Abstract
Identification of virulence determinants of viruses is of critical importance in virology. In search of such determinants, virologists traditionally utilize comparative genomics between a virulent and an avirulent virus strain and construct chimeras to map their locations. Subsequent comparison reveals sequence differences, and through analyses of site-directed mutants, key residues are identified. In the absence of a naturally occurring virulent strain, an avirulent strain can be functionally converted to a virulent variant via an experimental evolutionary approach. However, the concern remains whether experimentally evolved virulence determinants mimic those that have evolved naturally. To provide a direct comparison, we exploited a plant RNA virus, soybean mosaic virus (SMV), and its natural host, soybean. Through a serial in vivo passage experiment, the molecularly cloned genome of an avirulent SMV strain was converted to virulent variants on functionally immune soybean genotypes harboring resistance factor(s) from the complex Rsv1 locus. Several of the experimentally evolved virulence determinants were identical to those discovered through a comparative genomic approach with a naturally evolved virulent strain. Thus, our observations validate an experimental evolutionary approach to identify relevant virulence determinants of an RNA virus.
The rapid evolution of viruses, allowing adaptation to new hosts and new forms of resistance and/or drug therapies, is of critical importance in virology (7, 10, 19, 26). Identification of the responsible determinants has numerous applications in combating viruses of animals and plants. These include the development of next-generation antiviral drugs, prescription of antiviral medicines in the course of treatment of patients, and the generation of durable resistance against plant viruses (6, 16, 18).
Virulence determinants of viruses are typically identified by one of two approaches: (i) examination of naturally occurring variants, if in existence (9, 26), or (ii) experimental evolution in a laboratory setting involving passages under novel selection pressures (4, 17, 19). Comparative genomics of adapted and original viral genomes, followed by construction and analyses of recombinants and site-directed mutants, will subsequently identify the critical amino acid(s) involved (9, 26). The experimental approach has great advantages as the settings and selection pressures are controlled and allow for repeatability and statistical analysis. Moreover, in the course of development of the next generation of antiviral drugs, it is the only available approach (6, 18). However, the concern remains whether virulence determinants identified through serial-passage experiments accurately represent those evolving naturally in the virus of interest (18). Few, if any, viral systems have direct comparisons of adaptive responses to the same selection pressure in nature versus laboratory settings in the same host. Such experiments are particularly difficult to conduct with vertebrate viruses at the whole-organism level (4) due to constraints imposed by the culture of the biological hosts.
To provide a direct comparison, we took advantage of a plant RNA virus, soybean mosaic virus (SMV), and its natural host, soybean. SMV, a single-stranded positive-sense RNA virus, is a species within the genus Potyvirus belonging to the family Potyviridae (1). SMV is transmitted naturally via infected soybean seed and by several species of aphids (29). Its genome, linked to a virus-encoded protein (VPg) at its 5′ end and polyadenylated at its 3′ end, contains a long open reading frame (ORF) encoding a single large polypeptide, which is cleaved by three virus-encoded proteases to yield mature proteins including multifunctional helper component-proteinase (HC-Pro) and P3 (Fig. 1) (27). Additionally, a small ORF, termed pipo, is also embedded in the P3 cistron of SMV (30).
FIG. 1.
Identification of virulence determinants of soybean mosaic virus (SMV) on functionally immune Rsv1 genotype soybeans by comparative genomics followed by construction and analysis of chimeras and site-directed mutants. The genomes of avirulent SMV-N (avrN) and virulent SMV-G7 (virG7) each encode a polyprotein of 3,066 amino acids (aa) that is cleaved posttranslationally by virus-encoded proteases to produce the mature proteins (open rectangles) including multifunctional helper component-proteinase (HC-Pro) and P3. However, virG7 has 89 amino acid substitutions relative to avrN across the genome (red bars). Concurrent replacement of three amino acids of avrN with those of virG7, one in HC-Pro (R682M) and two in P3 (R787I, A947T), is necessary and sufficient to convert avrN to virulence (virN) on L78-379 (9) and other Rsv1 soybeans. The ability (+) or inability (−) to establish infection in susceptible (rsv1) or functionally immune Rsv1 soybeans following direct biolistic inoculation with plasmids containing infectious cDNA clones is indicated on the right. The intensity of the purple shading associated with each Rsv1 genotype soybean correlates with its strength of resistance against avrN measured by the number of amino acid substitutions required for virulence. UTR, untranslated region; P1, P1 proteinase; CI, cytoplasmic inclusion protein; 6K, kilodalton protein; NIa, nuclear inclusion protein a; NIb, nuclear inclusion protein b; CP, coat protein.
