Abstract
During virus particle assembly, the arenavirus nucleoprotein (NP) associates with the viral genome to form nucleocapsids, which ultimately become incorporated into new virions at the cell membrane. Virion release is facilitated by the viral matrix Z protein through its interaction with the cellular endosomal sorting complex required for transport (ESCRT) machinery. However, the mechanism of nucleocapsid incorporation into virions is not well understood. Here, we demonstrate that ALIX/AIP1, an ESCRT-associated host protein, is required for the incorporation of the NP of Mopeia virus, a close relative of Lassa virus, into Z-induced virus-like particles (VLPs). Furthermore, we show that the Bro1 domain of ALIX/AIP1 interacts with the NP and Z proteins simultaneously, facilitating their interaction, and we identify residues 342 to 399 of NP as being necessary for its interaction with ALIX/AIP1. Our observations suggest a potential role for ALIX/AIP1 in linking Mopeia virus NP to Z and the budding apparatus, thereby promoting NP incorporation into virions.
Mopeia virus is a member of the family Arenaviridae of enveloped single-stranded RNA viruses and is related to other Old World arenaviruses, including Lassa and Lujo hemorrhagic fever viruses and lymphocytic choriomeningitis virus (LCMV). While the natural reservoir of Mopeia virus is Mastomys natalensis, the multimammate rat found throughout sub-Saharan Africa, humans also carry antibodies to the virus; however, there is no indication that the virus is pathogenic to them (33).
The arenavirus genome is contained within two single-stranded RNA molecules, designated the S and L segments. The Mopeia virus S segment is 3,427 nucleotides long and encodes the major structural proteins, the nucleoprotein (NP) and the glycoprotein precursor (GPC) (33). The GPC is posttranslationally cleaved into GP1 and GP2, which are linked via ionic interactions and form spikes on the surface of the virions. The viral glycoproteins mediate viral attachment to cellular receptors and subsequent entry into the cell (3, 5). NP, the most abundant viral protein, associates with the viral genome and replicative intermediates and, together with viral RNA-dependent RNA polymerase L, forms the ribonucleoprotein (RNP) complex (26). The L segment is 7,271 nucleotides long and encodes two proteins, Z, which has both regulatory and structural roles in the viral life cycle, and L (4, 10, 14, 33, 34, 45).
The virion release of arenaviruses from the host cell membrane is promoted by Z, whose solitary expression was shown to induce the formation of virus-like particles (VLPs) (7, 34, 43, 45, 47). For several enveloped viruses, virion release requires a membrane fission event, which is promoted by viral matrix protein-containing short sequences termed “late domains” due to their critical role in the late stages of assembly (17, 22). Putative arenavirus late domains have been mapped to three tetrapeptide motifs, PT/SAP, PPXY, and YXXL, which represent docking sites for cellular proteins. PT/SAP-, PPXY-, and YXXL-type late domains interact with TSG101, NEDD4-like proteins, and ALIX/AIP1 (referred to here as AIP1), respectively, all of which function in the multivesicular body (MVB) pathway (1, 2, 11, 23). TSG101 is a component of ESCRT (endosomal sorting complex required for transport), a protein interaction network that helps sort endosomal cargo into vesicles budding into the MVB lumen (27). The ESCRT network comprises three multiprotein complexes: ESCRT-I, -II, and -III. The cytosolic ESCRT-I, with its core component of TSG101, recognizes protein cargo and activates ESCRT-II (23). ESCRT-II then activates ESCRT-III, which is located on endosomal membranes and is an essential part of the apparatus that induces MVB vesicle formation (1, 2). For several viruses, AIP1 is known to bind to the TSG101 of ESCRT-I and to components of ESCRT-III, joining the two complexes and eliminating the need for ESCRT-II (13, 28, 37). AIP1 also recruits NEDD4-like proteins to facilitate HIV-1 release (42).
Several studies have explored the involvement of the MVB proteins in arenavirus VLP release (32, 34, 46, 47). The cell depletion of TSG101 was shown previously to inhibit Lassa virus and LCMV VLP budding, whereas the VLP formation of another arenavirus, Tacaribe virus, was not TSG101 dependent (34, 45, 47). A mutation of the PPPY sequence within arenavirus Z proteins is detrimental to budding; however, NEDD4 is not involved in virion release (34, 46). Although arenavirus Z proteins contain a conserved YLCL sequence, AIP1 does not contribute to Lassa virus Z-induced VLP formation (19, 46, 47). These findings highlight the complexity of and differences in the protein interactions within the arenavirus budding machinery.
Despite advancements in our understanding of arenavirus VLP release, the factors that facilitate the incorporation of arenavirus nucleocapsids into virions remain largely unknown. Reports of interactions between the Z and NP proteins/nucleocapsids fuel speculation that such interactions play a central role in nucleocapsid recruitment to the cell membrane, resulting in the incorporation of the nucleocapsid into virions (7, 15, 40). However, our recent report demonstrated that the association between the Mopeia virus NP and Z proteins and the cell membrane is not sufficient for the efficient incorporation of NP into Z-driven VLPs, suggesting the need for an additional factor(s) (43). Here, we show that AIP1 has just such a role, linking Mopeia virus NP and Z to promote NP virion incorporation.
MATERIALS AND METHODS
Cells and antibodies.
Human embryonic kidney (HEK) 293T cells and African green monkey (Vero) cells were maintained in Dulbecco's modified Eagle's medium and minimal essential medium, respectively, supplemented with 10% fetal bovine serum (FBS), l-glutamine, and penicillin-streptomycin solution. Cells were maintained at 37°C with 5% CO2.
A mouse monoclonal antibody to the hemagglutinin (HA) tag (Covance, Princeton, NJ), rabbit or chicken polyclonal antibodies to the HA tag (Abcam, Cambridge, MA), mouse monoclonal and rabbit polyclonal antibodies to the FLAG tag (Sigma, St. Louis, MO), and a rabbit anti-PDC6I (anti-AIP1) antibody (Abcam, Cambridge, MA) were used according to the manufacturers' instructions.
Plasmids.
