Abstract
Human T-lymphotropic virus type 1 (HTLV-1) is the causative agent of adult T-cell leukemia/lymphoma (ATL), a malignancy of CD4+ T cells whose etiology is thought to be associated with the viral trans-activator Tax. We have shown recently that Tax can drastically upregulate the expression of p27Kip1 and p21CIP1/WAF1 through protein stabilization and mRNA trans-activation and stabilization, respectively. The Tax-induced surge in p21CIP1/WAF1 and p27Kip1 begins in S phase and results in cellular senescence. Importantly, HeLa and SupT1 T cells infected by HTLV-1 also arrest in senescence, thus challenging the notion that HTLV-1 infection causes cell proliferation. Here we use time-lapse microscopy to investigate the effect of Tax on cell cycle progression in two reporter cell lines, HeLa/18x21-EGFP and HeLa-FUCCI, that express enhanced green fluorescent protein (EGFP) under the control of 18 copies of the Tax-responsive 21-bp repeat element and fluorescent ubiquitin cell cycle indicators, respectively. Tax-expressing HeLa cells exhibit elongated or stalled cell cycle phases. Many of them bypass mitosis and become single senescent cells as evidenced by the expression of senescence-associated β-galactosidase. Such cells have twice the normal equivalent of cellular contents and hence are enlarged, with exaggerated nuclei. Interestingly, nocodazole treatment revealed a small variant population of HeLa/18x21-EGFP cells that could progress into mitosis normally with high levels of Tax expression, suggesting that genetic or epigenetic changes that prevent Tax-induced senescence can occur spontaneously at a detectable frequency.
Human T-lymphotropic virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia/lymphoma (ATLL). HTLV-1 oncoprotein Tax is known to induce DNA damage, micronuclei formation, multinucleation, and DNA aneuploidy. The genomic instability caused by Tax is thought to play an important role in ATLL development (6, 14, 27). We have shown recently that Tax prematurely activates the anaphase-promoting complex/cyclosome (APC/C), a multisubunit E3 ubiquitin ligase that controls metaphase to anaphase transition and mitotic exit. APC/C activation by Tax leads to polyubiquitination and degradation of Skp2 and inactivation of SCFSkp2, the E3 ligase that targets p27Kip1 (referred to as p27 herein), the G1/S and S cyclin-dependent kinase inhibitor, for degradation. This, in turn, causes stabilization of p27 (20). Tax also trans-activates the p21CIP1 (p21) promoter in a p53-independent manner (4) and increases p21CIP1 mRNA stability (54). The dramatic rise in p21 and p27 levels slows down cell cycle progression and induces rapid cellular senescence that we termed Tax-IRS (20). Importantly, HeLa and SupT1 cells infected by HTLV-1 also become senescent soon after infection (25). Similar activities of Tax have been observed for CD34+ hematopoietic progenitor cells by others (49, 50).
Consistent with the notion that p21 and p27 upregulation mediates Tax-IRS, p21 is mislocalized and p27 downregulated in HTLV-1-transformed T cells (HTxT cells) that express Tax (20, 25). The p21 and p27 deficiencies in HTxT cells correlate with the latter's capacity to avoid Tax-IRS (20). Indeed, HOS, a human osteosarcoma cell line dually deficient in p21 and p27, was able to sustain Tax expression and HTLV-1 infection without becoming arrested in senescence immediately (25). Interestingly, although Tax-transduced or HTLV-1-infected HOS cells continue to grow and divide, they show severe nuclear aberrations and double-stranded DNA breaks (25). Their growth rate was also significantly reduced. These results strongly suggest that productive HTLV-1 infection results in Tax-induced APC/C activation, p21 and p27 upregulation, DNA damage, and senescence. While the loss of p21 and p27 CKIs function and/or expression from HOS cells allows senescence to be delayed or prevented, the latter may continue to divide in the presence of Tax and develop DNA damage and genomic instability (25).
Several studies have shown that Tax can impair cellular response to DNA damage and induce genomic instability (9, 12, 38, 39). Tax-expressing cells resumed DNA synthesis more rapidly than control cells after ionizing radiation-induced S-phase checkpoint activation (3, 22). Tax has also been shown to attenuate ATM kinase activity and sequester huChk2 on chromatin (9). Finally, it has been reported recently that expression of Tax induces H2AX focus formation and H2AX phosphorylation (5). Here we use two HeLa reporter cell lines, HeLa/18x21-EGFP (53) and HeLa-FUCCI, that express enhanced green fluorescent protein (EGFP) under the control of 18 copies of the Tax-responsive 21-bp repeat element and Cdt1/geminin fluorescent ubiquitin cell cycle indicators, respectively, to investigate the effect of Tax on cell cycle progression. We found that Tax-expressing cells had elongated or stalled cell cycle phases. As previously reported, Tax dramatically upregulates the levels of the cyclin-dependent kinase inhibitors p21 and p27, while it downregulates those of Skp2 and cyclin B1. Tax-expressing cells often bypass mitosis and become arrested in senescence. This effect of Tax correlates with the drastic upregulation of p21 and p27 and a reduction in cyclin B1 levels. Unexpectedly, nocodazole treatment of Ad-Tax-transduced HeLa/18x21-EGFP cells revealed a subpopulation that expressed Tax to high levels but progressed normally to metaphase like non-Tax-expressing cells, suggesting that epigenetic and/or genetic changes in this subpopulation prevented Tax-induced mitotic abnormalities and senescence. Elucidation of the cellular pathways that lead to the prevention of Tax-IRS in these cells may shed light on how cancer cells evade p21 and p27-mediated senescence.
Tax-expressing HeLa cells stall in cell cycle progression.
