Abstract
Prion diseases are associated with the presence of PrPSc, a disease-associated misfolded conformer of the prion protein. We report that superparamagnetic nanoparticles bind PrPSc molecules efficiently and specifically, permitting magnetic separation of prions from a sample mixture. Captured PrPSc molecules retain the activity to seed protein misfolding cyclic amplification (PMCA) reactions, enabling the rapid concentration of dilute prions to improve detection. Furthermore, superparamagnetic nanoparticles clear contaminated solutions of PrPSc. Our findings suggest that coupling magnetic nanoparticle capture with PMCA could accelerate and improve prion detection. Magnetic nanoparticles may also be useful for developing a nontoxic prion decontamination method for biologically derived products.
Bovine spongiform encephalopathy, Creutzfeldt-Jakob disease, and other prion diseases are caused by an infectious agent that contains PrPSc, a misfolded conformer of the normal cellular prion protein (PrPC) (24). Low-abundance sources of prions, such as blood, may still transmit disease (17, 23). Prion diseases currently have no therapy, nor can prions be specifically removed from contaminated material.
Inoculation bioassay serves as the gold standard for specific detection of prion infectivity. For sensitive detection, protein misfolding cyclic amplification (PMCA) has emerged as a rapid alternative to bioassay. PMCA exploits prion multiplication mechanisms to amplify PrPSc in vitro using PrPC substrate (1, 5). Analogous to amplification of DNA sequence template by PCR, PrPSc template seeds the conversion of PrPC substrate in PMCA, resulting in propagation and amplification of the PrPSc conformation. Each serial PMCA round increases the detection sensitivity exponentially but requires ∼72 h (7). The application of PMCA is also limited by prion propagation inhibitors present in blood and other biological solutions (6). An effective method to concentrate prions would improve subsequent PMCA sensitivity and utility.
Nanotechnology presents many opportunities for fine control of molecular events. Certain iron oxide crystals less than ∼25 nm in diameter exhibit superparamagnetism, with a net magnetization only occurring in the presence of an external magnetic field (15). MagnaBind and Dynal superparamagnetic beads contain many iron oxide crystals, dispersed such that no permanent magnetic order can form. This enables the whole particles to be superparamagnetic, allowing them to be rapidly attracted to a magnet and to lose magnetic interactions upon removal of the magnet (28). In molecular biology, superparamagnetic beads are often conjugated to specifically bind a target molecule.
Using superparamagnetic nanoparticles, we have identified a novel binding interaction with PrPSc. Magnetic capture of PrPSc may be applied to prion detection and prion decontamination.
MATERIALS AND METHODS
Preparation of scrapie-infected and uninfected brain homogenate.
CD-1 mouse (prion strains RML, Me7, and 301C) and Syrian hamster (prion strains Sc237 and 139H) scrapie-infected brains were homogenized (Covidien tissue grinder; Covidien, Mansfield, MA) to 10% in phosphate-buffered saline (PBS), pH 7.4 (Cellgro, Manassas, VA). Uninfected CD-1 mouse and Syrian hamster brains (Biochemed, Winchester, VA) were homogenized in the same manner. The homogenates were initially clarified by centrifugation at 200 × g for 30 s and stored at −70°C. Freshly clarified 5% homogenate for each experiment was prepared by adding an equal volume of Tris-buffered saline (TBS; 50 mM Tris, 200 mM NaCl, pH 7.5), vortexing for 15 s, sonicating (Misonix 4000 with microplate horn; Qsonica, Newtown, CT) for 1 min, and centrifuging at 500 × g for 15 min.
Preparation of magnetic particles.
The superparamagnetic beads used in these studies were MagnaBind (Pierce, Rockford, IL) or Dynal (Invitrogen, Carlsbad, CA) bearing either protein A or streptavidin conjugates. All magnetic particles were separated from solution with a magnetic particle separator (PureBiotech, Middlesex, NJ).
