Abstract
We previously showed that the herpes simplex virus 1 (HSV-1) tegument protein VP11/12 activates the lymphocyte-specific Src family kinase (SFK) Lck and is tyrosine phosphorylated in an Lck-dependent manner during T cell infection. We now extend these findings to show that ectopic expression of Lck induces robust tyrosine phosphorylation of VP11/12 in Vero cells, strongly suggesting that VP11/12 participates in an Lck-mediated signaling pathway as a substrate of Lck or a kinase activated by Lck. We sought to elucidate signaling events downstream of VP11/12-SFK interactions. SFKs lie upstream of the canonical phosphoinositide 3-kinase (PI3K)-Akt pathway in signaling emanating from immune receptors, growth factor receptors, and polyomavirus middle T antigen. Here, we show that VP11/12 is required for virus-induced activation of PI3K-Akt signaling in HSV-infected Jurkat T cells and primary fibroblasts. VP11/12 interacts with PI3K or PI3K signaling complexes during infection, suggesting that VP11/12 activates PI3K directly. SFK activity is required for tyrosine phosphorylation of VP11/12, VP11/12-PI3K interactions, and Akt activation in infected fibroblasts, suggesting that SFK-dependent phosphorylation of VP11/12 is required for interactions with downstream signaling effectors. Akt controls many biological functions, including cell survival, cell motility, and translation, but it is currently unclear which Akt targets are modulated by VP11/12 during infection. Although the Akt target mTORC1 is activated during HSV-1 infection, VP11/12 is not required for this effect, implying that one or more additional viral proteins regulate this pathway. Further studies are therefore required to determine which Akt targets and associated biological functions are uniquely modulated by VP11/12.
Tegument proteins are layered between the capsid and the envelope in virions of the Herpesviridae family, including those of herpes simplex virus 1 (HSV-1). These proteins are delivered into the cytoplasm during viral entry, and from there, they translocate to various subcellular locations to manipulate host cell functions. Examples include virion protein 16 (VP16), a viral transcription factor that promotes immediate-early gene transcription during infection (reviewed in reference 68), and virion host shutoff protein, which contributes to an early blockade of host protein synthesis by destabilizing cellular mRNAs (reviewed in reference 55). Many tegument proteins likely play important roles in virus-host interactions, but the functions of most of these remain undefined.
VP11/12, one of the most abundant proteins in the HSV-1 tegument, lacks a well-established function during infection; however, studies from our laboratory suggest that it serves as a substrate and an activator of the lymphocyte-specific Lck tyrosine kinase (64, 72). Originally identified as two proteins, VP11 and VP12, based on its mobility relative to that of other virion proteins in a denaturing SDS-polyacrylamide gel (at ca. 87 and ca. 93 kDa) (57), VP11/12 is now known to be one protein encoded by the open reading frame UL46 (74). VP11/12 enhances immediate-early viral gene expression in transfection-based assays by augmenting the activity of the HSV-encoded transcriptional activator VP16 (19, 32). However, studies of VP11/12-null viruses have demonstrated that VP11/12 is not required for viral immediate-early gene expression during HSV infection (72, 75). VP11/12 is present in the membrane fraction of infected cells, although it is not membrane associated in mature virions (36). In addition, VP11/12 localizes to the plasma membrane immediately following viral entry (67). These studies suggest that VP11/12 may serve a membrane-associated function that is distinct from its role in virion assembly.
Our previous studies raised the possibility that VP11/12 modulates one or more cellular signaling pathways (64, 72). VP11/12 is highly tyrosine phosphorylated in lymphocytes, and in Jurkat T cells, tyrosine phosphorylation is largely dependent on the lymphocyte-specific Src family kinase (SFK) Lck (72). Lck is activated during HSV-1 infection of T cells, and VP11/12 is required for this effect; moreover, VP11/12 interacts with Lck or Lck signaling complexes (64). These data suggest that VP11/12 activates Lck to induce its own tyrosine phosphorylation, perhaps thereby inducing or modifying downstream signaling events. However, effects of VP11/12 on signaling downstream of Lck or other SFKs have not yet been documented. Despite the well-established role of Lck in initiating signaling through the T cell receptor (TCR) (reviewed in reference 56), VP11/12-dependent activation of Lck does not trigger TCR signaling (72). Indeed, HSV-1 infection inhibits TCR signaling (53), and VP11/12 is not required for this effect (72). Thus, the biological function of Lck-dependent tyrosine phosphorylation of VP11/12 has yet to be defined.
It is possible that VP11/12 also interacts with SFKs other than Lck. Robust tyrosine phosphorylation of VP11/12 is observed for infected T and B cells and natural killer lymphocytes, and lower levels of tyrosine phosphorylation are observed for epithelial cells and fibroblasts (72). B cells, epithelial cells, and fibroblasts lack Lck, raising the possibility that VP11/12 serves as an activator and a substrate for multiple Src family members.
In this paper, we describe a role for VP11/12 in the activation of the phosphoinositide 3-kinase (PI3K)-Akt signaling pathway. Signaling through this pathway controls many biological processes, such as cell motility, growth, proliferation, transcription, translation, and metabolism (reviewed in reference 29). Immune receptors, such as the TCR and BCR (reviewed in references 17 and 56), growth factor receptors, including platelet-derived growth factor receptor beta (PDGFR-β) and insulin receptor (reviewed in reference 38), and the viral protein polyomavirus middle T antigen (MTAg) (reviewed in reference 7) are all known to initiate signaling through this pathway. The PI3K-Akt signaling module begins with the membrane recruitment and activation of class IA PI3K. Class IA PI3K (here referred to as PI3K) consists of a catalytic subunit, p110, and a regulatory subunit, p85, which includes an Src homology 3 (SH3) and two SH2 adaptor domains. PI3K is brought to its substrate in the plasma membrane by interactions between the p85 subunit and membrane-associated proteins, such as activated PDGFR-β (69), the insulin receptor substrate 1 (IRS-1) adaptor protein (37), or polyomavirus MTAg (62). Once proximal to its substrate, PI3K converts phosphatidylinositol 4,5-biphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3), a secondary messenger that leads directly to Akt activation (26). PI3K activity is antagonized by a lipid phosphatase, phosphatase and tensin homologue (PTEN), an important negative regulator of PI3K-Akt signaling (26). PIP3 binds Akt and the Akt activator 3-phosphoinositide-dependent kinase 1 (PDK1) through their pleckstrin homology (PH) domains (39). Colocalization of Akt and PDK1 along with a conformational change induced by PIP3 binding to Akt allows PDK1 to phosphorylate Akt at T308 of the activation loop (58, 59). Phosphorylation at S473 of the Akt hydrophobic motif by an enzyme known as PDK2 is also required for full Akt kinase activity (47). Several kinases have been proposed to have PDK2 activity, with mammalian target of rapamycin complex 2 (mTORC2) currently being the favored candidate (47).
Several lines of evidence suggest that SFKs influence the PI3K-Akt signaling module; however, in some cases, the mechanisms underlying this link have not been clearly defined. The strongest correlation between SFK activity and Akt signaling is provided by studies of viral Src (v-Src) from Rous sarcoma virus. v-Src resembles a constitutively active mutant form of cellular Src (reviewed in reference 31). Transformation of cells by v-Src is accompanied by the formation of a v-Src-PI3K complex and an increase in PI3K activity (14), suggesting that an active SFK is sufficient to induce signaling through the PI3K-Akt pathway. Early studies suggested that SFKs, like v-Src, activate PI3K through a mechanism that involves direct binding, since the SH3 domains of v-Src (27), Lck (63), and Fyn (45) bind PI3K in vitro. However, in vivo data supporting this theory seem to be lacking.