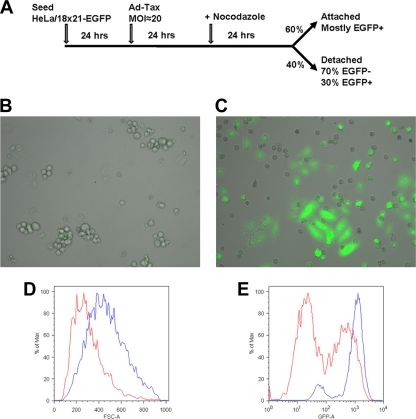
We have shown previously that many cells expressing Tax progress through S, G2, and M phases of the cell cycle with difficulties despite the mitogenic activities of Tax. This aberrant cell cycle is accompanied by a dramatic rise in the levels of G1/S cyclin-dependent kinase inhibitors p21 and p27, starting in S phase, persisting through G2 and M, and finally ending in cellular senescence. To investigate the impact of Tax on cell cycle progression from S through M, we transduced HeLa/18x21-EGFP cells with Ad-Tax or Ad-tTa at a multiplicity of infection (MOI) of approximately 20 for 24 h and then treated the transduced cells with nocodazole for an additional 24 h (see Fig. 1 A for the treatment protocol). It is important to note that the titers of the adenoviral vectors were determined in HEK293 cells and that their infectivity in HeLa or HeLa/18x21-EGFP cells is substantially lower. Under the condition used, approximately 60 to 70% of cells were transduced as judged by EGFP expression. Nocodazole disrupts microtubules and prevents mitotic spindle assembly, thereby activating the spindle checkpoint to cause metaphase arrest. Nocodazole-treated cells progress to M with a high level of cyclin B-Cdk activity and become round but fail to undergo metaphase to anaphase transition (see Fig. 1B for an example). Such metaphase-arrested round cells are easily detached from culture plates and can be isolated for further studies. As indicated in Fig. 1B, most, if not all, untransduced HeLa cells treated with nocodazole became round, as expected. They readily detached from the cell culture dish upon shaking. In contrast, no cell rounding was observed for the majority of EGFP-positive (and hence Tax-positive) Ad-Tax-transduced HeLa/18x21-EGFP cells after nocodazole treatment (Fig. 1C). These cells remained attached to the culture flask. Flow cytometry further indicated that they were larger (Fig. 1D, blue versus red trace) and expressed EGFP to a higher level than the detached population (Fig. 1C and E, blue versus red trace). Under the experimental conditions depicted in Fig. 1A, cells that became round (approximately 40% of total cell population) can be easily dislodged from the flask after shaking, and most were EGFP negative (≈70% of the detached population), as might be expected for cells not expressing Tax (Fig. 1C and E, red trace). Unexpectedly, a small but significant fraction (≈30% of the detached population, 12% of the total population) was EGFP positive (Fig. 1C and E, red trace, right peak).
FIG. 1.
Ad-Tax-transduced cells lag in S and G2 phases of the cell cycle. (A) Schematic diagram of the schedules of Ad-Tax transduction and nocodazole treatment. Under the conditions used, 60% of Ad-Tax-transduced cells remained attached to the culture dish after incubation with nocodazole for 24 h. (B and C) Fifty thousand HeLa/18x21-EGFP cells in 1 ml Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and antibiotic supplements were seeded in each well of a six-well plate and then grown at 37°C in 5% CO2 for 24 h. Culture medium was then removed by aspiration and replaced with 1 ml of the same medium with or without Ad-Tax or Ad-tTA. Where appropriate, each well received approximately 2 × 106 PFU of adenovirus vector (final MOI, ≈20). Under this condition, approximately 60% of cells became transduced. The cells were then incubated at 37°C in 5% CO2 for another 24 h. Again, the medium was removed by aspiration and replaced with 1 ml of the same medium containing 100 ng/ml nocodazole, incubated for another 24 h, and then photographed. The photographs are composites that merge both the phase-contrast and the EGFP images. Most, if not all, Ad-tTa-treated cells became round and detached from the culture dish after nocodazole treatment. (C) Ad-Tax-transduced HeLa/18x21-EGFP cells were treated with nocodazole as in panel A. The fractions of Ad-Tax-treated cells that remained attached or detached, respectively, are as detailed for panel A. (D and E) Histograms of cell sizes (D) and EGFP expression (E) as determined by forward light scattering (FSC-A) and fluorescence intensity (GFP-A). Blue and red traces represent, respectively, the attached and detached populations of Ad-Tax-transduced cells after nocodazole treatment.
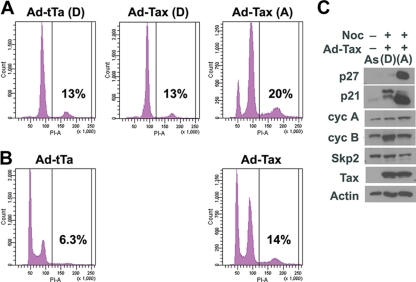
These results were further confirmed by flow cytometry. Indeed, the majority of the control Ad-tTa-/nocodazole-treated cells detached from the plate and were arrested in metaphase with twice the DNA content [Fig. 2 A, Ad-tTa (D)] of the G1 population seen in untreated and asynchronously grown cells (Fig. 2B, Ad-tTa). The Ad-Tax-/nocodazole-treated cells were also divided into attached and detached populations as indicated (Fig. 1A). Those that detached from the plate exhibited flow cytometry histograms very similar to those of the detached population of the control [Fig. 2A, compare Ad-Tax (D) and Ad-tTa (D)]. Surprisingly, one-third of the detached cells expressed Tax (as indicated by the EGFP signal; Fig. 1E). This point is elaborated upon below. In contrast, the majority of the adherent cells in the Ad-Tax-/nocodazole-treated population had DNA content consistent with late S or G2 [Fig. 2A, Ad-Tax (A)]. Two smaller but sizable populations of cells were found to have G1 and greater than two times G1 DNA contents [Fig. 2A Ad-Tax (A)]. The G1 and especially the G2 population with enlarged cell sizes likely represent Tax-expressing cells that are senescent or are becoming senescent, as reported before (20). Those cells with greater than G2 DNA content [20%; Fig. 2A, Ad-Tax (A)] are likely to have undergone DNA endoreduplication or hyperreplication and synthesized more than the normal equivalent of DNA. This population of cells is also seen in asynchronously grown Ad-Tax-transduced cells not treated with nocodazole (Fig. 2B, Ad-Tax, 14%) and in Ad-tTa-transduced cells (Fig. 2B, Ad-tTa, 6.3%) but at lower levels.