The plasmids for the expression of the viral proteins were generated based on the sequences of the Z, NP, and GP proteins of Mopeia virus strain AN20410 (NCBI accession numbers NC_006574 [Z] and NC_006575 [NP and GP]). pC-MopZ-HA and pC-MopNP-FLAG were described previously (43). Mopeia virus wild-type NP and NP deletion constructs (43), all containing an HA tag at their C termini, were cloned into the protein expression vector pCAGGS/MCS (24, 32) by using PCR and standard cloning techniques. Wild-type NP, NPΔ114-228, NPΔ229-341, NPΔ342-399, NPΔ400-457, and NPΔ458-514 were amplified with forward primer 5′-GCGC GAATTC ATG TCC AAT TCA AAG GAG GTG AAG TCC TTC-3′ and reverse primer 5′-GCGC CCCGGG TCA AGC GTA GTC TGG GAC GTC GTA TGG GTA CAG GAC AAC TCT GGG AGG ACC TGT TC-3′; NPΔ1-113 was amplified with forward primer 5′-GCGC GAATTC ATG ATC AGA GGT GAG AGG CCT CTT GCT G-3′ and reverse primer 5′-GCGC CCCGGG TCA AGC GTA GTC TGG GAC GTC GTA TGG GTA CAG GAC AAC TCT GGG AGG ACC TGT TC-3′; and NPΔ515-570 was amplified with forward primer 5′-GCGC GAATTC ATG TCC AAT TCA AAG GAG GTG AAG TCC TTC-3′ and reverse primer 5′-GCGC CTCGAG TCA AGC GTA GTC TGG GAC GTC GTA TGG GTA TAC AAT CCC CGT ATG CAT TCT ACA AAG GTG-3′. The generated plasmids were designated pC-MopNP-HA, pC-MopNPΔ1-113-HA, pC-MopNPΔ114-228-HA, pC-MopNPΔ229-341-HA, pC-MopNPΔ342-399-HA, pC-MopNPΔ400-457-HA, pC-MopNPΔ458-514-HA, and pC-MopNPΔ515-570-HA.
The wild-type Mopeia virus Z construct with the FLAG tag at its C terminus was PCR amplified with forward primer 5′-GCGC CCCGGG ATG GGG AAA ACG CAG TCC AAG G-3′ and reverse primer 5′-GCGC CTCGAG TCA CTT GTC GTC ATC GTC TTT GTA GTC GGG GCT GTA GGG TGG-3′ and cloned into pCAGGS/MCS; the resulting plasmid was designated pC-MopZ-FLAG. The Mopeia virus Z constructs containing the replacement of YLCL with either AAAA, ALCL, YACL, or YLCA and an HA tag at the C termini were generated by site-directed mutagenesis with forward primer 5′-GTC AGG TGC AAC GAT CAC GCC GCA GCT GCG AAC TGT CTT ACA C-3′ and reverse primer 5′-GTG TAA GAC AGT TCG CAG CTG CGG CGT GAT CGT TGC ACC TGA C-3′, forward primer 5′-GTC AGG TGC AAC GAT CAC GCC CTA TGT TTG AAC TGT CTT ACA C-3′ and reverse primer 5′-GTG TAA GAC AGT TCA AAC ATA GGG CGT GAT CGT TGC ACC TGA C-3′, forward primer 5′-GTC AGG TGC AAC GAT CAC TAC GCA TGT TTG AAC TGT CTT ACA C-3′ and reverse primer 5′-G TGT AAG ACA GTT CAA ACA TGC GTA GTG ATC GTT GCA CCT GAC-3′, or forward primer 5′-GAT CAC TAC CTA TGT GCG AAC TGT CTT ACA CTT TTA C-3′ and reverse primer 5′-G TAA AAG TGT AAG ACA GTT CGC ACA TAG GTA GTG ATC-3′, respectively, and cloned into pCAGGS/MCS to produce pC-MopZ-AAAA-HA, pC-MopZ-ALCL-HA, pC-MopZ-YACL-HA, and pC-MopZ-YLCA-HA, respectively.
The Mopeia virus GP gene containing a FLAG tag at its C terminus was amplified by use of reverse transcription (RT)-PCR using a total RNA extract from Mopeia virus-infected Vero cells and forward primer 5′-GCGC GAGCTC ATG GGG CAG ATA GTC ACC TTC TTT CAA G-3′ and reverse primer 5′-GCGC CCCGGG TCA CTT GTC GTC ATC GTC TTT GTA GTC CCT TTT CCA TTG TGT GGG GAG ACC TGG TTG C-3′. The PCR product was cloned into the protein expression vector pCAGGS/MCS, generating pC-MopGP-FLAG.
A cDNA encoding FLAG-tagged AIP1 at the N terminus was PCR amplified by using forward primer 5′-GCGC GAATTC ATG GAC TAC AAA GAC GAT GAC GAC AAG GCG ACA TTC ATC TCG ATG CAG CTG-3′, reverse primer 5′-GCGC GAATTC TTA CTG CTG TGG ATA GTA AGA CTG CTG TGG-3′, and the previously reported plasmid AIP1-WT (kindly provided by Takemasa Sakaguchi, Hiroshima University, Japan) as a template (20). The PCR product was inserted into pCAGGS/MCS, and the resulting plasmid was designated pC-FLAG-AIP1. The expression plasmid for the dominant negative (DN) form of AIP1, AIP1DN, was kindly provided by Paul Ahlquist (University of Wisconsin—Madison) (48). The constructs encoding the AIP1 Bro1 (residues 1 to 358), V (residues 362 to 702), and proline-rich region (PRR) (residues 717 to 868) domains (16) with the FLAG tag fused to their N termini were generated by PCR using AIP1-WT as a template and forward primer 5′-GCGC GAATTC ATG GAC TAC AAA GAC GAT GAC GAC AAG GCG ACA TTC ATC TCG ATG CAG CTG-3′ and reverse primer 5′-GCGC GAATTC TTA ATC AGT AAA TTT CTG ACT GAT GGG TAC ATT GAC-3′, forward primer 5′-GCGC GAATTC ATG GAC TAC AAA GAC GAT GAC GAC AAG GTT CCC GTG TCA GTA CAG CAG-3′ and reverse primer 5′-GCGC CTCGAG TTA TGC AAA AAC TAT ATC ACT GCA TTT GTT CTG G-3′, and forward primer 5′-GCGC GAATTC ATG GAC TAC AAA GAC GAT GAC GAC AAG CGG AAG ACA GAA AGA GAT GAA CTC TTA AAG G-3′ and reverse primer 5′-GCGC GAATTC TTA CTG CTG TGG ATA GTA AGA CTG CTG TGG-3′, respectively. The constructs were subsequently inserted into pCAGGS/MCS; the resulting plasmids were designated pC-FLAG-AIP1_Bro, pC-FLAG-AIP1_V, and pC-FLAG-AIP1_PRR, respectively.
The coding regions of the generated constructs were verified by DNA sequencing.
Western blotting.
All transfections were performed by using TransIT LT-1 (Mirus, Madison, WI) reagent according to the supplier's instructions. Forty-eight hours posttransfection, 293T cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-Cl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, and protease inhibitor mixture [Roche, Branford, CT]). After incubation on ice for 15 min, lysates were clarified by centrifugation. Samples were then mixed with SDS-PAGE sample buffer, incubated at 100°C for 5 min, and subjected to a 4%-to-20% SDS-PAGE gradient gel (Bio-Rad Laboratories, Hercules, CA). The resolved proteins were then electrotransferred onto Western iBlot nitrocellulose membranes (Invitrogen, San Diego, CA) and subsequently blocked for 1 h at room temperature with 5% skim milk in PBS-T (phosphate-buffered saline [PBS] containing 0.05% Tween 20 [Sigma, St. Louis, MO]). Blots were then incubated in PBS-T containing 0.5% skim milk and the corresponding primary antibodies at room temperature for 1 h and then incubated with either an anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (Zymed, San Francisco, CA) or an anti-mouse HRP-conjugated secondary antibody (Pierce, Rockford, IL) at room temperature for 1 h. Blots were washed 3 times with PBS-T after each incubation. Protein bands were developed with a chemiluminescence reagent (Roche, Branford, CT) and then exposed on Kodak Biomax films.