SFKs are also used to recruit PI3K to cell surface receptors or other membrane-associated proteins in a variety of signaling pathways. Examples include signaling through the TCR (56), BCR (17), PDGFR-β (3, 35, 70), insulin receptor (37, 38, 70), and polyomavirus MTAg (62). In the cases of the PDGFR-β, insulin receptor, and polyomavirus MTag, PI3K is brought to the membrane through interactions of the p85 SH2 domain and a phosphorylated p85 SH2 binding motif (consensus sequence YXXM). SFKs mediate phosphorylation of the p85 SH2 binding motif, either directly or indirectly. For example, active Src binds MTAg and directly phosphorylates the YXXM motif within MTAg (5, 8, 9, 48) to facilitate PI3K binding and PI3K-Akt signaling in vivo (60, 62). In signaling from the insulin receptor, Src phosphorylates and activates the insulin receptor kinase (70) to indirectly allow for YXXM phosphorylation in the adaptor protein IRS-1 (37).
One report further suggested that SFKs activate Akt signaling by disrupting the activity of PTEN (28). This second in vivo mechanism is not well established. However, the possibility that SFKs may simultaneously activate PI3K and suppress PTEN to induce in vivo Akt activity is intriguing.
Many viruses have been shown to manipulate Akt signaling for their benefit. Some activate Akt to prevent apoptosis and enhance infected cell survival; these include Epstein-Barr virus (EBV) (11, 49, 61), human papillomavirus (73), and hepatitis B virus (52). The EBV protein BRLF1 also activates Akt signaling to drive transcription of a subset of immediate-early and early genes, consequently triggering lytic viral replication in EBV-positive cell lines (10). Human immunodeficiency virus (HIV) virion production is enhanced by Akt signaling, which appears to be mediated by at least two viral proteins. HIV gp120 initiates this signaling during virion binding and entry, and Nef participates in the assembly of a PI3K signaling complex (13, 25). In addition, many viruses activate the downstream mTORC1 signaling module (reviewed in reference 6), which controls cell growth by integrating signals from growth factors, amino acids, and energy and oxygen levels (reviewed in references 12 and 43). The mTORC1 complex contains the mTOR kinase and the scaffolding protein, regulatory associated protein of mTOR (Raptor). Typically, activation of mTORC1 leads to phosphorylation and activation of p70 ribosomal S6 kinase (S6K) and hyperphosphorylation and inhibition of eukaryotic translation initiation factor 4E (eIF4E) binding protein 1 (4EBP1). These two events then converge to enhance translation. Active mTORC1 has also been reported to support functions other than translation, such as enhancement of viral DNA replication during adenovirus infection (41). For human cytomegalovirus (18) and adenovirus (41) infections, mTORC1 activation is known to require PI3K, but for many other viral infections, it is unknown whether mTORC1 is activated by the PI3K-Akt pathway. The mTORC1 complex may be activated directly by a virus-encoded protein or it may be targeted through an alternate cellular mechanism, such as the ill-defined pathway linking excess amino acids and mTORC1 activation (reviewed in references 12 and 43).
HSV-1 infection activates Akt and the mTORC1 pathway (4, 65); however, the viral gene products responsible for these effects have not been defined and it is not known whether these two pathways are linked in the context of HSV infection. HSV-1 activates Akt early in infection of Hep-2 epithelial cells (4). However, activation appears to be dampened (although not eliminated) by the viral serine-threonine kinase US3 at later times postinfection (4). It is possible that US3 negatively regulates Akt by directly phosphorylating cellular proteins that modulate Akt signaling, but the viral or cellular component(s) that acts with US3 to suppress Akt activation remains unknown. HSV-1 infection also activates the Akt target mTORC1 in primary fibroblasts and several cell lines (65), leading to rapamycin-sensitive hyperphosphorylation of 4EBP1 (65). Whether HSV-induced activation of Akt contributes to the mTORC1 activation observed during infection is yet to be investigated.
Our earlier studies demonstrated that HSV activates Lck in a VP11/12-dependent fashion and that tyrosine phosphorylation of VP11/12 is dependent largely on Lck. In the present study, we show that VP11/12 is required for HSV-induced activation of Akt, and we provide evidence that this effect requires SFK and PI3K activity.
MATERIALS AND METHODS
Cells and viruses.
Human embryonic lung (HEL) fibroblasts and Vero cells were obtained from the ATCC, while Jurkat 6.8 cells were donated by H. L. Ostergaard (University of Alberta). These cells were maintained as described previously (72). The HSV-1 KOS recombinants, GHSV-UL46 (67) and KOS-G (34), have been described previously. HSV-1 strain KOS37 was derived from the KOS37 bacmid (15). The KOS37-derived mutants ΔUL46galK (VP11/12−), ΔUL46 (VP11/12−), and RUL46 (VP11/12 repaired) have been described previously (72). All virus stocks were prepared from infected Vero cells.
Infection.
Jurkat and HEL cells were infected in serum-free RPMI medium and Dulbecco's modified Eagle's medium (DMEM), respectively, for 1 h at a multiplicity of infection (MOI) of 10. Cells were harvested at 9 to 20 h postinfection (hpi), as indicated in the figure legend.
Transfection and plasmids.
Vero cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. pSX-SR Lck Y505F has been described previously (66) and was kindly donated by D. N. Burshtyn (University of Alberta). pUL46 has been described previously (72). pGUL46 was constructed by inserting a green fluorescent protein (GFP) tag into pUL46 10 amino acids from the C terminus of VP11/12 (after L710), to give a VP11/12-GFP fusion protein analogous to that encoded by GHSV-UL46 (67). First, a portion of the multiple cloning site of pUL46 containing a HindIII restriction site was removed by excision of the NheI-EcoRI fragment. GFP cDNA was amplified from pEGFP-N1 (Clontech) using the primer pair gtcaaagcttAAGATGGTGAGCAAGGGCGA and cttgaagcttCTTGTACAGCTCGTCC (lowercase letters indicate restriction endonuclease cleavge sites, while uppercase letters indicate eGFP sequences). GFP cDNA was then inserted into the remaining HindIII site within UL46.
Western blotting.
Cell extracts were prepared at 4°C by a 20-min incubation in lysis buffer (1% Nonidet, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 10 mM NaF, 10 mM β-glycerol phosphate, 1 mM Na3VO4, 50 mM Tris-HCl [pH 7.4]) supplemented with a complete protease inhibitor cocktail (Roche). Western blotting was performed as described previously (64).
Immunoprecipitation.
Cell extracts were prepared as described for Western blotting. Immunoprecipitation was performed at 4°C as previously described (64).
Antibodies.
Immunoprecipitation was performed using anti-p85 (4 μg; Millipore), anti-rabbit IgG (4 μg; Sigma), and anti-GFP (2 μl; kind gift of L. Berthiaume). Antibodies used for primary detection by Western blotting included antibodies directed against p85 (1:10,000), GFP (1:3,000), phosphotyrosine (4G10, 1:30,000; Upstate), HSV-1 VP16 (LP1, 1:32,000; provided by Tony Minson), and actin (1:1,000; Sigma). Antibodies directed against the following were also used for primary detection by Western blotting (1:1,000; Cell Signaling): phospho-Akt (Thr308), phospho-Akt (Ser473), Akt, phospho-p38 (Thr180/Tyr182), p38, phospho-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, phospho-p70 S6 kinase (Thr389), p70 S6 kinase, 4E-BP1, phospho-GSK-3β (Ser9), and GSK-3β (27C10). The anti-phospho-Src family (Tyr 416) from Cell Signaling was used at a 1:2,000 dilution. Secondary antibodies included donkey anti-mouse IR800 (1:15,000; Rockland, Inc.), goat anti-rabbit Alexa Fluor 680 (1:15,000; Invitrogen), goat anti-mouse Alexa Fluor 680 (1:15,000; Invitrogen), goat anti-rabbit horseradish peroxidase (HRP) (1:3,000; Promega), and donkey anti-goat HRP (1:15,000; Jackson ImmunoResearch).
