Abstract
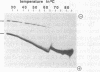
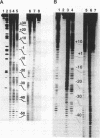
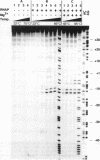
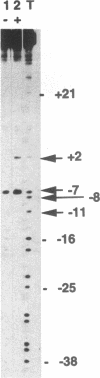
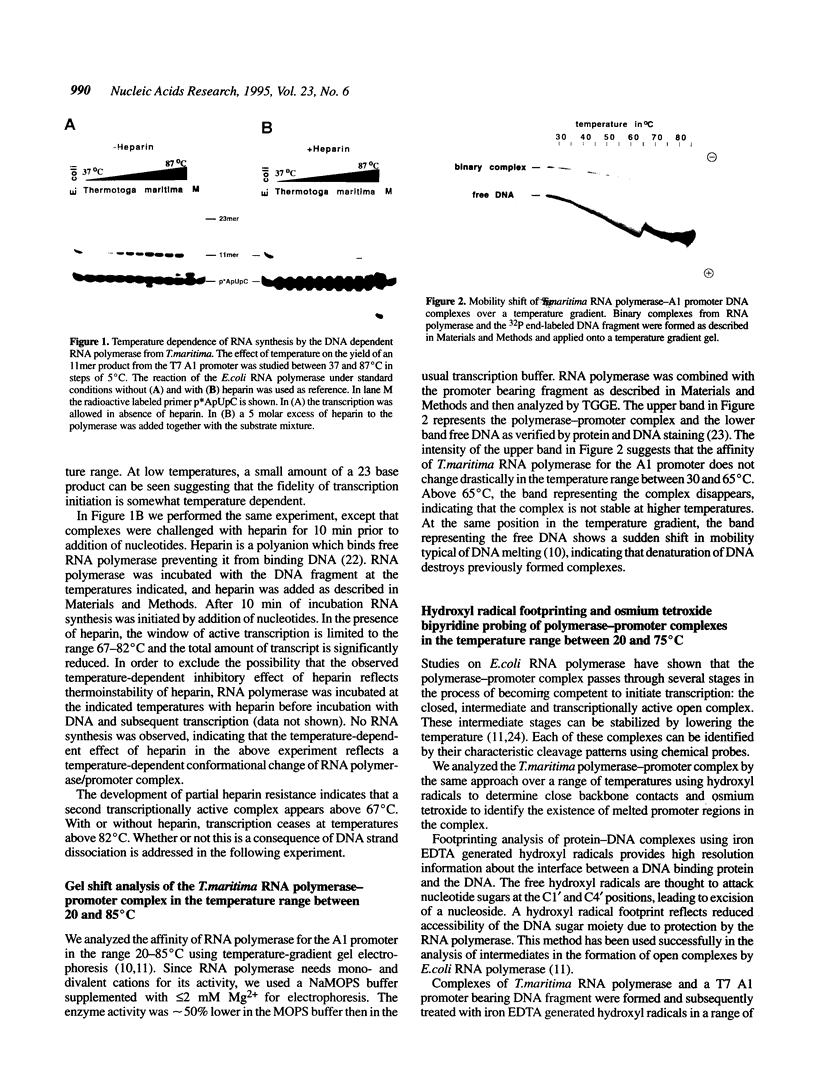
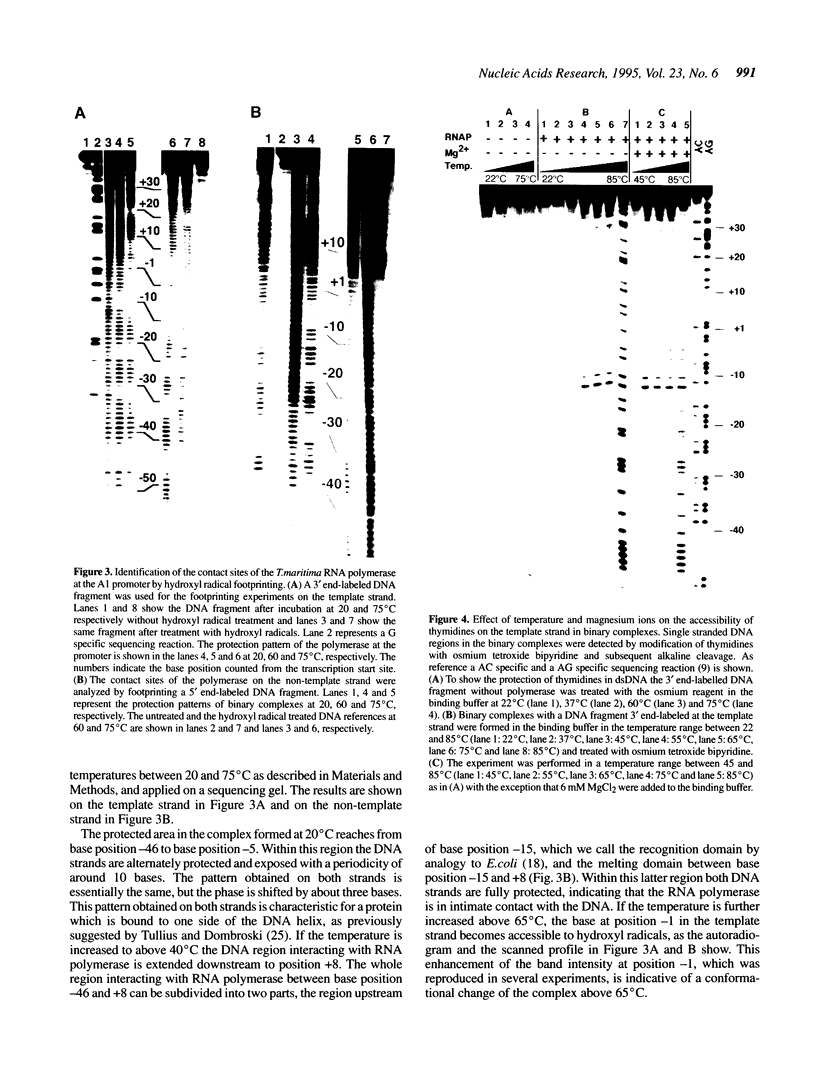
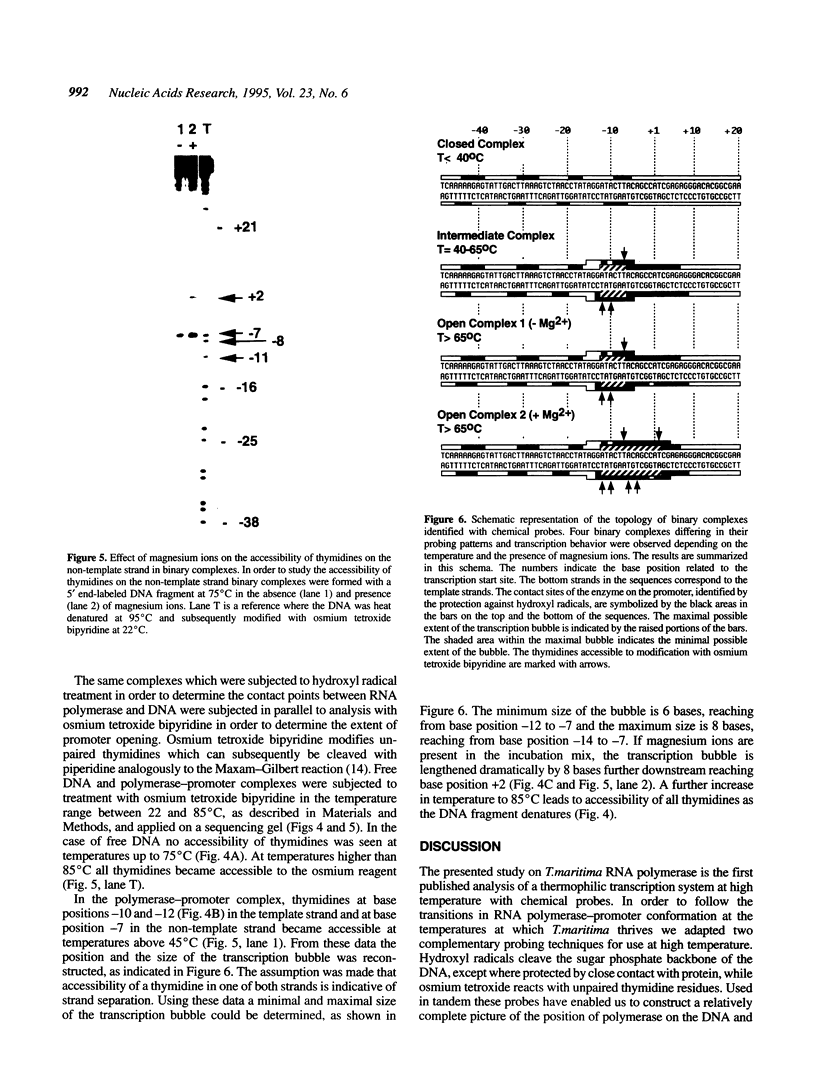
The interaction of DNA dependent RNA polymerase of the extreme thermophile bacteria Thermotoga maritima with a promoter bearing DNA fragment was investigated in the temperature range from 20 to 85 degrees C. We show that the T. maritima RNA polymerase recognizes and utilizes the Escherichia coli T7 A1 promoter with an efficiency similar to that of the E. coli polymerase. We have investigated the interaction of both polymerases with the same promoter over a wide range of temperatures using hydroxyl radical foot-printing and osmium tetroxide probing. This study revealed that the T. maritima polymerase goes through a series of isomerisation events very similar to the E. coli polymerase, i.e. the closed, intermediate and open complexes, but the transitions themselves occur at radically different temperatures. This indicates that conformational changes in the DNA that accompany initiation of transcription such as promoter melting are determined by the polymerase rather than the DNA sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achenbach-Richter L., Gupta R., Stetter K. O., Woese C. R. Were the original eubacteria thermophiles? Syst Appl Microbiol. 1987;9:34–39. doi: 10.1016/s0723-2020(87)80053-x. [DOI] [PubMed] [Google Scholar]
- Arndt K. M., Chamberlin M. J. RNA chain elongation by Escherichia coli RNA polymerase. Factors affecting the stability of elongating ternary complexes. J Mol Biol. 1990 May 5;213(1):79–108. doi: 10.1016/S0022-2836(05)80123-8. [DOI] [PubMed] [Google Scholar]