FIG. 2.
Variant population of HeLa cells resistant to Tax-induced senescence. (A) Flow cytometry histograms of nocodazole-treated, Ad-tTa- or Ad-Tax-transduced HeLa cells. For flow cytometry and immunoblots, 1 × 106 HeLa and HeLa/18x21-EGFP cells were grown, respectively, in 15 ml Dulbecco modified Eagle medium (DMEM) plus supplements in a T75 flask. Cells were then treated with adenovirus vectors and nocodazole. Round cells were detached from the flask by vigorous shaking, transferred to a 50-ml tube, and washed twice with 15 ml of phosphate-buffered saline (PBS) solution. Cells that remained attached were trypsinized, collected, and washed twice with PBS. Cell pellets were resuspended in 1 ml PBS, and the cell numbers in each population were counted using a hemacytometer. The majority of Ad-tTa-transduced cells detached from the plate after nocodazole treatment [Ad-tTa(D)]. Approximately one-third of the nocodazole-treated, Ad-Tax-transduced cells were detached from the flask. The Ad-Tax-transduced cells were divided into detached [Ad-Tax (D)] and attached [Ad-Tax(A)] populations. (B) Flow cytometry histograms of asynchronously grown HeLa cells transduced by Ad-tTa and Ad-Tax. For flow cytometry, HeLa cells were fixed in 1% paraformaldehyde, permeabilized with ethanol, treated with DNase- and protease-free RNase A (catalog number EN0531; Fermentas), and stained with propidium iodide (PI) as previously reported (20). Detection of EGFP and PI fluorescence was carried out using a fluorescence-activated cell sorter (Beckman-Coulter Epics XL-MCL flow cytometer). The fractions of cells in G0/G1, S, and G2/M were computed using the ModFit LT software package. (C) Immunoblots of cell lysates prepared from Ad-Tax-transduced HeLa/18x21-EGFP cells that detached from [lane (D)] or remained attached to [lane (A)] the culture dish after nocodazole treatment. The lysate of asynchronously grown cells (lane As) was included for comparison. The antibodies used for the blots are as indicated.
The attached and detached Ad-Tax-/nocodazole-treated cells were compared for the levels of p21, p27, cyclin A, cyclin B1, Skp2, Tax, and β-actin by immunoblotting. As anticipated, the levels of p21 and p27 were dramatically increased in the attached population, which consisted mostly of Tax-transduced cells, compared to those in the detached population and the asynchronously grown control (Tax-negative) cells [Fig. 2C, compare lane (A) to lanes (D) and As], consistent with the notion that the senescent program was activated in these Tax-positive adherent cells. The levels of cyclin B1 (Cyc B) and Skp1 were reduced in these cells (Fig. 2C) as previously described, a reduction attributed to the premature activation of the anaphase-promoting complex by Tax (20). The level of cyclin A was slightly higher in the same population, suggesting that some cells in this group likely remained in late S or G2 and had not progressed to mitosis.
The levels of p21 and p27 were low or barely detectable in the detached cell population [Fig. 2C, lane (D)], consistent with relatively normal cell cycle progression. Further, these cells had a high level of cyclin B1 that coincided with mitosis entry [Fig. 2C, compare lanes (D) and (A)]. As mentioned before, the flow cytometry histogram of the detached population of Ad-Tax-/nocodazole-treated cells was largely the same as that of the Ad-tTa control [compare Fig. 2A Ad-Tax (D) and Ad-tTa (D)]. Interestingly, the level of Tax expression in this cell population was similar to that of the attached fraction (Fig. 2C), even though only 30% of the population was transduced by Ad-Tax, as revealed by positive EGFP expression (Fig. 1C and E). The level of Tax expression per detached “senescence-resistant” cell is therefore higher. This may be related to the cellular changes in senescent cells that downregulate gene expression in general. These results suggest that while many Ad-Tax-transduced HeLa/18x21-EGFP cells did not enter mitosis and some became arrested in G1/senescence, a small but sizable variant population (12% of the total, 30% of the detached population) expressed Tax but were capable of progressing to metaphase normally much like the untransduced and the Ad-tTa-transduced cells. These variants appear to have spontaneously emerged in the HeLa/18x21-EGFP cell population. Indeed, multiple subclones of HeLa/18x21-EGFP with variable degrees of resistance to Tax-IRS have now been isolated (data not shown).
Tax-expressing cells bypass mitosis and become senescent.
Cdt1 (Cdc10-dependent transcript 1) and Cdc6 are two proteins that bind to a six-subunit protein complex known as the origin recognition complex (ORC), which assembles on the origins of DNA replication (Ori) of eukaryotic chromosomes. The assembly of the ORC-Cdt1-Cdc6 “prereplicative complex (pre-RC)” takes place during late M to G1 phase of the cell cycle and licenses DNA for a new round of replication. As a part of pre-RC, Cdt1 is responsible for recruiting the mini-chromosome maintenance (MCM) DNA helicase to the Ori to initiate DNA replication (47). Once DNA replication is initiated during G1/S transition, Cdt1 becomes the target of two E3 ligases, SCFSkp2 and Cul4-DDB1-Cdt2 (18, 35). Ubiquitin/proteasome-mediated degradation of Cdt1 prevents the relicensing of DNA replication (35). In contrast, illegitimate accumulation of Cdt1 leads to DNA endoreduplication or DNA hyperreplication (47). Cul4-DDB1-Cdt2 also directs Cdt1 proteolysis during M and G1 in response to DNA damage. Geminin, a protein expressed during S/G2/early M, further binds Cdt1 and prevents it from interacting with the MCM complex, thereby blocking the refiring of DNA replication origin (7, 47). Geminin is a substrate of APCCdh1, and its degradation during mitosis and early G1 allows Cdt1 activity to be restored in G1 to license a new round of DNA replication (47).
