Abstract
Histone H1 is an abundant and essential component of chromatin whose precise role in regulating gene expression is poorly understood. Here, we report that a major target of H1-mediated regulation in embryonic stem (ES) cells is the X-linked Rhox homeobox gene cluster. To address the underlying mechanism, we examined the founding member of the Rhox gene cluster—Rhox5—and found that its distal promoter (Pd) loses H1, undergoes demethylation, and is transcriptionally activated in response to loss of H1 genes in ES cells. Demethylation of the Pd is required for its transcriptional induction and we identified a single cytosine in the Pd that, when methylated, is sufficient to inhibit Pd transcription. Methylation of this single cytosine prevents the Pd from binding GA-binding protein (GABP), a transcription factor essential for Pd transcription. Thus, H1 silences Rhox5 transcription by promoting methylation of one of its promoters, a mechanism likely to extend to other H1-regulated Rhox genes, based on analysis of ES cells lacking DNA methyltransferases. The Rhox cluster genes targeted for H1-mediated transcriptional repression are also subject to another DNA methylation-regulated process: Xp imprinting. Remarkably, we found that only H1-regulated Rhox genes are imprinted, not those immune to H1-mediated repression. Together, our results indicate that the Rhox gene cluster is a major target of H1-mediated transcriptional repression in ES cells and that H1 is a candidate to have a role in Xp imprinting.
Epigenetic mechanisms tightly control gene expression to regulate a wide variety of events, including placental function, embryonic growth, tissue differentiation, and tissue remodeling (2). The only known epigenetic modification of DNA in mammals is methylation (11). DNA methylation plays a key role in many gene silencing events, including X-chromosome inactivation, genomic imprinting, and silencing of retrotransposons and heterochromatin (2, 50). DNA methylation also has the potential to be responsible for dictating the cell type-specific and developmentally regulated pattern of expression of some genes, but this possibility has remained largely unproven. Most of the evidence that DNA methylation represses gene transcription in particular cell types, tissues, and developmental stages comes from descriptive analysis of DNA methylation patterns in vivo. When cause-and-effect studies have been performed, they have typically been performed in cell lines that do not fully correspond to normal cells and are sometimes grown under conditions that favor aberrant methylation or demethylation. Furthermore, the cell lines used in most studies are immortalized or malignant, and thus they rely on aberrant gene regulation to maintain their growth.
Despite the uncertainty as to whether DNA methylation has a significant role in controlling the tissue- and cell type-specific transcription of genes in mammals, it is clear that DNA methylation has important roles during embryonic mammalian development. Studies of mice lacking DNA methyltransferases (DNMTs) have provided evidence that genome-wide loss of DNA methylation during early vertebrate development allows the blastocyst to acquire access to a full complement of genes so as to maintain totipotency (37). There are three principal enzymes that are responsible for catalyzing DNA methylation in mammals. DNMT1 is responsible for maintaining the pattern of DNA methylation after DNA replication; its loss leads to embryonic lethality in all vertebrates examined to date, including mice (27). DNMT3A and DNMT3B are responsible for the de novo DNA methylation of specific genes in embryonic stem (ES) cells, trophoblast tissues, and primordial germ cells (42). Targeted mutation of Dnmt3a causes postnatal lethality, whereas loss of Dnmt3b elicits embryonic lethality, which occurs earlier during embryonic development in Dnmt3a/Dnmt3b double-knockout (KO) mice (42). While these studies strongly suggest that DNA methylation is important for vertebrate development, neither the key targets of DNA methylation nor the factors that target DNA methylation to specific developmental genes are known.
A component of chromatin that has recently been suggested to have a role in targeting DNA methylation to specific genomic sites is the linker histone H1 (7, 49). H1 binds to nucleosome core particles and protects an additional ∼20 bp of DNA (linker DNA) from nuclease digestion. The precise functions of H1 have proven difficult to define. In higher eukaryotes, H1 is nearly as abundant as nucleosome core particles, suggesting that H1 plays an important role in the structure of the chromatin fiber. In vitro studies indicate that two principal functions of linker histones are to stabilize the DNA entering and exiting the core particle and to facilitate the folding of nucleosome arrays into more compact structures (46, 59). Combined with other studies showing that H1 reduces nucleosome sliding and access to transcription factors in vitro, this has led to the view that H1 globally represses transcription. However, elucidation of the biological role of H1 in mammals has been difficult owing to the existence of at least 11 distinct H1 subtypes in mice. While these subtypes differ in primary sequence and expression during development, it is likely that some of these subtypes also act redundantly, as mice carrying targeted mutations of one or even two copies of some subtypes have no obvious phenotypic defects (6). In contrast, we found that KO of three histone H1 genes (H1c, H1d, and H1e) leads to a significant reduction in H1 level (∼50% of normal) and causes a broad range of developmental defects and embryonic lethality (7). ES cells lacking functional copies of these three H1 genes have a global decrease in nucleosome spacing (by ∼15 bp), reduced local chromatin compaction, and decreased levels of some core histone modifications (7). These effects might have been expected to cause global changes in gene expression, but instead gene expression profiling showed that only 29 genes exhibited significantly altered expression in response to H1 depletion. The majority of the affected genes are upregulated in response to reduced H1 levels, consistent with them being direct targets of H1-mediated repression. While the precise mechanism responsible for repression is not known, H1 appears to act on some of its targets by promoting DNA methylation. The primary evidence for this is that depletion of H1 caused a decrease in DNA methylation at the H19/Igf2 and Gtl2/Dlk1 imprinting control regions and alterations in the expression of genes regulated by these control regions (7). This suggested that a certain threshold of H1 is necessary to establish and/or maintain a subset of gene-specific DNA methylation patterns.
Here, we report a major target of H1-mediated repression: a newly discovered homeobox gene cluster on the mouse X chromosome. This reproductive homeobox (Rhox) gene cluster contains over 30 genes that are selectively expressed in postnatal and adult reproductive tissues (18, 31, 32, 38, 56). The founding member of this complex, Pem (Rhox5), is also expressed in a cell type- and tissue-specific manner during embryonic development (14, 30, 58). It is likely that Rhox5 has redundant roles with other Rhox genes during embryonic development, as targeted deletion of Rhox5 does not result in embryonic defects, but instead, male subfertility (31, 45). Indeed, virtually all Rhox genes are expressed in extraembryonic tissues (31, 32, 38), and most Rhox genes are also expressed in the embryo proper, including fetal germ cells (4, 21, 54, 55). While the function of most Rhox genes remains to be determined, their expression patterns, coupled with the fact that they all possess a homeobox, suggest that they encode DNA-binding transcription factors that contribute to the formation of the early embryo, the generation and function of extraembryonic tissue, and different stages of gametogenesis. The well-characterized Hox homeobox genes also function in formation of the early embryo, but they are quite different from the Rhox homeobox genes in both sequence and evolutionary origin. Hox genes are arranged in relatively ancient gene clusters preserved in a wide range of eukaryotic species, whereas the Rhox gene cluster appears to have arisen relatively recently in mammals from an ancestral homeobox gene related to the single-copy Drosophila melanogaster aristaless gene (9, 31, 32, 35). The Rhox genes are likely to have both conserved and species-specific roles in mammals, as human, mouse, and rat Rhox genes have similar, but not identical, patterns of gene expression in the early embryo and during gametogenesis (12, 31, 34, 57).
Here, we demonstrate that most of the genes in the mouse Rhox gene cluster are repressed by an H1-dependent mechanism in mouse ES cells. Remarkably, the particular Rhox genes regulated by H1 are the ones that we find are also subject to paternal X chromosome (Xp) imprinting in the placenta (50) and uniparental ES cells. Conversely, the few Rhox genes not subject to H1-mediated transcriptional repression escape Xp imprinting. Using Rhox5 as an example to investigate the underlying regulatory mechanism, we demonstrate that H1 promotes site-specific methylation of a key regulatory region in Rhox5 and that this methylation event inhibits transcription from one of Rhox5's alternative promoters. We identify a single CpG within this regulatory region as being responsible and identify a positive-acting transcription factor whose binding to the Pd is prevented by methylation of this CpG. Finally, we provide evidence that H1 also regulates the other genes in the Rhox cluster by promoting their methylation. We propose that the Rhox gene cluster is a useful model system to understand how regulators of chromatin structure control gene expression and genomic imprinting.
MATERIALS AND METHODS
Mice and cell lines.
Mus musculus molossinus mice were purchased from Jackson Laboratory. All animal experiments and maintenance were carried out according to NIH and AALAC guidelines at the M. D. Anderson Cancer Center vivarium. Testis and placental tissues were collected, and RNA was extracted from ES cell pellets by the Trizol method (Invitrogen, Carlsbad, CA) and converted to cDNA by using iScript reverse transcription (Bio-Rad, Hercules, CA). Full-length Rhox cDNA from Mus musculus molossinus mice was cloned into pGEM-T (Promega, Madison, WI) and sequenced to identify polymorphisms from our previously described Mus musculus musculus Rhox genes. Histone H1-depleted, Dnmt3A/B knockout, and uniparental ES cells were prepared as described previously (5, 7, 27).