RESULTS
Lck enhances tyrosine phosphorylation of VP11/12.
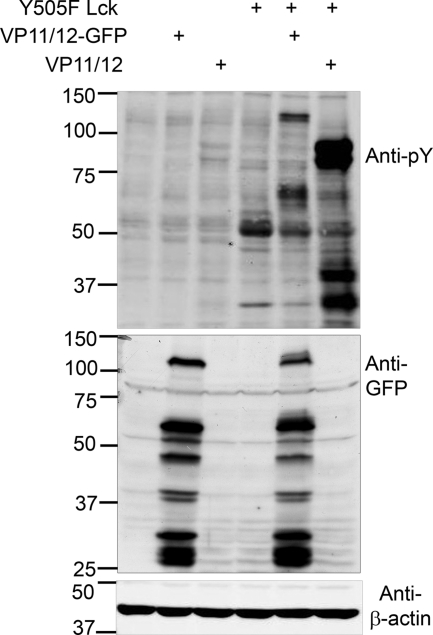
We previously reported that VP11/12 is highly tyrosine phosphorylated in lymphocytes and much less so in Vero epithelial cells (72). We also found that tyrosine phosphorylation in Jurkat T cells is heavily dependent on the lymphocyte-specific SFK Lck (72). Therefore, we asked if ectopic overexpression of Lck is sufficient to enhance tyrosine phosphorylation of VP11/12 in Vero cells. Cells were transfected with a plasmid(s) encoding VP11/12, a VP11/12-GFP fusion protein (VP11/12-GFP), and constitutively active Lck bearing a Y505F mutation. Constitutively active Lck was used in this experiment to ensure Lck activity in the absence of the leukocyte-specific phosphatase CD45 (21, 42, 50). Tyrosine-phosphorylated proteins were detected in transfected cell lysates by Western blot analysis with an anti-phosphotyrosine antibody (Fig. 1). Cells transfected with the VP11/12 and VP11/12-GFP expression plasmids did not display a greatly altered tyrosine phosphorylation profile relative to that of cells transfected with empty vector, while cells expressing Lck alone showed a prominent ca. 56-kDa tyrosine-phosphorylated band that we assume is Lck, as well as an additional ca. 30-kDa protein that remains to be identified (it is well known that Lck Y505F enhances cellular tyrosine phosphorylation, including the autophosphorylation of Lck [1, 30]). In contrast, cells transfected to coexpress Lck and VP11/12 displayed a prominent novel doublet at ca. 90 kDa, consistent with the mobility of tyrosine-phosphorylated VP11/12 (57), as well as additional bands at ca. 60, 40, and 30 kDa. As expected, the ca. 90-kDa VP11/12 doublet was replaced by the predicted ca. 120-kDa VP11/12-GFP fusion protein in cells coexpressing Lck and VP11/12-GFP. This sample also displayed additional bands at ca. 60 and 30 kDa. The ca. 60-, 40-, and 30-kDa proteins may be degradation products of VP11/12 and VP11/12-GFP or indicative of separate tyrosine phosphorylation events related to Lck-VP11/12-mediated signaling. Consistent with the former possibility, Western blotting with an anti-GFP antibody revealed several prominent GFP-reactive bands that migrated more rapidly than full-length VP11/12-GFP (Fig. 1). Overall, these data document that Lck is sufficient to enhance tyrosine phosphorylation of VP11/12 in vivo.
FIG. 1.
Constitutively active Lck enhances tyrosine phosphorylation of VP11/12 in Vero cells. Vero cells were transfected with plasmids encoding constitutively active Y505F Lck, VP11/12, and a VP11/12-GFP fusion protein, as indicated. Cell lysates were collected 46 h posttransfection. Phosphotyrosine, GFP, and β-actin were detected by Western blotting. In all figures, the numbers on the left are molecular size markers (in kilodaltons).
HSV requires VP11/12 for Akt activation during Jurkat T cell infection.
We sought to identify HSV-1-induced signaling events downstream of VP11/12-Lck interactions. Previous studies have documented that HSV-1 infection activates several host signaling effectors, including p38 (22, 33, 54, 71), JNK (22, 33, 54, 71), Akt (4), and the Akt target mTORC1 (65). Since HSV-induced activation of p38 and JNK has been described for Jurkat T cells (54), we first asked whether activation of these kinases requires VP11/12. To address this question, the activating phosphorylation of p38 and JNK was measured for Jurkat T cells infected with wild-type HSV-1 KOS37, two VP11/12-null HSV-1 mutants derived from KOS37, ΔUL46galK and ΔUL46, and a ΔUL46galK derivative with a repaired VP11/12 open reading frame, RUL46 (see Fig. S1 in the supplemental material). All four viruses enhanced the activating phosphorylation of these kinases, demonstrating that VP11/12 is not required for HSV-induced activation of p38 and JNK.
As reviewed in the introduction, SFKs lie upstream of PI3K-Akt in many signaling pathways. Therefore, we asked whether HSV-1 infection activates Akt in a VP11/12-dependent manner in Jurkat cells. Active Akt was detected by Western blotting using an antibody specific for Akt phosphorylated at the activating serine of the hydrophobic motif (anti-pS473) (Fig. 2). The ratio of the infrared signal generated by anti-pS473 relative to that of total anti-Akt was used to quantitatively compare Akt activation between samples. As a leukemic cell line, Jurkat T cells are PTEN deficient, a defect that promotes cell survival through constitutive activation of Akt (51). Accordingly, mock-infected Jurkat T cells displayed high levels of active phospho-S473 Akt. Nevertheless, KOS37 and RUL46 increased the levels of phospho-S473 Akt more than 2-fold relative to levels in mock-infected cells. Both infected samples also demonstrated a slight reduction in the electrophoretic mobility of total Akt. This change in the mobility of Akt is consistent with activation-induced posttranslational modifications, including phosphorylation of S473. In contrast, samples infected with the VP11/12-null mutants ΔUL46galK and ΔUL46 displayed lower levels of phospho-S473 Akt than those in the KOS37, RUL46, and mock-infected samples. ΔUL46galK and ΔUL46 also failed to induce a mobility shift in the total Akt signal relative to that for mock-infected cells. Importantly, the late viral protein VP16 accumulated to wild-type levels during infection with ΔUL46galK and ΔUL46, suggesting that defects observed did not arise from reduced expression of viral proteins other than VP11/12 (Fig. 2). These findings indicate that VP11/12 is required for HSV-induced activation of Akt. Furthermore, infection with VP11/12-null HSV appears to decrease phospho-S473 Akt levels relative to those for mock-infected cells, suggesting that one or more viral gene products inhibit Akt activity in the absence of VP11/12.
FIG. 2.
VP11/12 is necessary for Akt activation in HSV-infected Jurkat cells. Jurkat T cells were mock infected and infected with HSV-1 KOS37, the VP11/12-null mutants ΔUL46galK and ΔUL46, and the VP11/12-repaired virus RUL46. Cell lysates were collected at 9 hpi and analyzed for the activating phosphorylation of Akt at S473 (anti-pS473), total Akt, VP16, and β-actin by Western blotting.
VP11/12 is required for Akt activation in primary HEL fibroblasts.