In an elegant study, Sakaue-Sawano et al. have used fluorescent protein fusions of Cdt1 and geminin to monitor cell cycle progression in live cells (41). These derivatives of Cdt1 and geminin are known as fluorescent, ubiquitination-based cell cycle indicator (FUCCI) (41). They take advantage of the fact that the levels of Cdt1 and geminin are tightly regulated by Cul4-DDB1-Cdt2 and SCFSkp2 and by APCCdh1, respectively. In the FUCCI constructions, the region spanning amino acid residues 30 to 120 of Cdt1, which mediates Cdt1 ubiquitination and degradation, is fused to a fast-folding form of the monomeric Kusabira Orange (mKO) fluorescent protein. Similarly, the region encompassing amino acid residues 1 to 110 of geminin is fused to the monomeric Azami Green (mAG) fluorescent protein. When mKO-Cdt130-120 and mAG-geminin1-110 are stably coexpressed, the fluorescence signal localizes to the nucleus of a dividing cell and changes from red (during G1, when Cdt1 accumulates) to yellow (G1/S transition, when both Cdt1 and geminin are expressed) to green (S/G2/M, when geminin accumulates) and then disappears upon mitotic exit. These fluorescent fusion proteins are expressed from the translational elongation factor 2 promoter, and both expression cassettes have been cloned into a lentivirus vector, pCSII (41).
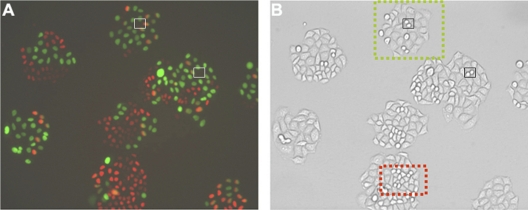
To investigate the effect of Tax on cell cycle progression, Cdt1 and geminin FUCCIs were stably coexpressed in HeLa cells through lentivirus vector-mediated gene transfer. Dual FUCCI-transduced HeLa cells were clonally isolated using a cell sorter based on the expression of both orange and green fluorescent proteins. Clones were then expanded into stable cell lines. The cycling of fluorescent reporters in HeLa FUCCI cells was as expected, red→yellow→green→colorless→red, reflecting progression through different cell cycle stages (G1→G1/S transition→S, G2→M→G1). A representative photograph of one such cell line is shown (Fig. 3). As indicated, cells expressing Cdt1-FUCCI were, in general, smaller (Fig. 3B, red box) and with smaller nuclei, consistent with their being in G1. On the other hand, cells expressing geminin-FUCCI were in S/G2 and, as might be expected, larger (Fig. 3B, green box) and with larger nuclei. Finally, rounding cells undergoing mitosis were colorless (see cells in small black and white boxes in Fig. 3A and B, respectively), in keeping with the loss of geminin during M phase of the cell cycle.
FIG. 3.
Derivation of a HeLa cell line expressing the Cdt1 and the geminin FUCCI reporters. The lentivirus FUCCI reporter vectors, LV-mKO2-hcdt1(30/120) and LV-mAG-Gem(1/110), were obtained from Atsushi Miyawaki (Laboratory for Cell Function and Dynamics, Brain Science Institute, RIKEN, Japan). LV-mKO2-hcdt1(30/120) and LV-mAG-Gem(1/110), carrying the Cdt1 and the geminin FUCCI reporter cassettes, respectively, were produced by DNA transfection of HEK293T cells as reported previously (20). HeLa cells were then transduced with LV-mKO2-hcdt1(30/120) and LV-mAG-Gem(1/110) at an MOI of 5, respectively, and grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) for 3 days. Doubly transduced HeLa cells were sorted, one cell per well, into 96-well plates using a BD FACSAria cell sorter and grown in the presence of 200 μl of DMEM containing 20% FBS, 2 mM l-glutamine, and antibiotics for 7 days. Clones of interest were identified by fluorescence microscopy and further expanded. A representative HeLa-FUCCI cell line was plated for single colonies. Cells were then visualized by fluorescence (A) and phase-contrast (B) microscopy. As indicated, cells expressing Cdt1 are generally smaller (represented by cells in the red box in panel B; see corresponding cells in panel A), consistent with their being in the G1 phase of the cell cycle. Cells expressing geminin are in S/G2 phases and are larger (represented by cells in the green box in panel B; see corresponding cells in panel A). Rounding cells are in mitosis and colorless (see representative cells in corresponding small black or white boxes).
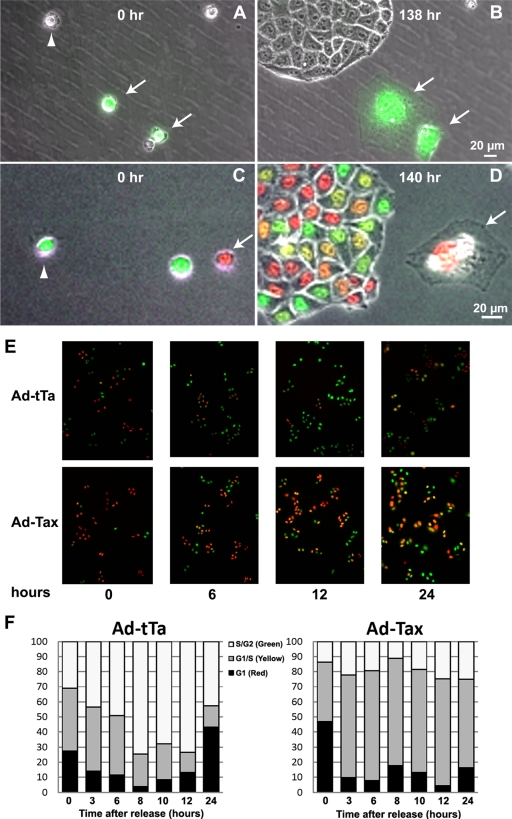
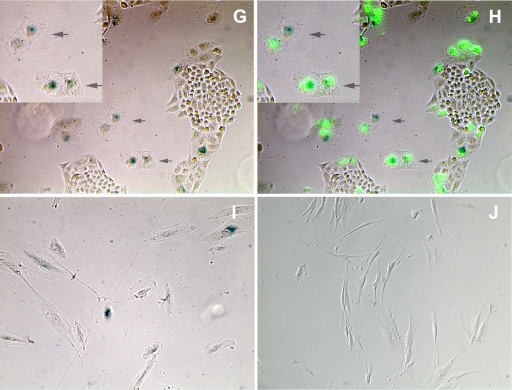
We had previously derived two reporter cell lines, HeLa/18x21-EGFP and SupT1/18x21-EGFP, for detecting Tax expression and HTLV-1 infection (25, 53). The transduction of the tax gene into these cells using a replication-defective adenovirus vector (Ad-Tax), using a lentivirus vector (LV-Tax), or after cocultivation with HTLV-1-producing MT2 cells results in trans-activation of EGFP expression driven by 18 copies of the Tax-responsive 21-bp repeat enhancer element and can be easily visualized by fluorescence microscopy (25, 53). To observe the cell cycle effect of Tax in single live cells, we infected sparsely seeded HeLa/18x21-EGFP with Ad-Tax at an MOI of 20 for 24 h. As mentioned above, under this condition, 60 to 70% of reporter cells became transduced by Ad-Tax. The cells were then monitored using time-lapse microscopy. As shown in Fig. 4 A and in the accompanying movie (see Movie S1 in the supplemental material), over the course of 138 h, the untransduced HeLa cell (colorless) in the visual field continued to proliferate and formed a sizable colony (compare Fig. 4A and B). The Ad-Tax-transduced cell (in green), however, became enlarged and showed significant cell movement but failed to undergo cell division and assumed a senescence phenotype (compare Fig. 4A and B). Similar time-lapse movies were made with Ad-Tax-transduced HeLa-FUCCI cells. In this instance, although Tax expression could not be directly scored using a fluorescence indicator, cells displaying