PCR and EMSA analyses.
For quantitative reverse transcription (RT)-PCR (qPCR) analysis, total cellular RNA was extracted from ES cell pellets by the Trizol method (Invitrogen) and converted to cDNA by using iScript reverse transcription (Bio-Rad, Hercules, CA). Relative gene expression was determined by SYBR green incorporation in a Bio-Rad myCycler as described previously (31; data not shown). An electrophoretic mobility shift assay (EMSA) was performed as previously described using adult mouse testes extracts (1). A 32P-end-labeled probe was generated by annealing two complementary oligonucleotides (see Fig. 2E; Table 1). Two micrograms of goat polyclonal anti-GA binding protein (GABP) or goat IgG control (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used in the supershift assay.
TABLE 1.
Primer sequences used in this study
| Gene | No. | Strand | Sequencea | Description |
|---|---|---|---|---|
| Esx1 | 3248 | Se | 5′-GTTGAACAGAGAGTTCTCCAT-3′ | Cloning |
| 3249 | As | 5′-TCAGTATCTGTATCTGGGAC-3′ | qPCR | |
| 3250 | Se | 5′-CCCTCCAGGATTCAGAATG-3′ | qPCR/cloning | |
| G6PD | 3251 | Se | 5′-ATGGCAGAGCAGGTGGCC-3′ | Cloning |
| 3252 | As | 5′-TCAGAGCTTGTGAGGGTTC-3′ | qPCR | |
| 3253 | Se | 5′-CCTGATGCCTATGAACGCC-3′ | qPCR/cloning | |
| HPRT | 3254 | Se | 5′-ATGCCGACCCGCAGTCC-3′ | Cloning |
| 3255 | As | 5′-TTAGGCTTTGTATTTGGCTTT-3′ | qPCR | |
| 3256 | Se | 5′-TGAAGATATAATTGACACTGG-3′ | qPCR/cloning | |
| Lamp2 | 3257 | Se | 5′-ATGTGCCTCTCTCCGGTTA-3′ | Cloning |
| 3258 | As | 5′-CTAAAATTGCTCATATCCAGTA-3′ | qPCR | |
| 3259 | Se | 5′-GGGAAGTTCTTATATGTGCA-3′ | qPCR/cloning | |
| Mcts1 | 3260 | Se | 5′-ATGTTCAAGAAATTTGATGAAAAA-3′ | Cloning |
| 3261 | As | 5′-TCATTTATATGTCTTCATATGC-3′ | qPCR | |
| 3262 | Se | 5′-GATAAAGGAGCCATCAAATTT-3′ | qPCR/cloning | |
| Ndufa1 | 3263 | Se | 5′-ATGTGGTTCGAGATTCTCC-3′ | qPCR/cloning |
| 3264 | As | 5′-TTAGTCAATGTTTTCCAGGC-3′ | qPCR/cloning | |
| Sept6g | 3265 | Se | 5′-ATGGCAGCGGCCGATATAG-3′ | Cloning |
| 3266 | As | 5′-TTAAAAAAAGTTCTTCTTCTCTTT-3′ | qPCR | |
| 3267 | Se | 5′-GATGTTTGTCCAGAGAGTCA-3′ | qPCR/cloning | |
| Rhox5 (Pd) | 4374 | Se | 5′-GGGTCTTCCGGGTCTCTGGAGGAA-3′ | WT |
| 4375 | As | 5′-TTCCTCCAGAGACCCGGAAGACCC-3′ | WT | |
| 4376 | Se | 5′-GGGTCTTCatGGTCTCTGGAGGAA-3′ | CpG mutant | |
| 4377 | As | 5′-TTCCTCCAGAGACCatGAAGACCC-3′ | CpG mutant | |
| 4378 | Se | 5′-GGGTCTTCoGGGTCTCTGGAGGAA-3′ | CpG methylation | |
| 4379 | As | 5′-TTCCTCCAGAGACCoGGAAGACCC-3′ | CpG methylation | |
| Se | 5′-TTTGTGTGTGTGTATATGTTTGTGTG-3′ | Bisulfite sequencing | ||
| As | 5′-CCTTAAATCTCTTTTCCTCCAAAA-3′ | Bisulfite sequencing | ||
| Rhox5 (CpG mutagenesis) | 3699 | 5′-GGTCTTCCHGGTCTCTGGAG-3′ | H = A or T or C at position −189 | |
| 3700 | 5′-CTCCAGAGACCDGGAAGACC-3′ | D = T or A or G | ||
| 3834 | 5′-GGGCCAATTGCTRGGTGGAAGGAAAAGG-3′ | Pd mutant at position −109 C-A/G | ||
| 3835 | 5′-CCTTTTCCTTCCACCYAGCAATTGGCCC-3′ | Pd mutant at position −109 C-A/G | ||
| 3836 | 5′-GGGCCAATTGCTCHGTGGAAGGAAAAGG-3′ | Pd mutant at position −109 G-C/A/T | ||
| 3837 | 5′-CCTTTTCCTTCCACDGAGCAATTGGCCC-3′ | Pd mutant at position −109 G-C/A/T |
Bold font indicates variable nucleotides as shown in the last column; lowercase letters indicate mutated nucleotides.
ChIP analysis.
Chromatin immunoprecipitation (ChIP) analysis was performed according to the protocol suggested by Millipore with minor modifications. Cells were cross-linked with 1% formaldehyde for 10 min at 37°C, and then the reaction was stopped by adding glycine for 5 min at room temperature. After being washed twice with cold phosphate-buffered saline (PBS), cells were then scraped off, resuspended in lysis buffer (50 mM Tris-HCl [pH 8.0], 10 mM EDTA, 1% SDS, and protease inhibitor cocktail), and incubated on ice for 10 min. Subsequently, chromatin was fragmented by sonication on ice using a Sonic Dismembrator MDL100 (Fisher Scientific), each for 30 s four times at 30% amplification. Soluble fractions were collected by centrifugation at 14,000 rpm in a bench-top centrifuge for 10 min at 4°C and were diluted 10-fold with dilution buffer (16.7 mM Tris-HCl [pH 8.0], 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, 0.01% SDS, and protease inhibitor cocktail). The diluted fractions were treated with protein A and G-agarose-salmon sperm DNA (Millipore) for 1 h at 4°C to preclear chromatin and then incubated with primary antibodies at 4°C overnight. The chromatin-antibody complex was incubated with protein A and G agarose/salmon sperm DNA for 1 h at 4°C. The beads were washed once with low-salt buffer (20 mM Tris-HCl [pH 8.0], 2 mM EDTA, 1% Triton X-100, 0.1% SDS, and 150 mM NaCl), once with high-salt buffer (20 mM Tris-HCl [pH 8.0], 2 mM EDTA, 1% Triton X-100, 0.1% SDS, and 500 mM NaCl), once with LiCl buffer (10 mM Tris-HCl [pH 8.0], 0.25 M LiCl, 1% IPEGAL CA630 [Nonidet P-40], 1% Na-deoxycholate, and 1 mM EDTA), and twice with Tris-EDTA (TE) buffer (10 mM Tris-HCl [pH 8.0] and 1 mM EDTA). All buffer used was supplemented with protease inhibitor cocktail. The beads were incubated with elution buffer (1% SDS and 0.1 M NaHCO3). To reverse protein-DNA cross-link, the NaCl concentration of the eluted sample was adjusted to 0.2 M, followed by incubation at 65°C for 4 h. The sample was then further incubated with proteinase K (100 μg/ml) for 2 h at 45°C. DNA was purified by phenol chloroform extraction and resuspended in TE buffer. DNA was quantified by real-time PCR as described previously (52). The primer sequences used for this assay are listed in Table 1.
Bisulfite sequencing and methylation analysis.
Genomic DNA was extracted using the DNeasy tissue kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Two micrograms of genomic DNA was denatured in 0.4 N NaOH for 10 min in a 50-μl volume and then deaminated by diluting it in 520 μl buffer containing 4.5 M sodium bisulfite and 0.5 mM hydroquinone (pH 5.0) and incubating it at 50°C for 16 h in the dark with mineral oil on top. The bisulfite-treated DNA was purified using the Wizard DNA cleanup kit (Promega, Madison, WI), and the minimal Pd promoter was amplified using primers designed using the MethPrimer software indicated in Table 1 (28). Touchdown PCR analysis was done with various annealing temperatures (3 cycles at 60°C, 5 cycles at 57°C, 7 cycles at 53°C, and 22 cycles at 51°C) in a 50-μl reaction volume consisting of 200 μM deoxynucleoside triphosphates (dNTPs), 1 unit of AmpliTaq DNA polymerase (Roche Applied Science), 1× buffer [16.6 mM (NH4)2·SO4; 67 mM Tris, pH 8.8; 6.7 mM MgCl2; 10 mM β-mercaptoethanol], and 0.2 μM each primer. All cycles included a denaturing step (94°C) for 1 min and an extension step (72°C) for 45 s. DNA from a minimum of 3 separate PCRs per cell line was mixed, purified by gel elution using the QIAquick gel extraction kit (Qiagen, Valencia, CA), and cloned into the pGEM-T Easy vector (Promega Inc., Madison, WI). Ligation products were transformed and plated on LB-Amp plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and IPTG (isopropyl-β-d-thiogalactopyranoside). White colonies were selected, and positive clones (determined by EcoRI digestion) were sequenced.