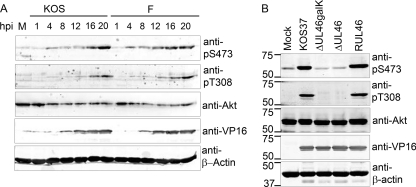
A thorough investigation of VP11/12-dependent Akt signaling in Jurkat T cells was not possible due to the PTEN defect in these cells (51). We therefore sought to examine Akt activation following infection of primary CD8+ T cells. Others have shown that although primary T cells cannot be infected by cell-free HSV, they are efficiently infected following exposure to HSV-infected fibroblasts (2, 20, 44). During the course of preliminary experiments along these lines, it became apparent that HSV-1-infected primary human embryonic lung (HEL) fibroblasts display high levels of active Akt. To determine the time course of activation, HEL fibroblasts were infected with wild-type HSV-1 strains F and KOS, and samples harvested at various times postinfection were analyzed by Western blotting using antibodies specific for Akt phosphorylated at each of the activating residues, threonine 308 in the activation loop and serine 473 in the hydrophobic motif (anti-pT308 and anti-pS473) (Fig. 3). The signals obtained with the anti-Akt antibody suggested that total Akt levels decline somewhat over the course of infection in HEL cells. Thus, any increases in the signal obtained with the anti-phospho-T308 and anti-phospho-S473 Akt antibodies reflect enhanced Akt activation. HSV-1 strains F and KOS both induced increased Akt phosphorylation, with the increase being detectable by 4 hpi and rising until 20 hpi.
FIG. 3.
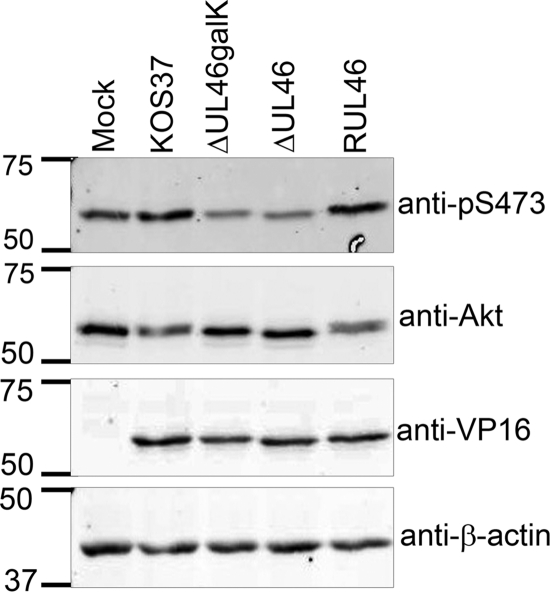
HSV requires VP11/12 to activate Akt late during HEL fibroblast infection. (A) HEL fibroblasts were mock infected (M) or infected with HSV-1 strain F or KOS. Cell lysates were collected at the indicated times postinfection and analyzed by Western blotting using anti-pS473, anti-pT308, anti-VP16, and anti-Akt. (B) HEL fibroblasts were mock infected and infected with HSV-1 KOS37, VP11/12-null viruses, ΔUL46galK and ΔUL46, and the VP11/12-repaired virus RUL46. Cell lysates were collected 15 hpi and analyzed by Western blotting using antibodies against the activating phosphorylation of Akt at T308 (anti-pT308) and S473 (anti-pS473), total Akt, VP16, and β-actin.
We asked if HSV-1-induced activation of Akt in HEL cells requires VP11/12 (Fig. 3B). Infection with KOS37 and RUL46 led to a large increase in the levels of active Akt compared to those in mock-infected cells, while ΔUL46galK and ΔUL46 had no such effect (Fig. 3B). Thus, VP11/12 is required for activation of Akt in primary HEL fibroblasts, providing a convenient system for studying the underlying mechanisms. It is interesting to note that although VP11/12 is an abundant component of the virion tegument, no increase in Akt phosphorylation was observed at 1 hpi (Fig. 3A). This observation suggests that the enhanced Akt phosphorylation that we have observed depends on de novo synthesis of VP11/12 rather than on VP11/12 delivered by the infecting virion.
HSV infection activates Akt through cellular PI3K.
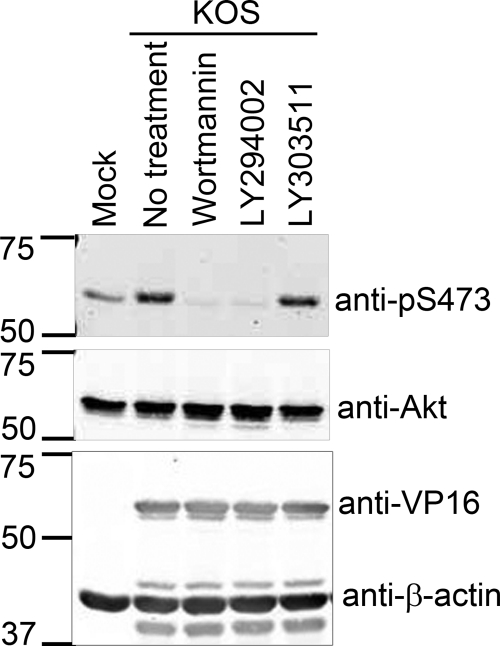
PI3K often lies upstream of Akt in signaling pathways triggered by host cell surface receptors. To assess whether PI3K activity is also required for the VP11/12-dependent activation of Akt observed late during HSV infection, we infected HEL fibroblasts with wild-type HSV-1 KOS and then treated the cultures with the PI3K-specific inhibitors wortmannin and Ly294002 (Fig. 4). The inactive LY294002 analogue LY303511 was used as a negative control. Wortmannin and LY294002 completely blocked HSV-induced activation of Akt as assessed by phosphorylation of S473, while LY303511 had no effect. Given that the inhibitors had little or no effect on expression of VP16, these results suggest that HSV infection triggers VP11/12-dependent PI3K-Akt signaling.
FIG. 4.
Infection-induced activation of Akt is PI3K dependent. HEL fibroblasts were mock infected or infected with HSV-1 KOS. Kinase inhibitors specific for PI3K, wortmannin, and LY294002 and the inactive analogue of LY294002, LY303511, were added to cultures at 11 h postinfection as indicated. Cell lysates were collected at 19 hpi and analyzed by Western blotting using the indicated antibodies.
VP11/12 interacts with the regulatory subunit of PI3K, p85.
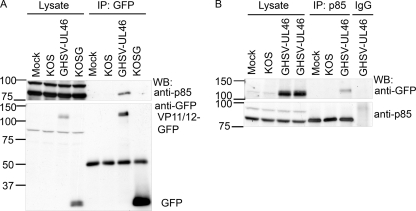
The Scansite 2.0 algorithm (40) identified a potential binding motif for the SH2 domain of the regulatory subunit of PI3K p85 in VP11/12 (YTHM, at Y519). In addition, VP11/12 has been reported to associate with infected cell membranes (36). Therefore, it seemed possible that direct interactions between PI3K and VP11/12 might activate cellular PI3K-Akt signaling, by recruiting PI3K to its substrate PIP2. As a test of this possibility, we used immunoprecipitation/Western blot assays to determine whether VP11/12 interacts with PI3K during infection (Fig. 5). Jurkat T cells were either mock infected or infected with HSV-1 KOS or the KOS derivatives GHSV-UL46 and KOS-G, which encode GFP-tagged VP11/12 or unmodified enhanced GFP (eGFP), respectively. Cell extracts were immunoprecipitated with an anti-GFP antibody and then analyzed by Western blotting for p85. We found that p85 was coimmunoprecipitated only from the GHSV-UL46-infected samples (Fig. 5A); note that the ca. 100-kDa species detected by the p85 antibody in the cell lysates was not consistently observed and was not coimmunoprecipitated with VP11/12. Conversely, the VP11/12-GFP fusion protein was pulled down by the anti-p85 antibody but not by control rabbit IgG (Fig. 5B). Collectively, these assays indicate that VP11/12 interacts with p85 either directly or indirectly during infection.