mitotic abnormalities (attributed to Tax) could be readily captured microscopically. Similar to what is shown in Fig. 4A, untransduced cells progressed through the cell cycle with the expected changes of fluorescence indicators (see Movie S2 in the supplemental material). As anticipated, during mitosis, the colorless cells became round and then divided into two daughter cells, which then entered into G1 (red). The “Ad-Tax-transduced” cell, however, progressed through G1 (red), G1/S transition (yellow), S (green), and G2 (green) slowly, as reflected by the FUCCI changes, but never underwent the cell rounding characteristic of mitosis. These cells lost geminin expression only about 44 h into the time-lapse series and directly entered G1/senescence (red) thereafter without undergoing mitosis (cell rounding) and cell division (see cells marked with arrows in Fig. 4C and D; Movie S2). A similar analysis of Ad-tTa and Ad-Tax-transduced FUCCI cells that had been synchronized by double thymidine treatment indicates that the Ad-Tax cells persisted in Cdt1 and geminin expression after release from the G1/S arrest (compare Ad-Tax versus Ad-tTa at 6, 12, and 24 h after release in Fig. 4E), consistent with a lengthening of G1/S transition, S, and G2 phases similar to what is revealed in the time-lapse movie. Direct counting of cells expressing Cdt1-FUCCI and geminin-FUCCI also indicates that FUCCI reporters faithfully reflected the cell cycle status of the Ad-tTa-transduced cells with most cells in G1/S before the release from the double thymidine block (Fig. 4F, left panel). After release, the cell population in S/G2 progressively increased (Fig. 4F, left panel). In contrast, the progression of Ad-Tax-transduced cells through the cell cycle was stalled, with many persisting in G1/S, S, and G2 states (Fig. 4F, compare the S/G2 populations of Ad-tTa- and Ad-Tax-transduced cells). These results indicate that Tax-expressing cells progressed through an aberrant cell cycle, bypassed mitosis, and entered into senescence. They further provide an explanation for the enlarged cellular and nuclear sizes or binucleated morphology of Tax-expressing or HTLV-1-infected HeLa or SupT1 cells (20, 25). Finally, the Tax-transduced cells that did undergo one cell division as reflected by two adjoining EGFP-positive cells also became arrested in senescence (Fig. 4G and H, marked by arrows and enlarged in insets). Because Ad-Tax transduction was done on sparsely plated single cells, the adjoining doublets of senescent cells were derived mostly from single Ad-Tax-transduced cells after one round of cell division rather than from two independently Ad-Tax-transduced cells fortuitously landing next to each other. Finally, the human diploid fibroblast cell line WI-38 also became senescent when transduced by Ad-Tax compared to those transduced by the Ad-tTa control (Fig. 4I and J). These data, together with our previous results showing that Tax causes SupT1 cells to become irreversibly arrested in cell cycle progression (25), demonstrate that Tax-IRS readily occurs in many cells.
FIG. 4.
(A and B) Time-lapse microscopy images of HeLa/18x21-EGFP transduced by Ad-Tax. Ad-Tax-transduced and untransduced cells were EGFP positive (marked by arrows) and EGFP negative, respectively. Cells in the same field were photographed at time zero (A) and at 138 h later (B). Phase and fluorescence images are merged. (C and D) Time-lapse photographs of HeLa-FUCCI cells transduced by Ad-Tax. HeLa-FUCCI cells were transduced by Ad-Tax and monitored by time-lapse photography (see Movies S1 and S2 in the supplemental material) for 140 h. Cells in the same field were photographed at time zero (panel C) and 140 h later (panel D). (E) Stalled cell cycle progression of Tax-transduced cells. HeLa-FUCCI cells were synchronized in G1/S by two cycles of thymidine treatment. At the start of the second thymidine cycle, cells were transduced with Ad-tTa or Ad-Tax vector at an MOI of 20. The transduced cells were then released into the S phase by thymidine removal and visualized for Cdt1 and geminin by fluorescence microscopy at 0, 3, 6, 8, 10, 12, and 24 h postrelease. Only photographs taken at 0, 6, 12, and 24 h are shown. (F) Cell cycle progression of Ad-tTa- and Ad-Tax-transduced HeLa-FUCCI cells as determined by FUCCI expression. HeLa-FUCCI cells from panel A were counted based on FUCCI expression. Fractions of cells expressing red (solid), yellow (shaded), and green (blank) fluorescence were calculated and plotted. (G and H) Tax-expressing cells become senescent. One hundred thousand HeLa/18x21-EGFP cells were plated sparsely as single cells in a six-well plate and simultaneously transduced with Ad-Tax at an MOI of 20. They were then grown for 5 days in culture. Ad-Tax-transduced HeLa/18x21-EGFP cells were fixed and stained for senescence-associated β-galactosidase (SA-β-Gal) activity per the manufacturer's protocol described in the senescence β-galactosidase staining kit purchased from Cell Signaling (catalog number 9860). Cells were visualized for EGFP expression and SA-β-Gal activity using an Olympus IX81 fluorescence microscope equipped with a tunable RGB filter (CRI Micro*Color; Cambridge Research & Instrumentation, Inc.) The colored phase (E) and fluorescence (F) images were merged to show a direct correlation of Tax expression and senescence. Doublet senescent cells are marked by arrows, and their images are enlarged in the insets. (I and J) Tax induces senescence in WI-38 cells. Human diploid fibroblast WI-38 cells were plated and infected with Ad-Tax (I) or Ad-tTa (J) and stained for SA-β-galactosidase as in panels G and H.