Imprinting analysis.
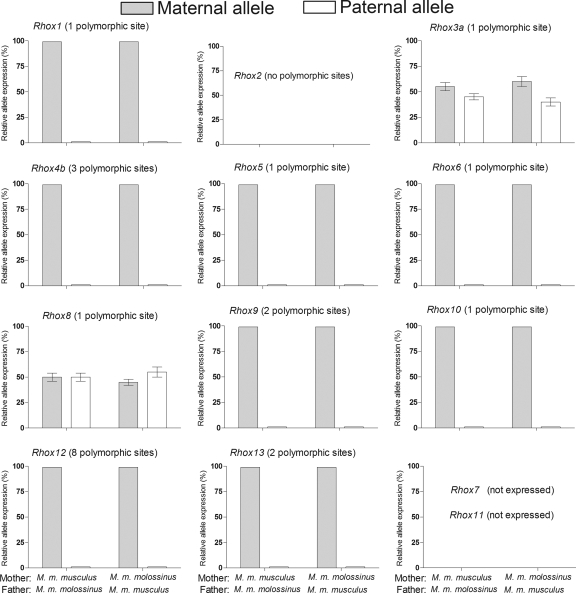
To examine parent-of-origin effects on differential expression of Rhox genes in placenta cells, Rhox genes were cloned and sequenced from Mus musculus molossinus placental and adult testis mRNA samples. Polymorphisms were identified in the coding sequence for every Rhox gene except Rhox2, which was identical in sequence in all paralogs examined. Paralog-specific polymorphisms were identified for Rhox3a, Rhox3c, Rhox3d, and Rhox4b that were clearly distinct from paralog-to-paralog sequence variations (32). We detected no polymorphisms in the control genes, Sept6, Ndufa1, Lamp2, Mcts1, Hprt1, and G6pdx, used for our studies with H1- and Dmnt-KO ES cells. To examine extraembryonic imprinting, placental tissues derived from Mus musculus molossinus and Mus musculus musculus crosses were obtained from Jackson Laboratory (6 mice from each cross) and the RNA was purified as previously described (30), converted to cDNA using Bio-Rad iScript RT kit, and amplified using specific primer pairs. RT-PCR products were subjected to direct sequencing using an Applied Biosystems 3730xl DNA sequencer, and colorimetric DNA sequence traces were examined for relative allele expression at the polymorphic positions. These analyses produced two outcomes: a single peak corresponding to the solely expressed polymorphic base pair or a mixture of two peaks corresponding to the presence of mRNA encoding both polymorphic base pairs. This method of analysis demonstrates the complete differential expression of the Rhox gene (or lack thereof) throughout the placental tissue without concerns for random selection of cDNA clones from a library that might be influenced by mRNA from cells of a maternal origin.
RESULTS
Site-specific demethylation and induction of the Rhox5 distal promoter in response to histone H1 depletion.
We previously described the generation of mouse ES cells depleted of H1 as a result of homozygous deletion of the three most highly expressed H1 genes (H1c, H1d, and H1e) in male-derived ES cells (7). Gene expression profiling of these triple H1 knockout (H1-TKO) ES cells revealed that the expression of a surprisingly small number of genes is affected. Among the 19 genes suggested by microarray analysis to be upregulated in level by 2-fold or more were two Rhox genes: Rhox5 and Rhox6 (7). Here, we verified this regulation, extended the regulation to other Rhox cluster genes, and elucidated the underlying mechanism. As a first step to examine the underlying mechanism for H1-mediated repression of Rhox gene expression, we chose to focus on Rhox5, as it is the only Rhox gene whose promoters have been defined in detail (1, 33). Rhox5 has two promoters: a distal promoter (Pd) expressed during embryogenesis and in the adult ovary, and a proximal promoter (Pp) expressed in somatic cells in adult testes and epididymides (Fig. 1A) (1, 33). Consistent with the expression of Pd, but not Pp, during mouse embryogenesis in vivo, quantitative RT-PCR (qPCR) analysis revealed that ES cells express Pd transcripts and almost no Pp transcripts (Fig. 1B). Pd expression was strongly upregulated as a result of H1 depletion (Fig. 1B). This effect of H1 is specific to the Pd, as Pp transcripts were not significantly upregulated in response to H1 depletion (Fig. 1B).
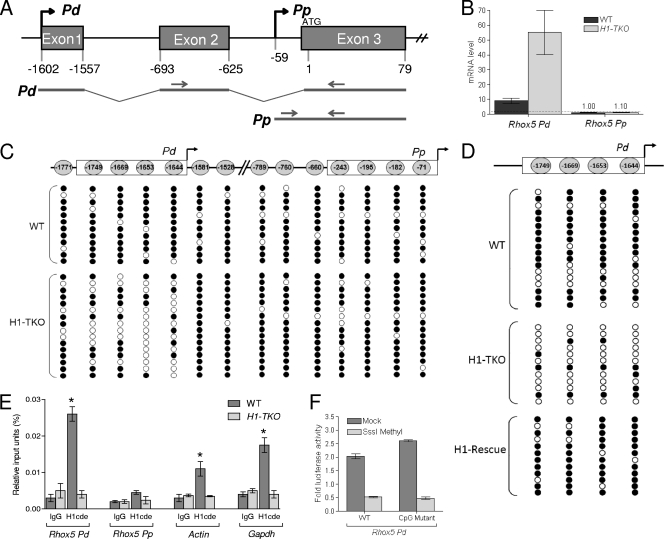
FIG. 1.
H1 promotes Rhox5 Pd methylation and transcriptional repression. (A) Schematic of Rhox5's two tissue-specific promoters. Nucleotide sequences are numbered relative to the translational start site, arbitrarily set to 1, which is shared by transcripts derived from both the distal (Pd) and proximal (Pp) promoters. Arrows indicate the relative positions of oligonucleotides used to distinguish Pd- and Pp-derived transcripts and ChIP signals. (B) qPCR analysis of total cellular RNA from wild-type (WT) and H1-depleted (H1-TKO) ES cells, normalized against L19 mRNA. (C) Methylation status of the CpGs in the Pd and Pp in the indicated ES cells. CpG positions are relative to the translation start site, which is shared by transcripts derived from either promoter. (D) Expression of H1D restores methylation of the Pd in H1-TKO ES cells. Results from bisulfite sequencing analysis of the indicated ES cells are shown (at least 16 cDNA clones were examined for each CpG). (E) ChIP analysis of H1 recruitment in the indicated ES cells. The negative control is immunoglobulin (IgG). (F) Luciferase activity from mouse ES cells transiently transfected with a WT Pd-Renilla luciferase reporter construct (WT; Pem-264) or a triple mutant version of this construct (CpG mutant) that has nucleotide substitutions at the −1749, −1669, and −1653 CpGs and thus only has the −1644 CpG. The Pd region in these two constructs was methylated in vitro with SssI methylase or mock treated, ligated in bulk into the renilla luciferase vector, and then cotransfected with a Firefly luciferase plasmid into ES (AB2.2) cells. Shown are mean values ± standard errors (SE) from three transfection experiments assayed in duplicate, normalized against Firefly luciferase.
We previously obtained evidence that one molecular mechanism by which H1 represses transcription is promoting DNA methylation. The primary evidence for this was the finding that depletion of H1 in ES cells led to hypomethylation of the imprinting control regions of the H19 and Gtl2 loci, as well as increased expression of resident genes within these loci (7). As a first step to determine whether H1 also represses Rhox5 transcription in a DNA methylation-dependent manner, we examined the methylation status of the Rhox5 promoters by bisulfite/sequencing analysis. The minimal region of the Pd that we previously showed is required for expression in transfected cell lines (33, 48) has four CpGs (Fig. 1C). We found that these four CpG sequences in the minimal Pd are hypermethylated in wild-type (WT) ES cells and that they become hypomethylated in H1-depleted ES cells (Fig. 1C). To determine whether this is reversible, we stably transfected the H1-TKO ES cells with an expression construct encoding H1d, the last gene inactivated during the sequential gene targeting procedure used to produce the H1-TKO ES cells (7). Several “H1 rescue” cell clones were obtained that had levels of H1D comparable to those of wild-type ES cells (B. J. Yang, B. J. Kim, and A. I. Skoultchi, unpublished data). Analysis of these cells showed that restoration of H1D levels also restored the Pd to a hypermethylated state (Fig. 1D).