Soybean has evolved surveillance and/or immunity mechanisms during coevolution with SMV, such as the Rsv1 dominant resistance locus, which detects SMV, mediates activation of host defense signaling pathway(s), and arrests the virus at a very early stage of infection (11). Soybean lines that contain Rsv1 are functionally immune to most SMV strains, including avirulent SMV-N (avrN), but not to naturally occurring virulent SMV-G7 (virG7) or experimentally evolved virulent SMV-G7d (virG7d) derived from virG7 (9, 11, 12). Both virG7 and virG7d infect Rsv1 soybeans but induce different systemic phenotypes (12). In contrast, Rsv1 soybean plants inoculated with avrN remain symptomless, and virus cannot be recovered from the inoculated leaves (11).
In this paper, data are presented on experimental adaptation of an infectious cDNA clone of avrN to soybean genotypes containing Rsv1. Interestingly, several of the virulence determinants observed in adapted viral genomes were identical to those discovered through a comparative genomic approach between avrN and the naturally occurring virG7. Our observation validates serial passage experiments for identifying relevant virulence determinants of SMV.
MATERIALS AND METHODS
Viruses, soybean genotypes, inoculation, and detection.
The plasmids pSMV-N (GenBank accession number D00507), pSMV-G7 (GenBank accession number AY216010), pSMV-G7d (GenBank accession number AY216987), pSMV-Nv, pSMV-N-GUS, pSMV-NR682M+R787I+A947T (a plasmid encoding SMV-N with point mutations in HC-Pro [R682M] and P3 [R787I and A947T]), pSMV-NR787I+A947T, pSMV-NT341I, pSMV-NR682M, pSMV-NR787I, and pSMV-NA947T have been described previously (9, 12, 14, 28). A plasmid encoding SMV-N tagged with the red fluorescent protein DsRed (pSMV-NDsR) was generated by replacing the uidA gene of pSMV-N-GUS with the PCR-amplified gene for DsRed from the plasmid pJH19 (24) using primers REPs and RFPa (see Table S1 in the supplemental material for oligonucleotide primer sequences). All plasmids were propagated in ElectroMax DH5α-E cells (Invitrogen, Carlsbad, CA) and purified using a QiaPrep Spin MiniPrep Kit (Qiagen, Valencia, CA).
Soybean cultivars Williams 82 (rsv1) and Lee 68 (rsv1), both universally susceptible to all SMV strains, and Rsv1 soybeans PI 96983, L800, and L78-379, all functionally immune to avrN, were used in this study (2, 15). PI 96983 (Rsv1) is a soybean plant introduction (PI) containing the Rsv1 resistance locus and was originally collected in Hwanghae Puk, South Korea, and introduced to the United States in 1932. Soybean L800 (Rsv1) is a recombinant line containing only one of the 6-nucleotide binding/leucine-rich repeat-type candidate resistance genes at the Rsv1 chromosomal region from PI 96983 (Rsv1) (15). L800 (Rsv1) was selected from a population resulting from crossing Lee 68 (rsv1) with PI 96983 (Rsv1) (15) and was subsequently advanced in the field. L78-379 (Rsv1) is an isoline of Williams (rsv1) resulting from the sixth generation of backcrossing PI 96983 (Rsv1) to Williams (rsv1). L78-379 (Rsv1) likely possesses an additional unknown genetic factor, in addition to the Rsv1 locus from PI 96983, which enhances resistance to certain SMV mutants (14). All soybean seeds originated from greenhouse or field-grown plants shown to be free of SMV by indexing.
Infection with plasmid DNA was initiated by biolistic inoculation of fully expanded primary leaves of soybean seedlings using a PDS-1000 helium gene gun system (Bio-Rad Laboratories, Hercules, CA) as described previously (12, 14). To inoculate viruses mechanically, viral progenies derived from cDNA clones were rub inoculated onto carborundum-dusted (600 mesh) primary leaves of soybeans (11). Plants were maintained in a growth chamber operating at 22°C with a photoperiod of 16 h. Detection of SMV in systemically infected secondary leaves of the inoculated plants was done by enzyme-linked immunosorbent assay (ELISA) (14), reverse transcription-PCR (RT-PCR), or visualization of DsRed expression at different time points (14 to 28 days postinoculation [dpi]) with an Olympus fluorescence stereomicroscope equipped with a filter set for excitation at 530 to 560 nm and emission at 590 to 650 nm.
Serial passage experiment.