In this study, we have used two reporter cell lines and time-lapse microscopy to investigate the complex cell cycle abnormalities induced by Tax. We found that many Tax-expressing cells progressed through elongated G1/S and S/G2 with difficulties and often bypassed mitosis and became irreversibly arrested in senescence. That Tax-expressing cells bypass mitosis explains why they manage to progress through S phase yet remain single senescent cells, often with enlarged nuclei or two nuclei. These results confirmed and extended earlier findings on the effect of Tax on cell cycle progression (20). Unexpectedly, a small but sizable fraction of HeLa/18x21-EGFP cells was found to be capable of dodging Tax-IRS, suggesting that during passage in culture, genetic or epigenetic changes that prevent senescence can spontaneously occur in a cell population.
Cellular senescence induced by Tax is due primarily to a dramatic rise in the levels of p21 and p27 associated with the transcriptional activation and stabilization of p21 mRNA and the stabilization of p27 protein. The transcriptional activation of p21 by Tax is p53 independent and is mediated by two Sp1 motifs near the start site of the p21 mRNA (4, 54). The mechanism responsible for Tax-mediated stabilization of p21 mRNA, however, remains unclear at present. The stabilization of p27 appears to be caused by the inappropriate activation of the mitotic exit program controlled by the E3 ubiquitin ligase activity of the APC/C and the premature loss of Skp2 (20, 24).
We have previously reported that Tax-expressing HTLV-1-transformed T-cell lines almost invariably express drastically lowered levels of p27 and have high levels of cytoplasmically localized and therefore functionally inactive p21 (references 20 and 25 and our unpublished results). Consistent with the role of these two cyclin-dependent kinase inhibitors in Tax-IRS, the variant HeLa population that progressed normally into metaphase with abundant Tax expression also had only low to moderate levels of p27 and p21. In-depth analyses of these variants may allow the mechanism responsible for Tax-IRS to be elucidated.
Our earlier studies indicate that the increase in p21 and p27 levels induced by Tax occurs in the S/G2 phases of the cell cycle after Tax is expressed (20). This may be the reason Tax-expressing cells do not arrest in G1 immediately after Tax expression and continue to progress slowly through G1/S, S, and G2. The premature APC/C-mediated degradation of Skp2 and cyclin B1 also occur during S/G2. As p27 can associate with and inhibit CDK1-cyclin A and CDK1-cyclin B1 complexes, the lowering of the level of cyclin B1 and the rise in the level of p27 likely act in concert to severely reduce cyclin B1-Cdk1 activity and disrupt transition to mitosis. Thus, most Tax-expressing cells simply do not have sufficient cyclin B-Cdk1 activity for mitosis and progress into senescence (G1) with G2/M DNA. This can explain the time-lapse microscopy showing that Tax-transduced (or HTLV-1-infected) cells bypassed mitosis and became arrested in G1 as oversized single cells with enlarged nuclei or sometimes two nuclei. It is important to point out that although many Tax-expressing cells could bypass mitosis altogether, some did complete mitosis only to enter into irreversible G1 arrest immediately after (see Fig. 2A, 4E, and 4F). How the latter population manage to divide without activating the spindle checkpoint is not clear.
As Tax is thought of as a viral oncoprotein, earlier studies have focused on demonstrating its functional resemblance to the oncoproteins of small DNA tumor viruses. It has been amply documented that Tax induces expression of genes encoding D-type cyclins, particularly cyclin D2, and CDK4 (1, 13, 21, 36, 42), due, in part, to the potent NF-κB activation by Tax (13). Tax also accelerates progression through G1 by binding to and stabilizing the enzymatic complexes formed by cyclin D family members and CDK4 and CDK6 (10, 11, 23, 32, 44). Furthermore, Tax has also been demonstrated to increase E2F production (17, 36). These activities of Tax impact tumor suppressor Rb to drive G1/S transition. Along this line of reasoning, Tax has been shown to inactivate p53 by facilitating the formation of a p65 RelA and p53 complex, which assembles on p53-responsive promoters to block p53-mediated transcription (15, 40). Notably, Tax has also been reported to reduce expression of p18INK4C and p19INK4D (13) and inactivates p16INK4 (26, 45) and p15INK4B via direct binding (46). Finally, in addition to the aforementioned impact of Tax on the G1/S restriction point, the potent activation of NF-κB by Tax is known to upregulate many anti-apoptotic proteins, including Bcl-xL (29, 31, 51), c-FLIP (19, 37), survivin (16, 30), HIAP-1 (33, 52), and Bcl-2 (2, 34). Curiously, the mitogenic and anti-apoptotic activities of Tax notwithstanding, few ATL cells continue to express Tax (48), and cell transformation by HTLV-1 or Tax in cell culture is inefficient (8). We think cellular senescence induced by Tax should be considered a host checkpoint mechanism against aberrant activation of potentially oncogenic signaling pathways. Our results and those of others showing that Tax expression leads to mitotic aberrations and irreversible cell cycle arrest support a model of ATL development in which the senescence-causing activity of Tax has to be effectively countered by cellular epigenetic or genetic changes (20, 25), by the silencing of HTLV-1 gene expression (48), or by other viral factors, such as the recently discovered HBZ protein, in order for the virus-infected cells to continue to divide and proliferate (28, 43).