The DNA demethylation event triggered by H1 depletion is specifically targeted to the Pd minimal promoter, as neither the immediately adjacent CpGs, nor the CpGs in the minimal Pp that we previously defined in vivo (48), are demethylated as a result of H1 depletion (Fig. 1C). To address whether H1 acts directly to promote methylation of the Pp, we performed ChIP analysis. We found that H1 was present at high levels at the Pd in control ES cells but was at background levels at the Pd in H1-TKO cells, consistent with the notion that H1 directly promotes methylation of the Pd (Fig. 1E). The level of H1 at the Pd was modestly higher than that at the β-actin and Gapdh loci (Fig. 1E), which had an average magnitude of H1 occupancy, based on our analysis of ∼30 gene loci (data not shown). H1 is recruited specifically to the Pd, as the Pp had undetectable levels of H1, even in control cells (Fig. 1E). We conclude that H1 specifically promotes methylation and silencing of the Pd.
Methylation of a single cytosine in the Rhox5 distal promoter is sufficient to inhibit its transcription.
To assess whether the methylation of the Pd has a causal role in inhibiting Pd transcription, we used a cassette methylation protocol in which the promoter of interest is in vitro methylated using SssI methylase, ligated with high efficiency into a reporter vector, and then transfected into cells to measure reporter activity (24). Using this procedure, we showed that targeted methylation of the Pd abolished ∼80% of its transcriptional activity in transfected ES cells (Fig. 1F). These data, combined with the methylation pattern profile of H1-depleted and H1-restored ES cells (Fig. 1C and D), strongly suggests that H1 has an essential role in a DNA methylation-dependent pathway that transcriptionally represses the Pd.
To determine which of the 4 CpGs in the Pd have a role in this repression mechanism, we first made single mutants at these 4 positions and then tested their transcriptional activities in the reporter vector (described above) in response to SssI versus mock treatment. Single mutants at three of the positions (−1653, −1669, and −1749 relative to the translation start site in exon 3 [Fig. 1A]; each with the cytosine replaced by a randomly selected purine) exhibited strongly repressed expression in response to SssI treatment (to a similar magnitude as that for the wild-type construct, as tested in the 10T1/2 mesenchymal stem cell line [data not shown]). This indicated that these three cytosines are not involved in methylation-dependent repression of Rhox5 Pd transcription. Mutation of the one remaining cytosine—at −1644—led to strongly reduced luciferase activity of the reporter construct, precluding analysis of the effect of DNA methylation (we found strongly reduced reporter expression regardless of whether we mutated the −1644 C or the −1643 G to any of the alternative nucleotides; i.e., 6 independent mutations [data not shown]). As an alternative approach to test the functional role of the −1644 cytosine, we made a triple mutant that lacked the other 3 cytosines and thus had only the −1644 cytosine. This triple mutant exhibited reduced reporter activity in ES cells after being methylated in vitro; the reduction in reporter activity was comparable to that of the methylated wild-type construct (Fig. 1F). Thus, methylation of the −1644 cytosine is sufficient to strongly repress Pd transcription.
DNA methylation of the −1644 cytosine in the Rhox5 distal promoter prevents the ETS factor GABP from activating Rhox5 transcription.
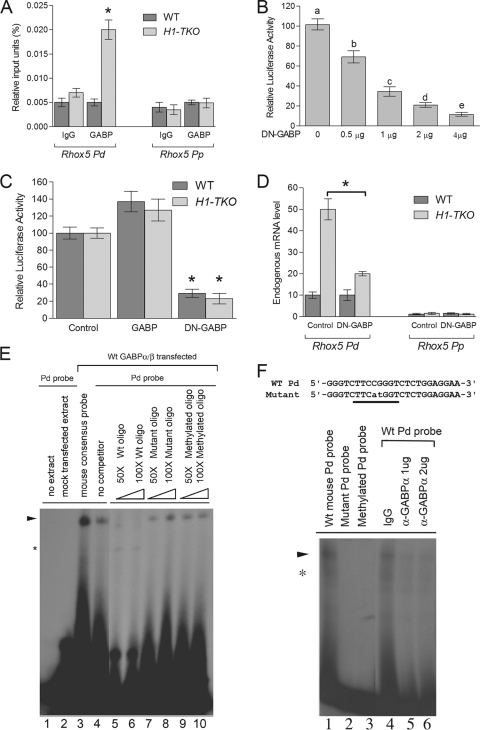
The −1644 cytosine overlaps with the binding site for the ETS factor GABP, which we previously showed was essential for Pd transcription in tumor cells, 10T1/2 mesenchymal stem cells, and primary granulosa cells (33, 47). This, along with the finding that H1-TKO ES cells exhibit reduced cytosine methylation at the −1644 position (Fig. 1B), led us to formulate a simple model in which H1 elicits transcriptional repression of the Pd by virtue of its ability to promote methylation of the −1644 cytosine, which in turn prevents recruitment of the Pd activator GABP. This model predicts that H1-depleted cells, which have a demethylated Pd, will have increased GABP occupancy at the Pd. Indeed, ChIP analysis revealed enrichment of GABP at the Pd in H1-TKO cells but not in control cells (Fig. 2A). To address whether GABP promotes Pd transcription in ES cells, we examined the effect of dominant negative (DN) forms of GABP-α and GABP-β on Pd-driven reporter expression (GABP normally forms an active heterotetramer of two subunits). We found that DN-GABP-α/β repressed Pd-driven luciferase expression in both H1-TKO and control ES cells (Fig. 2B and C), indicating that GABP is a positive regulator of Pd transcription in ES cells. We then examined the effect of DN-GABP-α/β on expression of the endogenous Pd. We found that DN-GABP-α/β repressed endogenous Pd expression in H1-TKO cells but not in control ES cells (Fig. 2D). The GABP-dependent expression of the Pd in H1-TKO cells is consistent with the fact that these cells harbor a hypomethylated GABP-binding site (−1644 position) (Fig. 1B), which we found recruits GABP, as determined by ChIP analysis (Fig. 2B). The GABP-independent expression of the Pd in control cells is consistent with the fact that these cells have a hypermethylated GABP-binding site (Fig. 1B) that does not detectably recruit GABP (Fig. 2A). The inability of control ES cells to recruit GABP also explains why these cells expressed only basal levels of Pd-derived transcripts (∼3% as much as H1-TKO cells) (data not shown). Finally, we note that we found that the effect of DN-GABP-α/β was specific, as it affected only Pd expression, not Pp expression (Fig. 2D). We conclude that GABP is a positive activator of the Pd in ES cells when this promoter is in a competent (hypomethylated and H1-depleted) state.
FIG. 2.
GABP selectively activates the Rhox5 Pd in a methylation-dependent manner. (A) ChIP analysis of GABP recruitment in the indicated ES cells. The negative control is immunoglobulin (IgG). (B) Luciferase activity from mouse WT ES cells transiently transfected with the Pd-Renilla luciferase reporter construct (Fig. 1F), Firefly luciferase internal control plasmid, and dominant negative (DN)-GABP-α and -β plasmids (the amount of the last two plasmids is indicated). The data are means ± SE from two transfection experiments, each with triplicate cultures for each condition, normalized against Firefly luciferase. Letters denote treatments that have statistically significant (P < 0.001) different levels from each other. (C) Luciferase activity from the indicated ES cells transiently transfected as described for panel B with either DN-GABP-α and -β plasmids (4 μg), WT GABP-α and -β plasmids, or the empty pCMX expression vector (4 μg). The data are means ± SE from two transfection experiments, each with triplicate cultures for each condition, normalized against Firefly luciferase (*, P < 0.005). (D) qPCR analysis of total cellular RNA from the indicated ES cells transiently transfected as described for panel C. The data are means ± SE from two transfection experiments, each with triplicate cultures for each condition, normalized against GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA (*, P < 0.005). (E) EMSA analysis showing GABP binding to the Pd. Extracts from mouse ES (mES) cells transfected with GABP-α/β expression vectors were incubated with probes containing either a consensus GABP site or the GABP-binding region in the Pd. The indicated cold competitor probes were incubated with the extract and hot probe shown in lanes 5 to 10. The presence of the major shifted complex is indicated with an arrow. *, a nonspecific product. (F) EMSA analysis showing GABP binding to the Pd. (Top) Pd probe harboring the GABP-binding site (underlined) corresponding to the region −1652 to −1629 upstream of the start ATG (indicated in Fig. 1A). The inactivating mutations (Mut) that were introduced in the mutant oligonucleotide probes are indicated by lowercase letters. (Bottom) EMSA analysis of the mouse Pd probe incubated with mES cell nuclear extracts. The presence of the major shifted complex is indicated with an arrow. *, a nonspecific product. Lanes 4 to 6 show pretreatment with anti-GABP-α antibody or control goat IgG.
