Abstract
Nuclear factor κB (NF-κB) transcription factors are involved in controlling numerous cellular processes, including inflammation, innate and adaptive immunity, and cell survival. Here we show that the immunosuppressive measles virus (MV; Morbillivirus genus, Paramyxoviridae) has evolved multiple functions to interfere with canonical NF-κB signaling in epithelial cells. The MV P, V, and C proteins, also involved in preventing host cell interferon responses, were found to individually suppress NF-κB-dependent reporter gene expression in response to activation of the tumor necrosis factor (TNF) receptor, RIG-I-like receptors, or Toll-like receptors. NF-κB activity was most efficiently suppressed in the presence of V, while expression of P or C resulted in moderate inhibition. As indicated by reporter gene assays involving overexpression of the IκB kinase (IKK) complex, which phosphorylates the inhibitor of κB to liberate NF-κB, V protein targets a downstream step in the signaling cascade. Coimmunoprecipitation experiments revealed that V specifically binds to the Rel homology domain of the NF-κB subunit p65 but not of p50. Notably, the short C-terminal domain of the V protein, which is also involved in binding STAT2, IRF7, and MDA5, was sufficient for the interaction and for preventing reporter gene activity. As observed by confocal microscopy, the presence of V abolished nuclear translocation of p65 upon TNF-α stimulation. Thus, MV V appears to prevent NF-κB-dependent gene expression by retaining p65 in the cytoplasm. These findings reveal NF-κB as a key target of MV and stress the importance of the V protein as the major viral immune-modulatory factor.
The innate immune response to viruses involves activation of pattern recognition receptors (PRRs) and transcriptional induction of type I interferons (IFNs) and proinflammatory cytokines. IFN genes are controlled mainly by the activities of interferon-regulatory factors 3 and 7 (IRF3 and -7, respectively), but activator protein 1 (AP1) and nuclear factor of the kappa light chain enhancer of B cells (NF-κB) are transcription factors joining the enhanceosome for efficient and regulated transcription of the IFN-β gene (31, 47). NF-κB, in addition, plays an important role in the innate immune system since it controls the transcription of a large variety of proinflammatory cytokines upon activation of diverse receptors, including not only PRRs like the toll-like receptors (TLRs) and retinoic acid-inducible gene I-like receptors (RLRs) but also members of the tumor necrosis factor receptor (TNFR) family (19). Moreover, NF-κB regulates numerous physiological processes, like immune cell development, proliferation, and homoeostasis of the adaptive immune system (24).
The mammalian NF-κB family comprises five members: p65 (RelA), p50 (NF-κB1), p52 (NF-κB2), cRel, and RelB. All family members share a structurally conserved N-terminal region of about 300 amino acids (aa), the Rel homology domain (RHD), which is critical for homo- or heterodimerization, binding to cognate DNA sequences, termed κB motifs, and interaction with specific inhibitory proteins. Rel proteins (p65/RelA, cRel, RelB) contain a C-terminal transactivation domain, which is lacking in p50 and p52. Thus, p50 and p52 form heterodimers with a Rel protein for gene activation or homodimers to function as repressors of promoters bearing κB motifs (35). The predominant form of NF-κB is a heterodimer of p65 and p50 subunits.
Most NF-κB dimers are located in the cytoplasm in an inactive form because of their association with inhibitor of κB (IκB) proteins, the most common of which is IκBα (2, 3). These regulatory proteins mask the NF-κB nuclear localization signal (NLS) within the NF-κB dimers and thus sequester them in the cytoplasm. A critical event in the so-called canonical activation of NF-κB is the phosphorylation of IκB proteins by IκB kinases (IKKs). The IKK complex involved contains two catalytic subunits, IKKα and IKKβ, as well as a regulatory subunit, IKKγ (NF-κB essential modulator [NEMO]). Upon activation, the IKK complex phosphorylates IκBα, which is the signal for ubiquitination and proteasomal degradation of the inhibitor. This leads to liberation of the NF-κB dimers, their nuclear translocation, and NF-κB-dependent gene transcription. Numerous upstream signaling cascades converge on the IKK complex (27), which is therefore the central mediator of canonical NF-κB activation.
RNA viruses like the Paramyxoviridae have developed multiple and powerful strategies to counteract IRF3/7-dependent IFN induction and signal transducers and activators of transcription (STAT)-dependent IFN signaling (21). While numerous recent studies on paramyxovirus innate immunity antagonistic activities have brought forth much knowledge on how control of IRF3/7 and STAT is achieved, their potential to interfere with NF-κB has been less well studied (25, 26).
Here, we assessed the ability of the immunosuppressive measles virus (MV) to interfere with NF-κB signaling. MV is a nonsegmented negative-strand RNA virus of the Paramyxoviridae family which typically triggers PRRs through interaction of viral RNA with TLR3 or TLR7 in the endosomes or with the RLRs, like RIG-I, in the cytoplasm (44). Induction of these pathways leads to the activation of both IRF3 and NF-κB and therefore to the transcription of IFN-β and inflammatory cytokines. Measles virus proteins have been shown to inhibit the IRF3- and IRF7-activating pathways as well as IFN signaling through different mechanisms (17). Specifically, the three phosphoprotein (P) gene products P, V, and C have been shown to act as the key players of MV-mediated immune evasion. A process called RNA editing, where an additional G is inserted into the mRNA of the P gene transcript, gives rise to the V protein (10). Thus, MV V has a unique, cysteine-rich C-terminal domain (VCTD) and an N-terminal domain which is identical to that of MV P (PVNTD) (Fig. 1A). Notably, the structure of the cysteine-rich and zinc-coordinated VCTD domain is conserved among paramyxovirus family members. Expression of the C protein is achieved through alternative translation initiation (5). In this study, we examined the effect of the MV P, V, and C proteins on canonical NF-κB activation. We found that any of the MV P gene products can interfere with NF-κB-dependent gene expression, illustrating that NF-κB is an important target of MV. The V protein displayed the strongest inhibitory effects and was found to specifically bind to the NF-κB subunit p65 and to preclude its nuclear accumulation. Intriguingly, the small VCTD, which is engaged in targeting multiple factors of IFN induction and IFN signaling pathways, was identified as responsible for p65 interaction.
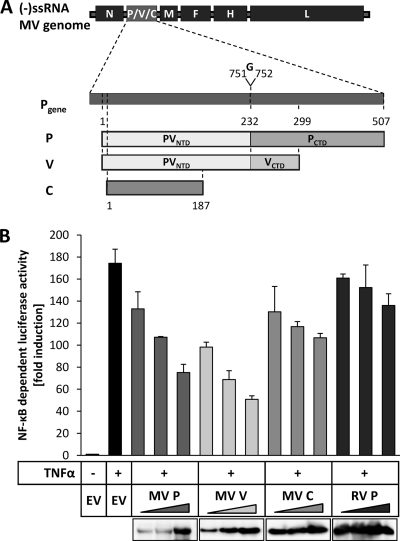
FIG. 1.