Nonbead nanoparticles were prepared as follows: 10-nm iron(II,III) oxide (Fe3O4, magnetite) nanoparticles (Sigma, St. Louis, MO) in toluene were mixed with an equal volume of methanol and magnetically separated. <50-nm iron(II,III) oxide (Fe3O4, magnetite) nanopowder was also obtained from Sigma. For silanization (21), nanoparticles or nanopowder was resuspended in methanol to 0.11 mg/ml, to which was added 1/10 volume 3-(trimethoxy-silyl)propyl methacrylate (Sigma). Each was sonicated for 1 min at 70% power and then incubated for 4.5 h at 25°C with 300 rpm shaking. Each was then rinsed in methanol and then ethanol.
Binding assays.
Unless otherwise noted, 25 μl of beads (5 mg/ml) or 2 mg of magnetite (10-nm nanoparticles or <50-nm nanopowder, as described above) was rinsed twice in 500 μl PBS plus 0.5% Triton X-100 and then incubated in 150 μl of assay buffer (TBS, 1% Triton X-100, 1% Tween 20) with 5 μl of clarified 5% brain homogenate overnight at room temperature with 10-rpm end-over-end rotation. IgG 89-112 anti-PrPSc antibody (18) was added to designated samples at 7.5 μg/ml. Particles were separated from the solution and rinsed twice in 500 μl of wash buffer (TBS, 0.05% Tween 20) before analysis of bound molecules. PrPSc/PrPC comparison reactions were carried out in TBS with 3% NP-40 and 3% Tween 20 for 2 h, followed by four 1-ml washes in TBS with 2% sarkosyl.
PMCA.
Following binding, samples were resuspended in 10% CD-1 mouse or Syrian hamster brain homogenate, which was prepared in conversion buffer (PBS, 1% Triton X-100, Roche Complete mini protease inhibitor) (6) with an additional 4 mM EDTA. One round of PMCA consisted of 30 s of microplate horn sonication pulses every 30 min for 24 h at 90% power.
Prion protein detection.
Bound PrPSc was detected by subjecting beads to limited proteolysis in 50 μl (25 μg/ml for mouse, 50 μg/ml for hamster) of proteinase K (Roche, Indianapolis, IN) in PBS, 1% Triton X-100. Proteolysis proceeded for 30 min (mouse) or 60 min (hamster) at 37°C and 750-rpm shaking and was terminated by the addition of 17 μl of 4× sample buffer (217 mM Tris, pH 6.8, 8.7% [wt/vol] sodium dodecyl sulfate, 21% [vol/vol] glycerol, 0.02% [wt/vol] bromophenol blue, 3 M β-mercaptoethanol) and 10 min of incubation at 95°C. PrP was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), semidry transfer to polyvinylidene difluoride (PVDF) membrane, immunoblot with anti-PrP antibody 6D11, horseradish peroxidase (HRP)-conjugated anti-mouse sheep antibody, and enhanced chemiluminescence (SuperSignal West Femto Substrate; Pierce, Rockford, IL). Signals were visualized by a Fuji (Fujifilm) LAS-3000 chemiluminescence documentation system.
Silver stain detection of total protein.
Following SDS-PAGE, the gel was fixed overnight in 50% ethanol-10% acetic acid and then treated with two 10-min washes in 10% ethanol to remove SDS. Next, the gel was incubated for 2 min in Farmer's solution (0.3 g sodium thiosulfate, 0.15 g potassium ferricyanide, 0.05 g sodium carbonate in 100 ml water), followed by four 20-min washes in water and then 12 min of silver staining (0.2 g silver nitrate in 100 ml water). The gel was then treated with developer (3 g sodium carbonate, 50 μl fresh 37% formaldehyde, 100 ml water) for a short rinse and subsequently incubated for approximately 8 min. The progression of the staining was halted by the addition of stop solution (5% acetic acid in water).