The serial passage experiment was started with a full-length infectious clone of the avrN genome. The strategy utilized previously for adaptation of molecularly cloned avirulent SMV-N (avrN)-derived P3 chimeras to Rsv1 soybeans (14) was adopted for this study. In order to generate genetic diversity, molecularly cloned avrN was initially inoculated biolistically to Williams 82 (rsv1). The derivative progenies were then passaged via mechanical inoculation through Williams 82 (rsv1) multiple times with an interval of 2 to 3 weeks. For each inoculation, infectious extract from symptomatic secondary leaves served as the inoculum. The passaged progenies in Williams 82 (rsv1) were eventually inoculated to L800 (Rsv1) and subsequently to PI 96983 (Rsv1) and L78-379 (Rsv1).
RNA extraction, RT-PCR, and sequencing.
RT-PCR and sequencing were used to identify mutations in the P3 and HC-Pro cistrons associated with the adaptation of viral progenies to Rsv1 soybeans. Total RNA was isolated from systemically infected secondary leaves by using an RNeasy Plant Mini Kit (Qiagen). The entire HC-Pro and P3 cistrons of the progeny viruses were reverse transcribed with SuperScript reverse transcriptase (Invitrogen, Carlsbad, CA) in the presence of random primers or SMV-3907a primer (see Table S1 in the supplemental material for the list of all primers). The resultant cDNAs were used as a template in the first PCR in the presence of Ex Taq DNA Polymerase (Takara Bio, Madison, WI) and primers G7-214s and SMV-3907a. Subsequently, the amplified fragments served as templates in a nested PCR using primers SMV-456s and SMV-3840a. The amplified products were purified by using a QIAquick PCR Purification Kit (Qiagen) and sequenced using primers SMV-1629a, SMV-1439s, SMV-2271s, and SMV-2919s. Sequencing was done at The University of Tennessee DNA Sequencing Facility, and sequences were edited and analyzed using Vector NTI (Invitrogen).
Construction of molecularly cloned viral variants.
The restriction sites used and their nucleotide positions in the SMV-N (avrN) genome were NotI (within the vector at 5′ end of the SMV cDNA), BglII (position 1605), KpnI (2335), SpeI (3234), and SalI (3782). The polyprotein amino acid positions encoded by the restriction sites in the SMV genome were 493, 736, 1036, and 1218, respectively. A PCR-based mutagenesis protocol (9, 13, 22) was used to reconstruct mutations associated with the adaptation of progeny viruses into the cloned SMV-N (pSMV-N) for assessment of their direct roles in adaptation. PrimeSTAR HS DNA polymerase (Takara Bio) was used during the synthesis of all point mutants (see Table S1 in the supplemental material for the list of all primers utilized).
SMV-N mutants with point mutations (indicated by subscripts) in HC-Pro, SMV-NG319S, SMV-NG319S+K321E, SMV-NK321E, and SMV-NK321E+T341I, were constructed by performing the initial PCR with the mutagenic primers N-1081s, N1080s, N-1081s.1st and N-1089s, respectively, and the primer N-1705a while pSMV-N served as the template. Subsequently, the amplified products were used as megaprimers in the presence of the vector primer V-13534s and template pSMV-N in the second PCRs. The resultant products were digested with NotI and BglII and ligated into pSMV-N.
SMV-N mutants with point mutations in P3, SMV-NR945G, SMV-NA947V, SMV-NP948L, SMV-NK952E, and SMV-NA947V+K952E, were constructed by performing the initial PCR with the primer N-3324a and the mutagenic primers N-2952s, N-2960s.v, N-2960s.1, N-2979s, and N2960s.ve, respectively, with pSMV-N as the template. Subsequently, the amplified fragments were used as megaprimers in the second PCRs in the presence of the primer N-2276s and pSMV-N. The resultant products were digested with KpnI and SpeI and ligated into pSMV-N. Mutants SMV-NV1045A and SMV-NM1157I were constructed by performing the initial PCRs with the mutagenic primers N-3255s and N-3593s, respectively, and the primer N-3892a in the presence of pSMV-N. The amplified products were subsequently used as megaprimers in the second PCRs in the presence of the primer N-2890s and pSMV-N. The resultant products were digested with SpeI and SalI and ligated into pSMV-N. Mutants SMV-G7I788R and SMV-G7dI788R were constructed by performing the initial PCRs with the mutagenic primer G7-2511a and the primer SMV-2271s while pSMV-G7 or pSMV-G7d served as the template, respectively. The amplified fragments were used subsequently as megaprimers in second PCRs in the presence of primer G7-3266a while pSMV-G7 or pSMV-G7d served as the template. The resultant products were digested with KpnI and SpeI and ligated into pSMV-G7 or pSMV-G7d.
