV-2 disease. (A) Mean disease scores for each vaccine group on each day postchallenge are shown by the symbols in the legend. (B) Disease scores from days 1 to 14 postchallenge were summed for each guinea pig (each symbol shows the value for one guinea pig), and the mean of each vaccine group is shown by the short horizontal line. The dotted line shows the acute disease-free baseline for clarity, and the numbers of guinea pigs in the groups with a cumulative score of 0 are 1, 6, 8, 8, and 8 for each group from left to right. Values that were significantly different from the value for the control group (pVAX-FI-Mock) by Kruskal-Wallis analysis plus Dunn's multiple-comparison test are indicated as follows: (*), P < 0.05; (**), P < 0.01; (***), P < 0.001).
To better assess the acute disease burden of each guinea pig in the vaccine groups, the cumulative acute disease scores of individual guinea pigs are shown in Fig. 3B. Compared with the pVAX-FI-Mock group, all of the other groups had statistically significant reductions in cumulative acute disease scores (P < 0.05 by Kruskal-Wallis analysis plus Dunn's multiple-comparison test), but there were no significant differences among the protected groups. It is noteworthy that the prime-boost immunization with UL5, UL30, gD2t DNA-FI-HSV2 resulted in a greater than 99.5% reduction in the mean cumulative acute disease score relative to the vehicle-only control (P < 0.001).
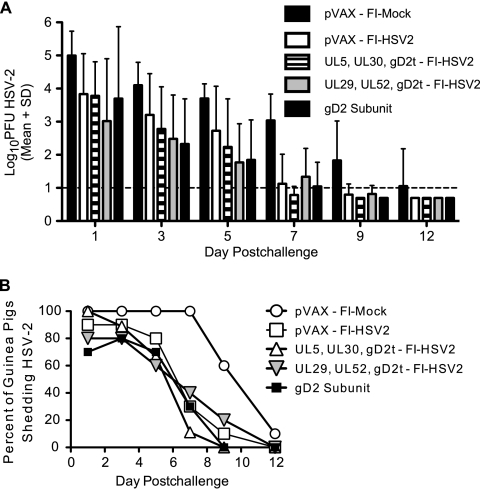
Protection against acute virus shedding.
Vaginal HSV-2 shedding was measured by plaque assay of intravaginal swabs performed on the days postchallenge shown in Fig. 4. The peak of virus shedding in the pVAX-FI-Mock group was 105 PFU on day 1 postchallenge (Fig. 4A), with all 10 guinea pigs shedding detectable virus through day 7 postchallenge. In the pVAX-FI-Mock group, 6 of 10 guinea pigs and 1 of 10 guinea pigs were still shedding detectable virus on days 9 and 12, respectively (Fig. 4B). On day 1 postchallenge, the group primed or immunized with UL29, UL52, and gD2t DNAs and boosted with FI-HSV2 (UL29, UL52, gD2t DNA-FI-HSV2 group) had a 2-log-unit reduction in HSV-2 levels compared with the pVAX-FI-Mock group, with the remaining groups showing a ca. 1.2-log-unit reduction (Fig. 4A). Both the UL29, UL52, gD2t DNA-FI-HSV2- and gD2 subunit-immunized guinea pigs showed similar mean titer reductions of 2.4 and 1.8 log units on days 3 and 5, respectively, compared to the mock-immunized guinea pigs. Mean titer reductions in the pVAX-FI-HSV2 and UL5, UL30, gD2t DNA-FI-HSV2 groups tended to be lower through day 5 postchallenge than those found in the other vaccine groups (Fig. 4A). No statistical differences between the HSV-2 titers in the mock and vaccine groups were found by Kruskal-Wallis analysis of the virus titers on each of the days postchallenge. With the exception of the UL5, UL30, gD2t DNA-FI-HSV2 group on day 1 postchallenge, all of the vaccines resulted in reductions in the percentage of guinea pigs in each group shedding virus relative to the mock control group (Fig. 4B). Of note, 1 guinea pig in each of the UL29, UL52, gD2t DNA-FI-HSV2 and gD2 subunit groups had no detectable virus on any of the swab days through day 12 postchallenge (data not shown).
FIG. 4.
