Abstract
Levels of herpes simplex virus 1 (HSV-1) and HSV-2 DNA in dorsal root ganglia (DRG) and spinal cord (SC) were quantified after inoculation of guinea pig genitals and footpads. In genital infection, viral DNA reached SC and DRG simultaneously (at 2 to 3 days after inoculation) but was more abundant in SC than in DRG. After inoculation of footpads, which lack parasympathetic innervation, the viruses spread more efficiently to DRG than to SC. These results show important differences between genital and footpad infections, including independence of spread to DRG and SC, and imply that autonomic neurons may play an important role in the pathogenesis of viral latency after genital inoculation.
The related species herpes simplex virus 1 (HSV-1) and HSV-2 exhibit differences in latency and recurrence patterns. While both viruses establish latency in sensory ganglia and reactivate to cause recurrent disease, HSV-1 reactivates more efficiently from trigeminal ganglia to cause cold sores or keratitis and HSV-2 reactivates more efficiently from lumbosacral dorsal root ganglia (DRG) to cause genital herpes (8). HSV-1 and HSV-2 also display different tropisms for nociceptive sensory neurons, with HSV-1 more likely to be detected during latency in murine neurons displaying the surface marker A5 and HSV-2 in those displaying KH10 (9). Both of these findings are associated with HSV-1 or HSV-2 viral species-specific latency-associated transcript (LAT) sequences (9, 18).
Although most studies have focused on viral latency in sensory neurons of either the dorsal root or trigeminal ganglia, we recently showed that levels of virus DNA in spinal cord exceeded those in the corresponding DRG, suggesting that other sites of latency may be important (1). After genital infection of guinea pigs, the quantities of latent HSV-1 DNA are greater in the thoracolumbar spinal cord than in the sacral spinal cord, while latent HSV-2 DNA is more abundant in the sacral spinal cord. Mutations in the LAT did not influence this tropism for different spinal cord levels. Because sympathetic neurons have their origin in the thoracolumbar cord and parasympathetic neurons arise in the sacral cord, we hypothesized that these findings may be related to viral infection of autonomic ganglia (2). Both HSV-1 and HSV-2 are able to infect autonomic neurons (3, 5, 7, 10, 12, 14-17). HSV-2 infection of autonomic neurons can give rise to transient bladder paralysis in humans, and latently infected murine parasympathetic neurons express the latency-associated transcript (12). Viral infection of autonomic ganglia is difficult to study directly in animal models of HSV-2 infection because perigenital ganglia are very small, are located in the perigenital fat, and are difficult to identify. In the present study, we used genital (6) and footpad models of HSV infection to further examine these phenomena that are potentially related to differences in autonomic neuronal infections with HSV-1 and HSV-2.
To further investigate the spread of virus to the spinal cord, we examined the time course of the appearance of viral nucleic acid in DRG relative to that in the spinal cord. Because there are two anatomically feasible neuronal pathways to the spinal cord, either through the DRG or through the autonomics, we first examined DRG and spinal cord from guinea pigs over the first 3 days after intravaginal inoculation. We reasoned that if virus reached the spinal cord via the DRG, we would observe evidence of virus in DRG at times prior to its detection in the spinal cord.
Female Hartley guinea pigs (Charles River, Wilmington, MA) were inoculated intravaginally with 2 × 105 PFU of wild-type HSV-2 (strain 333; obtained from Gary Hayward, Johns Hopkins University, Baltimore, MD) or HSV-1 (strain 17+; obtained from John Hay, SUNY-Buffalo, Buffalo, NY) in two independent experiments. Results presented are composites obtained using a total of 8 guinea pigs in each group. No external lesions were observed in any of the animals over the 3-day course of the experiments. Left and right lumbar and sacral dorsal root ganglia and lumbar and sacral spinal cord sections at each level (L2 to L6, S1 to S2) were removed from infected and uninfected control animals at the first, second, and third days after inoculation and flash frozen. Frozen tissues were homogenized, and DNA and RNA were extracted with a Qiagen AllPrep DNA/RNA minikit. DNA was quantified by TaqMan PCR using primers and probes specific for sequences within HSV-1 or HSV-2 thymidine kinase, and values were normalized to that for the 18S rRNA gene (2).