VLP formation assay.
293T cells in 60-mm dishes were transfected with 1 μg of plasmid; pCAGGS/MCS was used to adjust the total DNA amount for transfection. Culture supernatants were collected at 48 h posttransfection, clarified, laid over a cushion of 20% sucrose in PBS, and ultracentrifuged at 50,000 rpm for 1 h at 4°C. The pellets were resuspended in 40 μl of STE buffer (0.01 M Tris-Cl [pH 7.5], 0.01 M NaCl, 0.001 M EDTA [pH 8.0]) overnight at 4°C. Subsequently, the samples were treated with 0.1 mg/ml trypsin (Worthington, Lakewood, NJ) for 30 min at room temperature and mixed with SDS-PAGE sample buffer, and the VLP composition was analyzed by use of Western blotting. The relative amount of protein in the VLPs was estimated based on the intensity of the protein bands. The VLP formation efficiency of Z was calculated as the amount of Z in the VLP sample divided by that in the lysates; the NP incorporation efficiency was calculated as the ratio of the amount of the NP protein relative to that of the Z protein detected in the VLPs. The experiments were performed in triplicate.
siRNA.
To deplete cells of AIP1, we designed three sets of oligonucleotides: (i) sense sequence 5′-GCCGCUGGUGAAGUUCAUCTT-3′ and antisense sequence 5′-GAUGAACUUCACCAGCGGCTT-3′, corresponding to nucleotides 57 to 75 of the AIP1 gene; (ii) sense sequence 5′-GAAGGAUGCUUUCGAUAAATT-3′ and antisense sequence 5′-UUUAUCGAAAGCAUCCUUCTT-3′, corresponding to nucleotides 285 to 303 of the AIP1 gene; and (iii) sense sequence 5′-CCUAGUGCUCCUUCAAUUCTT-3′ and antisense sequence 5′-GAAUUGAAGGAGCACUAGGTT-3′, corresponding to nucleotides 2164 to 2182 of the AIP1 gene. A BLAST search confirmed that these sequences were unique to AIP1. These oligonucleotides, synthesized by Qiagen (Germantown, MD), were pooled to generate a working stock. As a negative control, we used the AllStar small interfering RNA (siRNA) negative control (Qiagen, Germantown, MD).
On the day of the siRNA transfection, 293T cells were seeded into 60-mm dishes. siRNA duplexes were transfected into cells to a final concentration of 35 nM with HiPerFect transfection reagent (Qiagen, San Diego, CA) according to the manufacturer's protocol. After 24 h, the cells were retransfected with siRNAs as described above and incubated for another 24 h. The cells were then transfected with 1 μg of each of the viral constructs, and the VLP assay was performed 48 h later. AIP1 gene silencing was verified by using an anti-PDC6I antibody.
Immunoprecipitation.
To immunoprecipitate NP or Z with AIP1, 293T cells grown in 60-mm dishes were transfected with 3 μg of each of the plasmids, with pCAGGS/MCS being used to maintain the total amount of transfected DNA. Forty-eight hours later, cells were collected in PBS, pelleted at 3,500 rpm for 5 min, and lysed in RIPA buffer. Dynabeads protein G (50 μl; Invitrogen, San Diego, CA) was resuspended in RIPA buffer containing rabbit anti-HA antibody (Abcam, Cambridge, MA). After incubation with rotation for 5 min at room temperature, the buffer was removed, and the Dynabeads were washed with RIPA buffer. The Dynabeads-antibody complexes were then combined with the lysates, and the mixtures were incubated with rotation for 5 min at room temperature. The Dynabeads were then washed 4 times in RIPA buffer and resuspended in SDS-PAGE sample buffer. After being boiled for 5 min, the mixtures were centrifuged to pellet the beads. The supernatants were resolved on SDS-PAGE gels and subjected to Western analysis with mouse anti-HA (Covance, Princeton, NJ) and mouse anti-FLAG (Sigma, St. Louis, MO) antibodies.
To cross-link protein complexes, the transfected 293T cells were washed with PBS to remove the medium and then resuspended in fresh PBS. Dithiobis(succinimidyl propionate) (DSP) (Thermo Fisher Scientific, Waltham, MA), dissolved in dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO), was added to the cells to a final concentration of 1 mM. After incubation at room temperature for 30 min, the cells were pelleted, lysed in RIPA buffer, and subjected to immunoprecipitation followed by Western analysis, as described above.
Confocal immunofluorescence.
Vero cells grown on glass coverslips in 35-mm dishes were transfected with 0.25 μg of plasmids. Twenty-four hours later, the cells were fixed in 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 in PBS for 5 min, and blocked with 10% goat serum in PBS. The samples were then incubated with primary antibodies for 1 h and subsequently incubated with Alexa Fluor 488-conjugated anti-mouse, Alexa Fluor 546-conjugated anti-rabbit, and Alexa Fluor 633-conjugated anti-chicken secondary antibodies (Molecular Probes, Eugene, OR). Images were obtained by using a Zeiss LSM 510 laser scanning confocal microscope.
RESULTS
The Mopeia virus Z and NP proteins weakly interact.
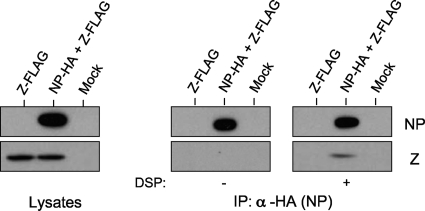
We previously reported that the association between the Mopeia virus Z and NP proteins was required for NP incorporation into Z-induced VLPs, although the two proteins were not coimmunoprecipitated (43). However, their interaction may be too weak to be detected by a regular coimmunoprecipitation assay. To test this possibility, we treated lysates of cells expressing the Z and NP proteins with a membrane-permeable protein cross-linker, DSP, to enhance the interaction between NP and Z (if any) and then performed an immunoprecipitation assay. DSP is thiol cleavable, and therefore, cross-linked protein complexes are cleaved upon β-mercaptoethanol treatment. As shown in Fig. 1, DSP treatment resulted in the Z protein coprecipitating with NP, suggesting a weak interaction between the viral proteins.
FIG. 1.
The Mopeia virus Z and NP proteins weakly interact. pC-MopZ-FLAG, pC-MopNP-HA/pC-MopZ-FLAG, or pCAGGS was transfected into 293T cells. Forty-eight hours later, the lysates were treated with DSP or DMSO for 30 min and then mixed with Dynabeads protein G preincubated with an anti-HA antibody. The immunoprecipitated (IP) proteins were detected by using Western blot analysis with anti-HA and anti-FLAG antibodies.