RESULTS
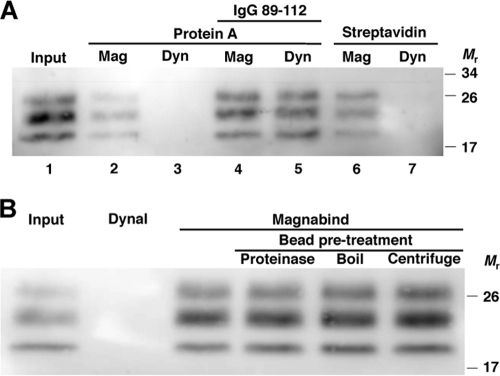
In the course of performing immunoprecipitation reactions, we observed that protein A- and streptavidin-conjugated MagnaBind magnetic particles bound PrPSc (Fig. 1 A). The binding did not depend on the presence of anti-PrP antibodies, as antibody-free control reaction mixtures also precipitated PrPSc (Fig. 1A, lane 2). The interaction was not due to the protein A moiety conjugated to the particles, as protein A-Dynabeads did not bind PrPSc without the addition of an anti-PrP antibody (Fig. 1A, lane 3). Furthermore, MagnaBind beads conjugated to streptavidin also bound PrPSc, while streptavidin-conjugated Dynabeads did not (Fig. 1A, lanes 6 and 7). We performed various protein disruption treatments to further test whether protein A played any role in MagnaBind-protein A interaction with PrPSc. Neither protease treatment nor boiling abrogated MagnaBind's ability to bind PrPSc molecules (Fig. 1B), further suggesting that MagnaBind beads alone bind PrPSc.
FIG. 1.
Binding of PrPSc to MagnaBind superparamagnetic beads. Bound PrPSc was detected by proteinase K digestion and anti-PrP (6D11) immunoblot. (A) RML scrapie-infected mouse brain homogenate was incubated with MagnaBind (Mag) or Dynal (Dyn) magnetic beads linked to protein A or streptavidin. One set of magnetic bead-protein A reaction mixtures was coincubated with IgG 89-112, which recognizes PrPSc. (B) Binding of PrPSc to MagnaBind-protein A beads treated for protein disruption. MagnaBind-protein A beads were pretreated by proteinase K digestion (25 μg/ml), boiling (95°C for 10 min), or centrifugation (14,000 × g for 10 min). Untreated MagnaBind-protein A and control Dynal-protein A beads were also tested. Following these treatments, beads were washed and incubated overnight with RML scrapie-infected mouse brain homogenate.
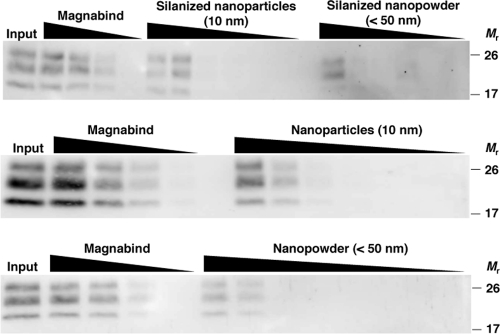
MagnaBind beads are composed of silanized superparamagnetic iron oxide. Dynabeads also contain superparamagnetic iron oxide but are completely enveloped by a polystyrene coat of uniform thickness, which presumably prevents interaction with PrPSc. Superparamagnetism is exhibited only by small nanoparticles. To further dissect the MagnaBind-PrPSc interaction and to identify simple reagents for prion capture, we tested defined particles with a composition similar to the MagnaBind silane-coated iron oxide. Silanized magnetite (Fe3O4) nanoparticles captured PrPSc in a dose-dependent manner (Fig. 2). Interestingly, magnetite nanoparticles alone (nonsilanized) also captured PrPSc, with 10-nm nanoparticles and <50-nm nanopowder performing similarly. This suggests that the prion-capturing activity of MagnaBind beads can be recapitulated by magnetite nanoparticles. Moreover, PrPSc appears to interact directly with the Fe3O4 metal surface.
FIG. 2.
Binding of PrPSc to MagnaBind, silanized nanomagnetite, and unsilanized nanomagnetite. RML scrapie-infected mouse brain homogenate was incubated with various quantities of MagnaBind protein A beads (0.005 to 0.125 mg), silanized magnetite nanoparticles (10 nm in size, 0.005 to 2 mg), silanized magnetite nanopowder (<50 nm in size, 0.005 to 2 mg), unsilanized magnetic nanoparticles (0.0015 to 2 mg), or unsilanized magnetic nanopowder (0.0015 to 2 mg). PrPSc molecules were detected by proteinase K digestion and anti-PrP (6D11) immunoblot.