Suppression of TNF-α-mediated NF-κB activation by measles virus P, V, and C proteins. (A) The P gene of MV encodes the P protein and the nonstructural proteins V and C. The V mRNA is generated by insertion of an additional guanosine between nucleotides 751 and 752 of the mRNA by RNA editing. Therefore, the MV P and V proteins share an amino-terminal domain (PVNTD) stretching from aa 1 to 231 but have distinct carboxy-terminal domains (PCTD; VCTD). The C protein is produced by translation of an alternative open reading frame (ORF) initiated 19 nucleotides downstream of the P/V start codon. (-)ssRNA, negative-sense single-stranded RNA. (B) Increasing amounts (200 ng, 400 ng, 600 ng) of expression plasmids encoding measles virus (MV) proteins (P, V, C), rabies virus (RV) P protein, or an empty vector (EV) were cotransfected into HEK-293T cells with the NF-κB-dependent reporter plasmid p55A2-luc and pRL-CMV for normalization. After 18 h, cells were stimulated with 10 ng/ml recombinant human TNF-α and incubated for an additional 6 h, followed by cell lysis. NF-κB-driven luciferase activity was determined by a dual-luciferase assay. Values given are averages and standard deviations of results from two independent experiments. Depicted are the results of a representative experiment of four repeats. (Lower panel) Cell lysates were subjected to SDS-PAGE, and separated proteins were probed with anti-MV P/V, anti-MV C, or anti-RV P antibodies by Western blotting to determine the expression levels.
(This work was conducted by K. M. Schuhmann in partial fulfillment of the requirements for a Ph.D. from Ludwig Maximilians University Munich.)
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney HEK-293T cells and HEp2 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 1× l-glutamine, and penicillin-streptomycin (Gibco, Invitrogen).
Plasmids and reagents.
The generation of expression vectors encoding individual P gene products of the MV Schwarz vaccine strain and their immunoglobulin (Ig)- and flag-tagged versions and fragments were described recently (38). The plasmid encoding rabies virus P (RV P) was described previously (6). The pCR3-Ig vector (8) was used to generate a plasmid expressing an N-terminally Ig-tagged NEMO binding domain peptide (Ig-NBD) (34). Oligodeoxynucleotides specifying NBD (forward, 5′ ATA GAA TTC CTA GAC TGG AGC TGG TTA CTC GAG ATA 3′; reverse, 5′ TAT CTC GAG TAA CCA GCT CCA GTC TAG GAA TTC TAT 3′) were annealed and cloned into pCR3-Ig using XhoI/EcoRI restrictions sites. The NF-κB firefly luciferase reporter plasmid p55A2-luc, comprising three repeats of the PRDII domain of the IFN-β promoter (50), and the ΔRIG-I plasmid were kindly provided by T. Fujita, Kyoto, Japan (49). Expression vectors for IKKα, IKKβ, and TRIF (TIR domain-containing adapter inducing IFN-β) were kindly provided by K. Ruckdeschel, Munich, Germany, the plasmid for IKKγ was provided by F. Randow, Glasgow, United Kingdom, and vectors expressing IPS-1 and MyD88 were provided by S. Akira, Osaka, Japan. pRL-CMV was purchased from Promega, and expression plasmids for p65 (catalog no. 21966) and p50 (catalog no. 21965) were purchased from Addgene (4). A plasmid encoding C-terminally flag-tagged p65 was generated by PCR amplification from p65 using the primers 5′ ATA AAG CTT GCC ACC ATG GAC GAA CTG TTC CCC 3′ (forward) and 5′ ATA CTC GAG CTA TTT ATC GTC ATC GTC TTT GTA GTC GGA GCT GAT CTG ACT CAG 3′ (reverse), followed by cloning into pCR3 using HindIII/XhoI restriction sites. The N-terminal fragment of p65 (aa 1 to 309) representing the Rel homology domain (RHD) was amplified with the 5′ ATA AAG CTT GCC ACC ATG GAC GAA CTG TTC CCC 3′ (forward) and 5′ ATA CTC GAG TTA GAA GGT CTC ATA TGT 3′ (reverse) primers and cloned into pCR3 using HindIII/XhoI restriction sites. The vector expressing the flag-tagged version of RHD p65 (flag-RHD p65) was generated by PCR amplification using the primers 5′ ATA AAG CTT GCC ACC ATG GAC GAA CTG TTC CCC 3′ (forward) and 5′ ATA CTC GAG TTA TTT ATC GTC ATC GTC TTT GTA GTC GAA GGT CTC ATA TGT 3′ (reverse), followed by cloning into pCR3 using HindIII/XhoI restriction sites. A plasmid encoding the flag-tagged version of p50 was constructed using the primers 5′ ATA AAG CTT GCC ACC ATG GAC TAC AAA GAC GAT GAC GAT AAA GCA GAA GAT GAT CCA 3′ (forward) and 5′ ATA CTG GAG TTA AAC TTT CCC AAA GAG GTT 3′ (reverse) and pCR3 restricted with HindIII/XhoI. The flag-tagged RHD of p50 (aa 1 to 366) was amplified with 5′ ATA AAG CTT GCC ACC ATG GAC TAC AAA GAC GAT GAC GAT AAA GCA GAA GAT GAT CCA 3′ (forward) and 5′ ATA CTC CAG TTA CTT CTG ACG TTT CCT 3′ (reverse) and cloned into pCR3 using HindIII/XhoI restriction sites. Recombinant human tumor necrosis factor alpha (TNF-α) was purchased from Biomol.
Reporter gene assay.
HEK-293T cells in 24-well plates were transfected with p55A2-luc (100 ng), pRL-CMV (10 ng), and the amounts of the expression vectors and Lipofectamine 2000 (Invitrogen) indicated in the figure legends. The total amounts of transfected DNA were adjusted by adding an empty vector. In case of external stimulation, cells were treated with 10 ng/ml TNF-α. Cells were harvested in passive lysis buffer (Promega) at the time points indicated in the figure legends, and lysates were subjected to reporter gene assay using the dual-luciferase reporter system (Promega). Luciferase activity was measured with a luminometer (Berthold Lumat LB 960) according to the manufacturer's instructions. For Western blotting, reporter assay lysates were mixed 1:1 with SDS sample buffer (62.5 mM Tris, 2% SDS, 10% glycerol, 6 M urea, 5% β-mercaptoethanol, 0.01% bromophenol blue, 0.01% phenol red).