- Babinet C. A new method for the purification of RNA-polymerase. Biochem Biophys Res Commun. 1967 Mar 21;26(6):639–644. doi: 10.1016/s0006-291x(67)80119-0. [DOI] [PubMed] [Google Scholar]
- Buc H., McClure W. R. Kinetics of open complex formation between Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry. 1985 May 21;24(11):2712–2723. doi: 10.1021/bi00332a018. [DOI] [PubMed] [Google Scholar]
- Buckle M., Buc H. Fine mapping of DNA single-stranded regions using base-specific chemical probes: study of an open complex formed between RNA polymerase and the lac UV5 promoter. Biochemistry. 1989 May 16;28(10):4388–4396. doi: 10.1021/bi00436a040. [DOI] [PubMed] [Google Scholar]
- Hayes J. J., Tullius T. D. The missing nucleoside experiment: a new technique to study recognition of DNA by protein. Biochemistry. 1989 Nov 28;28(24):9521–9527. doi: 10.1021/bi00450a041. [DOI] [PubMed] [Google Scholar]
- Heumann H., Lederer H., Kammerer W., Palm P., Metzger W., Baer G. Large-scale preparation of a DNA fragment containing the strong promoter A1 of the phage T7. Biochim Biophys Acta. 1987 Jul 14;909(2):126–132. doi: 10.1016/0167-4781(87)90034-0. [DOI] [PubMed] [Google Scholar]
- Heumann H., Metzger W., Niehörster M. Visualization of intermediary transcription states in the complex between Escherichia coli DNA-dependent RNA polymerases and a promoter-carrying DNA fragment using the gel retardation method. Eur J Biochem. 1986 Aug 1;158(3):575–579. doi: 10.1111/j.1432-1033.1986.tb09793.x. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Zentner P. G., Geiduschek E. P. Transcription at bacteriophage T4 variant late promoters. An application of a newly devised promoter-mapping method involving RNA chain retraction. J Biol Chem. 1986 Oct 25;261(30):14256–14265. [PubMed] [Google Scholar]