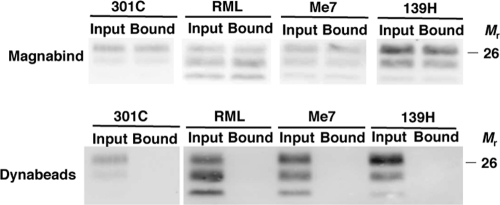
Distinct strains of prions infect different brain regions and display various biochemical properties, despite possessing identical PrP amino acid sequences (4). We assessed whether multiple prion strains can be magnetically captured and found that MagnaBind beads bound all strains examined, including mouse strains RML, 301C, and Me7 and hamster 139H (Fig. 3). This result suggests that magnetic nanoparticles could be used as a general prion capture reagent, including for strain 301C, which are mouse-adapted bovine spongiform encephalopathy (BSE) prions (3). Magnetic particle capture of prions appears to target a general feature of PrPSc that is conserved between strains and shared among prions from different animal species, which bear different prion protein sequences.
FIG. 3.
Binding of diverse strains of PrPSc molecules to MagnaBind or Dynal beads. Brain homogenates from animals infected with various prion strains (mouse 301C, mouse RML, mouse Me7, and hamster 139H) were incubated overnight with MagnaBind-protein A or protein A-Dynal beads. Input and bound PrPSc molecules were detected by proteinase K digestion and anti-PrP (6D11) immunoblot.
We next examined the selectivity of magnetic capture for PrPSc. Magnetic particles did not capture the normal prion protein conformer PrPC from uninfected mouse brain tissue (Fig. 4 A), suggesting specificity for the disease-associated conformer, PrPSc. Furthermore, silver stain analysis of total protein indicated that magnetic particles bound minimal protein from both uninfected and scrapie-infected brain (Fig. 4B). Other metals, minerals, and resins have been found to interact with normal and disease-associated prion protein (2, 12, 16, 25). Our findings indicate that superparamagnetic iron oxide particles capture PrPSc selectively and efficiently.
FIG. 4.
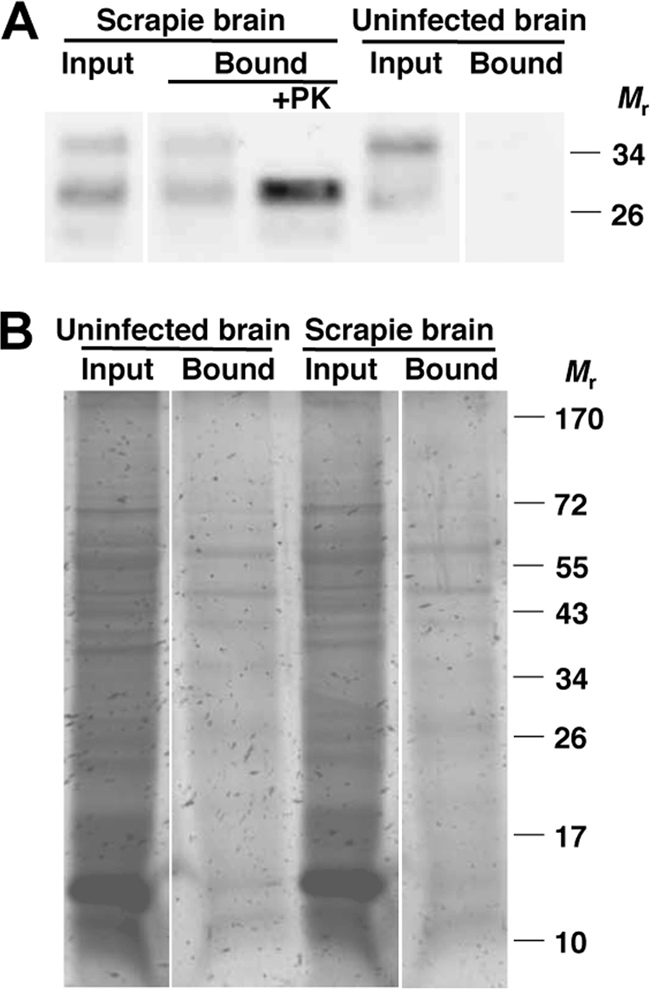
Binding specificity of MagnaBind beads. Binding of PrPSc and PrPC molecules to MagnaBind-protein A beads. RML scrapie-infected or uninfected mouse brain homogenates were incubated with MagnaBind-protein A beads for 2 h. (A) Input and bound fractions were analyzed for PrP molecules by anti-PrP (6D11) immunoblot. The scrapie brain bound fraction was also analyzed for PrPSc by proteinase K digestion (+PK). (B) Input and bound fractions were analyzed for total protein by silver staining. All samples were analyzed on the same gel, with white lines indicating excised intervening lanes.