Confocal microscopy and antibodies.
HEp2 cells grown on coverslips in 24-well plates were transfected 24 h postseeding with 500 ng of plasmids for flag-MV V or pCR3 using Lipofectamine 2000, incubated for an additional 24 h, and then treated with 10 ng/ml TNF-α. Cells were fixed after 30 min in 3% paraformaldehyde for 20 min at room temperature, quenched with 50 mmol ammonium chloride for 10 min at room temperature, and permeabilized in 0.5% Triton X-100 in phosphate-buffered saline (PBS). After being blocked with 2.5% milk in 0.1% Triton X-100-PBS, fixed cells were incubated with the primary antibodies anti-p65 (rabbit, sc-109; Santa Cruz), diluted 1:200, and anti-flag (mouse; Sigma), diluted 1:200 in 0.1% Triton X-100-PBS for 1 h at 4°C, followed by incubation with fluorescence-labeled secondary antibodies (goat anti-rabbit Alexa Fluor 488 and anti-mouse tetramethylrhodamine, both from Molecular Probes) at a dilution of 1:200 in 0.1% Triton X-100-PBS for 1 h at 4°C. Nuclear chromatin was stained by adding TO-PRO-3-iodide (Molecular Probes; 1:2,000) to the secondary antibodies. Confocal laser scanning microscopy was performed with a Zeiss LSM510 Meta laser system using a Zeiss Axiovert 200 microscope. Excitation of Alexa Fluor 488, tetramethylrhodamine, and TO-PRO-3-iodide occurred at wavelengths of 488 nm, 543 nm, and 633 nm, respectively.
CoIP.
For coimmunoprecipitation (CoIP), HEK-293T cells were grown in 6-cm dishes and cotransfected with the plasmids indicated in the figure legends (3 μg each) using polyethylenimine (PEI). Cells were lysed under native conditions 24 h posttransfection, and IP assays were performed as described recently (7) using protein A-conjugated Sepharose beads to pull down Ig-tagged proteins or anti-flag M2 affinity gel to immunoprecipitate flag-tagged proteins.
Western blotting and antibodies.
Cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore) using a semidry blotter (Peq-Lab). Membranes were incubated overnight at 4°C with primary antibodies. Protein signals were visualized with horseradish peroxidase-conjugated secondary antibodies and an enhanced-chemiluminescence (ECL) kit (Perkin Elmer) and detected by applying a film (Amersham Hyperfilm ECL; GE Healthcare) or using the Fusion-FX7 imaging system (Vilber Lourmat). Anti-flag-M2 (Sigma), anti-p65 (sc-109; Santa Cruz), anti-p65 N terminus (C22B4; Cell Signaling), and anti-p50 (catalog no. 3035; Cell Signaling) were purchased, and anti-MV P/V, anti-MV C, and anti-RV P antibodies were kindly provided by D. Gerlier, Lyon, France (11), R. Cattaneo, Rochester, MN, and S. Finke, Greifswald-Insel Riems, Germany, respectively. Anti-MV VCTD is a polyclonal rabbit serum raised against a synthetic peptide conjugated to KLH (keyhole limpet hemocyanin) corresponding to the C-terminal domain of V as described in reference 15.
RESULTS
Suppression of TNF-α-mediated NF-κB activation by measles virus P, V, and C proteins.
The canonical NF-κB activation pathway can be initiated by various stimuli, including tumor necrosis factor alpha (TNF-α), a key cytokine regulating immune functions as well as inflammatory responses (18). In order to identify MV proteins influencing NF-κB activity, we performed dual-luciferase reporter gene assays with cells treated with TNF-α. Increasing amounts of expression vectors for the MV Schwarz proteins, including P, V, and C, were cotransfected into HEK-293T cells with a plasmid encoding the firefly luciferase reporter gene under the control of a trimeric repeat of the NF-κB-binding motif (PRDII) of the IFN-β promoter (50) and a Renilla luciferase expression plasmid. The start codon of the C protein was changed by site-directed silent mutagenesis to prevent expression of MV C in the cases of all P- or V-expressing plasmids, as described previously (38). The P protein of rabies virus (RV P), which suppresses activation of IRF3 and STAT1/STAT2 nuclear import but which has no influence on NF-κB signaling (6, 7, 46), was used as a negative control. Cells were stimulated with TNF-α 6 h prior to cell lysis, and NF-κB-dependent luciferase activity was determined. Intriguingly, all three P gene products, MV P, V, and C, exerted a substantial and dose-dependent inhibitory effect on TNF-α-mediated NF-κB activation. The V protein showed the strongest suppression (Fig. 1B). The inhibitory effects of the P and C proteins were less prominent. In contrast to V, P, and C, NF-κB-dependent luciferase activity was not affected in the presence of MV N, F, H, or L (not shown) or of RV P protein. These results indicate that the MV P, V, and C proteins can interfere with canonical NF-κB activation but to different extents.
NF-κB activation by pattern recognition receptors is suppressed by MV P gene products.
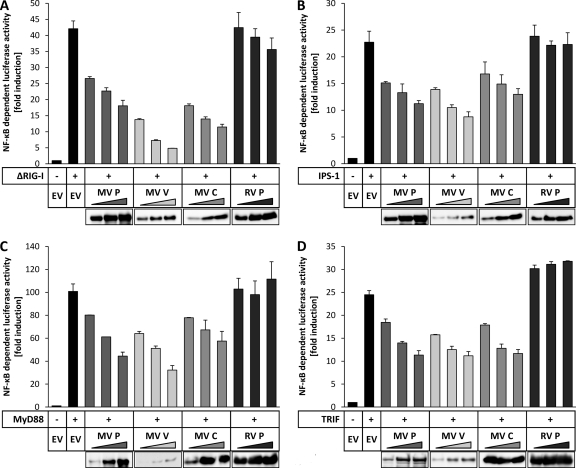
Triggering of PRRs, such as RLRs or TLRs, can also lead to the activation of NF-κB. To test the capacity of MV P gene products to downregulate NF-κB activation mediated by RIG-I signaling, a C-terminal deletion mutant, the ΔRIG-I mutant (comprising aa 1 to 284), which was previously shown to constitutively activate NF-κB (49), was cotransfected with increasing amounts of the MV proteins in HEK-293T cells, followed by dual-luciferase assays. As a control, increasing amounts of a plasmid encoding RV P were transfected. Expression of the ΔRIG-I mutant led to a >40-fold induction of NF-κB activity. In the presence of MV V at the highest dose, activity was suppressed to nearly basal level (Fig. 2A). MV P protein, which was expressed at much higher levels, as indicated by Western blotting using an antibody recognizing both P and V (Fig. 2A, bottom panel), had a less pronounced inhibitory potency. The C protein had intermediate capacity, while RV P had no significant influence on NF-κB activation.