The Mopeia virus Z protein YLCL motif is required for NP incorporation into Z-induced VLPs.
Several enveloped RNA viruses use PT/SAP, PPXY, and YXXL sequences in their matrix proteins to recruit cellular TSG101, NEDD4-like, and AIP1 proteins, respectively, for efficient VLP release (8, 9, 15, 17, 20, 22, 28, 29, 34, 38, 49). The sequence of the Mopeia virus matrix Z protein contains conserved arenavirus PTAP, PPPY, and YLCL motifs (Fig. 2A). The YLCL motif could potentially interact with AIP1, which was also demonstrated to bind viral nucleocapsid proteins (31, 37) and to regulate the transport of nucleocapsids from endosomes to the cytosol (25). To test whether this YLCL motif plays a role in VLP release or NP incorporation into VLPs, we generated a Z mutant in which YLCL was changed to AAAA (Fig. 2A). To compare the stability between wild-type Z and Z-AAAA, we replaced the glycine residues at position 2 with alanines, generating Z-G2A and Z-AAAA/G2A, respectively. Because arenavirus VLP release requires an intact glycine at this position (35), Z-G2A and Z-AAAA/G2A could not induce the formation of VLPs and, therefore, were confined to the host cell. This modification of the Z protein is necessary to properly evaluate the stability of the mutant protein; otherwise, intracellular Z is lost due to budding. The constructs were individually transfected into 293T cells, and after 16, 32, and 48 h, cellular lysates were examined for Z protein expression. As shown in Fig. 2B, Z-G2A and Z-AAAA/G2A were expressed at similar levels, prompting us to conclude that Z-AAAA was stable.
FIG. 2.
The Mopeia virus Z protein YLCL motif is required for NP incorporation into Z-induced VLPs. (A) Alignment of the following arenavirus Z protein sequences showing the conserved YLCL region (the corresponding NCBI accession numbers are shown in parentheses): wild-type Mopeia virus (WT MOPV) Z (accession number AAV54106), mutant Mopeia virus (Mut MOPV) Z containing alanine substitutions at amino acids 53 to 56, Lassa virus (LASV) Z (accession number NP_694871), lymphocytic choriomeningitis virus (LCMV) Z (accession number P18541), Lujo virus (LUJV) Z (accession number YP_002929492), Junin virus (JUNV) Z (accession number Q6IVU5), Machupo virus (MACV) Z (accession number AAY27823), Guanarito virus (GTOV) Z (accession number NP_899220), Chapare virus (CV) Z (accession number YP_001816784), and Tacaribe virus (TCRV) Z (accession number Q88470). The numbers indicate the numbers of amino acids within the respective Z proteins. (B) Comparison of the stability between wild-type Z and Z-AAAA. We replaced the glycine residues at position 2 with alanines, generating Z-G2A and Z-AAAA/G2A, respectively. The constructs were individually transfected into 293T cells. After 16, 32, and 48 h, cellular lysates were examined for Z protein expression by use of Western blotting with an anti-HA antibody. An equal number of cells in the samples was confirmed by using Western blotting with an anti-actin antibody. (C) pC-MopZ-HA or pC-MopZ-AAAA-HA was individually transfected into 293T cells. VLP isolation and analysis by use of Western blotting with an anti-HA antibody were performed 48 h after transfection. The VLP formation efficiency of Z was calculated as the amount of Z in the VLPs divided by that in the lysates. The budding efficiency for wild-type Z was set at 1, and the efficiency for mutant Z is reported relative to the wild-type result. Three independent experiments were performed, and standard deviations were calculated. Representative data are shown. (D) 293T cells were cotransfected with plasmids encoding pC-MopNP-FLAG and either the wild type or Z-AAAA. After 48 h, VLPs were isolated and analyzed by Western blotting with anti-FLAG and anti-HA antibodies. The VLP formation efficiency of Z was calculated as described above (C). The NP incorporation efficiency was calculated as the ratio of the amount of the NP protein relative to that of the Z protein detected in the VLPs. The averages of data from three independent experiments are shown. (E) pC-MopZ-HA, pC-MopZ-AAAA-HA, pC-MopZ-ALCL-HA, pC-MopZ-YACL-HA, or pC-MopZ-YLCA-HA was cotransfected with pC-MopNP-FLAG into 293T cells. VLP isolation and analysis were performed as described above (D).
We next transfected a plasmid expressing either the wild type or the Z-AAAA protein, either individually or in combination with a plasmid expressing NP, into 293T cells and examined the efficiency of VLP production. All VLP samples were treated with trypsin prior to Western blotting to ensure that the mutant Z protein detected was inside the lipid-enveloped particles. We found that the mutant Z protein promoted VLP formation when expressed alone (Fig. 2C) and that its budding efficiency was not affected by the presence of NP (Fig. 2D). The mutant Z protein facilitated VLP formation approximately 15 times more efficiently than did wild-type Z (Fig. 2C and D); however, the amount of mutant Z in the cell lysate was lower than that of the wild type, probably due to the efficient release of the former (Fig. 2B). The mutant Z protein, however, failed to support NP VLP incorporation (Fig. 2D). These results indicate that the Mopeia virus Z protein YLCL motif is dispensable for VLP formation but is required for NP incorporation into the VLPs.
The cysteine residue within the arenavirus YLCL motif is a part of the RING domain of the Z protein (10). The perturbation of this domain therefore could affect the overall structure of the Z protein, eliminating NP recruitment into VLPs. To address this possibility, we generated Z-ALCL, Z-YACL, and Z-YLCA mutant constructs and examined them for their ability to support NP incorporation. We found that although the mutants differed in the extent to which they supported VLP release, none of them could incorporate NP (Fig. 2E). These data suggest that the perturbation of the zinc finger is an unlikely explanation for the absence of NP incorporation into mutant VLPs.
We also tested whether the wild-type Z or Z-AAAA protein could interact with the Mopeia virus glycoprotein GP during VLP formation. The coexpression of GP with the Z proteins proved to be toxic to the cells, making VLP production inefficient and analysis difficult (data not shown).
AIP1 is required for NP incorporation into VLPs.
To assess a role for AIP1 in Mopeia virus VLP formation, we examined the effect of depleting cellular AIP1 on the efficiency of VLP formation and NP incorporation. As shown in Fig. 3A, NP VLP incorporation was reduced by approximately two-thirds upon the treatment of cells with siRNA specific to AIP1 but not with a nonspecific siRNA, although VLP formation was not affected. We also tested the effect of a dominant negative (DN) form of AIP1 (48) on the efficiency of VLP formation and NP incorporation into VLPs. The DN construct lacks the proline-rich region that is located at the C terminus and, therefore, does not associate with TSG101, a component of the ESCRT-1 complex (6, 16, 48). As shown in Fig. 3B, DN AIP1 significantly inhibited NP VLP incorporation in a dose-dependent manner but had no effect on VLP release. These data suggest that AIP1 is involved in NP incorporation into Z-induced VLPs.