Supplementary Material
Acknowledgments
We thank D. DeBiaso for technical assistance, K. M. Wolcott and K. Lund of the Biomedical Instrumentation Center (BIC) of the Uniformed Services University (USU) for help with flow cytometry and cell sorting, and A. Miyawaki of the Brain Science Institute, RIKEN, Japan, for the FUCCI lentivirus vectors.
This work was supported by a grant (R01CA115884) from the National Institutes of Health (NIH) to C.-Z.G. and an NIH shared instrument grant (1S10RR019083) for a BD FACSAria Flow Cytometer Cell Sorter to the USU BIC.
Footnotes
Published ahead of print on 5 January 2011.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Akagi, T., H. Ono, and K. Shimotohno. 1996. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sdi1. Oncogene 12:1645-1652. [PubMed] [Google Scholar]
- 2.Akita, K., S. Kawata, and K. Shimotohno. 2005. p21WAF1 modulates NF-kappaB signaling and induces anti-apoptotic protein Bcl-2 in Tax-expressing rat fibroblast. Virology 332:249-257. [DOI] [PubMed] [Google Scholar]
- 3.Chandhasin, C., R. I. Ducu, E. Berkovich, M. B. Kastan, and S. J. Marriott. 2008. Human T-cell leukemia virus type 1 Tax attenuates the ATM-mediated cellular DNA damage response. J. Virol. 82:6952-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhury, I. H., et al. 2003. Human T-cell leukemia virus type 1 Tax activates cyclin-dependent kinase inhibitor p21/Waf1/Cip1 expression through a p53-independent mechanism: inhibition of cdk2. Int. J. Cancer 107:603-611. [DOI] [PubMed] [Google Scholar]
- 5.Durkin, S. S., et al. 2008. HTLV-1 Tax oncoprotein subverts the cellular DNA damage response via binding to DNA-dependent protein kinase. J. Biol. Chem. 283:36311-36320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giam, C. Z., and K. T. Jeang. 2007. HTLV-1 Tax and adult T-cell leukemia. Front. Biosci. 12:1496-1507. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, M. A., et al. 2006. Geminin is essential to prevent endoreduplication and to form pluripotent cells during mammalian development. Genes Dev. 20:1880-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graziano, S. L., et al. 1987. Quantitative assay of human T-cell leukemia/lymphoma virus transformation. Cancer Res. 47:2468-2473. [PubMed] [Google Scholar]
- 9.Gupta, S. K., et al. 2007. Human T-cell leukemia virus type 1 Tax oncoprotein prevents DNA damage-induced chromatin egress of hyperphosphorylated Chk2. J. Biol. Chem. 282:29431-29440. [DOI] [PubMed] [Google Scholar]
- 10.Haller, K., et al. 2000. Tax-dependent stimulation of G1 phase-specific cyclin-dependent kinases and increased expression of signal transduction genes characterize HTLV type 1-transformed T cells. AIDS Res. Hum. Retroviruses 16:1683-1688. [DOI] [PubMed] [Google Scholar]
- 11.Haller, K., et al. 2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 22:3327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haoudi, A., R. C. Daniels, E. Wong, G. Kupfer, and O. J. Semmes. 2003. Human T-cell leukemia virus-I tax oncoprotein functionally targets a subnuclear complex involved in cellular DNA damage-response. J. Biol. Chem. 278:37736-37744. [DOI] [PubMed] [Google Scholar]
- 13.Iwanaga, R., K. Ohtani, T. Hayashi, and M. Nakamura. 2001. Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 20:2055-2067. [DOI] [PubMed] [Google Scholar]
- 14.Jeang, K. T., C. Z. Giam, F. Majone, and M. Aboud. 2004. Life, death, and Tax: role of HTLV-I oncoprotein in genetic instability and cellular transformation. J. Biol. Chem. 279:31991-31994. [DOI] [PubMed] [Google Scholar]
- 15.Jeong, S. J., M. Radonovich, J. N. Brady, and C. A. Pise-Masison. 2004. HTLV-I Tax induces a novel interaction between p65/RelA and p53 that results in inhibition of p53 transcriptional activity. Blood 104:1490-1497. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami, H., et al. 2005. Transcriptional activation of survivin through the NF-kappaB pathway by human T-cell leukemia virus type I Tax. Int. J. Cancer 115:967-974. [DOI] [PubMed] [Google Scholar]
- 17.Kehn, K., et al. 2005. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation. Oncogene 24:525-540. [DOI] [PubMed] [Google Scholar]
- 18.Kim, Y., and E. T. Kipreos. 2007. Cdt1 degradation to prevent DNA re-replication: conserved and non-conserved pathways. Cell Div. 2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krueger, A., et al. 2006. HTLV-1 Tax protects against CD95-mediated apoptosis by induction of the cellular FLICE-inhibitory protein (c-FLIP). Blood 107:3933-3939. [DOI] [PubMed] [Google Scholar]
- 20.Kuo, Y. L., and C. Z. Giam. 2006. Activation of the anaphase promoting complex by HTLV-1 tax leads to senescence. EMBO J. 25:1741-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemasson, I., S. Thebault, C. Sardet, C. Devaux, and L. H. J. M. Mesnard. 1998. Activation of E2F-mediated transcription by human T-cell leukemia virus type I Tax protein in a p16(INK4A)-negative T-cell line. J. Biol. Chem. 273:23598-23604. [DOI] [PubMed] [Google Scholar]
- 22.Lemoine, F. J., and S. J. Marriott. 2001. Accelerated G(1) phase progression induced by the human T cell leukemia virus type I (HTLV-I) Tax oncoprotein. J. Biol. Chem. 276:31851-31857. [DOI] [PubMed] [Google Scholar]
- 23.Li, J., H. Li, and M. D. Tsai. 2003. Direct binding of the N-terminus of HTLV-1 tax oncoprotein to cyclin-dependent kinase 4 is a dominant path to stimulate the kinase activity. Biochemistry 42:6921-6928. [DOI] [PubMed] [Google Scholar]
- 24.Liu, B., S. Hong, Z. Tang, H. Yu, and C. Z. Giam. 2005. HTLV-I Tax directly binds the Cdc20-associated anaphase-promoting complex and activates it ahead of schedule. Proc. Natl. Acad. Sci. U. S. A. 102:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, M., et al. 2008. Human T-cell leukemia virus type 1 infection leads to arrest in the G1 phase of the cell cycle. J. Virol. 82:8442-8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low, K. G., et al. 1997. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J. Virol. 71:1956-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka, M., and K. T. Jeang. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7:270-280. [DOI] [PubMed] [Google Scholar]