FIG. 2.
NF-κB activation by pattern recognition receptors is suppressed by MV P gene products. Increasing amounts (200 ng, 400 ng, 600 ng) of vectors encoding the indicated measles virus (MV) proteins, rabies virus (RV) P protein, or an empty vector (EV) were cotransfected into HEK-293T cells with either 200 ng of an expression plasmid for the ΔRIG-I mutant (A), MyD88 (B), IPS-1 (C), or TRIF (D) and the NF-κB-dependent reporter system (100 ng p55A2, 10 ng pRL-CMV). After 24 h, cells were lysed and NF-κB activity was determined by a dual-luciferase assay. Values given are averages and standard deviations of results from two independent experiments. Depicted are the results of representative experiments (of three repeats). (Lower panels) Expression of the viral proteins was assessed by Western blotting.
Signal transduction by the receptors RIG-I and MDA5 is transmitted via IPS-1, while that of TLRs involves the adaptor MyD88 (for TLR1, -2, -4, -5, -6, -7, -8, -9) or TRIF (for TLR3) (29). To examine whether MV P, V, and C are able to generally counteract NF-κB activation by RLRs and TLRs, dual-luciferase reporter gene assays involving overexpression of IPS-1 (Fig. 2B), MyD88 (Fig. 2C), and TRIF (Fig. 2D) for stimulation of NF-κB activity were performed. Analogously with the previous experiments, all MV P gene products were able to suppress the adaptor-induced NF-κB activation in a dose-dependent way, whereas expression of RV-P had no effect. The levels of reduction of NF-κB activity achieved by the V and P proteins appeared to be similar in these experiments; however, Western blotting revealed a substantially lower expression of the V protein (Fig. 2B to D, bottom panels), supporting the previous finding that the V protein is the most potent inhibitor of NF-κB activation among the MV P gene products.
MV V inhibits NF-κB signaling downstream of the IKK complex.
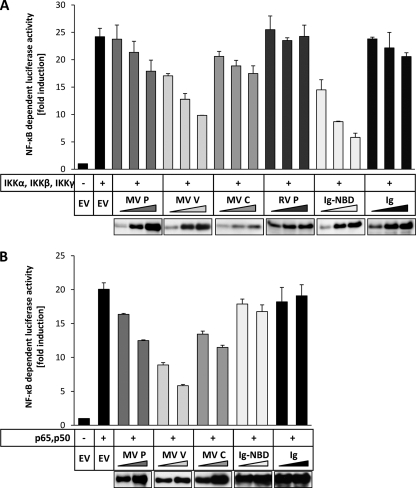
Stimulation of TLR, RLR, and TNFR triggers pathways leading to canonical NF-κB activation. These pathways converge on the IKK complex, which is composed of the kinases IKKα, IKKβ, and IKKγ (NEMO). To test whether the inhibition by MV proteins occurs at or downstream of these kinases, NF-κB-dependent luciferase expression was activated by overexpressing IKKα, IKKβ, and IKKγ in HEK-293T cells (Fig. 3A). As a positive control, an Ig-tagged NEMO-binding domain (NBD) was used (Ig-NBD). The NBD peptide binds to IKKγ and thereby inhibits the formation of the IKK complex, which is essential for canonical NF-κB activation (34). Expression of Ig-NBD resulted in a dose-dependent inhibition of IKK complex-induced NF-κB activity, while the Ig tag expressed individually did not decrease NF-κB activation. Expression of MV V led to a dose-dependent and effective suppression of NF-κB activity, comparable to that achieved by expression of Ig-NBD (Fig. 3A), indicating a target at the level of, or downstream of, the IKK complex. The presence of MV P and C proteins had less pronounced effects on NF-κB activation by the IKK complex.
FIG. 3.
MV V inhibits NF-κB signaling downstream of the IKK complex. (A) HEK-293T cells were cotransfected with expression plasmids encoding IKKα, IKKβ, and IKKγ (100 ng each) together with increasing amounts (200 ng, 400 ng, 600 ng) of vectors for the indicated proteins or an empty vector (EV) and the NF-κB-dependent reporter system (100 ng p55A2, 10 ng pRL-CMV). After 12 h, the cells were lysed and the luciferase activity was measured by a dual-luciferase assay. Values given are averages and standard deviations of results from two independent experiments. The results of a representative experiment (out of three repeats) are shown. (Lower panel) Expression levels were determined by Western blotting. (B) Plasmids encoding p65 and p50 (150 ng each) and increasing amounts (300 ng, 600 ng) of vectors for the indicated proteins or an empty vector (EV) were cotransfected into HEK-293T cells together with a dual-luciferase reporter system. Cells were lysed 12 h after transfection, followed by determination of normalized NF-κB-dependent luciferase activity. The given values are averages and standard deviations of results from two independent experiments. Shown are the results of a representative experiment (of three repeats). (Lower panel) Expression levels of viral proteins were assessed by Western blotting.
To further spot the step where MV V inhibits canonical NF-κB activation, we induced NF-κB-dependent luciferase activity by coexpression of the NF-κB subunits p65 and p50, which build the main heterodimer of NF-κB. In the presence of MV V, the NF-κB activity induced by p65/p50 was reduced dose dependently and significantly, whereas MV P and C showed only minor inhibitory capacities (Fig. 3B). As expected, coexpression of Ig-NBD had no effect on p65/p50-mediated NF-κB activity, as this inhibitor acts upstream of the transcription factor p65/p50. We conclude from these experiments that the V protein of MV can inhibit canonical NF-κB signaling downstream of the IKK complex, whereas P and C may act upstream in the signal transduction cascade.
MV V binds the NF-κB subunit p65.