Protection against acute-phase vaginal HSV-2 shedding. On the days shown postchallenge, each guinea pig was swabbed intravaginally, and the swabs were placed in 1 ml of DNG and frozen until standard plaque assay on Vero cells as described in Materials and Methods. (A) Levels of vaginal virus in the swabs are shown as group mean log10 PFU, and error bars represent the standard deviations (n = 10 for all groups except for the UL5, UL30, gD2t-FI-HSV2 group where n = 9). The dotted line shows the limit of assay sensitivity. (B) Kinetics of viral clearance are represented as the percentage of guinea pigs in each group shedding virus on the indicated days postchallenge.
Protection against recurrent HSV-2 disease.
The recurrent lesion rates and mean number of lesion episodes in each immunization group are summarized in Table 2. In the control group, all 6 observable guinea pigs developed at least one recurrent lesion through day 100 postchallenge. However, only 1 of 10 guinea pigs immunized with pVAX-FI-HSV2 (the animal that had acute-phase lesions and virus shedding persisting to day 9 postchallenge) developed recurrent lesions. Two of 9 animals in the UL5, UL30, gD2t DNA-FI-HSV2 group developed recurrent lesions, and 3 or 4 (out of 10 animals each) of the gD2 subunit group or UL29, UL52, gD2t DNA-FI-HSV2 groups respectively, developed recurrent lesions. Compared with the pVAX-FI-Mock group, all of the other vaccine groups had statistically significantly lower recurrence rates (P < 0.05 by Fisher's exact test), but there were no significant differences between the protective vaccine groups. The mean number of recurrent lesion episodes in the pVAX-FI-Mock group was 3.67. Although the pVAX-FI-HSV2 group had only 1 animal that developed lesions, it had 7 episodes of recurrent lesions, resulting in a mean number of 0.70 episodes per guinea pig (Table 2, P < 0.01 compared with the value for the pVAX-FI-Mock group). Most strikingly, the mean number of recurrent lesion episodes in the UL5, UL30, gD2t DNA-FI-HSV2 group was 0.22, representing 1 lesion day observed for each of 2 guinea pigs (P < 0.01 compared with the value for the pVAX-FI-Mock group). There were no statistical differences between the FI-HSV2-immunized groups.
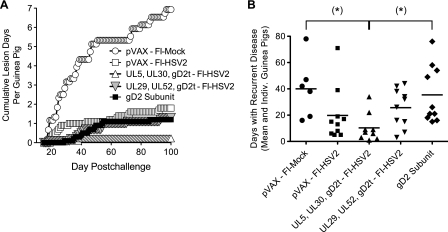
The kinetics of recurrent lesion development in each group are shown in Fig. 5A as the cumulative number of lesion days averaged over the number of guinea pigs in the group. All of the guinea pigs in the pVAX-FI-Mock group and 1 of the guinea pigs in the pVAX-FI-HSV2 group developed lesions toward the beginning of the recurrent phase (days 17 and 18, respectively), which continued to appear through day 97 and 93, respectively. In contrast, the 2 guinea pigs in the UL5, UL30, gD2t DNA-FI-HSV2 group that developed lesions each had a single lesion on 1 day only during the recurrent phase (day 20 or 39 postchallenge). The lesion rates and kinetics in the gD2 subunit and UL29, UL52, gD2t DNA-FI-HSV2 groups were very similar. As summarized in Table 2, all immunization groups showed statistically significant reductions in the number of days with recurrent lesions compared with mock-immunized controls. No significant differences were found between any of the protected vaccine groups. Remarkably, the UL5, UL30, gD2t DNA-FI-HSV2 group had a 97% reduction in the number of days with recurrent lesions (P < 0.01 compared with the value for the pVAX-FI-Mock group by Kruskal-Wallis analysis plus Dunn's multiple-comparison test).
FIG. 5.
Protection against recurrent lesions and disease. HSV-2-challenged guinea pigs were scored for disease during the recurrent phase of infection, defined as days 15 to 100 postchallenge. (A) A lesion day was scored for a guinea pig that developed at least one lesion (disease score of 2 or rarely 3), and the cumulative number of lesion days for each vaccine group was averaged over the number of guinea pigs in each group. The pVAX-FI-HSV2 group, UL29, UL52, gD2t DNA-FI-HSV2 group, and gD2 subunit group each had 10 guinea pigs. The UL5, UL30, gD2t DNA-FI-HSV2 group had 9 guinea pigs, and as shown in Table 2 and as described in the text, the pVAX-FI-Mock group had 6 guinea pigs until day 34 and then 5 guinea pigs through day 100. (B) Each symbol represents the number of days in the recurrent phase that an individual (Indiv.) guinea pig had a score of 0.5 or above, and the vaccine group means are shown by the short horizontal lines. Note that the guinea pig in the pVAX-FI-Mock group that died on day 34 had resolved its primary disease and then accumulated 16 recurrent disease days before death. This was in contrast to an additional animal in this group that was omitted from the recurrent-phase study since it never resolved its primary disease. Values that were significantly different (P < 0.05) from the value for the control group (pVAX-FI-Mock) by Kruskal-Wallis analysis plus Dunn's multiple-comparison tests are indicated (*).