- Koller G., Reeve J. N., Frey G., Thomm M. Transcription in vitro and in vivo of the 7S RNA gene associated with the ribosomal RNA operon in the hyperthermophilic archaeon Methanothermus fervidus. FEMS Microbiol Lett. 1992 Nov 1;77(1-3):95–101. doi: 10.1016/0378-1097(92)90138-e. [DOI] [PubMed] [Google Scholar]
- Krummel B., Chamberlin M. J. Structural analysis of ternary complexes of Escherichia coli RNA polymerase. Deoxyribonuclease I footprinting of defined complexes. J Mol Biol. 1992 May 20;225(2):239–250. doi: 10.1016/0022-2836(92)90918-a. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Frey H. E., Inniss W. E. Thermal analysis of bacteria by differential scanning calorimetry: relationship of protein denaturation in situ to maximum growth temperature. Biochim Biophys Acta. 1990 Oct 15;1055(1):19–26. doi: 10.1016/0167-4889(90)90086-s. [DOI] [PubMed] [Google Scholar]
- Levin J. R., Krummel B., Chamberlin M. J. Isolation and properties of transcribing ternary complexes of Escherichia coli RNA polymerase positioned at a single template base. J Mol Biol. 1987 Jul 5;196(1):85–100. doi: 10.1016/0022-2836(87)90512-2. [DOI] [PubMed] [Google Scholar]
- Liao D., Dennis P. P. The organization and expression of essential transcription translation component genes in the extremely thermophilic eubacterium Thermotoga maritima. J Biol Chem. 1992 Nov 15;267(32):22787–22797. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger W., Schickor P., Heumann H. A cinematographic view of Escherichia coli RNA polymerase translocation. EMBO J. 1989 Sep;8(9):2745–2754. doi: 10.1002/j.1460-2075.1989.tb08416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Palecek E. Probing DNA structure with osmium tetroxide complexes in vitro. Methods Enzymol. 1992;212:139–155. doi: 10.1016/0076-6879(92)12010-n. [DOI] [PubMed] [Google Scholar]