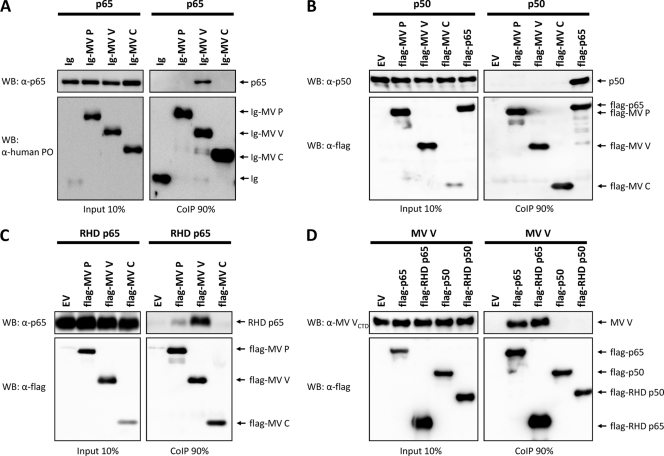
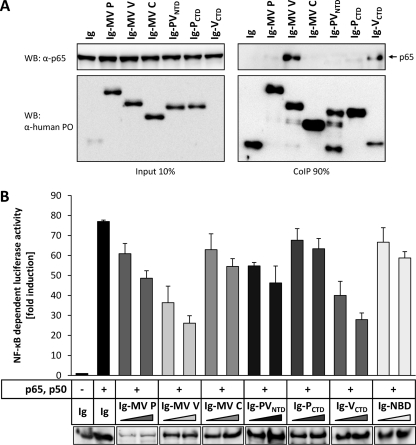
In order to clarify the molecular mechanism of MV proteins to suppress NF-κB activation, we analyzed the NF-κB subunits p65 and p50 for potential interactions with viral proteins in coimmunoprecipitation (CoIP) experiments. Extracts from HEK-293T cells coexpressing Ig-tagged MV P, V, or C protein and p65 from transfected plasmids were purified with protein A-conjugated Sepharose beads, and precipitates were analyzed by Western blotting using antibodies against p65 or human IgG. Indeed, the NF-κB subunit p65 was specifically coprecipitated with Ig-MV V, whereas no interaction of p65 with the P or C construct was detectable (Fig. 4A).
FIG. 4.
MV V binds the NF-κB subunit p65. (A) HEK-293T cells were used to express p65 in combination with the indicated Ig-tagged proteins or the Ig tag (Ig) itself (3 μg each). After 24 h, cells were lysed under native conditions and Ig-tagged proteins were pulled down using protein A-conjugated Sepharose beads. Binding of p65 to measles proteins was visualized by Western blotting. Depicted are the results of a representative experiment (of four repeats). PO, horseradish peroxidase. (B) p50 was coexpressed in HEK-293T cells with the indicated flag proteins or an empty vector (EV) (3 μg each). Cells were lysed 24 h posttransfection, and flag-tagged measles proteins were immunoprecipitated using anti-flag M2 affinity gel. The interaction of p50 and flag-tagged proteins was analyzed by Western blotting (WB). The results of a representative experiment (of three) is shown. (C) HEK-293T cells were cotransfected with vectors encoding the indicated flag-tagged constructs or an empty vector (EV) and the RHD of p65 (aa 1 to 309). A CoIP assay was performed as described, and RHD-p65 was stained using anti-p65 (Cell Signaling; catalog no. 3035). The results of a representative experiment (of three) are shown. (D) MV V was coexpressed with the indicated flag-tagged proteins or an empty vector (EV) (3 μg each) in HEK-293T cells. CoIP experiments were performed as described above, and MV V was stained using anti-MV VCTD. Depicted are the results of a representative experiment (of three repeats).
In further experiments, binding of p50 (NF-κB1) to flag-tagged MV proteins was assessed. Flag-p65 was included as a positive control. Cell extracts were analyzed by immunoblotting using antibodies specific for p50 and flag. While flag-p65 efficiently coprecipitated p50, no interactions of the p50 NF-κB subunit with any of the flag-MV proteins could be demonstrated (Fig. 4B). Taken together, these experiments revealed that the V protein of MV specifically interacts with the NF-κB subunit p65 but not with p50.
To determine if MV V binds to the N-terminal RHD of p65, which is responsible for dimerization, DNA binding, and nuclear import, we constructed the fragment of p65 spanning aa 1 to 309 (RHD p65) and performed CoIP experiments with flag-MV P, V, and C. As revealed by Western blotting with an antibody specific for the N terminus of p65, the p65 RHD was efficiently pulled down by flag-MV V (Fig. 4C). Notably, RHD p65 also showed some affinity to MV P, though it was considerably weaker than that to MV V. A minor affinity of the MV P protein to bind p65 was observed occasionally in pulldown experiments with flag-tagged p65 and authentic, untagged MV proteins (data not shown). Considering these things together, we observed that MV V binds with a strong affinity to the RHD of p65, while a weak interaction of MV P with RHD p65 was suggested.
To further verify the specificity of MV V to the RHD of p65, we tested binding of flag-tagged p65, RHD p65, p50, and RHD p50 to V within one experiment. Therefore, we constructed the RHD of p50 spanning aa 1 to 366 and the other constructs with a flag tag. CoIP experiments of the flag-tagged proteins with authentic, untagged MV V revealed specific binding of V to flag-p65 and flag-RHD p65, while no interaction of V and flag-p50 or flag-RHD p50 could be detected (Fig. 4D). These findings confirm the specificity of MV V to the RHD of p65.
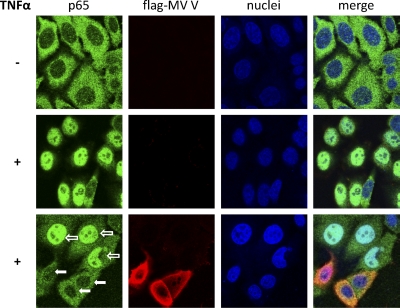
V prevents nuclear translocation of p65.
Since MV V is a cytoplasmic protein, binding of V to p65 might interfere with the trafficking of this NF-κB subunit. In order to address this hypothesis, HEp2 cells were transfected with a flag-MV V-encoding plasmid or an empty vector and stimulated with TNF-α for 30 min, followed by immunostaining of p65 and flag-tagged MV V. Although in unstimulated cells transfected with the empty vector, p65 is located predominantly in the cytoplasm (Fig. 5, upper panel), TNF-α treatment resulted in almost complete translocation of p65 from the cytoplasm to the nucleus (Fig. 5, middle panel). In contrast, accumulation of p65 in the nucleus upon TNF-α stimulation in cells expressing flag-MV V was severely impaired (Fig. 5, lower panel, filled arrows), while cells failing to express detectable flag-MV V showed a normal nuclear accumulation of p65 (Fig. 5B, lower panel, open arrows). Thus, binding of the MV V protein to p65 is sufficient to prevent the nuclear accumulation of NF-κB and thereby to preclude its transcriptional activity.
FIG. 5.
V prevents nuclear translocation of p65. HEp2 cells were transfected with a vector for flag-MV V or an empty vector. Twenty-four hours posttransfection, cells were either treated with 10 ng/ml TNF-α for 30 min (+) or left untreated (−). Subsequently, cells were fixed and stained with the indicated antibodies. Green, p65 (stained with specific antibody); red, flag-MV V (stained with anti-FLAG M2); blue, ToPro3 nuclear staining; open arrow, not a flag-MV V-expressing cell; filled arrow, flag-MV V-expressing cell. Depicted are the results of a representative experiment (of three repeats).