In addition to lesion formation, many animals in the study showed less-severe forms of recurrent disease, such as erythema, swelling, and/or papules that were worth noting. Some guinea pigs showed recurrent disease (score of ≥0.5) on half or more days in the recurrent phase. The cumulative number of days with vaginal disease (including lesions) was calculated for each guinea pig and plotted in Fig. 5B. Note particularly that immunization with UL5, UL30, gD2t DNA-FI-HSV2 was the most protective against any recurrent disease through day 100 postchallenge. This group had a statistically significant 71% reduction in disease days compared to the gD2 subunit group and a 74% reduction in disease days compared with mock-immunized controls (P < 0.05 for both values by Kruskal-Wallis analysis plus Dunn's multiple-comparison test). There were no statistically significant differences between any of the other vaccine groups compared to the FI-Mock group or each other. Taken together, prime-boost immunization with UL5, UL30, gD2t DNA-FI-HSV2 was the most strongly protective against recurrent HSV-2 lesion development and days with recurrent disease. The pVAX-FI-HSV2 immunization resulted in the fewest number of guinea pigs that developed recurrent lesions.
Protection against latent HSV-2 load in the DRG.
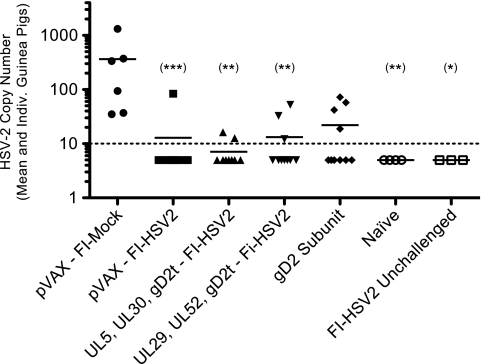
To determine whether the protection against recurrent lesions or vaginal HSV-2 disease was associated with differences in the number of latent viral genomes in the DRG or in postreactivation immunity, the lumbosacral DRG from each surviving guinea pig were harvested. The levels of HSV-2 DNA were quantified by real-time quantitative PCR as described in Materials and Methods. As shown in Fig. 6, HSV-2 DNA was detected in all 6 of the pVAX-FI-Mock-immunized guinea pigs, with a mean of 365 copies of HSV-2 DNA per 250 ng of DRG DNA (range, 35 to 1,316 copies). Importantly, all immunization groups, except for the gD2 subunit group, had significantly reduced loads of latent HSV-2 DNA compared to the mock-immunized group (pVAX-FI-Mock group). No significant differences were observed when the FI-HSV2 or gD2 subunit groups were compared with each other. Only 1 of the 10 guinea pigs immunized with pVAX-FI-HSV2 had detectable HSV-2 DNA (84 copies). This was the same animal that had detectable virus shedding through day 9 postchallenge followed by 7 recurrent lesion episodes. In the UL5, UL30, gD2t DNA-FI-HSV2 group, 2 of 9 guinea pigs had barely detectable levels (13 to 16 copies) of HSV-2 DNA. Similarly, 3 of 10 guinea pigs in the UL29, UL52, gD2t DNA-FI-HSV2 group had detectable levels of HSV-2 DNA, and 2 of these animals had developed recurrent lesions. Finally, 4 of the 10 gD2 subunit-immunized guinea pigs had detectable HSV-2 DNA levels, and 1 of these guinea pigs had developed recurrent lesions. No HSV-2 DNA was detectable in any of the PCRs using the DRG from the guinea pigs that were immunized with FI-HSV2 but not challenged, indicating that the HSV-2 DNA detected in the DRG of the FI-HSV2-immunized groups was that of the challenge virus and not the inactivated-virus vaccine. In addition, three replicates of quantitative PCR using β-actin-specific primers and probe showed that similar levels of the cellular gene could be amplified from every DRG preparation, including those that had undetectable HSV-2 DNA (data not shown). Finally, while no lesions were observed in any of the animals at the time of the DRG harvest, we cannot exclude the possibility that the DRG DNA level measured was affected by a coincidental recurrence episode.