FIG. 5.
VP11/12 interacts with p85 during infection. Jurkat T cells were mock infected or infected with KOS or KOS-derived viruses which express either GFP (KOS-G) or a VP11/12-GFP fusion protein (GHSV-UL46). (A) Western blot analysis of p85 and GFP in anti-GFP immunoprecipitates. Lysate controls were analyzed by Western blotting directly. The ca. 100-kDa band in the cell lysate that cross-reacts with anti-p85 antibody was not consistently observed. (B) Western blot analysis of p85 and GFP in immunoprecipitates obtained with anti-p85 or rabbit IgG. Lysate controls were analyzed by Western blotting directly. WB, Western blot; IP, immunoprecipitation.
SFK activation in HEL fibroblasts does not strictly require VP11/12.
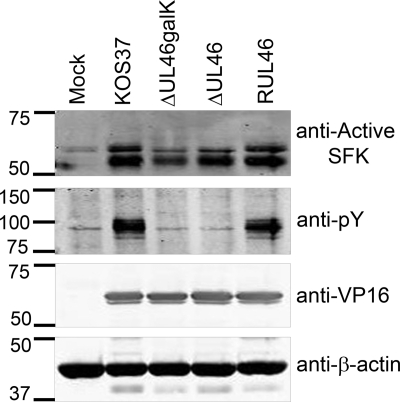
We previously demonstrated that VP11/12 is required for HSV-induced activation of Lck (64) and that Lck activity contributes to tyrosine phosphorylation of VP11/12 (72). It therefore seemed plausible that Lck may be required for activation of Akt in T cells and that other SFKs may support VP11/12-dependent Akt activation in HEL fibroblasts and other cell types that do not express Lck. To address this hypothesis, we first asked whether VP11/12 is required for activation of SFKs in HEL fibroblasts, as it is for Lck activation in Jurkat T cells (64). To detect SFK activation, we probed Western blots with an antibody specific for SFKs phosphorylated at the activating tyrosine residue (the equivalent of Y416 of Src) (Fig. 6). SFKs phosphorylated at this residue increased relative to β-actin during HSV infection. However, this increase was only partially impaired during infection with the VP11/12-null viruses ΔUL46galK and ΔUL46. These results indicate that although VP11/12 may contribute to SFK activation in HEL fibroblasts, other viral gene products are able to activate SFKs in its absence. In this context, it is interesting to note that a previous report implicated ICP0, US3, and UL13 in activating SFKs other than Lck in Vero cells (24).
FIG. 6.
VP11/12 is tyrosine phosphorylated and promotes SFK activation in infected HEL fibroblasts. HEL fibroblasts were infected with HSV-1 KOS37, ΔUL46galK, ΔUL46, and RUL46. Cell lysates were collected at 21 h postinfection and analyzed by Western blotting. Western blot detection of SFKs phosphorylated at the activating tyrosine (the equivalent of phospho-Y416 of Src, anti-active SFK), phosphotyrosine (anti-pTyr), VP16, and β-actin.
SFK activity is required for VP11/12-PI3K interactions and Akt activation.
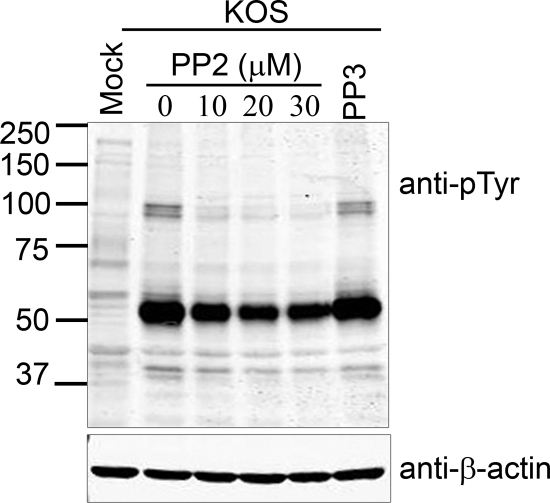
Although tyrosine phosphorylation of VP11/12 is weaker in HEL fibroblasts than in lymphocytes (72), it is nevertheless detectable in these cells by Western blot analysis using an anti-phosphotyrosine antibody (Fig. 6 and additional data not shown). To determine whether SFK activity is required for tyrosine phosphorylation of VP11/12 in HEL cells, we examined the effects of the SFK-specific inhibitor PP2 (Fig. 7). Cells were infected with wild-type HSV-1 KOS and then treated with PP2 at a concentration of 10, 20, or 30 μM at 11 hpi, and tyrosine phosphorylation was analyzed by Western blotting at 20 hpi. The inactive PP2 analogue PP3 (30 μM) served as a control. PP2 greatly reduced the ca. 90-kDa phosphotyrosine signal arising from VP11/12, while exposure to PP3 (30 μM) had no effect. Inasmuch as PP2 has little or no effect on the levels of (GFP-tagged) VP11/12 (Fig. 8), these data suggest that tyrosine phosphorylation of VP11/12 is dependent largely on SFKs in HEL fibroblasts, just as it is in Jurkat cells. HSV infection also greatly enhanced the intensity of a ca. 55-kDa tyrosine-phosphorylated band (Fig. 7). Some of this signal presumably arises from activated SFKs. Whether additional ca. 55-kDa proteins are also tyrosine phosphorylated remains to be determined.
FIG. 7.
SFK activity is required for tyrosine phosphorylation of VP11/12. HEL fibroblasts were mock infected or infected with HSV-1 KOS. At 11 h postinfection, the specific SFK inhibitor PP2 was added at 10, 20, and 30 μM, and the inactive analogue of PP2, PP3, was added at 30 μM as indicated. Cell lysates were collected at 16 hpi and analyzed by Western blotting using anti-phosphotyrosine and anti-β-actin.
FIG. 8.
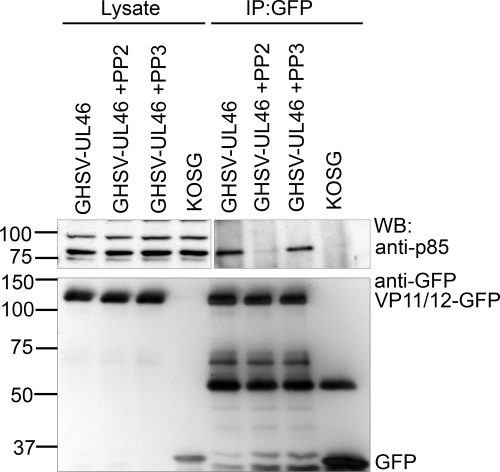
SFK activity is required for VP11/12-p85 interaction(s). HEL fibroblasts were infected with the HSV-1 KOS-derived viruses KOSG and GHSV-UL46. At 11 h postinfection, PP2 (10 μM) and PP3 (10 μM) were added to cultures as indicated. Cell lysates were collected at 18 hpi. Anti-GFP immunoprecipitates were analyzed by Western blotting using the indicated antibodies. Lysate controls were analyzed directly.