To directly test whether DNA methylation inhibits GABP recruitment, we performed EMSA analysis with an oligonucleotide containing the portion of the Pd harboring the GABP-binding site. We first verified our previous analysis (47) demonstrating that GABP binds to this Pd site. Using lysates from cells transfected with expression plasmids encoding the α and β GABP subunits, we found that the Pd oligonucleotide probe generated a single shifted band (Fig. 2E, lane 4). Several lines of evidence indicated that this band contains GABP. First, it was not present when we used lysates from mock-transfected cells (Fig. 2E, lane 2). Second, a band with an identical migration pattern was generated with an oligonucleotide probe harboring a consensus GABP-binding site (Fig. 2E, lane 3). Third, this band was eliminated when the reaction mixture was incubated with wild-type cold competitor Pd probe (Fig. 2E, lanes 5 and 6), but was not eliminated when incubated with mutant cold competitor Pd probe (with the GABP site mutated) (Fig. 2E, lanes 7 and 8). Fourth, the mutant Pd probe did not detectably generate the band (Fig. 2F, lane 2). Finally, the intensity of this band was decreased by coincubation with a GABP-α antisera (Fig. 2F, lanes 5 and 6). The only cytosine in a context to be methylated in this Pd oligonucleotide probe is in the core of the GABP-binding site (position −1644) (Fig. 2C). To elucidate whether methylation of this −1644 cytosine interferes with GABP binding, we in vitro methylated it. We found that methylation of the −1644 cytosine prevented the Pd from binding to GABP (Fig. 2F, lane 3), and it inhibited the ability of the Pd probe to compete with the nonmethylated Pd probe for GABP binding (Fig. 2E, lanes 9 and 10). We conclude that methylation of the −1644 cytosine potently inhibits binding of GABP to the Pd, at least in vitro.
Selective repression of the Rhox homeobox gene cluster by histone H1.
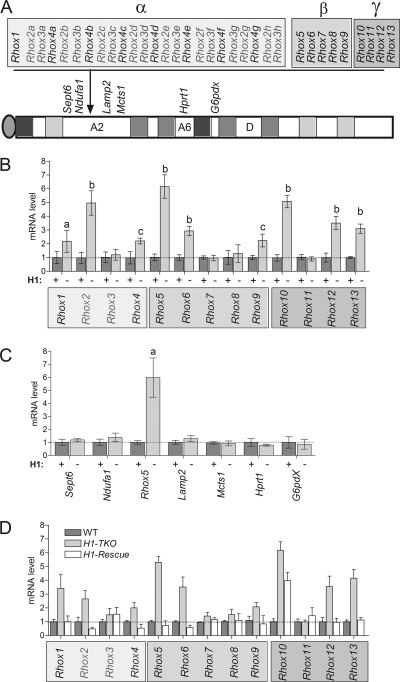
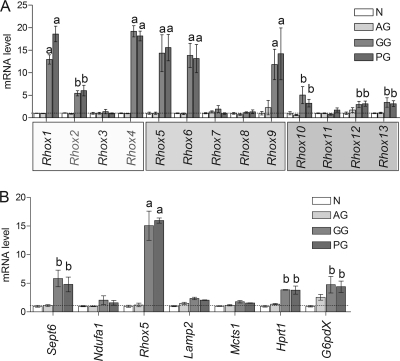
To determine whether other members of the Rhox gene cluster are regulated by H1, we compared the mRNA levels of other Rhox family members in wild-type and H1-TKO ES cells. Figure 3A shows a schematic diagram of the mouse Rhox gene cluster. This gene cluster contains single copies of nine genes (Rhox1 and Rhox5 to Rhox13) and several highly related copies (paralogs) of three genes (Rhox2, Rhox3, and Rhox4). For qPCR analysis we used primer pairs specific for each of the single-copy genes and primers possessing 100% complementarity with all members of the Rhox2, Rhox3, and Rhox4 paralog families. Figure 3B shows that most of the Rhox genes were upregulated in the H1-TKO ES cells. Our previous microarray analysis (7) did not identify these Rhox genes as being upregulated in H1-TKO ES cells because either they were not annotated in the microarray, did not have probes in the microarray, or were expressed at too low a level for accurate detection by microarray analysis. In addition to several of the single-copy Rhox genes, one or more members of the Rhox2 and Rhox4 paralog families were upregulated. Although distinguishing the mRNAs for most individual paralog family members was not possible because their sequences are almost identical (18, 31, 32, 38, 56), we were able to design a specific primer set for Rhox4c (Ehox), which had previously been shown to be upregulated during ES differentiation (17), and found that Rhox4c mRNA was upregulated in H1-TKO ES cells (data not shown). The only Rhox genes that are not upregulated in H1-TKO ES cells are Rhox3, Rhox7, Rhox8, and Rhox11.
FIG. 3.
H1 reversibly represses the expression of Rhox cluster genes. (A) Schematic of the Rhox gene cluster, immediately neighboring genes (Sept6, Ndufa1, Lamp2, and Mcts1), and distant genes (Hprt1 and G6pdx) on the mouse X chromosome. (B) qPCR analysis of Rhox gene expression levels in WT (+) and H1-TKO (−) ES cell clones (n = 5 clones per genotype). The expressions of the Rhox2, Rhox3, and Rhox4 gene paralogs (A) were detected with primers that were 100% complementary with all members of a given paralog set (e.g., the Rhox2 primer pair recognizes Rhox2a to Rhox2g). Relative gene expression was normalized to ribosomal Rpl19 transcripts which we previously demonstrated to be nonvariant in ES cells, and WT levels were arbitrarily given a value of 1. Letters indicate statistically significant differences from the control (a, P < 0.001; b, P < 0.005; c, P < 0.01). (C) qPCR analysis performed with the indicated genes as described for panel B. (D) qPCR analysis, performed with control, H1-TKO, and H1-TKO cell clones stably expressing an H1d transgene (H1-Rescue) as described for panel B (see the text for details).
To address the selectivity of H1-mediated repression, we examined genes adjacent to the Rhox gene cluster (Fig. 3A). qPCR analysis showed that neither genes just upstream of the α subcluster (Sept6 and Ndufa1) nor those just downstream of the γ subcluster (Lamp2 and Mcts1) had significantly altered expression in response to H1 depletion (Fig. 3C). To further evaluate selectivity, we examined expression of two genes distal to the Rhox gene cluster on the X chromosome—G6pdx and Hprt1—that are imprinted in bovine embryos (13, 44). Neither of these genes, nor an autosomal control gene—Itgb1bp1—exhibited significantly altered expression in H1-TKO ES cells. Collectively, our data indicate that H1-mediated repression is selectively directed toward genes in the Rhox gene cluster.
To determine whether activation of Rhox gene expression by H1 depletion is reversible, we examined Rhox gene expression in the H1 rescue clones described above. Analysis of these cells showed that restoration of H1D levels also fully restored repression of all the Rhox genes except Rhox10 (Fig. 3D shows the average values from several cell clones). Even though the Rhox10 mRNA level did not return to wild-type levels in the rescued clones, it was significantly reduced compared to that of H1-TKO cells. In striking contrast, Rhox genes whose expression was not upregulated by H1 depletion (Rhox3, Rhox7, Rhox8, and Rhox11) were not downregulated in the H1 rescue clones (Fig. 3D). We conclude that the activation of Rhox gene expression triggered by H1 depletion is a highly specific and reversible event.
Histone H1-regulated Rhox genes are repressed by DNA methyltransferases and imprinted.
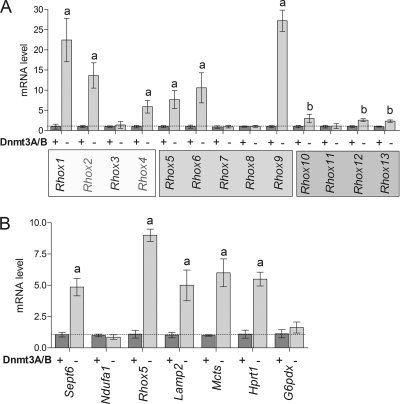
If H1 generally mediates transcriptional repression by promoting DNA methylation, this predicts that all the Rhox genes regulated by H1 will also be regulated by DNA methylation. To test this prediction, we examined Rhox gene expression in Dnmt3a/Dnmt3b-null ES cells, which lack de novo methylation activity and have an ∼50% reduction in global levels of DNA methylation (41, 42). Strongly supporting the model, we found that loss of Dnmt3a and Dnmt3b upregulated all of the H1-regulated Rhox genes (many by 5-fold or more) (compare Fig. 3B with Fig. 4). Conversely, the H1-independent Rhox genes (Rhox3, Rhox7, Rhox8, and Rhox11) were not upregulated in the Dnmt-deficient cells. These results are consistent with the notion that H1 selectively represses the transcription of a large but specific subset of homeobox genes in the Rhox gene cluster by a mechanism involving DNA methylation.
FIG. 4.
Loss of DNMT3A/3B upregulates the expression of H1-regulated Rhox genes. (A) qPCR analysis of Rhox gene expression in WT (+) and Dnmt3a/Dnmt3b-null (−) ES cells. (B) Effect of loss of DNMT3A/B on X-linked genes. qPCR analysis of the expression of Rhox5 and genes flanking the Rhox cluster (see legend to Fig. 1) in control or Dnmt3a/3b-KO ES cells. Letters indicate mean expression values that were significantly different from those of control cells (a, P < 0.001; b, P < 0.01).