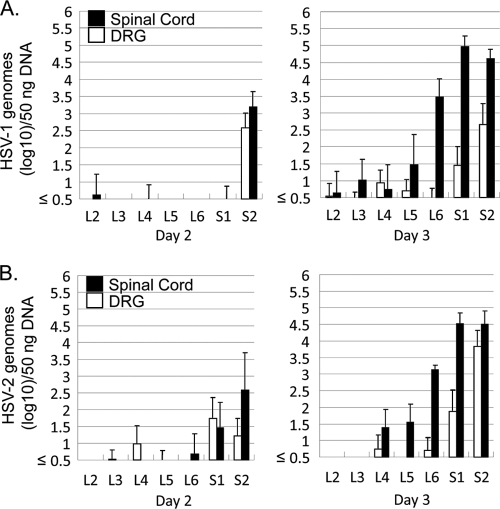
Neither HSV-1 nor HSV-2 was detected in any DRG or spinal cord sample 1 day after intravaginal inoculation (data not shown). HSV-1 DNA became detectable in the spinal cord at day 2, before it was detectable in the DRG (Fig. 1 A). HSV-2 DNA became detectable in the spinal cord and DRG at day 2, with higher levels of virus DNA observed in the spinal cord than in the DRG (Fig. 1B). This pattern continued for both viruses, with higher levels observed in the spinal cord than in the DRG at day 3. This strongly suggests that HSV does not reach the spinal cord via infection of the DRG, implying that there is an alternate pathway to the cord, likely via infection of autonomic neurons. By day 3, we had not yet observed the previously described pattern of increased relative quantities of HSV-2 in sacral and HSV-1 in lumbar spinal cord, implying that this phenotype may develop later in infection.
FIG. 1.
Quantities of viral DNA in the DRG, sacral spinal cord, and lumbar spinal cord of vaginally infected guinea pigs, quantified by TaqMan PCR assay and normalized to that of the 18S rRNA gene. Results are expressed as geometric means ± standard errors (SEs; log 10) of the amounts of viral DNA per 50 ng of total DNA. (A) HSV-1 DNA copy numbers quantified in guinea pig tissues on day 2 and day 3 after inoculation. (B) HSV-2 DNA copy numbers quantified in guinea pig tissues on day 2 and day 3 after inoculation.
To further investigate the pathway for virus spread and potential differences between HSV-1 and HSV-2 in autonomic nervous system infection, we performed studies with the guinea pig footpad model. Unlike the genital tract, the footpad does not possess direct parasympathetic innervation, and sympathetic innervation is reduced compared to that in the genitalia. Comparing viral spread to the DRG and spinal cord between the genital and footpad models also provided insight into the relative contributions of the autonomic and sensory pathways toward spread to the spinal cord.
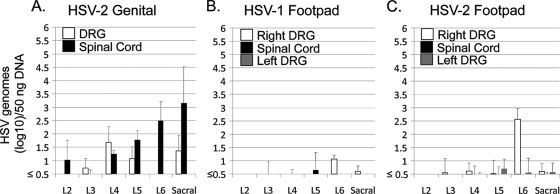
Female Hartley guinea pigs (n = 3 per group) were inoculated in the right footpad in the L6 dermatome with 2 × 106 PFU of wild-type HSV-2 (strain 333) or HSV-1 (strain 17+) or were intravaginally inoculated with 2 × 105 PFU of HSV-2 (strain 333). Lumbar and sacral DRG and spinal cord were removed at day 3 after inoculation, and DNA was extracted and quantified as described above. Results from intravaginally inoculated guinea pigs (Fig. 2 A; DRG results shown combine left and right sides) were consistent with those presented above and shown in Fig. 1. After footpad injection, both HSV-1 and HSV-2 DNA were detected most prominently in the right L6 DRG, corresponding to the inoculation site. Unlike in the genital model, in the footpad model HSV-1 (Fig. 2B) and HSV-2 (Fig. 2C) DNAs were detected in spinal cord at quantities considerably below those in the corresponding DRG, showing that DRG infection does not inevitably lead to high levels of virus DNA in spinal cord and indicating that the viruses spread independently to these locations. In genital infection, virus thus likely reaches the spinal cord via infection of autonomic neurons, not via the DRG.