- 28.Mesnard, J. M., B. Barbeau, and C. Devaux. 2006. HBZ, a new important player in the mystery of adult T-cell leukemia. Blood 108:3979-3982. [DOI] [PubMed] [Google Scholar]
- 29.Mori, N., et al. 2001. Human T-cell leukemia virus type I tax protein induces the expression of anti-apoptotic gene Bcl-xL in human T-cells through nuclear factor-kappaB and c-AMP responsive element binding protein pathways. Virus Genes 22:279-287. [DOI] [PubMed] [Google Scholar]
- 30.Mori, N., et al. 2001. Expression of survivin in HTLV-I-infected T-cell lines and primary ATL cells. Biochem. Biophys. Res. Commun. 282:1110-1113. [DOI] [PubMed] [Google Scholar]
- 31.Nakashima, K., et al. 2003. Protection of mitochondrial perturbation by human T-lymphotropic virus type 1 tax through induction of Bcl-xL expression. J. Lab. Clin. Med. 142:341-347. [DOI] [PubMed] [Google Scholar]
- 32.Neuveut, C., et al. 1998. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol. 18:3620-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng, P. W., et al. 2001. Genome-wide expression changes induced by HTLV-1 Tax: evidence for MLK-3 mixed lineage kinase involvement in Tax-mediated NF-kappaB activation. Oncogene 20:4484-4496. [DOI] [PubMed] [Google Scholar]
- 34.Nicot, C., T. Astier-Gin, and B. 6. Guillemain. 1997. Activation of bcl-2 expression in human endothelial cells chronically expressing the human t-cell lymphotropic virus type I. Virology 236:47-53. [DOI] [PubMed] [Google Scholar]
- 35.Nishitani, H., et al. 2006. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 25:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtani, K., et al. 2000. Cell type-specific E2F activation and cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. J. Biol. Chem. 275:11154-11163. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto, K., J. Fujisawa, M. Reth, and S. Yonehara. 2006. Human T-cell leukemia virus type-I oncoprotein Tax inhibits Fas-mediated apoptosis by inducing cellular FLIP through activation of NF-kappaB. Genes Cells 11:177-191. [DOI] [PubMed] [Google Scholar]
- 38.Park, H. U., J. H. Jeong, J. H. Chung, and J. N. Brady. 2004. Human T-cell leukemia virus type 1 Tax interacts with Chk1 and attenuates DNA-damage induced G2 arrest mediated by Chk1. Oncogene 23:4966-4974. [DOI] [PubMed] [Google Scholar]
- 39.Park, H. U., S. J. Jeong, J. H. Jeong, J. H. Chung, and J. N. Brady. 2006. Human T-cell leukemia virus type 1 Tax attenuates gamma-irradiation-induced apoptosis through physical interaction with Chk2. Oncogene 25:438-447. [DOI] [PubMed] [Google Scholar]
- 40.Pise-Masison, C. A., et al. 2000. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-kappaB pathway and is dependent on p53 phosphorylation. Mol. Cell. Biol. 20:3377-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaue-Sawano, A., et al. 2008. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132:487-498. [DOI] [PubMed] [Google Scholar]
- 42.Santiago, F., et al. 1999. Transcriptional up-regulation of the cyclin D2 gene and acquisition of new cyclin-dependent kinase partners in human T-cell leukemia virus type 1-infected cells. J. Virol. 73:9917-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satou, Y., J. Yasunaga, M. Yoshida, and M. Matsuoka. 2006. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. U. S. A. 103:720-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitt, I., O. Rosin, P. Rohwer, M. Gossen, and R. Grassmann. 1998. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 72:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki, T., S. Kitao, H. Matsushime, and M. Yoshida. 1996. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 15:1607-1614. [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki, T., T. Narita, M. Uchida-Toita, M. L. Yoshida, and 214-Current 8. 1999. Down-regulation of the INK4 family of cyclin-dependent kinase inhibitors by tax protein of HTLV-1 through two distinct mechanisms. Virology 259:384-391. [DOI] [PubMed] [Google Scholar]
- 47.Tada, S. 2007. Cdt1 and geminin: role during cell cycle progression and DNA damage in higher eukaryotes. Front. Biosci. 12:1629-1641. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi, Y., et al. 2005. Silencing of human T-cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology 2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tripp, A., et al. 2005. Induction of cell cycle arrest by human T-cell lymphotropic virus type 1 Tax in hematopoietic progenitor (CD34+) cells: modulation of p21cip1/waf1 and p27kip1 expression. J. Virol. 79:14069-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tripp, A., et al. 2003. Human T-cell leukemia virus type 1 tax oncoprotein suppression of multilineage hematopoiesis of CD34+ cells in vitro. J. Virol. 77:12152-12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsukahara, T., et al. 1999. Induction of Bcl-xL expression by human T-cell leukemia virus type 1 Tax through NF-κB in apoptosis-resistant T-cell transfectants with Tax. J. Virol. 73:7981-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waldele, K., et al. 2006. Requirement of the human T-cell leukemia virus (HTLV-1) Tax-stimulated HIAP-1 gene for the survival of transformed lymphocytes. Blood 107:4491-4499. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, L., M. Liu, R. Merling, and C. Z. Giam. 2006. Versatile reporter systems show that transactivation by human T-cell leukemia virus type 1 Tax occurs independently of chromatin remodeling factor BRG1. J. Virol. 80:7459-7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, L., H. Zhi, M. Liu, Y. L. Kuo, and C. Z. Giam. 2009. Induction of p21(CIP1/WAF1) expression by human T-lymphotropic virus type 1 Tax requires transcriptional activation and mRNA stabilization. Retrovirology 6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.