FIG. 3.
AIP1 is required for the incorporation of Mopeia virus NP into VLPs. (A) 293T cells were treated with siRNA for AIP1 or AllStar (nonspecific) siRNA or were left untreated. After 24 h, the treatment was repeated, and after another 24 h, the cells were cotransfected with pC-MopZ-HA and pC-MopNP-FLAG. VLP isolation and analysis with anti-HA and anti-FLAG antibodies by Western blotting were performed 48 h posttransfection with plasmids. The AIP1 silencing in the cell lysates was assessed with an anti-PDC6I (i.e., anti-AIP1) antibody, and an equal number of cells in each sample was confirmed with an anti-actin antibody. The VLP formation efficiencies were determined as described in the legend of Fig. 1C. The NP incorporation efficiencies were calculated as the amount of NP divided by that of Z in the VLP sample. The incorporation efficiencies for the NPs in the siRNA-treated samples are reported relative to the results for the untreated sample. The estimates of the VLP formation and NP incorporation efficiencies are averages of data from three independent experiments, and standard deviations are reported. (B) 293T cells were cotransfected with plasmids expressing Z and NP and increasing concentrations of DN AIP1. Forty-eight hours later, VLPs were subjected to ultracentrifugation, and their compositions were examined by Western blotting with anti-HA and anti-FLAG antibodies. The expression of dominant negative AIP1 was verified with an anti-PDC6I (i.e., anti-AIP1) antibody. The NP incorporation efficiencies were estimated as described above (A). (C) pC-MopZ-HA, pC-MopNP-FLAG, and increasing amounts of pC-FLAG-AIP1 were cotransfected into 293T cells. After 48 h, VLPs were pelleted and examined by Western blotting as described above (A).
We next tested the effect of wild-type AIP1 overexpression on Mopeia virus VLP formation. For several retroviruses whose budding depends on functional AIP1, the overexpression of wild-type AIP1 affects virion release (13, 41). While HIV-1 VLP formation is inhibited in the presence of coexpressed AIP1 (13), the VLP formation of murine leukemia virus (MLV) is enhanced by coexpression with wild-type AIP1 (41). Our results suggest that the overexpression of AIP1 stimulated NP incorporation into Mopeia virus VLPs, although the VLP release itself was not affected (Fig. 3C). These data further support a role for AIP1 in NP incorporation into VLPs.
NP and Z interact with AIP1.
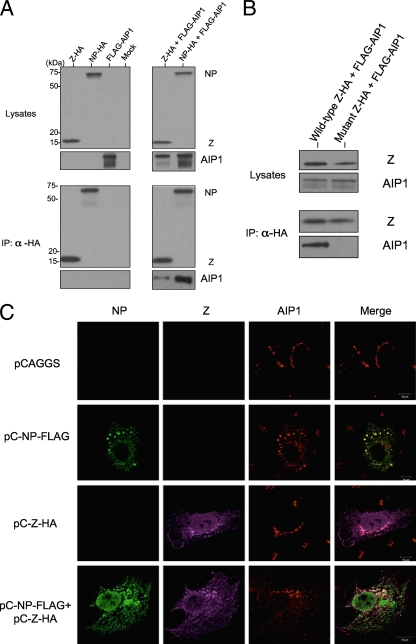
Our findings that AIP1 is required for NP incorporation into VLPs prompted us to ask whether AIP1 interacts with NP, Z, or both. To answer this question, we performed coimmunoprecipitation assays. We separately transfected each plasmid expressing either FLAG-AIP1, NP-HA, or Z-HA or cotransfected plasmids expressing FLAG-AIP1 and NP-HA, or FLAG-AIP1 and Z-HA. As shown in Fig. 4A, both NP and Z coprecipitated AIP1, with more AIP1 being coimmunoprecipitated with NP than with Z. The overexpression of AIP1 resulted in the detection of two protein species, where the minor band is likely to be a degradation product (Fig. 4A).
FIG. 4.
Mopeia virus Z and NP interact with AIP1. (A) 293T cells were transfected with pC-MopZ-HA, pC-MopNP-HA, pC-FLAG-AIP1, pC-MopZ-HA/pC-FLAG-AIP1, or pC-MopNP-HA/pC-FLAG-AIP1. Forty-eight hours later, cell lysates were mixed with Dynabeads protein G preincubated with the anti-HA antibody. The immunoprecipitated proteins were detected by Western blot analysis with anti-HA and anti-FLAG antibodies. (B) 293T cell lysates cotransfected with pC-FLAG-AIP1 and either pC-MopZ-HA or pC-MopZ-AAAA-HA were immunoprecipitated with anti-HA and analyzed 48 h after transfection as described above (A). (C) pC-MopNP-FLAG and pC-MopZ-HA were cotransfected into Vero cells. Twenty-four hours later, cells were fixed, permeabilized, and incubated with anti-FLAG (green, Alexa Fluor 488), anti-HA (magenta, Alexa Fluor 633), and anti-PDC6I (AIP1) (red, Alexa Fluor 546) antibodies. Confocal immunofluorescence microscopy was used to examine NP, Z, and AIP1 fluorescences separately; the images were then merged to assess colocalization.
Figure 2D shows that the mutation within the Z YLCL motif abolished the ability of Z to support NP incorporation into virions. To investigate the relationship among Z, NP, and AIP1 in terms of NP incorporation into Z-induced VLPs, we next examined the effect of the mutation within the Z YLCL motif on the interaction between Z and AIP1 by using coimmunoprecipitation. We cotransfected plasmids expressing FLAG-AIP1 and either wild-type or mutant Z-HA into 293T cells. As shown in Fig. 4B, the wild-type, but not the mutant, Z protein coprecipitated AIP1, suggesting that the lack of the Z-AIP1 interaction may affect NP incorporation.
Next, we examined the colocalization of NP, Z, and endogenous AIP1 in Vero cells by immunofluorescence microscopy. As shown in Fig. 4C, AIP1 was found predominantly in a perinuclear region. Interestingly, upon NP expression, AIP1 redistributed from the perinuclear region into NP-positive clusters. In contrast, in the presence of the Z protein, AIP1 colocalized with NP and Z only in the perinuclear region and not in the other NP-positive compartments (Fig. 4C), suggesting an association of AIP1 with both Z and NP.
The Bro1 domain of AIP1 binds NP and Z.
Crystal structure analysis has identified three domains within the AIP1 protein: the N-terminal Bro1 domain (residues 1 to 358), the central V domain (residues 362 to 702), and the C-terminal proline-rich region (PRR) (residues 717 to 868) (50). To investigate which of these regions interacts with the Mopeia virus Z and NP proteins, we generated constructs encoding the AIP1 domains with a FLAG tag fused to their N termini (Fig. 5A). Each construct was coexpressed with plasmids expressing either Z or NP in 293T cells, and each domain was examined for its ability to coimmunoprecipitate with Z or NP. We found that both NP and Z pulled down the Bro1 domain but not the V or PRR domain, as shown in Fig. 5B and C, respectively, suggesting that the Bro1 domain of AIP1 is sufficient for the interaction with both viral proteins.