FIG. 6.
Protection against HSV-2 latent DNA load in the DRG. On weeks 46 to 48 of the study (28 to 30 weeks postchallenge), the lumbosacral DRG from each surviving guinea pig were removed, pooled, and frozen. The DRG DNA from each pool was extracted and quantified by spectrophotometry. HSV-2 DNA was quantified by TaqMan real-time quantitative PCR using primers and a probe specific for gG2. Each reaction mixture contained 250 ng of DRG DNA, and each standard curve reaction mixture contained 250 ng of DRG DNA from naïve guinea pigs and between 10 and 104 copies of HSV-2 DNA isolated from purified HSV-2 virions. Each symbol represents the mean of 3 independent assays for an individual (Indiv.) guinea pig, and the horizontal lines represent vaccine group means. Two additional control groups consisted of the DRG from 4 naïve guinea pigs (Naïve) and from 3 guinea pigs that were immunized with FI-HSV2 but were not challenged with HSV-2 (FI-HSV2 Unchallenged). The broken line represents the detection limit of the assay of 10 copies of HSV-2 DNA per 250 ng of DRG DNA. Statistical significance levels for the values for each group compared with the pVAX-FI-Mock group are as described in the legend to Fig. 3.
DISCUSSION
In this study, we employed a DNA prime and whole, inactivated virus in MPL-alum boost strategy to protect guinea pigs against acute and recurrent HSV-2 disease. This strategy was developed using a mouse model of MCMV protection in which DNA vaccines were used to elicit T cell responses against nonstructural antigens and to prime B cell responses against viral targets of neutralizing antibodies (33, 34). Immunization with these DNA vaccines protected mice against MCMV challenge, but the highest levels of protection were in DNA-primed mice boosted with formalin-inactivated virus in adjuvant. Furthermore, only DNA prime-inactivated-virus boost vaccination could provide suppression of virus replication in target organs to undetectable levels in all mice tested (34). We found that inactivated-virus boosting resulted in both neutralizing antibody levels at least as high as those elicited by MCMV infection and recall CD8+ T cell responses to the virion-associated antigens (34). In a recent study using this prime-boost vaccination strategy for protection against HSV-2 in mice, we found that nearly complete protection against lethal, intravaginal challenge of mice could be conferred by a prime-boost vaccine consisting of the UL5, UL30, and gD2t DNAs followed by FI-HSV2 together with MPL-alum (data not shown).
The gD2t DNA-containing cocktails and the FI-HSV2 were found to elicit similar, strong HSV-2 virion-specific IgG and neutralizing antibody responses. In both groups primed with the gD2t DNA, the boosting effect of the FI-HSV2 on HSV-2 antibody levels was most evident after two injections. At this time, the levels of HSV-2-specific IgG antibodies were 14-fold greater in these two groups than in the gD2 subunit group. In contrast, the gD2 subunit and gD2t plasmid were found to be much better stimulators of gD2-specific antibodies than the FI-HSV2. These data, together with a proteomic analysis that estimated that HSV-1 gD1 comprises 1 to 4% of the HSV-1 virion-associated protein (28), suggest that the gD2 protein content of the FI-HSV2 is relatively low. We observed that FI-HSV2 elicited low-level gD2-specific antibodies as a primary immunogen and only slightly increased the gD2-specific responses as a boost in the gD2t DNA groups. Since FI-HSV2 boosted the levels of virion-specific antibodies to a greater extent than gD2-specific antibodies, it is likely that FI-HSV2 mainly elicited non-gD2-specific antibodies. Conversely, immunization with the gD2 subunit elicited high levels of gD2-specific antibodies but lower levels of HSV-2 virion-specific antibodies. Interestingly, 2 doses of gD2 subunit with adjuvant were required to produce virion-specific antibody titers as high as those elicited by the DNA immunization. The multivalent nature of FI-HSV2 likely accounts for its ability to generate higher virion-specific responses than the gD2 subunit.
The analysis of neutralizing antibody levels showed that sera from the gD2 subunit-immunized guinea pigs had the highest mean titer by plaque reduction assay. Peak neutralizing antibody titers were detected either following DNA immunization or following a single dose of FI-HSV2, and these titers were slightly lower than the titers following 2 doses of gD2 subunit vaccine. Together, these data suggest that similar levels of neutralizing antibodies can be achieved either by gD2-specific antibodies or the mainly non-gD2-specific antibodies elicited by FI-HSV2.