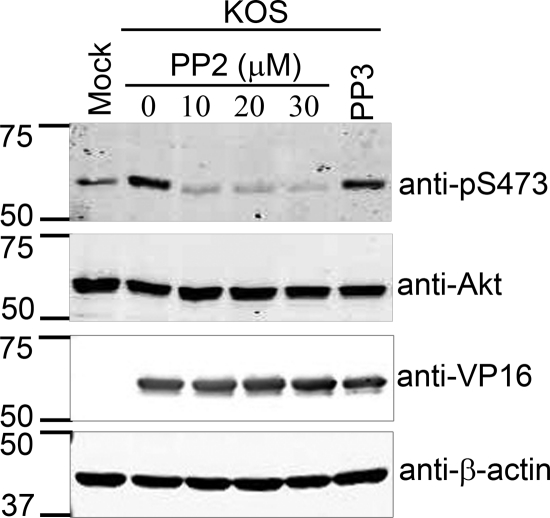
To determine whether SFK-dependent phosphorylation of VP11/12 is required for the interaction between VP11/12 and PI3K, we assessed whether inhibiting SFK activity reduces the association of these two proteins (Fig. 8). HEL fibroblasts were infected with the HSV-1 KOS derivatives GHSV-UL46 and KOS-G. Some of the cultures infected with GHSV-UL46 were treated at 11 hpi with PP2 (10 μM) and PP3 (10 μM). Cell lysates were prepared 7 h later and immunoprecipitated with the anti-GFP antibody, and the precipitates were analyzed for p85 by Western blotting. PP2 treatment greatly reduced the association between p85 and the VP11/12-GFP fusion protein encoded by GHSV-UL46, while PP3 had no effect. A similar result was also seen for Jurkat T cells (data not shown). These data indicate that SFK activity is required for p85-VP11/12 interactions and suggest that SFK activation is one of the initial steps in VP11/12-dependent PI3K-Akt signaling. Consistent with this hypothesis, PP2 treatment severely reduced HSV-induced activation of Akt as assessed by phosphorylation of Akt S473, while PP3 had no effect (Fig. 9).
FIG. 9.
Inhibition of SFK activity prevents Akt activation during HSV infection. HEL fibroblasts were mock infected or infected with HSV-1 KOS. At 11 h postinfection, the SFK inhibitor PP2 was added at a concentration of 10, 20, and 30 μM, and the inactive PP2 analogue PP3 was added at a 30 μM concentration. Cell lysates were collected at 16 hpi and analyzed by Western blotting using the indicated antibodies.
VP11/12 is not required for HSV-induced activation of mTORC1.
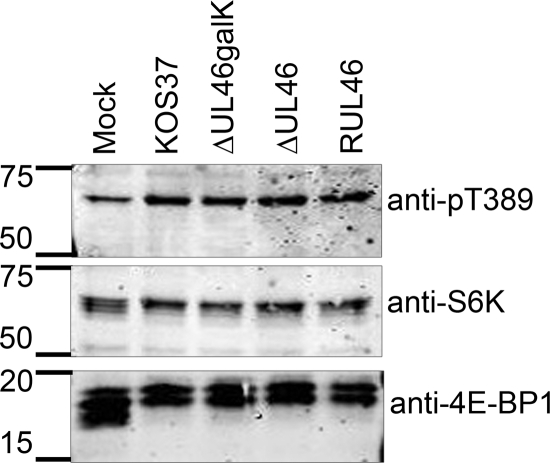
A previous report by Walsh and Mohr demonstrated that HSV infection activates mTORC1, resulting in hyperphosphorylation and inactivation of the mTORC1 target 4EBP1 (65). Because Akt is a well-known activator of mTORC1, we asked if VP11/12 contributes to mTORC1 signaling and examined the effects of VP11/12-null mutations on phosphorylation of the downstream mTORC1 targets 4EBP1 and S6 kinase (S6K) (Fig. 10). As seen previously (65), infection with wild-type HSV-1 KOS37 and RUL46 induced hyperphosphorylation of 4EBP1, as was evident by a decrease in its electrophoretic mobility. KOS37 and RUL46 also increased the fraction of S6K phosphorylated at the activating residue, T389 (anti-pT389) compared to that seen with mock infection. These changes are consistent with the previously reported HSV-induced activation of mTORC1. Interestingly, the VP11/12-null mutants ΔUL46galK and ΔUL46 displayed wild-type activity levels in these assays, indicating that VP11/12 is not required for activation of mTORC1.
FIG. 10.
VP11/12 is not required for infection-induced phosphorylation of the mTORC1 substrates S6K and 4E-BP1. HEL fibroblasts were infected with HSV-1 KOS37, ΔUL46galK, ΔUL46, and RUL46. At 20 h postinfection, lysates were collected and analyzed for phosphorylation of S6K at T389 (anti-pT389), total S6K, and total 4E-BP1 by Western blotting.
Phosphorylation of another Akt substrate, glycogen synthetase kinase 3β (GSK-3β), was also examined. Akt phosphorylates GSK-3β at residue S9, inactivating the enzyme and promoting glycogen synthesis and protein synthesis by releasing inhibition of glycogen synthase and eIF2B (46). Phosphorylation of GSK-3β S9 was enhanced during infection with wild-type HSV KOS37, ΔUL46galK, ΔUL46, and RUL46 (see Fig. S2 in the supplemental material). Thus, HSV infection enhances GSK-3β phosphorylation, but it does not require VP11/12 to do so.
DISCUSSION
A previous study documented that HSV-1 infection leads to activation of Akt (4); however, the viral gene product responsible was not identified. Here, we demonstrate that the HSV-1 tegument protein VP11/12 is essential for this process, and we present evidence that VP11/12 acts by recruiting and activating cellular PI3K. Along with our previous reports, the results presented in the present study support a model in which VP11/12 recruits Lck and other SFKs to initiate signaling through the PI3K-Akt signaling module. VP11/12 contains a putative high-affinity SFK SH2 binding motif, YEEI, at residue Y624, and we have previously suggested that this motif binds and activates Lck and other SFKs (64), leading to tyrosine phosphorylation of VP11/12 (72). Based on the results of the present study, we further propose that the activated SFK then promotes binding of PI3K to membrane-associated VP11/12, most likely by triggering phosphorylation of VP11/12 residue Y519 within the predicted p85 SH2 binding motif, YTHM. According to this model, VP11/12 activates PI3K-Akt signaling by mimicking an activated growth factor receptor, such as PDGFR-β. In support of this theory, VP11/12 is found in a complex with PI3K (Fig. 5) and associates with cellular membranes during infection (36). Thus, binding of PI3K to VP11/12 may recruit PI3K to its substrate PIP2. Also consistent with this model, SFK activity is required for VP11/12 tyrosine phosphorylation (Fig. 7), PI3K binding to VP11/12 (Fig. 8), and activation of Akt (Fig. 9). It is interesting to note that the YEEI and YTHM motifs are conserved in the VP11/12 orthologues of several closely related members of the Simplexvirus genus of the Alphaherpesvirinae, including HSV-2, cercopithecine herpesvirus 1 (CHV-1), CHV-2, and CHV-16, suggesting that these proteins may also participate in SFK- and PI3K-dependent signaling. We currently seek to directly test the roles of the YEEI and YTHM motifs in SFK activation and PI3K recruitment, respectively, and determine whether VP11/12 is sufficient to trigger activation of Akt when it is expressed in isolation.
VP11/12 interacts with PI3K, suggesting that it directly activates PI3K. However, it is unclear whether VP11/12-mediated PI3K activation fully accounts for the Akt activation observed during HSV-1 infection. For example, full activation of Akt requires phosphorylation of S473 in the hydrophobic motif by PDK2 in addition to phosphorylation of the activation loop by PDK1 (47), and the identity of PDK2 and the trigger for its activation have yet to be conclusively determined. To date, the best candidate for PDK2 is mTORC2 (47). Thus, determining whether and how HSV-1 activates mTORC2 is of great interest. It is also possible that HSV-1 infection stimulates Akt by inhibiting the PI3K antagonist PTEN in addition to activating PI3K. In this context, it is interesting to note that overexpression of active Src interferes with PTEN activity (28), although it is unclear whether active SFKs generally disrupt PTEN activity in other signaling systems. It will therefore be interesting to determine whether HSV-1 infection inhibits PTEN, and if so, whether VP11/12 is involved.