The CTD of MV V is required and sufficient for p65 binding and suppression of p65/p50-mediated NF-κB activity.
Since MV P and V have identical amino-terminal domains (PVNTD) but distinct carboxy-terminal domains (PCTD; VCTD) (see Fig. 1A), we reasoned that p65 binding is mediated via the V-specific CTD. To verify this, HEK-293T cells were transfected with expression plasmids encoding the individual protein domains fused to an Ig tag (Ig-MV PVNTD, PCTD, VCTD) or the full-length proteins, together with p65. Indeed, Ig-MV VCTD was sufficient for precipitation of p65, with a binding affinity apparently similar to that of full-length Ig-MV V, while the other constructs did not reveal interaction with the NF-κB subunit (Fig. 6A). To clarify whether binding of the small VCTD is also sufficient for inhibition of NF-κB transcriptional activity, p65 and p50 were overexpressed in HEK-293T cells along with Ig-MV VCTD or Ig-MV V. A similar and dose-dependent reduction of NF-κB-dependent luciferase expression confirmed that binding of VCTD is sufficient for full inhibition (Fig. 6B). In contrast, expression of the C-terminal portion of the P protein had no considerable effect on p65/p50-mediated luciferase activity. In summary, the C-terminal domain of the V protein is sufficient for mediating p65 binding and inhibition of p65/p50-mediated NF-κB activity.
FIG. 6.
The CTD of MV V is required and sufficient for p65 binding and suppression of p65/p50-mediated NF-κB activity. (A) HEK-293T cells were cotransfected with vectors encoding the indicated Ig-tagged constructs or the Ig tag itself (Ig) and p65. A CoIP assay was performed as described in the text. The results of a representative experiment of four are shown. (B) Increasing amounts (300 ng, 600 ng) of the indicated Ig-tagged constructs were coexpressed in HEK-293T cells together with p65, p50 (150 ng each), and the NF-κB reporter system (100 ng p55A2, 10 ng pRL-CMV). Twelve hours posttransfection, cells were lysed and NF-κB activity was determined by a dual-luciferase assay. Values given are averages and standard deviations of results from two independent experiments. Depicted are the results of a representative experiment (of three repeats). (Lower panel) Expression levels were determined by Western blotting.
DISCUSSION
NF-κB is a key mediator of antiviral host responses and inflammation, as well as of immune cell development, survival, and function (24), and therefore a prime candidate for viral interference (26). In particular, hematotropic viruses like the immune-suppressive and immune-modulatory measles virus (22) should have means to interfere with NF-κB signaling. In fact, recent work showed upregulation of the ubiquitin-modifying enzyme A20 in monocytes, but not in epithelial cells, infected with MV or expressing the MV P protein (48), indicating that MV has at least an indirect means of affecting NF-κB signaling. Our present data revealed that all of the MV Schwarz P gene products, including the essential P protein, and the “accessory” proteins V and C, which are established MV virulence factors, are able to interfere with NF-κB activation. The V protein, in particular, revealed a potent inhibitory capacity. This is correlated with the specific binding of V to the central NF-κB subunit, p65, and therefore a lack of p65 nuclear accumulation. P and V of an MV wild-type isolate (genotype D5) bear some point mutations in their common PVNTD domain, while the unique PCTD has two conservative amino acid exchanges compared to the Schwarz strain. VCTD, however, which was shown to be responsible for p65 binding, is completely conserved in the wild-type isolate and the Schwarz strain. Consistently with this fact, binding of wild-type V to p65 and similar levels of inhibition of reporter gene activity were also observed (data not shown). The C protein of the D5 strain shows only some amino acid exchanges, which seem to have no effect on the suppression of NF-κB activity, since all wild-type P gene products showed the same suppression pattern as MV Schwarz proteins in the NF-κB-dependent luciferase assays (data not shown). Thus, the inhibitory capacity of MV P gene products seems not to be affected in the vaccine strain compared to that of the wild type.
Expression of the individual MV proteins P, V, and C was sufficient to suppress NF-κB-mediated reporter gene transcription triggered through different signaling cascades, including the TNFR, RLR, and TLR. Importantly, upregulation of the NF-κB inhibitor A20 was not observed in HEK-293T cells with any of the MV proteins (not shown), excluding the possibility of a contribution of this recently described mechanism (48). In any of the pathways investigated, the specific inhibitory capacity of V was greater than that of P or C, while it appeared to be particularly pronounced in cells stimulated by overexpression of the ΔRIG-I mutant. Since RIG-I is thought to be the main sensor of paramyxovirus infection (28, 39), interference with RLR signaling cascades is a promising mechanism for evading host immune responses. MV V is known to strongly bind to the helicase domain of MDA5 but not to RIG-I (1, 12, 13). In addition, genetic knockout of the MDA5 gene was previously shown to reduce RIG-I-mediated IFN induction in transgenic mice (20), indicating that MDA5 is synergistic to RIG-I signaling. Although the ΔRIG-I mutant consists only of the RIG-I CARD domains, a potential ΔRIG-I-MDA5 interplay, which is disturbed by V binding, cannot be formally excluded and might contribute to the potent inhibition of ΔRIG-I mutant-mediated NF-κB activity by V. However, we suggest that binding of MV V to the downstream transcription factor p65 is the major mechanism for suppression of RLR-mediated NF-κB activation.
Signaling cascades initiated by TNFR, TLR, and RLR or their respective adaptor proteins, MyD88, TRIF, and IPS-1, converge on the IKK complex, which controls the phosphorylation-dependent proteasomal degradation of the inhibitor IκB and therefore is the central regulator of the canonical NF-κB pathway (23, 27). The finding that MV V is able to suppress not only IKK-mediated NF-κB activity but also the activity of overexpressed NF-κB (p65/p50) revealed a universal, downstream inhibitory mechanism of the V protein (Fig. 3).