The gD2 subunit-MPL-alum vaccine used in this study provided protection against acute and recurrent disease and acute shedding that was comparable to that provided by FI-HSV2 alone and the UL29, UL52, gD2t DNA-FI-HSV2 vaccine. The gD2 subunit vaccination also showed a significant 82% reduction in recurrent lesion days relative to the mock-immunized controls. However, it did not significantly reduce the mean number of days with all recurrent disease, such as erythema, swelling, papules, or lesion formation (40 recurrent disease days for vehicle control versus 35.4 for gD2 subunit) (Fig. 5B). These results were unexpected, as the published reports of the efficacy of gD2 subunit vaccination in guinea pigs only show measures of cumulative lesion days and the number of guinea pigs with recurrent lesions. In the study presented here, the protective immunity provided by the gD2 subunit may not have been sufficient to suppress the early stages of reactivation, leading to anogenital inflammation and possibly low-level pathology of the mucosa, but it was sufficient to prevent the majority of recurrent lesion episodes, as evidenced by the large reduction in lesion days compared to controls. Ideally, an effective HSV-2 vaccine would be protective against all of the genital symptoms that are associated with HSV-2 disease in human subjects. We found that only the UL5, UL30, gD2t DNA-FI-HSV2 group had a significant reduction in all levels of recurrent HSV-2 disease. While the gD2 subunit group had reduced levels of latent HSV-2 DNA in the DRG relative to the vehicle control (16.5-fold mean reduction) and 6 of the 10 guinea pigs had undetectable HSV-2 DNA, this vaccine group demonstrated the least protection against all recurrent disease, and the reduction in latent viral genomes was not statistically significant. It should be noted that the gD2 subunit immunization strategy that was used as a comparative control was designed with dosing and formulation similar to those of previously published studies. Thus, our results cannot exclude the possibility that 5 immunizations with gD2 subunit in this group (instead of 2) would result in enhanced protection comparable to that seen in the animals primed with DNA and boosted with FI-HSV2. In addition, boosting of guinea pigs that were primed with UL5 and UL30 DNAs (with or without gD2t DNA) with gD2 subunit vaccine may also provide protection comparable to boosting with FI-HSV2. Other studies have shown that protein boost vaccination can elicit enhanced protection against HSV-2 disease and shedding in the guinea pig compared with either vaccine component alone (14).
Previous studies of naïve or immunized guinea pigs that were infected with HSV-1 or HSV-2 suggest that the level of latent HSV-2 DNA in the DRG is positively correlated with the rate of recurrent disease (18, 27). A successful HSV vaccine could be effective by either decreasing the level of latent HSV-2 load established in the DRG, presumably through suppressing the level of the acute infection (18), or by providing control after the establishment of the latent load and during the steps associated with reactivation. In our study, we found that the UL5, UL30, gD2t DNA-FI-HSV2 group had the lowest levels of acute and recurrent disease, recurrent lesion days, and latent HSV-2 load in the DRG. There was also a trend showing that the other immunization groups that had the lowest recurrent lesion or disease days had the lowest levels of latent HSV-2 DNA. On a per guinea pig basis, however, these correlations did not occur in every instance. Although in general, our data suggest that protection against recurrent disease was due to the decreased loads of latent HSV-2 in the DRG, only in the gD2 subunit group could a significant inverse correlation be demonstrated between the prechallenge HSV-2 virion-specific IgG levels and the resultant HSV-2 DNA loads (P = 0.037). In this group, there were also trends toward positive correlations of the HSV-2 DNA load in each guinea pig with the number of recurrent episodes (P = 0.057), the number of recurrent lesion days (P = 0.058), and the number of recurrent disease days (P = 0.064) (data not shown).
A recent study by Bernstein et al. (3) compared the efficacy of gD2 subunit used either alone, with MPL-alum, or with a novel cationic liposome-DNA complex (CLDC) adjuvant. This study showed that while gD2-MPL-alum or gD2-CLDC immunization resulted in similar numbers of guinea pigs with detectable HSV-2 DNA in the DRG, the gD2-CLDC-immunized animals had a lower percentage of animals with recurrent disease, a lower number of cumulative lesion days, and a lower mean number of recurrent shedding days (3). Although the levels of HSV-2 were not quantified in this study, these results suggest that vaccine-induced immunity from different vaccines (or in this case adjuvants) may exert different levels of protection that can act at times subsequent to the establishment of the latent load. Since the protective mechanisms or antigen specificities that control the disease or shedding during acute infection may not necessarily be the same as those that control reactivation and/or recurrent infection, a successful vaccine strategy may need to incorporate components that address each of these phases of infection. Additionally, varying the challenge dose given to the immunized animals, rather than using a single dose, may reveal more obvious qualitative or quantitative differences in the resultant protection outcomes than those seen in this study. Specifically, greater differences in the vaccine efficacies may be observed by either (i) reducing the challenge dose to one that is more physiological and less likely to overwhelm preexisting immunity in all of the vaccine groups or (ii) increasing the challenge dose to a level that overcomes the immunity elicited by the less-protective, but not by the more-protective, vaccines.