FIG. 2.
Quantities of viral DNA in the DRG, sacral spinal cord, and lumbar spinal cord 3 days after inoculation of guinea pigs with HSV-2 via the vaginal route (A) or via right footpad infection with HSV-1 (B) or HSV-2 (C), quantified by TaqMan PCR assay and normalized to that of the 18S rRNA gene. Results are expressed as geometric means ± SEs (log 10) of the amounts of viral DNA per 50 ng of total DNA.
The present study shows clear differences in the abilities of HSV-1 and HSV-2 to reach the spinal cord in genital and footpad infections. Thus, footpad infection does not mimic genital infection. The larger concentration of viral DNA observed in spinal cord than in DRG in genitally infected animals strongly implies a prominent infection of autonomic neurons in acutely infected guinea pigs and further suggests that for genital infection, autonomic neurons may be as important a site of viral latency as sensory neurons.
While autonomic neurons have been recognized as a site for HSV infection for many years, the relevance of these neurons to the establishment of viral latency and their contribution to viral recurrence have not been as well studied. Most studies of infection of the autonomic neurons have involved HSV-1 in ocular infection, where it is relatively easy to dissect the autonomic ganglia (3,10,14). Primary autonomic ganglion infections have also been used as an entry to the spinal cord to trace neuronal connections in animal models, for example, infections using reporter gene-expressing pseudorabiesvirus (4).
While autonomic infection has been recognized as relevant to acute HSV-2 infection in humans (for example, genital herpes is sometimes associated with urinary retention attributed to autonomic dysfunction due to infection of these neurons [11]), only a few investigators have performed studies that attempted to directly examine autonomic infection in an animal genital herpes model (12,15). In these experiments, performed in mice, the virus reached autonomic ganglia during acute infection, though the virus was not detected in the spinal cord as rapidly as in our experiment in guinea pigs.
Our studies are the first to use real-time PCR to quantify the relative amounts of HSV nucleic acid present in DRG and spinal cord. We were surprised to find that the quantity of virus observed in spinal cord (as measured by copy number/quantity of cellular DNA) was similar to or greater than that in DRG, suggesting that DRG might not be the only important site of latency in genital herpes. The ability of the viral nucleic acid in spinal cord to transcribe the LAT (1) implies that this nucleic acid is intact and thus potentially reactivation competent. Spinal cord HSV could potentially explain Mollaret's meningitis, a syndrome in which recurrent meningitis (usually without genital lesions) is caused by recurrent HSV-2 (13). The observation that HSV antigen may be present in very well defined peripheral locations for relatively long periods of time (19) may also be better explained by autonomic than by ganglionic reactivations.
The relative importance of sensory and autonomic neurons in viral reactivation, as well as in human latency and reactivation, remain to be understood. The findings in the present study, showing that HSV independently reaches DRG and spinal cord, strongly suggest the utility of developing improved models to evaluate latency in autonomic neurons to improve understanding of the pathogenesis of genital herpes, as well as to aid in the development of appropriate countermeasures, including vaccines and antivirals.
Acknowledgments
We gratefully acknowledge helpful discussions with Shuang Tang, Santosh Nanda, and Christine Uhlenhaut.