Our previous microarray analysis revealed that known imprinted genes are overrepresented in the small group of genes with altered expression in the H1-TKO ES cells (7). This suggested the hypothesis that H1 preferentially targets imprinted genes. We therefore determined whether Rhox genes are subject to Xp imprinting, a form of genomic imprinting that selectively represses the paternal copy of X-linked genes in extraembryonic tissues (43). To assess this, we determined the allele-specific expression pattern of Rhox genes in placenta cells from Mus musculus musculus/Mus musculus molossinus hybrid mice. To assay the expression of M. musculus molossinus Rhox genes, we cloned and sequenced cDNAs corresponding to each M. musculus molossinus Rhox gene so that we could generate species-specific primers for qPCR analysis. Sequence analysis revealed nucleotide differences in the M. musculus molossinus and M. musculus musculus versions of most of the Rhox genes, including single members of the Rhox3 and Rhox4 paralog groups (Rhox3b and Rhox4c, respectively). We also found sequence differences in the Rhox2 paralog group between these two species, but we were not able to determine whether these represented species-specific differences or different Rhox2 paralogs, so we excluded Rhox2 from our analysis. We also excluded Rhox7 and Rhox11, as they were not detectably expressed in placenta cells using four distinct primer sets for each gene (31; data not shown).
Using primers specific for the M. musculus molossinus and M. musculus musculus versions of the Rhox genes, we found that most of the Rhox genes displayed exclusive expression from the maternal allele, not the paternal allele (Fig. 5). This was the case regardless of whether the maternal allele came from M. musculus molossinus or M. musculus musculus. The Rhox genes displaying this expression pattern (Rhox1, Rhox4b, Rhox5, Rhox6, Rhox9, Rhox10, and Rhox12) were precisely the same Rhox genes regulated by DNMT3a/3b and H1 in ES cells (Fig. 3B and Fig. 4). The only Rhox genes not preferentially expressed from the maternal allele in placenta cells, Rhox3b and Rhox8, were approximately equally expressed from the maternal and paternal alleles (Fig. 1). These two genes were also exempt from regulation by DNMTs (Fig. 4) and histone H1 (Fig. 3B). These results indicate that H1-mediated repression is specifically directed at imprinted Rhox genes.
FIG. 5.
H1-regulated Rhox cluster genes are paternally imprinted in placenta cells. To examine parent-of-origin effects on Rhox gene expression in placenta cells, Mus musculus molossinus and Mus musculus musculus mice were crossed and the relative expression of maternally and paternally specific polymorphic alleles was determined by direct cDNA sequencing of RT-PCR products from placental tissue (n = 6 for each cross). The expressions of Rhox7 and Rhox11 were not examined because they are not significantly expressed in placenta cells (31).
As another model system to examine monoallelic expression, we used uniparental ES cell lines that have either paternal or maternal chromosomes. We found that the Rhox genes subject to Xp imprinting had a higher level of expression (2.5- to ∼20-fold) in ES cells containing two sets of maternal chromosomes (from gynogenetic [GG] or parthenogenetic [PG] embryos) than in ES cells containing two sets of paternal chromosomes (from androgenetic [AG] embryos) (Fig. 6A). In contrast, the Rhox genes refractory to Xp imprinting in placenta cells did not exhibit significantly higher expression in the GG/PG-derived ES cells compared to that in the AG-derived ES cells (Fig. 6A). This result was confirmed by microarray analysis of uniparental ES clones, which showed that Rhox2a, Rhox4e, Rhox5, Rhox6, and Rhox9 were upregulated in the maternally derived (GG/PG) cell clones relative to the paternally derived (AG) cell clones (3- to 20-fold, varying by both gene and cell clone), whereas Rhox3a and Rhox11 exhibited similar expression in both maternally and paternally derived cell clones (data not shown). Thus, we observed a perfect concordance between parent-specific expression pattern of Rhox genes in uniparental ES and placenta cells in vivo. As a control, we tested other X-linked genes and found that many, but not all, displayed higher expression in maternal chromosome- than paternal chromosome-containing ES cells (Fig. 6B).
FIG. 6.
H1-regulated Rhox genes are preferentially expressed from the maternal allele in ES cells. (A and B) qPCR analysis of the indicated genes in uniparental ES cell clones obtained from blastocysts harboring 2 copies of male (AG) or female (GG or PG) genomes. “N” cells are normal (control) ES cells that harbor one maternal copy and one paternal copy of the genome. Data represent fold expression above WT ES cells, which was arbitrarily given a value of 1. Letters indicate mean values that were significantly different from control (N) cells (a, P < 0.001; b, P < 0.005).
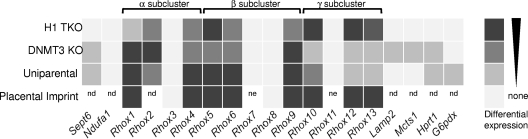
Figure 7 summarizes the expression pattern of the Rhox gene cluster, as determined in this paper. What is evident from inspection of this figure is that one can divide the Rhox genes into two distinct categories: (i) those repressed by histone H1 and DNA methylation and displaying allele-specific expression and (ii) those not repressed by histone H1 and DNA methylation and not displaying allele-specific expression.
FIG. 7.
H1-regulated genes are selectively subject to regulation by DNA methylation and imprinting control. The chart summarizes data from Fig. 1 to 6. The top two panels depict the relative derepression of each gene in response to depletion of H1 (H1 TKO) (Fig. 3) or loss of Dnmt3a/3b (DNMT3 KO) (Fig. 4). The bottom two panels depict differential expression between the paternal and maternal alleles in placenta cells (Fig. 5) and uniparental ES cells (Fig. 6). nd, not determined; ne, not expressed.
DISCUSSION
In this paper, we demonstrate that the Rhox homeobox gene cluster is a major target of H1-mediated repression in ES cells. Most of the single-copy Rhox genes and two of the three multiple-copy Rhox gene paralogs—which together represent 21 genes (Fig. 4A)—are upregulated in ES cells stably depleted of H1 to ∼50% of wild-type levels (Fig. 4B). Thus, our study clearly demonstrates that the genes in the Rhox cluster are particularly dependent on H1 for transcriptional repression; i.e., only a modest reduction in H1 level elicits their release from transcriptional silencing. The finding that H1 acts in a highly targeted manner to transcriptionally silence genes in mouse ES cells is consistent with the action of H1 in other organisms. For example, elimination of the linker histone in Tetrahymena protozoa in vivo leads to altered expression of only a small subset of polymerase II (Pol II)-derived transcripts and does not affect the steady-state levels of RNA Pol I- and III-derived transcripts (52). In Saccharomyces cerevisiae, deletion of the linker histone elicits a modest reduction in transcript levels (∼2-fold) from only a very small fraction of genes (15). Finally, Xenopus laevis embryos depleted of H1 have a specific defect in 5S rRNA gene expression rather than global changes in gene expression (3, 20). Together, these results indicate more specific roles for H1 in regulating gene transcription than was suggested by earlier in vitro studies (see the introduction).
Our paper provides several lines of evidence that H1 represses Rhox gene cluster expression through a DNA methylation-dependent mechanism. First, we show that the Rhox5 promoter expressed in ES cells—the Pd—is both demethylated and transcriptionally induced upon H1 depletion (Fig. 1A and B). This is a localized demethylation event, as it does not occur at the alternative Rhox5 promoter, the Pp, which remains inactive in ES cells even when H1 is depleted (Fig. 1A). Second, we found that Pd expression is also upregulated in Dnmt3a/Dnmt3b-null ES cells (Fig. 5). Together with the first line of evidence, this indicates that H1 promotes Pd methylation and that, in turn, methylation of the Pd has a causal role in transcriptionally silencing Rhox5. Third, we showed using a cassette methylation procedure that DNA methylation directly represses Pd transcription (Fig. 1F). Finally, we used mutational analysis coupled with the cassette methylation procedure to demonstrate that a single CpG in the Pd is responsible for the DNA methylation-dependent silencing of the Rhox5 gene. These data extend that of previous studies which suggested that Rhox5 is regulated by DNA methylation (19, 29, 40, 51). These studies all showed that blockade or elimination of DNMTs upregulates Rhox5 in certain cell types, but unlike the study herein, these previous studies did not address whether DNA methylation directly regulates Rhox5 transcription. Our paper also demonstrates which of Rhox5's two alternative promoters are targeted for repression by DNA methylation, and it identifies for the first time the specific CpGs involved. In particular, we identified a CpG within the binding site for GABP, a factor essential for Rhox5 Pd transcription (Fig. 1E). Indeed, we provided evidence that methylation of this site inhibits Pd transcription by inhibiting GABP recruitment (Fig. 2). We propose that H1 also represses other genes in the Rhox cluster in ES cells through a mechanism involving DNA methylation. While there is no direct evidence for this model (i.e., the involvement of GABP at other Rhox promoters has not been examined), it is supported by our finding that all H1-regulated Rhox genes are subject to DNA methylation control, whereas all Rhox genes immune to H1 regulation are immune to regulation by DNA methylation (based on studies of Dnmt3a/Dnmt3b-null ES cells) (Fig. 5 and 7). It is also supported by the evidence from other laboratories that some Rhox cluster genes are subject to DNA methylation control in cell types other than ES cells (10, 29, 40).