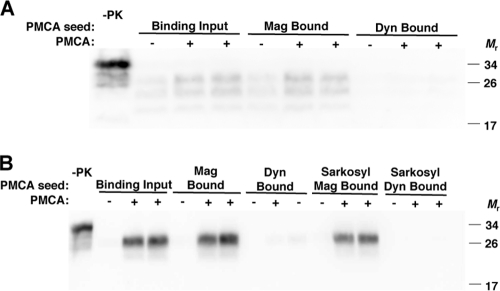
Detection of prions by PMCA may be made more efficient, rapid, and sensitive if samples are concentrated for PrPSc prior to amplification. This depends on the concentration procedure leaving PrPSc with the autocatalytic ability to seed the conversion of PrPC. To determine whether magnetic particle-captured PrPSc retained seeding ability, we used bound PrPSc to seed PMCA reaction mixtures containing normal brain homogenate substrate. RML prion-infected mouse PrPSc successfully seeded the conversion of PrPC to PrPSc, causing amplification of PrPSc (Fig. 5 A). We found that captured hamster Sc237 PrPSc also seeded PMCA, even after the bound PrPSc was washed stringently with the ionic detergent sarkosyl (Fig. 5B). Thus, magnetically concentrated PrPSc is competent for amplification by PMCA.
FIG. 5.
Protein misfolding cyclic amplification (PMCA) reactions seeded with MagnaBind-bound PrPSc. (A and B) RML-infected mouse (A) or Sc237-infected hamster (B) brain homogenates before (input) or after binding by MagnaBind-protein A beads (Mag) or by Dynal-protein A beads (Dyn) were used to seed PMCA reactions. Normal mouse (A) or hamster (B) brain homogenates were used as substrate. Prior to PMCA, one set of beads was washed with sarkosyl detergent (B). Each reaction mixture was analyzed before (−) or after (+) PMCA. PrPSc molecules were detected by proteinase K digestion and anti-PrP (6D11) immunoblot. Control samples were not digested with proteinase K (−PK), to show the amount of PrPC substrate in each PMCA reaction.
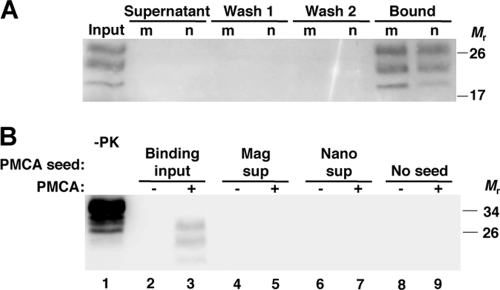
Magnetic capture of PrPSc could be useful to remove prions from potentially contaminated biological solutions. We treated a prion-contaminated solution with magnetic nanoparticles and detected no PrPSc in the supernatant (Fig. 6 A). Both MagnaBind beads and magnetite nanoparticles captured all input PrPSc, leaving none in the remaining fluid. To test for small amounts of residual PrPSc not detected by immunoblot, we used nanoparticle-treated supernatants to seed PMCA reaction mixtures with normal brain homogenate as substrate (Fig. 6B). Input PrPSc was amplified upon performing PMCA (Fig. 6B, lane 3); in contrast, supernatants treated with MagnaBind and ∼10-nm nanoparticles showed no detectable PrPSc, even after amplification by PMCA (Fig. 6B, lanes 5 and 7). This suggests that nanoparticle treatments are highly effective in removing PrPSc from contaminated samples.
FIG. 6.