FIG. 5.
The Bro1 domain of AIP1 binds to Z and NP. (A) Schematic representation of wild-type AIP1 and the constructs encoding the AIP1 domains used in this study. The numbers indicate the positions of the domains within wild-type AIP1. Each construct was fused with a FLAG tag at its N terminus. Bro, Bro1 domain; V, “V” domain; PRR, proline-rich region. (B and C) pC-MopNP-HA (B) or pC-MopZ-HA (C) was cotransfected with pC-FLAG-AIP1_Bro, pC-FLAG-AIP1_V, or pC-FLAG-AIP1_PRR into 293T cells. After 48 h, proteins were precipitated with Dynabeads/anti-HA antibody complexes and analyzed by Western blotting with anti-HA and anti-FLAG antibodies.
Overexpression of the Bro1 domain facilitates the Z-NP interaction.
To verify whether the overexpression of AIP1 or the Bro1 domain could facilitate the Z-NP interaction, we cotransfected plasmids expressing NP-HA/Z-FLAG, NP-HA/Z-FLAG/FLAG-AIP1, or NP-HA/Z-FLAG/FLAG-Bro1. The lysates were prepared and immunoprecipitated with an anti-HA antibody, and the precipitated proteins were subjected to SDS-PAGE followed by Western blotting with an anti-FLAG antibody. As shown in Fig. 6, no coprecipitation of Z was detected in the absence of wild-type AIP1 or the Bro1 domain, which is in agreement with data from our previous report (43). The overexpression of AIP1 or the Bro1 domain, however, resulted in the precipitation of Z, suggesting that AIP1, through its Bro1 domain, facilitates the Z-NP interaction (Fig. 6, right, DSP −). Since the endogenous AIP1 was not detected by coimmunoprecipitation with NP (data not shown), this interaction might be weak (if any). Thus, we next treated cells with the cross-linker DSP to enhance the interaction between NP and Z through AIP1 (if any) and performed immunoprecipitation under the conditions described above. DSP treatment led to the detection of the interaction between Z and NP even without the overexpression of AIP1 or the Bro1 domain and enhanced this interaction when AIP1 or the Bro1 domain was overexpressed (Fig. 6, right, DSP +).
FIG. 6.
The Bro1 domain facilitates the Z-NP interaction. pC-MopNP-HA/pC-MopZ-FLAG/pC-FLAG-AIP1 or pC-MopNP-HA/pC-MopZ-FLAG/pC-FLAG-AIP1_Bro was cotransfected into 293T cells. After 48 h, the lysates were treated with DSP or DMSO for 30 min. The immunoprecipitated proteins were analyzed by Western blotting with anti-HA and anti-FLAG antibodies.
NP interacts with AIP1 through residues 342 to 399.
To determine which NP region(s) interacts with AIP1, we created a series of NP deletion mutants containing an HA tag at their C terminus and examined their ability to coimmunoprecipitate AIP1 from lysates of 293T cells coexpressing AIP1 and the individual NP deletion mutant proteins. We found that all mutants, except for NPΔ342-399, precipitated AIP1 (Fig. 7A), suggesting that residues 342 to 399 of NP are important for the interaction between NP and AIP1.
FIG. 7.
The NP protein interacts with AIP1 through residues 342 to 399. (A) 293T cells were cotransfected with plasmids encoding FLAG-AIP1 and either wild-type NP or an NP deletion mutant. After 48 h, the proteins precipitated with an anti-HA antibody were analyzed by Western blotting with anti-HA and anti-FLAG antibodies. (B) Alignment of amino acids 342 to 399 of the Mopeia virus NP protein with several arenavirus NP protein sequences as follows (the corresponding NCBI accession numbers for the sequences are shown in parentheses): Mopeia virus (MOPV) NP (accession number YP_170710), Lassa virus (LASV) NP (accession number AAT49003), lymphocytic choriomeningitis virus (LCMV) NP (accession number AAA46257), Lujo virus (LUJV) NP (accession number YP_002929491), Junin virus (JUNV) NP (accession number P14239), Machupo virus (MACV) NP (accession number AY571959), Guanarito virus (GTOV) NP (accession number AF485258), Chapare virus (CV) NP (accession number YP_001816783), and Tacaribe virus (TCRV) NP (accession number AAA47903). The numbers indicate the terminal residues of the respective NP sequences. Highly conserved amino acids are shown in boldface type.
Well-characterized AIP1-interacting domains within viral proteins include the tyrosine-based YXXL and LYPX(n)LXXL motifs and zinc fingers (8, 11, 13, 16, 20, 36-38, 44, 50). To verify whether such motifs are present in the region comprising amino acids 342 to 399 of Mopeia virus NP and to test the conservation of this region among arenaviruses, we aligned the Mopeia virus sequence with those of several arenavirus NP proteins. As shown in Fig. 7B, despite the presence of highly conserved residues, mostly at the carboxyl end of the region, there are no tyrosine-based motifs or zinc fingers within the region.
DISCUSSION
The arenavirus matrix protein Z plays a pivotal role in virion formation and viral nucleocapsid incorporation into virions (7, 34, 43, 45, 47). In this study, we determined that the ESCRT-associated protein AIP1 is critical for the incorporation of Mopeia virus NP into Z-induced VLPs. We also found that the Bro1 domain of AIP1 was sufficient to interact with both Z and NP. Moreover, we found that residues 342 to 399 of NP and the YLCL motif (residues 53 to 56) of Z were essential for the NP-AIP1 and Z-AIP1 interactions, respectively.
For several enveloped viruses, the viral matrix proteins are known to use the cellular ESCRT pathway via their late domains to facilitate virus particle formation (6, 9, 11, 17, 20-22, 29, 30, 34, 38, 45, 46, 48, 49). Recently, a role for the viral nucleocapsid proteins, in cooperation with the matrix proteins and the ESCRT pathway, has been suggested for Marburg virus and HIV-1 (12, 13). Analysis of Marburg virus NP revealed several putative late domains, one of which recruits TSG101 to the budding sites, resulting in enhanced VLP formation (12). Similarly, the zinc finger of the HIV-1 nucleocapsid domain of Gag has been shown to bind AIP1, cooperating with the late domains to employ the ESCRT pathway for viral budding (13, 37). Here, we demonstrated that Mopeia virus uses an ESCRT-associated protein, AIP1, for NP incorporation into virions; the use of the ESCRT pathway for NP incorporation into virions has not been shown for any other virus. We also identified residues 342 to 399 of the Mopeia virus NP protein as being necessary to interact with AIP1, although this region does not contain YXXL or zinc finger motifs (Fig. 7B). It is therefore possible that this NP region contains a novel AIP1-interacting motif. It is also possible that the deletion of these amino acids simply disrupted the overall structural integrity of the protein, resulting in its failure to interact with AIP1.