Establishing the immune correlates that protect against acute and recurrent HSV-2 disease and shedding in humans has been difficult, and the use of animal models has thus far shown only limited success for the rational design of an effective HSV-2 vaccine (24). Besides the differences in HSV-2 antigenic peptides differentially binding to human leukocyte antigen (HLA) or major histocompatibility complex (MHC) class I complexes in humans or animals, respectively, HSV-2 mechanisms of MHC class I downregulation that function in the human host may be poorly active or inactive in the tissues of mice and guinea pigs. This possibility was evidenced both by the low activity of infected-cell protein 47 (ICP47) for mouse TAP 1/2 complex inhibition (TAP stands for transporter associated with antigen processing) (1, 45) and the increased frequency of reactivation in mouse eyes and trigeminal ganglia by ectopic expression of the m152 immunoevasin of MCMV by a chimeric HSV-1 (37). Thus, the predictive abilities of the animal models of HSV-2 vaccination, especially for T cell immunity, have known limitations. In our studies, the choice of using the essential HSV-2 genes that are conserved among herpesviruses as T cell targets was based upon the rationale that these targets must be expressed for virus replication and that the high conservation may limit immune escape and provide cross-strain protection. In addition, genome-wide screening studies of the T cell specificities against the antigens of HCMV and MCMV have shown that as a class, these antigens generally elicit either undetectable or very low T cell levels in the virus-infected host (36, 44). Thus, high T cell responses against these targets resulting from DNA immunization may provide better immunity than does natural infection, in which immunity still allows for the life-long establishment of latency and viral shedding. Ultimately, the protective efficacy of any HSV-2 vaccine will need to be specifically addressed in clinical trials.
In general, inactivated-HSV-2 vaccines have fallen out of favor due to many years of clinical studies with poor methodologies, such as the lack of a placebo arm, as well as disappointing protective efficacies in placebo control studies, especially regarding long-term protection against recurrent disease, (reviewed in reference 47). More recently, subunit vaccines based on gD2 or gB2 were tested for clinical efficacy trials, and although the study designs of these trials were more rigorous than for the inactivated-virus vaccines, significant protective efficacy was not readily achieved (24). It was not until the GSK gD2 vaccine using a novel adjuvant (AS04) containing aluminum hydroxide and 3-O-deacylated MPL was tested in two clinical trials were there consistent and significant rates of 73% and 74% protection against genital disease, and this was observed only for women who were HSV-1 and HSV-2 seronegative (41). However, a recent statement by the National Institutes of Health regarding a subsequent phase 3 trial of this vaccine in HSV-1/2-seronegative women showed an estimated protection rate of 20%, an efficacy that did not reach statistical significance. The adjuvant activity of MPL is thought to be derived from its ability to drive Th1-type cytokine secretion of interleukin 2 (IL-2) and IFN-γ, its Toll-like receptor 4 (TLR4) agonist activity, its induction of NF-κB, and its subsequent activation of antigen-loaded dendritic cells and monocytes in the draining lymph nodes (10). Combining the MPL with alum—an adjuvant that had been separately tested in subunit and inactivated-HSV-2 vaccines—was later demonstrated to prolong the cytokine secretion due to MPL (10).