M.O. acknowledges financial support from the Japan Herpesvirus Infectious Forum Scholarship Awards in Herpesvirus Infections Research.
Footnotes
Published ahead of print on 15 December 2010.
REFERENCES
- 1.Bertke, A. S., et al. 2009. Latency-associated transcript (LAT) exon 1 controls herpes simplex virus species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT. J. Virol. 83:10007-10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertke, A. S., A. Patel, and P. R. Krause. 2007. Herpes simplex virus latency-associated transcript sequence downstream of the promoter influences type-specific reactivation and viral neurotropism. J. Virol. 81:6605-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustos, D. E., and S. S. Atherton. 2002. Detection of herpes simplex virus type 1 in human ciliary ganglia. Invest. Ophthalmol. Vis. Sci. 43:2244-2249. [PubMed] [Google Scholar]
- 4.Enquist, L. W., and J. P. Card. 2003. Recent advances in the use of neurotropic viruses for circuit analysis. Curr. Opin. Neurobiol. 13:603-606. [DOI] [PubMed] [Google Scholar]
- 5.Gilden, D. H., et al. 2001. Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes 23:145-147. [DOI] [PubMed] [Google Scholar]
- 6.Krause, P. R., et al. 1995. Expression of the herpes simplex virus type 2 latency-associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J. Exp. Med. 181:297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labetoulle, M., et al. 2000. Neuronal pathways for the propagation of herpes simplex virus type 1 from one retina to the other in a murine model. J. Gen. Virol. 81:1201-1210. [DOI] [PubMed] [Google Scholar]
- 8.Lafferty, W. E., R. W. Coombs, J. Benedetti, C. Critchlow, and L. Corey. 1987. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N. Engl. J. Med. 316:1444-1449. [DOI] [PubMed] [Google Scholar]
- 9.Margolis, T. P., Y. Imai, L. Yang, V. Vallas, and P. R. Krause. 2007. Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts. J. Virol. 81:1872-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin, J. R., and S. Suzuki. 1989. Targets of infection in a herpes simplex-reactivation model. Acta Neuropathol. 77:402-411. [DOI] [PubMed] [Google Scholar]
- 11.Mertz, G., and L. Corey. 1984. Genital herpes simplex virus infections in adults. Urol. Clin. North Am. 11:103-119. [PubMed] [Google Scholar]
- 12.Parr, M. B., and E. L. Parr. 2003. Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J. Neurovirol. 9:594-602. [DOI] [PubMed] [Google Scholar]
- 13.Picard, F. J., G. A. Dekaban, J. Silva, and G. P. Rice. 1993. Mollaret's meningitis associated with herpes simplex type 2 infection. Neurology 43:1722-1777. [DOI] [PubMed] [Google Scholar]
- 14.Richter, E. R., J. K. Dias, J. E. Gilbert, and S. S. Atherton. 2009. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J. Infect. Dis. 200:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanjuan, N. A., and E. F. Lascano. 1986. Autonomic nervous system involvement in experimental genital infection by herpes simplex virus type 2. Arch. Virol. 91:329-339. [DOI] [PubMed] [Google Scholar]
- 16.Sanjuan, N. A., and M. N. Zimberlin. 2001. Pathogenesis of herpes simplex virus type 2 experimental genital infection in pregnant mice. FEMS Immunol. Med. Microbiol. 30:197-202. [DOI] [PubMed] [Google Scholar]
- 17.Vann, V. R., and S. S. Atherton. 1991. Neural spread of herpes simplex virus after anterior chamber inoculation. Invest. Ophthalmol. Vis. Sci. 32:2462-2472. [PubMed] [Google Scholar]
- 18.Yoshikawa, T., et al. 1996. The characteristic site-specific reactivation phenotypes of HSV-1 and HSV-2 depend upon the latency-associated transcript region. J. Exp. Med. 184:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu, J., et al. 2009. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat. Med. 15:886-892. [DOI] [PMC free article] [PubMed] [Google Scholar]