We showed that all of our NP deletion mutants, with the exception of NPΔ342-399, interacted with AIP1 (Fig. 7A), although our previous study suggested that the region important for NP incorporation into Z-induced VLPs extends to the whole C-terminal half of NP (residues 342 to 570) (43). Because the deletion of NP residues 400 to 570 did not affect the association of the mutants with Z or their recruitment to the membranes, it is possible that additional cellular factors may associate with NP through these residues for efficient NP virion incorporation, perhaps at the budding sites. A more detailed analysis of the association of Z and NP with the components of the MVB pathway and its associated proteins will potentially reveal the nature of such cellular determinants.
We demonstrated that the Mopeia virus Z protein interacts with AIP1 (Fig. 4A) and that this interaction depends on the Z protein YLCL motif (Fig. 4B), the putative AIP1-interacting late domain. However, Z-induced VLP formation did not appear to depend on AIP1 or the YLCL motif, in agreement with data from previous reports (19, 46, 47). Our data suggest that a mutation of this motif results in an altered intracellular distribution, where mutant Z cannot localize to the perinuclear region of the cell (Fig. 8). Because Z associates with AIP1 primarily in regions adjacent to the cell nucleus (Fig. 4C), it is possible that the mutation within the motif results in Z's inability to traffic to AIP1-containing sites and therefore in its inability to recruit NP into VLPs.
FIG. 8.
Mutation of the Mopeia virus Z YLCL motif affects the intracellular distribution of Z. Vero cells were transfected with either pC-MopZ-HA or pC-MopZ-AAAA-HA. Twenty-four hours later, cells were fixed and stained with an anti-HA antibody (green, Alexa Flour 488). The images were obtained by using confocal immunofluorescence.
The AIP1 protein has three distinct regions: the Bro1 domain (residues 1 to 358), the V domain (residues 362 to 702), and the proline-rich region (PRR) (residues 717 to 868) (16). The PRR interacts with the cellular ESCRT-I-associated TSG101 and also with endophilins (16, 50). The V domain mediates the interaction with the YPXnL late domains of HIV-1 p6Gag and equine infectious anemia virus (EIAV) p9Gag, which is necessary for VLP release (8, 16, 18, 28). The N-terminal elongated, banana-shaped Bro1 domain interacts with the ESCRT-III complex-associated CHMP4 and binds various viral proteins to facilitate virion formation (13, 20, 37, 39). We found that both the Mopeia virus Z and NP proteins interacted with the Bro1 domain, prompting us to propose that these two viral proteins are linked through this domain during assembly, similarly to the Sendai virus matrix M and accessory C proteins (20, 39).
In their recent study, Groseth et al. demonstrated that the Tacaribe virus Z YLCL motif is required for the recruitment of NP into VLPs and for the NP-mediated enhancement of Tacaribe virus VLP release, suggesting that this motif is important for the interaction with NP. Those authors speculated that the YLCL motif may play a direct role in the recognition of or interaction with NP (19). Our work takes the research on arenavirus VLP formation one step further by implicating AIP1 in arenavirus NP incorporation. Our data suggest that Mopeia virus NP binds AIP1 in the cytosol (Fig. 4A and C). Subsequently, the NP-AIP1 complex is recruited to the budding sites through the interaction between the Mopeia virus Z YLCL motif and AIP1 (Fig. 4A to C). It is important to note that the weak Z-AIP1 interaction (Fig. 4A) does not appear to be sufficient for recruitment. The dominant negative form of AIP1, which interacts with both NP and Z but not with TSG101, significantly inhibits NP incorporation, suggesting that the TSG101-AIP1 interaction is also essential for NP incorporation. Once the NP-AIP1-Z-TSG101 complex is assembled, NP is incorporated into nascent viral particles.
In summary, we have demonstrated that the ESCRT-associated protein AIP1 interacts with the Mopeia virus NP and Z proteins and is required for NP incorporation into Z-induced VLPs, suggesting that AIP1 may couple virus nucleocapsids to Z and the budding apparatus. A more detailed analysis of the interplay between Z, NP, and the components of the MVB pathway will advance our understanding of the mechanism of incorporation of arenavirus nucleocapsids into virions.
Acknowledgments
We thank Susan Watson for editing the manuscript and Krisna Wells, Martha McGregor, and Kelly Moore for excellent technical assistance. We also thank Takemasa Sakaguchi for kindly providing us with the wild-type AIP1 construct and Paul Ahlquist for providing us with the dominant negative form of AIP1.
This work was supported by a U.S. National Institutes of Health and National Institute of Allergy and Infectious Diseases Public Health Service grant.
Footnotes
Published ahead of print on 19 January 2011.
REFERENCES
- 1.Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo, and S. D. Emr. 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell 3:271-282. [DOI] [PubMed] [Google Scholar]
- 2.Babst, M., D. J. Katzmann, W. B. Snyder, B. Wendland, and S. D. Emr. 2002. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3:283-289. [DOI] [PubMed] [Google Scholar]
- 3.Beyer, W. R., D. Popplau, W. Garten, D. von Laer, and O. Lenz. 2003. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 77:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borden, K. L., E. J. C. Dwyer, and M. S. Salvato. 1998. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J. Virol. 72:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., and M. B. Oldstone. 1992. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J. Virol. 66:7270-7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlton, J. G., and J. Martin-Serrano. 2007. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316:1908-1912. [DOI] [PubMed] [Google Scholar]
- 7.Casabona, J. C., J. M. L. Macleod, M. E. Loureiro, G. A. Gomez, and N. Lopez. 2009. The RING domain and the L79 residue of Z protein are involved in both the rescue of nucleocapsids and the incorporation of glycoproteins into infectious chimeric arenavirus-like particles. J. Virol. 83:7029-7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C., O. Vincent, J. Jin, O. A. Weisz, and R. C. Montelaro. 2005. Functions of early (AP-2) and late (AIP1/ALIX) endocytic proteins in equine infectious anemia virus budding. J. Biol. Chem. 280:40474-40480. [DOI] [PubMed] [Google Scholar]
- 9.Ciancanelli, M. J., and C. F. Basler. 2006. Mutation of YMYL in the Nipah virus matrix protein abrogates budding and alters subcellular localization. J. Virol. 80:12070-12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornu, T. I., and J. C. de la Torre. 2001. RING finger Z protein of lymphocytic choriomeningitis virus (LCMV) inhibits transcription and RNA replication of an LCMV S-segment minigenome. J. Virol. 75:9415-9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demirov, D. G., and E. O. Freed. 2004. Retrovirus budding. Virus Res. 106:87-102. [DOI] [PubMed] [Google Scholar]