Inspired by the fact that MV V and P are cytoplasmic proteins and interfere with the import of STAT1 and STAT2 to the nucleus (9, 16, 36), we performed coimmunoprecipitation assays, which revealed the specific binding of V to p65 (Fig. 4), and immunofluorescence assays, which indicated V-mediated retention of p65 in the cytoplasm (Fig. 5). We found that binding of MV V to p65 is mediated through the Rel homology domain (RHD) of this NF-κB subunit, while no interaction with the RHD of p50 was detected (Fig. 4D). The N-terminal RHD is characteristic of all NF-κB subunits (19) and is responsible for the homo- and heterodimerization of the NF-κB proteins and the binding to IκB, as well as sequence-specific DNA binding. The RHD also contains the nuclear localization signal (NLS), which is masked by the IκBs in nonstimulated cells. Upon stimulation of the NF-κB signaling cascade, IκB is degraded and the NLS is liberated, which results in the nuclear translocation of NF-κB. We propose that binding of V to the RHD domain of p65 shields the NLS of the NF-κB subunit such that translocation of NF-κB into the nucleus is impaired, therefore suggesting an IκB-like function for the MV V protein.
We recently described the binding of MV V to IKKα (38), which is involved in the activation of IRF7 through TLR7/8/9 but is also a subunit of the canonical IKK complex. We could show in in vitro kinase assays that the IKKα-dependent phosphorylation of IRF7 was diminished in the presence of MV V but that the phosphorylation of the NF-κB inhibitor IκBα by IKKα was not altered (38). Furthermore, activation of NF-κB by overexpression of either IKKα or IKKβ was inhibited equally by MV V (not shown). Therefore, we suggest that the binding of MV V to IKKα suppresses IRF7 activation but does not affect the activation of NF-κB. Only the binding of V to p65 interferes with NF-κB signaling. This is also emphasized by the fact that IKKα plays only a minor role in the canonical NF-κB pathway, since IKKα may support canonical NF-κB activation but is dispensable, whereas IKKβ is the essential kinase (27).
Intriguingly, the short, 68-aa C-terminal domain of the V protein (VCTD) was found to be responsible and sufficient for the specific binding of V to the p65 RHD and the inhibition of canonical NF-κB activation (Fig. 6). The structure of the cysteine-rich and zinc-coordinated VCTD is the conserved part of Paramyxovirinae V proteins and responsible for most of the host-antagonistic functions described for V so far (21). Therefore, it is not surprising that VCTDs of different Paramyxovirinae family members have common functions and binding partners, such as STAT2 (42, 43, 45) and MDA5 (1, 12, 37). However, it appears that the VCTDs of Paramyxovirinae can further adapt to different targets, according to their requirements and niches. While the VCTD of the respiratory pathogen parainfluenza virus type 5 (PIV5) and of related rubulaviruses were found to bind IKKɛ and thereby to prevent IRF3 activation and RLR-dependent IFN-β induction (33), we recently showed that the MV VCTD instead binds IKKα and IRF7 and thereby prevents TLR7/8/9-mediated IFN-α induction, which is instrumental in hematopoietic cells like pDC (38). Similarly, a general inhibition of canonical NF-κB activation due to binding to p65, as observed here for MV, seems not to be a common feature of Paramyxovirinae V proteins. Though the V protein of PIV5 was reported to suppress NF-κB activation upon being triggered with synthetic double-stranded RNA (dsRNA) or due to viral infection, inhibition of lipopolysaccharide (LPS)- or TNF-α-dependent NF-κB activity was not observed (32, 40). This is in accordance with our own NF-κB reporter gene experiments where expression of PIV5 V was ineffective in preventing TNF-α-mediated NF-κB activity (data not shown). Further experiments should reveal whether NF-κB p65 targeting by V is specific for the human MV or also applied by the related animal morbilliviruses.
As indicated by reporter gene experiments, in addition to MV V, both MV P and C interfered with canonical NF-κB signaling, though their interference was for the most part less prominent. However, neither the C nor the P protein of MV revealed a pronounced interaction with p65. We therefore presume that the P and C proteins contribute to MV-mediated NF-κB escape by targeting other steps of the canonical activation pathways which remain to be elucidated. In the case of P, the PVNTD appears to be required for counteracting NF-κB signaling in epithelial cells, since the PCTD displayed no inhibition of TNF-α-mediated NF-κB reporter gene activation (data not shown). Notably, the PVNTD of P and V is also involved in an association with STAT1 and thereby contributes to the inhibition of IFN signaling (9, 42). In MV-infected monocytes, P may in addition lead to upregulation of the NF-κB inhibitor A20 (48). As far as C is concerned, an explanation is not close at hand. C proteins of other Paramyxovirinae family members also show an NF-κB-inhibitory capacity, as illustrated by the Sendai virus C proteins which suppress dsRNA- and Newcastle disease virus-mediated NF-κB activation; however, the mechanism is also elusive (30).
In summary, we demonstrated that measles virus applies multiple mechanisms in manipulating NF-κB signaling pathways. The major activity could be attributed to the V protein and more specifically to the VCTD, which interferes with canonical NF-κB activation by binding to the RHD of the NF-κB subunit p65 and therefore prevents nuclear accumulation of the transcriptionally active NF-κB subunit. The V protein is a well-established virulence factor, and the VCTD turns out to be a hub for the specific binding of numerous cellular proteins. This includes not only targets of innate immunity but also proteins related to proliferation and cell death, like the p53 family member p73, which downregulates expression of the proapoptotic target gene PUMA and might therefore function as a viral antiapoptotic factor (14). Revealing the exact binding sites for specific proteins, as was recently done for MDA5 and STAT2 (41, 42), and generating recombinant MV strains deficient in only single functions of V should help in elucidating the contributions of individual V functions to measles virus cell biology and immune modulation.
Acknowledgments
We thank Nadin Hagendorf for perfect technical assistance. Antibodies were kindly provided by D. Gerlier, R. Cattaneo, and S. Finke and cDNAs by S. Akira, T. Fujita, K. Ruckdeschel, and F. Randow.
K. M. Schuhmann was supported by the Deutsche Forschungsgemeinschaft through grants GraKo 1202 (Oligonucleotides in Cell Biology and Therapy) and SFB 455 (Viral Functions and Immune Modulation).
Footnotes
Published ahead of print on 26 January 2011.