Crucial questions for the future revolve around determining what mechanisms control the specificity of H1-mediated repression. For example, what is the molecular mechanism by which H1 promotes the methylation of specific CpGs within a select set of genes within ES cells? Since H1 is present at a very large number of loci throughout the genome, it is not clear how it would lead to recruitment of repressive transcriptional complexes to specific loci. One possibility is that H1 forms complexes with sequence-specific DNA binding proteins and other proteins (25, 39). Thus, these interactions might target DNMTs to specific H1-containing loci in chromatin. A possible contributing factor is that targets of H1 are marked by inhibitory isoforms of H3 and H4 that are ultimately displaced by H1 (11). Another question is: how general is the ability of H1 to promote DNA methylation-dependent transcriptional repression? One possibility is that H1 is responsible for promoting the methylation and transcriptional repression of only a select set of genes in ES, including most Rhox cluster genes, but excluding most other genes transcriptionally repressed by DNA methylation in ES cells. In support of this, we identified several genes regulated by DNMTs whose expression is not affected by depletion of H1, including one gene just upstream of the Rhox gene cluster (Sept6), two downstream genes (Lamp2 and Mcts1), and one X-linked gene distant from the Rhox gene cluster (Hprt1) with known parent-of-origin effects (Fig. 4A and B) (13). An alternative possibility is that H1 actually has widespread repressive effects on gene expression in ES cells even though we observed only selective gene regulatory effects in H1-TKO cells. H1 protein levels are reduced to only ∼50% of the normal level in H1-TKO cells, and thus it is possible that a more dramatic reduction in H1 levels will impact a greater number of genes, including some or all of those repressed by DNA methylation in ES cells. While this is an attractive idea, it is technically difficult to address since there are several other H1 genes besides the three we targeted for mutation and because a further reduction in H1 levels could have broad pleiotropic and toxic effects in ES cells. Indeed, it is clear that even a 50% reduction in the level of H1 has profound effects, as it causes severe biological defects in mice in vivo (8) and elicits dramatic genome-wide alterations in chromatin structure in ES cells (7).
An unexpected finding of our study was the discovery that most Rhox cluster genes exhibit paternal allele-specific repression in both placenta and ES cells (Fig. 5 and 6). While the placenta and other extraembryonic tissues are known to specifically repress the paternal copy of X-linked genes (36, 53), ES cells are derived from the epiblast and thus would not necessarily be expected to transcriptionally repress paternal copies of X-linked genes (5). It will be interesting to determine whether other genes on the X chromosome display this unusual form of Xp imprinting. Another surprising outcome of our study was the discovery that the particular members of the Rhox gene cluster that undergo Xp imprinting are also subject to H1- and DNA methylation-dependent transcriptional silencing (Fig. 6). As described above, the finding that most Rhox genes are transcriptionally silent on the Xp conforms to the rule established in the 1970s that the Xp is preferentially inactivated in mouse extraembryonic tissue (36, 53). What is surprising is that some Rhox genes escape this form of imprinting and that these specific Rhox genes are the ones that also escape regulation by H1 and DNA methylation (Fig. 7). The “escapers” were not confined to a particular region of the Rhox cluster, leading to the question of how this “checkerboard” pattern of expression within the Rhox cluster is achieved. Experiments addressing this issue may lead to a better understanding of how local versus global gene repression is achieved; i.e., how gene-specific regulation occurs in the context of broader regulation exerted on an entire gene cluster. Of note, X-chromosome inactivation is known to not be absolute in the mouse placenta, with ∼15% of X-linked genes escaping Xp imprinting (26). While it is clear that Xp imprinting serves as a means to impart dosage compensation, it is not known how or why it occurs preferentially on the paternal allele and why some Rhox genes escape this form of imprinting. One possible function for Xp imprinting is that it reduces the frequency of mutations, as the paternal genome undergoes many more mitotic divisions during spermatogenesis than does the maternal genome during oogenesis (22). Another non-mutually exclusive function for Xp imprinting is that it reduces the risk of immune rejection of the placenta because it prevents the expression of paternal alleles of X-linked genes, many of which could encode immunogenic proteins (22). Our finding that the paternal copy of Rhox5 is silent in placenta cells (Fig. 6) is intriguing in light of the finding that the paternal copy of Rhox5 is preferentially expressed in the early embryo (23). Whether Rhox5 is differentially imprinted in different cell lineages, as appears to be the case for the Grb10 gene (60), or whether it instead undergoes an “imprint switch” as cells differentiate remains to be determined. While we showed a strong linkage between H1-mediated regulation and Xp imprinting, it remains for future studies to determine whether these two events have a cause-and-effect relationship. We believe this is likely, as genomic imprinting is known to be mediated by DNA methylation (2), which we show herein is, in turn, promoted by H1 (Fig. 1). If indeed H1 is involved in Xp imprinting, this will have broad implications, as this form of imprinting occurs in extraembryonic tissues of several mammals and in all tissues of marsupials (16).
Acknowledgments
We thank Naohiro Terada (University of Florida) for providing mRNA from Dnmt3A/3B-null ES cells.
J.M., S.S., A.B., and M.F.W. were supported by NIH grants HD53808 and HD45595. J.M. was supported by NIH grant HD55268. S.-M.Y., B.J.K., Y.F., and A.I.S. were supported by NIH grant CA079057.
Footnotes
Published ahead of print on 18 January 2011.
REFERENCES
- 1.Bhardwaj, A., M. K. Rao, R. Kaur, M. R. Buttigieg, and M. F. Wilkinson. 2008. GATA factors and androgen receptor collaborate to transcriptionally activate the Rhox5 homeobox gene in Sertoli cells. Mol. Cell. Biol. 28:2138-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird, A. 2002. DNA methylation patterns and epigenetic memory. Genes Dev. 16:6-21. [DOI] [PubMed] [Google Scholar]
- 3.Bouvet, P., S. Dimitrov, and A. P. Wolffe. 1994. Specific regulation of Xenopus chromosomal 5S rRNA gene transcription in vivo by histone H1. Genes Dev. 8:1147-1159. [DOI] [PubMed] [Google Scholar]
- 4.Daggag, H., et al. 2008. The Rhox homeobox gene family shows sexually dimorphic and dynamic expression during mouse embryonic gonad development. Biol. Reprod. 79:468-474. [DOI] [PubMed] [Google Scholar]
- 5.Eckardt, S., et al. 2007. Hematopoietic reconstitution with androgenetic and gynogenetic stem cells. Genes Dev. 21:409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan, Y., et al. 2003. H1 linker histones are essential for mouse development and affect nucleosome spacing in vivo. Mol. Cell. Biol. 23:4559-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan, Y., et al. 2005. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell 123:1199-1212. [DOI] [PubMed] [Google Scholar]
- 8.Fan, Y., and A. I. Skoultchi. 2004. Genetic analysis of H1 linker histone subtypes and their functions in mice. Methods Enzymol. 377:85-107. [DOI] [PubMed] [Google Scholar]
- 9.Ferrier, D. E., and P. W. Holland. 2001. Ancient origin of the Hox gene cluster. Nat. Rev. Genet. 2:33-38. [DOI] [PubMed] [Google Scholar]
- 10.Fouse, S. D., et al. 2008. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell 2:160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan, Q., T. Yoshida, O. G. McDonald, and G. K. Owens. 2007. Concise review: epigenetic mechanisms contribute to pluripotency and cell lineage determination of embryonic stem cells. Stem Cells 25:2-9. [DOI] [PubMed] [Google Scholar]
- 12.Geserick, C., B. Weiss, W. D. Schleuning, and B. Haendler. 2002. OTEX, an androgen-regulated human member of the paired-like class of homeobox genes. Biochem. J. 366:367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez-Adán, A., M. Oter, B. Martínez-Madrid, B. Pintado, and J. De La Fuente. 2000. Differential expression of two genes located on the X chromosome between male and female in vitro-produced bovine embryos at the blastocyst stage. Mol. Reprod. Dev. 55:146-151. [DOI] [PubMed] [Google Scholar]
- 14.Hamatani, T., M. G. Carter, A. A. Sharov, and M. S. Ko. 2004. Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell 6:117-131. [DOI] [PubMed] [Google Scholar]