There is a rapidly increasing body of evidence that demonstrates that the stimulation of innate immunity, such as that mediated by specific TLR agonists, has subsequent effects on the type, strength, and durability of adaptive immunity (reviewed in reference 16). These new insights, coupled with some positive efficacy of the GSK MPL-alum adjuvanted gD2 vaccine and the historical safety record of inactivated-HSV-2 vaccines, provides a renewed stimulus to revisit the use of inactivated-HSV-2 vaccines when administered together with the newest generation of adjuvants that stimulate one or more TLRs. In our study, when the FI-HSV2-MPL-alum vaccine was given to the pVAX-primed guinea pigs, with the exception of one “nonresponder” animal in the group, we found complete protection against both acute and recurrent lesion development, undetectable levels of HSV-2 DNA in the DRG, lower cumulative acute disease scores, and lower numbers of recurrent disease days that were comparable to those for the UL5, UL30, gD2t DNA-FI-HSV2 group. Follow-up studies in the guinea pig using higher numbers of animals and short- and long-term challenge groups may further delineate the impact of the UL5, UL30, and gD2t DNA prime on the strength and durability of the observed protective responses to FI-HSV2-MPL-alum and may help to provide the basis for human trials.
Acknowledgments
We thank David M. Knipe for providing virus, Timothy Dudek for technical instruction, protocols, and insight into the animal models of HSV-2, Yo Hoshino for helpful discussions regarding the guinea pig model, and Shelle Malkmus, Mario Brock, Tony Yaksh, and Nigel Calcutt for instructions on the DRG dissection.
This work was supported by NIH NIAID grant 1R21 AI073585 (D.H.S.). The research described in this paper was also sponsored in part by King Abdulaziz City for Science and Technology (KACST) in Saudi Arabia. The authors thank KACST for their generous support of this program.
Footnotes
Published ahead of print on 26 January 2011.
REFERENCES
- 1.Ahn, K., et al. 1996. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 15:3247-3255. [PMC free article] [PubMed] [Google Scholar]
- 2.Baldridge, J. R., and R. T. Crane. 1999. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods 19:103-107. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein, D. I., et al. 2010. The adjuvant CLDC increases protection of a herpes simplex type 2 glycoprotein D vaccine in guinea pigs. Vaccine 28:3748-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2010. Seroprevalence of herpes simplex virus type 2 among persons aged 14-49 years—United States, 2005-2008. MMWR Morb. Mortal. Wkly Rep. 59:456-459. [PubMed] [Google Scholar]
- 5.Challberg, M. D. 1986. A method for identifying the viral genes required for herpesvirus DNA replication. Proc. Natl. Acad. Sci. U. S. A. 83:9094-9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corey, L., et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 282:331-340. [DOI] [PubMed] [Google Scholar]
- 7.Da Costa, X., M. F. Kramer, J. Zhu, M. A. Brockman, and D. M. Knipe. 2000. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J. Virol. 74:7963-7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da Costa, X. J., N. Bourne, L. R. Stanberry, and D. M. Knipe. 1997. Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital disease. Virology 232:1-12. [DOI] [PubMed] [Google Scholar]
- 9.Da Costa, X. J., C. A. Jones, and D. M. Knipe. 1999. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc. Natl. Acad. Sci. U. S. A. 96:6994-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Didierlaurent, A. M., et al. 2009. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 183:6186-6197. [DOI] [PubMed] [Google Scholar]
- 11.Dudek, T., L. C. Mathews, and D. M. Knipe. 2008. Disruption of the U(L)41 gene in the herpes simplex virus 2 dl5-29 mutant increases its immunogenicity and protective capacity in a murine model of genital herpes. Virology 372:165-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupuis, S., et al. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33:388-391. [DOI] [PubMed] [Google Scholar]
- 13.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 14.Fotouhi, F., H. Soleimanjahi, M. H. Roostaee, and F. Behzadian. 2008. Enhancement of protective humoral immune responses against herpes simplex virus-2 in DNA-immunized guinea-pigs using protein boosting. FEMS Immunol. Med. Microbiol. 54:18-26. [DOI] [PubMed] [Google Scholar]
- 15.Freeman, E. E., et al. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73-83. [DOI] [PubMed] [Google Scholar]
- 16.Guy, B. 2007. The perfect mix: recent progress in adjuvant research. Nat. Rev. Microbiol. 5:505-517. [DOI] [PubMed] [Google Scholar]
- 17.Higgins, T. J., et al. 2000. Plasmid DNA-expressed secreted and nonsecreted forms of herpes simplex virus glycoprotein D2 induce different types of immune responses. J. Infect. Dis. 182:1311-1320. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino, Y., et al. 2005. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 79:410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino, Y., et al. 2009. Protection from herpes simplex virus (HSV)-2 infection with replication-defective HSV-2 or glycoprotein D2 vaccines in HSV-1-seropositive and HSV-1-seronegative guinea pigs. J. Infect. Dis. 200:1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshino, Y., et al. 2008. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs. Vaccine 26:4034-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimberlin, D. W. 2007. Management of HSV encephalitis in adults and neonates: diagnosis, prognosis and treatment. Herpes 14:11-16. [PubMed] [Google Scholar]