- Palm P., Schleper C., Arnold-Ammer I., Holz I., Meier T., Lottspeich F., Zillig W. The DNA-dependent RNA-polymerase of Thermotoga maritima; characterisation of the enzyme and the DNA-sequence of the genes for the large subunits. Nucleic Acids Res. 1993 Oct 25;21(21):4904–4908. doi: 10.1093/nar/21.21.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesner D., Steger G., Zimmat R., Owens R. A., Wagenhöfer M., Hillen W., Vollbach S., Henco K. Temperature-gradient gel electrophoresis of nucleic acids: analysis of conformational transitions, sequence variations, and protein-nucleic acid interactions. Electrophoresis. 1989 May-Jun;10(5-6):377–389. doi: 10.1002/elps.1150100516. [DOI] [PubMed] [Google Scholar]
- Schickor P., Metzger W., Werel W., Lederer H., Heumann H. Topography of intermediates in transcription initiation of E.coli. EMBO J. 1990 Jul;9(7):2215–2220. doi: 10.1002/j.1460-2075.1990.tb07391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh W. C., Ross W., Record M. T., Jr Two open complexes and a requirement for Mg2+ to open the lambda PR transcription start site. Science. 1993 Jan 15;259(5093):358–361. doi: 10.1126/science.8420002. [DOI] [PubMed] [Google Scholar]
- Thomm M., Sandman K., Frey G., Koller G., Reeve J. N. Transcription in vivo and in vitro of the histone-encoding gene hmfB from the hyperthermophilic archaeon Methanothermus fervidus. J Bacteriol. 1992 Jun;174(11):3508–3513. doi: 10.1128/jb.174.11.3508-3513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullius T. D., Dombroski B. A. Hydroxyl radical "footprinting": high-resolution information about DNA-protein contacts and application to lambda repressor and Cro protein. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5469–5473. doi: 10.1073/pnas.83.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Zillig W., Palm P., Fuchs E. Initiation of DNA-dependent RNA synthesis and the effect of heparin on RNA polymerase. Eur J Biochem. 1967 Dec;3(2):194–201. doi: 10.1111/j.1432-1033.1967.tb19515.x. [DOI] [PubMed] [Google Scholar]