- 15.Hellauer, K., E. Sirard, and B. Turcotte. 2001. Decreased expression of specific genes in yeast cells lacking histone H1. J. Biol. Chem. 276:13587-13592. [DOI] [PubMed] [Google Scholar]
- 16.Huynh, K. D., and J. T. Lee. 2005. X-chromosome inactivation: a hypothesis linking ontogeny and phylogeny. Nat. Rev. Genet. 6:410-418. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, M., et al. 2002. Cloning and characterization of Ehox, a novel homeobox gene essential for embryonic stem cell differentiation. J. Biol. Chem. 277:38683-38692. [DOI] [PubMed] [Google Scholar]
- 18.Jackson, M., et al. 2006. A murine specific expansion of the Rhox cluster involved in embryonic stem cell biology is under natural selection. BMC Genomics 7:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson-Grusby, L., et al. 2001. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat. Genet. 27:31-39. [DOI] [PubMed] [Google Scholar]
- 20.Kandolf, H. 1994. The H1A histone variant is an in vivo repressor of oocyte-type 5S gene transcription in Xenopus laevis embryos. Proc. Natl. Acad. Sci. U. S. A. 91:7257-7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang, Y. L., et al. 2004. A novel PEPP homeobox gene, TOX, is highly glutamic acid rich and specifically expressed in murine testis and ovary. Biol. Reprod. 70:828-836. [DOI] [PubMed] [Google Scholar]
- 22.Keverne, B. 2009. Monoallelic gene expression and mammalian evolution. Bioessays 31:1318-1326. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, S., et al. 2006. Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X-linked gene, Rhox5/Pem, at preimplantation stages. Curr. Biol. 16:166-172. [DOI] [PubMed] [Google Scholar]
- 24.Kudo, S. 1998. Methyl-CpG-binding protein MeCP2 represses Sp1-activated transcription of the human leukosialin gene when the promoter is methylated. Mol. Cell. Biol. 18:5492-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, H., R. Habas, and C. Abate-Shen. 2004. MSX1 cooperates with histone H1b for inhibition of transcription and myogenesis. Science 304:1675-1678. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J. T. 2000. Disruption of imprinted X inactivation by parent-of-origin effects at Tsix. Cell 103:17-27. [DOI] [PubMed] [Google Scholar]
- 27.Li, E., T. H. Bestor, and R. Jaenisch. 1992. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69:915-926. [DOI] [PubMed] [Google Scholar]
- 28.Li, L. C., and R. Dahiya. 2002. MethPrimer: designing primers for methylation PCRs. Bioinformatics 18:1427-1431. [DOI] [PubMed] [Google Scholar]
- 29.Li, Q., D. L. Bartlett, M. C. Gorry, M. E. O'Malley, and Z. S. Guo. 2009. Three epigenetic drugs up-regulate homeobox gene Rhox5 in cancer cells through overlapping and distinct molecular mechanisms. Mol. Pharmacol. 76:1072-1081. [DOI] [PubMed] [Google Scholar]
- 30.Lin, T. P., et al. 1994. The Pem homeobox gene is X-linked and exclusively expressed in extraembryonic tissues during early murine development. Dev. Biol. 166:170-179. [DOI] [PubMed] [Google Scholar]
- 31.MacLean, J. A., II, et al. 2005. Rhox: a new homeobox gene cluster. Cell 120:369-382. [DOI] [PubMed] [Google Scholar]
- 32.MacLean, J. A., II, et al. 2006. Rhox homeobox gene cluster: recent duplication of three family members. Genesis 44:122-129. [DOI] [PubMed] [Google Scholar]
- 33.MacLean, J. A., II, M. K. Rao, K. M. Doyle, J. S. Richards, and M. F. Wilkinson. 2005. Regulation of the Rhox5 homeobox gene in primary granulosa cells: preovulatory expression and dependence on SP1/SP3 and GABP. Biol. Reprod. 73:1126-1134. [DOI] [PubMed] [Google Scholar]
- 34.Maiti, S., et al. 1996. The Pem homeobox gene. Androgen-dependent and -independent promoters and tissue-specific alternative RNA splicing. J. Biol. Chem. 271:17536-17546. [DOI] [PubMed] [Google Scholar]
- 35.Maiti, S., et al. 1996. The Pem homeobox gene: rapid evolution of the homeodomain, X chromosomal localization, and expression in reproductive tissue. Genomics 34:304-316. [DOI] [PubMed] [Google Scholar]
- 36.McBurney, M. W., and E. D. Adamson. 1976. Studies on the activity of the X chromosomes in female teratocarcinoma cells in culture. Cell 9:57-70. [DOI] [PubMed] [Google Scholar]
- 37.Morgan, H. D., F. Santos, K. Green, W. Dean, and W. Reik. 2005. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 14:R47-R58. [DOI] [PubMed] [Google Scholar]
- 38.Morris, L., J. Gordon, and C. C. Blackburn. 2006. Identification of a tandem duplicated array in the Rhox alpha locus on mouse chromosome X. Mamm. Genome 17:178-187. [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama, M., et al. 2009. CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis. Nat. Cell Biol. 11:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oda, M., et al. 2006. DNA methylation regulates long-range gene silencing of an X-linked homeobox gene cluster in a lineage-specific manner. Genes Dev. 20:3382-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oka, M., et al. 2005. De novo DNA methyltransferases Dnmt3a and Dnmt3b primarily mediate the cytotoxic effect of 5-aza-2′-deoxycytidine. Oncogene 24:3091-3099. [DOI] [PubMed] [Google Scholar]
- 42.Okano, M., D. W. Bell, D. A. Haber, and E. Li. 1999. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99:247-257. [DOI] [PubMed] [Google Scholar]
- 43.Patrat, C., et al. 2009. Dynamic changes in paternal X-chromosome activity during imprinted X-chromosome inactivation in mice. Proc. Natl. Acad. Sci. U. S. A. 106:5198-5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peippo, J., et al. 2002. Sex-chromosome linked gene expression in in-vitro produced bovine embryos. Mol. Hum. Reprod. 8:923-929. [DOI] [PubMed] [Google Scholar]
- 45.Pitman, J. L., T. P. Lin, J. E. Kleeman, G. F. Erickson, and C. L. MacLeod. 1998. Normal reproductive and macrophage function in Pem homeobox gene-deficient mice. Dev. Biol. 202:196-214. [DOI] [PubMed] [Google Scholar]
- 46.Ramakrishnan, V. 1997. Histone H1 and chromatin higher-order structure. Crit. Rev. Eukaryot. Gene Expr. 7:215-230. [DOI] [PubMed] [Google Scholar]
- 47.Rao, M. K., S. Maiti, H. N. Ananthaswamy, and M. F. Wilkinson. 2002. A highly active homeobox gene promoter regulated by Ets and Sp1 family members in normal granulosa cells and diverse tumor cell types. J. Biol. Chem. 277:26036-26045. [DOI] [PubMed] [Google Scholar]
- 48.Rao, M. K., C. M. Wayne, M. L. Meistrich, and M. F. Wilkinson. 2003. Pem homeobox gene promoter sequences that direct transcription in a Sertoli cell-specific, stage-specific, and androgen-dependent manner in the testis in vivo. Mol. Endocrinol. 17:223-233. [DOI] [PubMed] [Google Scholar]
- 49.Rupp, R. A., and P. B. Becker. 2005. Gene regulation by histone H1: new links to DNA methylation. Cell 123:1178-1179. [DOI] [PubMed] [Google Scholar]
- 50.Sado, T., and A. C. Ferguson-Smith. 2005. Imprinted X inactivation and reprogramming in the preimplantation mouse embryo. Hum. Mol. Genet. 14:R59-R64. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki, A. W., et al. 1991. The oncofetal gene Pem encodes a homeodomain and is regulated in primordial and pre-muscle stem cells. Mech. Dev. 34:155-164. [DOI] [PubMed] [Google Scholar]
- 52.Shen, X., and M. A. Gorovsky. 1996. Linker histone H1 regulates specific gene expression but not global transcription in vivo. Cell 86:475-483. [DOI] [PubMed] [Google Scholar]
- 53.Takagi, N., and M. Sasaki. 1975. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256:640-642. [DOI] [PubMed] [Google Scholar]
- 54.Takasaki, N., R. McIsaac, and J. Dean. 2000. Gpbox (Psx2), a homeobox gene preferentially expressed in female germ cells at the onset of sexual dimorphism in mice. Dev. Biol. 223:181-193. [DOI] [PubMed] [Google Scholar]
- 55.Takasaki, N., T. Rankin, and J. Dean. 2001. Normal gonadal development in mice lacking GPBOX, a homeobox protein expressed in germ cells at the onset of sexual dimorphism. Mol. Cell. Biol. 21:8197-8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, X., and J. Zhang. 2006. Remarkable expansions of an X-linked reproductive homeobox gene cluster in rodent evolution. Genomics 88:34-43. [DOI] [PubMed] [Google Scholar]
- 57.Wayne, C. M., J. A. MacLean, G. Cornwall, and M. F. Wilkinson. 2002. Two novel human X-linked homeobox genes, hPEPP1 and hPEPP2, selectively expressed in the testis. Gene 301:1-11. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson, M. F., J. Kleeman, J. Richards, and C. L. MacLeod. 1990. A novel oncofetal gene is expressed in a stage-specific manner in murine embryonic development. Dev. Biol. 141:451-455. (Erratum, 146:263, 1991.) [DOI] [PubMed] [Google Scholar]
- 59.Wolffe, A. P. 1997. Histone H1. Int. J. Biochem. Cell Biol. 29:1463-1466. [DOI] [PubMed] [Google Scholar]
- 60.Yamasaki-Ishizaki, Y., et al. 2007. Role of DNA methylation and histone H3 lysine 27 methylation in tissue-specific imprinting of mouse Grb10. Mol. Cell. Biol. 27:732-742. [DOI] [PMC free article] [PubMed] [Google Scholar]