- 22.Knipe, D. M. 1989. The role of viral and cellular nuclear proteins in herpes simplex virus replication. Adv. Virus Res. 37:85-123. [DOI] [PubMed] [Google Scholar]
- 23.Koelle, D. M., and L. Corey. 2008. Herpes simplex: insights on pathogenesis and possible vaccines. Annu. Rev. Med. 59:381-395. [DOI] [PubMed] [Google Scholar]
- 24.Koelle, D. M., and L. Corey. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 16:96-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koelle, D. M., et al. 1994. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68:2803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koelle, D. M., et al. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Invest. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lekstrom-Himes, J. A., L. Pesnicak, and S. E. Straus. 1998. The quantity of latent viral DNA correlates with the relative rates at which herpes simplex virus types 1 and 2 cause recurrent genital herpes outbreaks. J. Virol. 72:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loret, S., G. Guay, and R. Lippe. 2008. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J. Virol. 82:8605-8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertz, G. J., et al. 1985. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex. Transm. Dis. 12:33-39. [DOI] [PubMed] [Google Scholar]
- 30.Milligan, G. N., and D. I. Bernstein. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 31.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 32.Morello, C. S., L. A. Kelley, M. W. Munks, A. B. Hill, and D. H. Spector. 2007. DNA immunization using highly conserved murine cytomegalovirus genes encoding homologs of human cytomegalovirus UL54 (DNA polymerase) and UL105 (helicase) elicits strong CD8 T-cell responses and is protective against systemic challenge. J. Virol. 81:7766-7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morello, C. S., M. Ye, S. Hung, L. A. Kelley, and D. H. Spector. 2005. Systemic priming-boosting immunization with a trivalent plasmid DNA and inactivated murine cytomegalovirus (MCMV) vaccine provides long-term protection against viral replication following systemic or mucosal MCMV challenge. J. Virol. 79:159-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morello, C. S., M. Ye, and D. H. Spector. 2002. Development of a vaccine against murine cytomegalovirus (MCMV), consisting of plasmid DNA and formalin-inactivated MCMV, that provides long-term, complete protection against viral replication. J. Virol. 76:4822-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison, L. A., X. J. Da Costa, and D. M. Knipe. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243:178-187. [DOI] [PubMed] [Google Scholar]
- 36.Munks, M. W., et al. 2006. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J. Immunol. 176:3760-3766. [DOI] [PubMed] [Google Scholar]
- 36a.National Institutes of Health. 2000. Public Health Service policy on humane care and use of laboratory animals. National Institutes of Health, Bethesda, MD.
- 37.Orr, M. T., M. A. Mathis, M. Lagunoff, J. A. Sacks, and C. B. Wilson. 2007. CD8 T cell control of HSV reactivation from latency is abrogated by viral inhibition of MHC class I. Cell Host Microbe 2:172-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parr, M. B., and E. L. Parr. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schacker, T., J. Zeh, H. L. Hu, E. Hill, and L. Corey. 1998. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J. Infect. Dis. 178:1616-1622. [DOI] [PubMed] [Google Scholar]
- 40.Stanberry, L. R., D. I. Bernstein, R. L. Burke, C. Pachl, and M. G. Myers. 1987. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J. Infect. Dis. 155:914-920. [DOI] [PubMed] [Google Scholar]
- 41.Stanberry, L. R., et al. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 42.Strasser, J. E., R. L. Arnold, C. Pachuk, T. J. Higgins, and D. I. Bernstein. 2000. Herpes simplex virus DNA vaccine efficacy: effect of glycoprotein D plasmid constructs. J. Infect. Dis. 182:1304-1310. [DOI] [PubMed] [Google Scholar]
- 43.Straus, S. E., et al. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J. Infect. Dis. 176:1129-1134. [DOI] [PubMed] [Google Scholar]
- 44.Sylwester, A. W., et al. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomazin, R., et al. 1996. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 15:3256-3266. [PMC free article] [PubMed] [Google Scholar]
- 46.Wald, A., and K. Link. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 185:45-52. [DOI] [PubMed] [Google Scholar]
- 47.Whitley, R. J. 2001. Herpes simplex viruses, p. 2461-2509. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 48.Zhu, J., et al. 2009. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat. Med. 15:886-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu, J., et al. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 204:595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]