Clearance of PrPSc from solution by magnetic beads or nanoparticles. RML scrapie-infected mouse brain homogenate was incubated with MagnaBind-protein A beads (m) or 10-nm magnetite nanoparticles (n). Particles were then washed twice and resuspended in buffer. (A) Equal proportions of input, supernatant, each wash, and bound fractions were analyzed for PrPSc. (B) Binding input, MagnaBind-treated supernatant (Mag sup), nanoparticle-treated supernatant (Nano sup), and buffer alone (no seed) were analyzed for autocatalytic PrPSc by diluting in uninfected mouse brain homogenate and performing PMCA (+). Aliquots of each sample were not subjected to PMCA (−). A control brain homogenate sample was not digested with proteinase K (−PK), to show the amount of PrPC substrate in each PMCA reaction. All other samples shown in panels A and B were analyzed for PrPSc by proteinase K digestion. Prion protein was detected by anti-PrP (6D11) immunoblot.
DISCUSSION
Various materials have been reported to bind prion protein. Prion infectivity adheres to stainless steel (29), promoted by nickel and molybdenum, which in isolation also bind to PrPC and PrPSc (16). Stainless steel has been proposed for prion concentration, for use in a coupled concentration-cell culture detection scheme (9). Prions also adsorb to various minerals found in soil (14, 20, 27). Phosphotungstic acid has been used to precipitate PrPSc in the laboratory (26) and may also be used to concentrate prions for PMCA detection (10). Superparamagnetic nanoparticles present a novel method for binding and concentrating PrPSc, making use of specific and efficient capture.
Capture of prions by magnetic nanoparticles holds great potential to improve current methods of prion detection. Though the PMCA technique is very sensitive, particularly when serial amplifications are performed, each round requires 24 to 72 h (7). Nanoparticle-bound PrPSc is competent to seed PMCA reactions, facilitating the coupling of magnetic nanoparticle concentration with PMCA detection. Another technique, immunoprecipitation, may also be able to concentrate prions (18, 22), but antibodies directed against PrP may inhibit prion propagation (19), precluding such a detection scheme. Coupling of magnetic concentration with PMCA would improve current methods to detect low concentrations of prions, valuable in safeguarding biological materials for consumption and in medicine.
Prions may be transmitted to humans by transfusion of infected blood (23). Magnetic nanoparticle capture presents an opportunity to decontaminate biological products derived from potentially contaminated sources. Other methods, such as sodium hydroxide, sodium hypochlorite, and phosphotungstic acid treatments, destroy or remove prions (11, 26) but also damage the material of interest. In contrast, magnetic nanoparticles capture PrPSc with specificity. Innovative methods, such as filtration, have been proposed to remove prions from blood (13). Magnetic capture could potentially reduce the prion load in contaminated samples while at the same time facilitating detection. Following treatment with magnetic nanoparticles, we detected no remaining PrPSc, even after amplification, indicating that nanoparticle capture is effective for prion removal. The safety of treating biologically derived pharmaceutical products with iron oxide nanoparticles is further supported by their nontoxicity, demonstrated in clinical studies. The small size of nanoparticles enables passage through capillary beds, and iron oxide nanoparticles have been approved by the American Food and Drug Administration for use as a magnetic resonance imaging contrast agent (8). Thus, superparamagnetic nanoparticles may be used to simultaneously detect and decontaminate prion-contaminated materials.
Acknowledgments
We thank Andrew Giustini for critical reading of the manuscript.
This work was supported by National Institutes of Health grants 2R01 NS046478 and R01 NS055875 to S.S. M.B.M. is supported by a Kirschstein M.D./Ph.D. NRSA fellowship (no. F30 NS064637).
Footnotes
Published ahead of print on 12 January 2011.