HSV-1 may also encode Akt activators in addition to VP11/12. One report demonstrated that expression of HSV-1 latency-associated transcript in neuroblastoma cells was sufficient to increase levels of total Akt and phosphorylated Akt (23). Another report suggested that the serine/threonine kinase activity of HSV-2 ICP10 activates Akt (16). We have yet to investigate whether ICP6 (the HSV-1 homologue of HSV-2 ICP10), the latency-associated transcript, or other HSV-1-encoded signaling modulators are able to contribute to the VP11/12-dependent activation of Akt.
Previously, the HSV-1 serine threonine kinase US3 was shown to contribute to the loss of the active phosphorylated form of Akt late during HSV-1 infection of Hep-2 cells (4). Combined with our findings, these results suggest that HSV-1 encodes both positive and negative regulators of Akt. Supporting this theory, the levels of phospho-S473 Akt were greatly reduced compared to those for mock-infected cells after infection of Jurkat T cells with VP11/12-null HSV-1 (Fig. 2). Further experimentation is required to determine whether US3 is responsible for this inhibition. If HSV does indeed encode both an activator and an inhibitor of Akt, then the status of this key signaling kinase is likely to be subject to complex control, implying that it modulates one or more important aspects of the viral life cycle.
Although HSV-1 infection clearly induces VP11/12-dependent activation of Akt, as indicated by phosphorylation of Akt on its activation loop and hydrophobic motif, we have yet to identify downstream Akt targets that are phosphorylated in a VP11/12-dependent fashion during HSV-1 infection. Although HSV-1 infection activates the Akt target mTORC1 (65), our data indicate that VP11/12 is not required for this effect (Fig. 10). Likewise, HSV-1 infection enhances phosphorylation of the Akt substrate GSK-3β, but in a VP11/12-independent fashion (Fig. S1). These surprising data raise the possibility that these Akt substrates are phosphorylated via an alternative Akt-independent pathway during HSV-1 infection. Further studies are required to test this hypothesis and determine which (if any) Akt targets are phosphorylated in a VP11/12-dependent manner during infection.
Supplementary Material
Acknowledgments
We thank Holly Saffran and Rob Maranchuk for technical support.
This research was supported by an operating grant from the Canadian Institutes for Health Research (FRN 12172). M.J.W. was supported by a graduate studentship from the Alberta Heritage Foundation for Medical Research, and J.R.S. is a Canada Research Chair in Molecular Virology.
Footnotes
Published ahead of print on 12 January 2011.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Amrein, K. E., and B. M. Sefton. 1988. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 85:4247-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert, M., M. Yoon, D. D. Sloan, P. G. Spear, and K. R. Jerome. 2009. The virological synapse facilitates herpes simplex virus entry into T cells. J. Virol. 83:6171-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter, R. M., J. P. Secrist, R. R. Vaillancourt, and A. Kazlauskas. 1998. Full activation of the platelet-derived growth factor beta-receptor kinase involves multiple events. J. Biol. Chem. 273:17050-17055. [DOI] [PubMed] [Google Scholar]
- 4.Benetti, L., and B. Roizman. 2006. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J. Virol. 80:3341-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolen, J. B., et al. 1984. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell 38:767-777. [DOI] [PubMed] [Google Scholar]
- 6.Buchkovich, N. J., Y. Yu, C. A. Zampieri, and J. C. Alwine. 2008. The TORrid affairs of viruses: effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat. Rev. Microbiol. 6:266-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, J., J. A. DeCaprio, M. M. Fluck, and B. S. Schaffhausen. 2009. Cellular transformation by simian virus 40 and murine polyoma virus T antigens. Semin. Cancer Biol. 19:218-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtneidge, S. A. 1985. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 4:1471-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtneidge, S. A., and A. E. Smith. 1983. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature 303:435-439. [DOI] [PubMed] [Google Scholar]
- 10.Darr, C. D., A. Mauser, and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation. J. Virol. 75:6135-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson, C. W., G. Tramountanis, A. G. Eliopoulos, and L. S. Young. 2003. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J. Biol. Chem. 278:3694-3704. [DOI] [PubMed] [Google Scholar]
- 12.Efeyan, A., and D. M. Sabatini. 2010. mTOR and cancer: many loops in one pathway. Curr. Opin. Cell Biol. 22:169-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.François, F., and M. E. Klotman. 2003. Phosphatidylinositol 3-kinase regulates human immunodeficiency virus type 1 replication following viral entry in primary CD4+ T lymphocytes and macrophages. J. Virol. 77:2539-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukui, Y., and H. Hanafusa. 1989. Phosphatidylinositol kinase activity associates with viral p60src protein. Mol. Cell. Biol. 9:1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gierasch, W. W., et al. 2006. Construction and characterization of bacterial artificial chromosomes containing HSV-1 strains 17 and KOS. J. Virol. Methods 135:197-206. [DOI] [PubMed] [Google Scholar]
- 16.Gober, M. D., J. M. Laing, S. M. Thompson, and L. Aurelian. 2006. The growth compromised HSV-2 mutant DeltaRR prevents kainic acid-induced apoptosis and loss of function in organotypic hippocampal cultures. Brain Res. 1119:26-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harwood, N. E., and F. D. Batista. 2008. New insights into the early molecular events underlying B cell activation. Immunity 28:609-619. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, R. A., X. Wang, X. L. Ma, S. M. Huong, and E. S. Huang. 2001. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J. Virol. 75:6022-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato, K., et al. 2000. Synthesis, subcellular localization and VP16 interaction of the herpes simplex virus type 2 UL46 gene product. Arch. Virol. 145:2149-2162. [DOI] [PubMed] [Google Scholar]
- 20.Kirchner, H., C. Kleinicke, and H. Northoff. 1977. Replication of herpes simplex virus in human peripheral T lymphocytes. J. Gen. Virol. 37:647-649. [Google Scholar]
- 21.Koretzky, G. A., J. Picus, M. L. Thomas, and A. Weiss. 1990. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature 346:66-68. [DOI] [PubMed] [Google Scholar]
- 22.Kummer, M., A. T. Prechtel, P. Muhl-Zurbes, N. M. Turza, and A. Steinkasserer. 2009. HSV-1 upregulates the ARE-binding protein tristetraprolin in a STAT1- and p38-dependent manner in mature dendritic cells. Immunobiology 214:852-860. [DOI] [PubMed] [Google Scholar]
- 23.Li, S., D. Carpenter, C. Hsiang, S. L. Wechsler, and C. Jones. 2010. Herpes simplex virus type 1 latency-associated transcript inhibits apoptosis and promotes neurite sprouting in neuroblastoma cells following serum starvation by maintaining protein kinase B (AKT) levels. J. Gen. Virol. 91:858-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang, Y., and B. Roizman. 2006. State and role of SRC family kinases in replication of herpes simplex virus 1. J. Virol. 80:3349-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linnemann, T., Y. H. Zheng, R. Mandic, and B. M. Peterlin. 2002. Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology 294:246-255. [DOI] [PubMed] [Google Scholar]
- 26.Liu, P., H. Cheng, T. M. Roberts, and J. J. Zhao. 2009. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 8:627-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, X., L. E. Marengere, C. A. Koch, and T. Pawson. 1993. The v-Src SH3 domain binds phosphatidylinositol 3′-kinase. Mol. Cell. Biol. 13:5225-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu, Y., et al. 2003. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J. Biol. Chem. 278:40057-40066. [DOI] [PubMed] [Google Scholar]
- 29.Manning, B. D., and L. C. Cantley. 2007. AKT/PKB signaling: navigating downstream. Cell 129:1261-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marth, J. D., et al. 1988. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol. Cell. Biol. 8:540-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin, G. S. 2001. The hunting of the Src. Nat. Rev. Mol. Cell Biol. 2:467-475. [DOI] [PubMed] [Google Scholar]
- 32.McKnight, J. L., P. E. Pellett, F. J. Jenkins, and B. Roizman. 1987. Characterization and nucleotide sequence of two herpes simplex virus 1 genes whose products modulate alpha-trans-inducing factor-dependent activation of alpha genes. J. Virol. 61:992-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean, T. I., and S. L. Bachenheimer. 1999. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 73:8415-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minaker, R. L., K. L. Mossman, and J. R. Smiley. 2005. Functional inaccessibility of quiescent herpes simplex virus genomes. Virol. J. 2:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori, S., et al. 1993. Identification of two juxtamembrane autophosphorylation sites in the PDGF beta-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J. 12:2257-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, M. A., M. A. Bucks, K. J. O'Regan, and R. J. Courtney. 2008. The HSV-1 tegument protein pUL46 associates with cellular membranes and viral capsids. Virology 376:279-289. [DOI] [PubMed] [Google Scholar]
- 37.Myers, M. G. Jr., et al. 1992. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc. Natl. Acad. Sci. U. S. A. 89:10350-10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson, J. 2008. Structure and function in cell signalling. John Wiley & Sons Canada, Ltd., Mississauga, Canada.