REFERENCES
- 1.Andrejeva, J., et al. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. U. S. A. 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle, P. A. 1998. I[kappa]B-NF-[kappa]B structures: at the interface of inflammation control. Cell 95:729-731. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle, P. A., and D. Baltimore. 1988. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 242:540-546. [DOI] [PubMed] [Google Scholar]
- 4.Ballard, D. W., et al. 1992. The 65-kDa subunit of human NF-kappa B functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc. Natl. Acad. Sci. U. S. A. 89:1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellini, W. J., G. Englund, S. Rozenblatt, H. Arnheiter, and C. D. Richardson. 1985. Measles virus P gene codes for two proteins. J. Virol. 53:908-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brzózka, K., S. Finke, and K. K. Conzelmann. 2005. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J. Virol. 79:7673-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brzózka, K., S. Finke, and K. K. Conzelmann. 2006. Inhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2. J. Virol. 80:2675-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bubeck, A., et al. 2004. Comprehensive mutational analysis of a herpesvirus gene in the viral genome context reveals a region essential for virus replication. J. Virol. 78:8026-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caignard, G., et al. 2007. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology 368:351-362. [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo, R., K. Kaelin, K. Baczko, and M. A. Billeter. 1989. Measles virus editing provides an additional cysteine-rich protein. Cell 56:759-764. [DOI] [PubMed] [Google Scholar]
- 11.Chen, M., J. C. Cortay, and D. Gerlier. 2003. Measles virus protein interactions in yeast: new findings and caveats. Virus Res. 98:123-129. [DOI] [PubMed] [Google Scholar]
- 12.Childs, K., et al. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359:190-200. [DOI] [PubMed] [Google Scholar]
- 13.Childs, K. S., J. Andrejeva, R. E. Randall, and S. Goodbourn. 2009. Mechanism of mda-5 inhibition by paramyxovirus V proteins. J. Virol. 83:1465-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz, C. D., et al. 2006. Measles virus V protein inhibits p53 family member p73. J. Virol. 80:5644-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaux, P., and R. Cattaneo. 2004. Measles virus phosphoprotein gene products: conformational flexibility of the P/V protein amino-terminal domain and C protein infectivity factor function. J. Virol. 78:11632-11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaux, P., V. von Messling, W. Songsungthong, C. Springfeld, and R. Cattaneo. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology 360:72-83. [DOI] [PubMed] [Google Scholar]
- 17.Gerlier, D., and H. Valentin. 2009. Measles virus interaction with host cells and impact on innate immunity. Curr. Top. Microbiol. Immunol. 329:163-191. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-[kappa]B puzzle. Cell 109:S81-S96. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 20.Gitlin, L., et al. 2010. Melanoma differentiation-associated gene 5 (MDA5) is involved in the innate immune response to Paramyxoviridae infection in vivo. PLoS Pathog. 6:e1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodbourn, S., and R. E. Randall. 2009. The regulation of type I interferon production by paramyxoviruses. J. Interferon Cytokine Res. 29:539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin, D. E. 2010. Measles virus-induced suppression of immune responses. Immunol. Rev. 236:176-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayden, M. S., and S. Ghosh. 2008. Shared principles in NF-kappaB signaling. Cell 132:344-362. [DOI] [PubMed] [Google Scholar]
- 24.Hayden, M. S., A. P. West, and S. Ghosh. 2006. NF-kappaB and the immune response. Oncogene 25:6758-6780. [DOI] [PubMed] [Google Scholar]
- 25.Hiscott, J., H. Kwon, and P. Genin. 2001. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J. Clin. Invest. 107:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiscott, J., T. L. A. Nguyen, M. Arguello, P. Nakhaei, and S. Paz. 2006. Manipulation of the nuclear factor-[kappa]B pathway and the innate immune response by viruses. Oncogene 25:6844-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Israel, A. 2010. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2:a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato, H., et al. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 29.Kawai, T., and S. Akira. 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21:317-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komatsu, T., K. Takeuchi, J. Yokoo, and B. Gotoh. 2004. C and V proteins of Sendai virus target signaling pathways leading to IRF-3 activation for the negative regulation of interferon-beta production. Virology 325:137-148. [DOI] [PubMed] [Google Scholar]
- 31.Lenardo, M. J., C. M. Fan, T. Maniatis, and D. Baltimore. 1989. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell 57:287-294. [DOI] [PubMed] [Google Scholar]
- 32.Lin, Y., et al. 2007. Inhibition of interleukin-6 expression by the V protein of parainfluenza virus 5. Virology 368:262-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu, L. L., M. Puri, C. M. Horvath, and G. C. Sen. 2008. Select paramyxoviral V proteins inhibit IRF3 activation by acting as alternative substrates for inhibitor of IκB kinase ɛ (IKKɛ)/TBK1. J. Biol. Chem. 283:14269-14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.May, M. J., et al. 2000. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science 289:1550-1554. [DOI] [PubMed] [Google Scholar]
- 35.May, M. J., and S. Ghosh. 1997. Rel/NF-kappa B and I kappa B proteins: an overview. Semin. Cancer Biol. 8:63-73. [DOI] [PubMed] [Google Scholar]
- 36.Palosaari, H., J.-P. Parisien, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 77:7635-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parisien, J. P., et al. 2009. A shared interface mediates paramyxovirus interference with antiviral RNA helicases MDA5 and LGP2. J. Virol. 83:7252-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaller, C. K., and K. K. Conzelmann. 2008. Measles virus V protein is a decoy substrate for IkappaB kinase alpha and prevents Toll-like receptor 7/9-mediated interferon induction. J. Virol. 82:12365-12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plumet, S., et al. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS One 2:e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-[beta]. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 41.Ramachandran, A., and C. M. Horvath. 2010. Dissociation of paramyxovirus interferon evasion activities: universal and virus-specific requirements for conserved V protein amino acids in MDA5 interference. J. Virol. 84:11152-11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramachandran, A., J. P. Parisien, and C. M. Horvath. 2008. STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition. J. Virol. 82:8330-8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothlisberger, A., et al. 2010. Two domains of the V protein of virulent canine distemper virus selectively inhibit STAT1 and STAT2 nuclear import. J. Virol. 84:6328-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeuchi, O., and S. Akira. 2010. Pattern recognition receptors and inflammation. Cell 140:805-820. [DOI] [PubMed] [Google Scholar]
- 45.Ulane, C. M., et al. 2005. Composition and assembly of STAT-targeting ubiquitin ligase complexes: paramyxovirus V protein carboxyl terminus is an oligomerization domain. J. Virol. 79:10180-10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vidy, A., M. Chelbi-Alix, and D. Blondel. 2005. Rabies virus P protein interacts with STAT1 and inhibits interferon signal transduction pathways. J. Virol. 79:14411-14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, J., et al. 2010. NF-κB RelA subunit is crucial for early IFN-β expression and resistance to RNA virus replication. J. Immunol. 185:1720-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokota, S., T. Okabayashi, N. Yokosawa, and N. Fujii. 2008. Measles virus P protein suppresses Toll-like receptor signal through up-regulation of ubiquitin-modifying enzyme A20. FASEB J. 22:74-83. [DOI] [PubMed] [Google Scholar]
- 49.Yoneyama, M., et al. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 50.Yoneyama, M., et al. 1996. Autocrine amplification of type I interferon gene expression mediated by interferon stimulated gene factor 3 (ISGF3). J. Biochem. 120:160-169. [DOI] [PubMed] [Google Scholar]