REFERENCES
- 1.Atarashi, R., et al. 2007. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat. Methods 4:645-650. [DOI] [PubMed] [Google Scholar]
- 2.Brown, D. R., et al. 1997. The cellular prion protein binds copper in vivo. Nature 390:684-687. [DOI] [PubMed] [Google Scholar]
- 3.Bruce, M., et al. 1994. Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 343:405-411. [DOI] [PubMed] [Google Scholar]
- 4.Castilla, J., et al. 2008. Cell-free propagation of prion strains. EMBO J. 27:2557-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castilla, J., P. Saa, C. Hetz, and C. Soto. 2005. In vitro generation of infectious scrapie prions. Cell 121:195-206. [DOI] [PubMed] [Google Scholar]
- 6.Castilla, J., et al. 2006. Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol. 412:3-21. [DOI] [PubMed] [Google Scholar]
- 7.Chen, B., R. Morales, M. A. Barria, and C. Soto. 2010. Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat. Methods 7:519-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries, I. J., et al. 2005. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 23:1407-1413. [DOI] [PubMed] [Google Scholar]
- 9.Edgeworth, J. A., et al. 2010. Spontaneous generation of mammalian prions. Proc. Natl. Acad. Sci. U. S. A. 107:14402-14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eiden, M., et al. 2010. Biochemical and immunohistochemical characterization of feline spongiform encephalopathy in a German captive cheetah. J. Gen. Virol. 91:2874-2883. [DOI] [PubMed] [Google Scholar]
- 11.Fichet, G., et al. 2004. Novel methods for disinfection of prion-contaminated medical devices. Lancet 364:521-526. [DOI] [PubMed] [Google Scholar]
- 12.Gregori, L., et al. 2006. Reduction in infectivity of endogenous transmissible spongiform encephalopathies present in blood by adsorption to selective affinity resins. Lancet 368:2226-2230. [DOI] [PubMed] [Google Scholar]
- 13.Gregori, L., et al. 2004. Effectiveness of leucoreduction for removal of infectivity of transmissible spongiform encephalopathies from blood. Lancet 364:529-531. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, C. J., et al. 2006. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, X., et al. 2006. Preparation and characterization of hydrophobic superparamagnetic magnetite gel. J. Magnetism Magn. Mater. 306:248-253. [Google Scholar]
- 16.Luhr, K. M., P. Low, A. Taraboulos, T. Bergman, and K. Kristensson. 2009. Prion adsorption to stainless steel is promoted by nickel and molybdenum. J. Gen. Virol. 90:2821-2828. [DOI] [PubMed] [Google Scholar]
- 17.Mathiason, C. K., et al. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133-136. [DOI] [PubMed] [Google Scholar]
- 18.Moroncini, G., et al. 2004. Motif-grafted antibodies containing the replicative interface of cellular PrP are specific for PrPSc. Proc. Natl. Acad. Sci. U. S. A. 101:10404-10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller-Schiffmann, A., et al. 2009. Complementarity determining regions of an anti-prion protein scFv fragment orchestrate conformation specificity and antiprion activity. Mol. Immunol. 46:532-540. [DOI] [PubMed] [Google Scholar]
- 20.Nagaoka, K., et al. 2010. Sensitive detection of scrapie prion protein in soil. Biochem. Biophys. Res. Commun. 397:626-630. [DOI] [PubMed] [Google Scholar]
- 21.Norén, K., and M. Kempe. 2009. Multilayered magnetic nanoparticles as a support in solid-phase peptide synthesis. Int. J. Pept. Res. Ther. 15:287-292. [Google Scholar]
- 22.Paramithiotis, E., et al. 2003. A prion protein epitope selective for the pathologically misfolded conformation. Nat. Med. 9:893-899. [DOI] [PubMed] [Google Scholar]
- 23.Peden, A. H., M. W. Head, D. L. Ritchie, J. E. Bell, and J. W. Ironside. 2004. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364:527-529. [DOI] [PubMed] [Google Scholar]
- 24.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. U. S. A. 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rees, H. C., B. C. Maddison, J. P. Owen, G. C. Whitelam, and K. C. Gough. 2009. Concentration of disease-associated prion protein with silicon dioxide. Mol. Biotechnol. 41:254-262. [DOI] [PubMed] [Google Scholar]
- 26.Safar, J., et al. 1998. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 4:1157-1165. [DOI] [PubMed] [Google Scholar]
- 27.Saunders, S. E., J. C. Bartz, and S. L. Bartelt-Hunt. 2009. Prion protein adsorption to soil in a competitive matrix is slow and reduced. Environ. Sci. Technol. 43:7728-7733. [DOI] [PubMed] [Google Scholar]
- 28.Tarcha, P. J., and T. E. Rohr. 2004. Diagnostics and Biomaterials, p. 687-688. In B. D. Ratner (ed.), Biomaterials science: an introduction to materials in medicine. Elsevier, London, United Kingdom.
- 29.Zobeley, E., E. Flechsig, A. Cozzio, M. Enari, and C. Weissmann. 1999. Infectivity of scrapie prions bound to a stainless steel surface. Mol. Med. 5:240-243. [PMC free article] [PubMed] [Google Scholar]