- 39.Newton, A. C. 2009. Lipid activation of protein kinases. J. Lipid Res. 50(Suppl.):S266-S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obenauer, J. C., L. C. Cantley, and M. B. Yaffe. 2003. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 31:3635-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Shea, C., et al. 2005. Adenoviral proteins mimic nutrient/growth signals to activate the mTOR pathway for viral replication. EMBO J. 24:1211-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pingel, J. T., and M. L. Thomas. 1989. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell 58:1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polak, P., and M. N. Hall. 2009. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 21:209-218. [DOI] [PubMed] [Google Scholar]
- 44.Posavad, C. M., J. J. Newton, and K. L. Rosenthal. 1993. Inhibition of human CTL-mediated lysis by fibroblasts infected with herpes simplex virus. J. Immunol. 151:4865-4873. [PubMed] [Google Scholar]
- 45.Prasad, K. V., et al. 1993. Src-homology 3 domain of protein kinase p59fyn mediates binding to phosphatidylinositol 3-kinase in T cells. Proc. Natl. Acad. Sci. U. S. A. 90:7366-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rayasam, G. V., V. K. Tulasi, R. Sodhi, J. A. Davis, and A. Ray. 2009. Glycogen synthase kinase 3: more than a namesake. Br. J. Pharmacol. 156:885-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098-1101. [DOI] [PubMed] [Google Scholar]
- 48.Schaffhausen, B. S., H. Dorai, G. Arakere, and T. L. Benjamin. 1982. Polyoma virus middle T antigen: relationship to cell membranes and apparent lack of ATP-binding activity. Mol. Cell. Biol. 2:1187-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholle, F., K. M. Bendt, and N. Raab-Traub. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seavitt, J. R., et al. 1999. Expression of the p56(Lck) Y505F mutation in CD45-deficient mice rescues thymocyte development. Mol. Cell. Biol. 19:4200-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shan, X., et al. 2000. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol. Cell. Biol. 20:6945-6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shih, W. L., M. L. Kuo, S. E. Chuang, A. L. Cheng, and S. L. Doong. 2000. Hepatitis B virus X protein inhibits transforming growth factor-beta-induced apoptosis through the activation of phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 275:25858-25864. [DOI] [PubMed] [Google Scholar]
- 53.Sloan, D. D., et al. 2006. Inhibition of TCR signaling by herpes simplex virus. J. Immunol. 176:1825-1833. [DOI] [PubMed] [Google Scholar]
- 54.Sloan, D. D., and K. R. Jerome. 2007. Herpes simplex virus remodels T-cell receptor signaling, resulting in p38-dependent selective synthesis of interleukin-10. J. Virol. 81:12504-12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith-Garvin, J. E., G. A. Koretzky, and M. S. Jordan. 2009. T cell activation. Annu. Rev. Immunol. 27:591-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spear, P. G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J. Virol. 9:143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stephens, L., et al. 1998. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279:710-714. [DOI] [PubMed] [Google Scholar]
- 59.Stokoe, D., et al. 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277:567-570. [DOI] [PubMed] [Google Scholar]
- 60.Summers, S. A., L. Lipfert, and M. J. Birnbaum. 1998. Polyoma middle T antigen activates the Ser/Thr kinase Akt in a PI3-kinase-dependent manner. Biochem. Biophys. Res. Commun. 246:76-81. [DOI] [PubMed] [Google Scholar]
- 61.Swart, R., I. K. Ruf, J. Sample, and R. Longnecker. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-Kinase/Akt pathway. J. Virol. 74:10838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talmage, D. A., et al. 1989. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell 59:55-65. [DOI] [PubMed] [Google Scholar]
- 63.Vogel, L. B., and D. J. Fujita. 1993. The SH3 domain of p56lck is involved in binding to phosphatidylinositol 3′-kinase from T lymphocytes. Mol. Cell. Biol. 13:7408-7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner, M. J., and J. R. Smiley. 2009. Herpes simplex virus requires VP11/12 to induce phosphorylation of the activation loop tyrosine (Y394) of the Src family kinase Lck in T lymphocytes. J. Virol. 83:12452-12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walsh, D., and I. Mohr. 2004. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 18:660-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wange, R. L., et al. 1995. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J. Biol. Chem. 270:18730-18733. [DOI] [PubMed] [Google Scholar]
- 67.Willard, M. 2002. Rapid directional translocations in virus replication. J. Virol. 76:5220-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wysocka, J., and W. Herr. 2003. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem. Sci. 28:294-304. [DOI] [PubMed] [Google Scholar]
- 69.Yu, J. C., et al. 1991. Tyrosine mutations within the alpha platelet-derived growth factor receptor kinase insert domain abrogate receptor-associated phosphatidylinositol-3 kinase activity without affecting mitogenic or chemotactic signal transduction. Mol. Cell. Biol. 11:3780-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu, K. T., D. K. Werth, I. H. Pastan, and M. P. Czech. 1985. src kinase catalyzes the phosphorylation and activation of the insulin receptor kinase. J. Biol. Chem. 260:5838-5846. [PubMed] [Google Scholar]
- 71.Zachos, G., B. Clements, and J. Conner. 1999. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J. Biol. Chem. 274:5097-5103. [DOI] [PubMed] [Google Scholar]
- 72.Zahariadis, G., et al. 2008. Cell-type-specific tyrosine phosphorylation of the herpes simplex virus tegument protein VP11/12 encoded by gene UL46. J. Virol. 82:6098-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, B., D. F. Spandau, and A. Roman. 2002. E5 protein of human papillomavirus type 16 protects human foreskin keratinocytes from UV B-irradiation-induced apoptosis. J. Virol. 76:220-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, Y., and J. L. McKnight. 1993. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J. Virol. 67:1482-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, Y., D. A. Sirko, and J. L. McKnight. 1991. Role of herpes sim- plex virus type 1 UL46 and UL47 in alpha TIF-mediated transcriptional induction: characterization of three viral deletion mutants. J. Virol. 65:829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.