- 12.Dolnik, O., L. Kolesnikova, L. Stevermann, and S. Becker. 2010. Tsg101 is recruited by a late domain of the nucleocapsid protein to support budding of Marburg virus-like particles. J. Virol. 84:7847-7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dussupt, V., et al. 2009. The nucleocapsid region of HIV-1 Gag cooperates with the PTAP and LYPXnL late domains to recruit the cellular machinery necessary for viral budding. PLoS Pathog. 5:e1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwyer, E. J. C., H. Lai, R. C. MacDonald, M. S. Salvato, and K. L. Borden. 2000. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner. J. Virol. 74:3293-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichler, R., et al. 2004. Characterization of the Lassa virus matrix protein Z: electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP). Virus Res. 100:249-255. [DOI] [PubMed] [Google Scholar]
- 16.Fisher, R. D., et al. 2007. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell 128:841-852. [DOI] [PubMed] [Google Scholar]
- 17.Freed, E. O. 2002. Viral late domains. J. Virol. 76:4679-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geminard, C., A. De Gassart, L. Blanc, and M. Vidal. 2004. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic 5:181-193. [DOI] [PubMed] [Google Scholar]
- 19.Groseth, A., S. Wolff, T. Strecker, T. Hoenen, and S. Becker. 2010. Efficient budding of the Tacaribe virus matrix protein Z requires the nucleoprotein. J. Virol. 84:3603-3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irie, T., Y. Shimazu, T. Yoshida, and T. Sakaguchi. 2007. The YLDL sequence within Sendai virus M protein is critical for budding of virus-like particles and interacts with Alix/AIP1 independently of C protein. J. Virol. 81:2263-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jasenosky, L. D., G. Neumann, I. Lukashevich, and Y. Kawaoka. 2001. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J. Virol. 75:5205-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayakar, H. R., K. G. Murti, and M. A. Whitt. 2000. Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release. J. Virol. 74:9818-9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzmann, D. J., M. Babst, and S. D. Emr. 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145-155. [DOI] [PubMed] [Google Scholar]
- 24.Kobasa, D., M. E. Rodgers, K. Wells, and Y. Kawaoka. 1997. Neuraminidase hemadsorption activity, conserved in avian influenza A viruses, does not influence viral replication in ducks. J. Virol. 71:6706-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Blanc, I., et al. 2005. Endosome-to-cytosol transport of viral nucleocapsids. Nat. Cell Biol. 7:653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, K. J., I. S. Novella, M. N. Teng, M. B. Oldstone, and J. C. de La Torre. 2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 74:3470-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahul-Mellier, A. L., F. J. Hemming, B. Blot, S. Fraboulet, and R. Sadoul. 2006. Alix, making a link between apoptosis-linked gene-2, the endosomal sorting complexes required for transport, and neuronal death in vivo. J. Neurosci. 26:542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Serrano, J., A. Yarovoy, D. Perez-Caballero, and P. D. Bieniasz. 2003. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. U. S. A. 100:12414-12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 30.Munshi, U. M., J. Kim, K. Nagashima, J. H. Hurley, and E. O. Freed. 2007. An Alix fragment potently inhibits HIV-1 budding: characterization of binding to retroviral YPXL late domains. J. Biol. Chem. 282:3847-3855. [DOI] [PubMed] [Google Scholar]
- 31.Nishio, M., M. Tsurudome, H. Ishihara, M. Ito, and Y. Ito. 2007. The conserved carboxyl terminus of human parainfluenza virus type 2 V protein plays an important role in virus growth. Virology 362:85-98. [DOI] [PubMed] [Google Scholar]
- 32.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 33.Oldstone, M. B. 2002. Arenaviruses. I. The epidemiology molecular and cell biology of arenaviruses. Introduction. Curr. Top. Microbiol. Immunol. 262:V-XII. [PubMed] [Google Scholar]
- 34.Perez, M., R. C. Craven, and J. C. de la Torre. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc. Natl. Acad. Sci. U. S. A. 100:12978-12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez, M., D. L. Greenwald, and J. C. de la Torre. 2004. Myristoylation of the RING finger Z protein is essential for arenavirus budding. J. Virol. 78:11443-11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pires, R., et al. 2009. A crescent-shaped ALIX dimer targets ESCRT-III CHMP4 filaments. Structure 17:843-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popov, S., E. Popova, M. Inoue, and H. G. Gottlinger. 2008. Human immunodeficiency virus type 1 Gag engages the Bro1 domain of ALIX/AIP1 through the nucleocapsid. J. Virol. 82:1389-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puffer, B. A., L. J. Parent, J. W. Wills, and R. C. Montelaro. 1997. Equine infectious anemia virus utilizes a YXXL motif within the late assembly domain of the Gag p9 protein. J. Virol. 71:6541-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi, T., et al. 2005. AIP1/Alix is a binding partner of Sendai virus C protein and facilitates virus budding. J. Virol. 79:8933-8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salvato, M. S., K. J. Schweighofer, J. Burns, and E. M. Shimomaye. 1992. Biochemical and immunological evidence that the 11 kDa zinc-binding protein of lymphocytic choriomeningitis virus is a structural component of the virus. Virus Res. 22:185-198. [DOI] [PubMed] [Google Scholar]
- 41.Segura-Morales, C., et al. 2005. Tsg101 and Alix interact with murine leukemia virus Gag and cooperate with Nedd4 ubiquitin ligases during budding. J. Biol. Chem. 280:27004-27012. [DOI] [PubMed] [Google Scholar]
- 42.Sette, P., J. A. Jadwin, V. Dussupt, N. F. Bello, and F. Bouamr. 2010. The ESCRT-associated protein Alix recruits the ubiquitin ligase Nedd4-1 to facilitate HIV-1 release through the LYPXnL L domain motif. J. Virol. 84:8181-8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shtanko, O., et al. 2010. A role for the C terminus of Mopeia virus nucleoprotein in its incorporation into Z protein-induced virus-like particles. J. Virol. 84:5415-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strack, B., A. Calistri, S. Craig, E. Popova, and H. G. Gottlinger. 2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114:689-699. [DOI] [PubMed] [Google Scholar]
- 45.Strecker, T., et al. 2003. Lassa virus Z protein is a matrix protein sufficient for the release of virus-like particles. J. Virol. 77:10700-10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urata, S., T. Noda, Y. Kawaoka, H. Yokosawa, and J. Yasuda. 2006. Cellular factors required for Lassa virus budding. J. Virol. 80:4191-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urata, S., J. Yasuda, and J. C. de la Torre. 2009. The Z protein of the New World arenavirus Tacaribe virus has bona fide budding activity that does not depend on known late domain motifs. J. Virol. 83:12651-12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe, T., et al. 2007. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc. Natl. Acad. Sci. U. S. A. 104:10205-10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wills, J. W., et al. 1994. An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J. Virol. 68:6605-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhai, Q., et al. 2008. Structural and functional studies of ALIX interactions with YPX(n)L late domains of HIV-1 and EIAV. Nat. Struct. Mol. Biol. 15:43-49. [DOI] [PubMed] [Google Scholar]