To construct mutants with substitutions in both HC-Pro and P3, standard molecular biological techniques were used to exchange restriction fragments between various SMV cDNAs (9, 14, 22). To generate SMV-NK321E+R945G and SMV-NK321E+A947V, a KpnI/SpeI fragment was released from pSMV-NR945G and pSMV-NA947V and ligated into pSMV-NK321E. To construct SMV-NG319S+K321E+A947V and SMV-NG319S+K321E+A947V+K952E, a KpnI/SpeI fragment was released from pSMV-NA947V and pSMV-NA947V+K952E and ligated into pSMV-NG319S+K321E. SMV-NK321E+T341I+A947V was generated by releasing a KpnI/SpeI fragment encoding the A947V mutation from pSMV-NA947V and ligating it into pSMV-NK321E+T341I. SMV-NK321E+T341I+A947V+V1045A was constructed by releasing an SpeI/SalI fragment encoding V1045A from pSMV-NV1045A and ligating it into pSMV-NK321E+T341I+A947V. To generate SMV-NG319S+K321E+R682M+A947V+K952E+M1157I, a BglII/KpnI fragment encoding the R682M mutation was initially released from pSMV-NR682M+R787I+A947T and ligated into pSMV-NG319S+K321E+A947V+K952E to generate pSMV-NG319S+K321E+R682M+A947V+K952E. Subsequently, an SpeI/SalI fragment encoding the M1157I mutation was released from pSMV-NM1157I and ligated into pSMV-NG319S+K321E+R682M+A947V+K952E to yield SMV-NG319S+K321E+R682M+A947V+K952E+M1157I.
To construct SMV-NK321E+T341I+A947V tagged with DsRedT4, K321E and T341I mutations were introduced into pSMV-Nv (28) as described above while pSMV-Nv served as the template. The subsequent PCR was done using the amplified fragments as a megaprimer in the presence of primer V-13534s and pSMV-Nv. The resultant PCR product was digested with NotI and BglII and ligated into pSMV-Nv to construct pSMV-NvK321E+T341I. A KpnI/SpeI fragment encoding the A947V mutation was released from pSMV-NA947V and ligated into pSMV-NvK321E+T341I to generate pSMV-NvK321E+T341I+A947V. Finally, DsRedT4 was released by AvrII digestion of pSMV-NDsR and ligated into pSMV-NvK321E+T341I+A947V to generate pSMV-NK321E+T341I+A947VDsR.
To synthesize SMV-NG319S+K321E+R682M+A947V+K952E+M1157IDsR, the mutations G319S and K321E were first introduced into pSMV-Nv as described above to generate pSMV-NvG319S+K321E. Subsequently, a KpnI/SpeI fragment was released from pSMV-NA947V+K952E and ligated into pSMV-NvG319S+K321E to generate pSMV-NvK321E+T341I+A947V+K952E. A BglII/KpnI fragment encoding the R682M mutation was released from pSMV-NR682M+R787I+A947T and ligated into pSMV-NvK321E+T341I+A947V+K952E to construct pSMV-NvK321E+T341I+R682M+A947V+K952E. Subsequently, an SpeI/SalI fragment encoding the M1157I mutation was released from pSMV-NM1157I and ligated into pSMV-NvK321E+T341I+R682M+A947V+K952E to generate pSMV-NvG319S+K321E+R682M+A947V+K952E+M1157I. Finally, DsRedT4 was released by AvrII digestion of pSMV-NDsR and ligated into pSMV-NvG319S+K321E+R682M+A947V+K952E+M1157I to yield SMV-NG319S+K321E+R682M+A947V+K952E+M1157IDsR.
The presence of the introduced mutation(s) and the absence of any unwanted PCR-generated substitution in each of the infectious cDNAs were verified by sequencing of the entire amplified regions of the cloned viruses.
Evaluation of the stability of the mutant viruses in infection.
The stability of mutations in planta following infection with molecularly cloned variant viruses was verified by RT-PCR and sequencing as above. RNA for RT-PCR was purified from the soybean line or cultivar with the greatest resistance to SMV variants. For example, if a molecularly cloned variant established infection only in susceptible Williams 82 (rsv1), total RNA was extracted from this genotype. However, if a molecularly cloned variant was also virulent on L800 (Rsv1), the tissue from this genotype was targeted for analysis instead of that of Williams 82 (rsv1). If a molecularly cloned variant was virulent on both L800 (Rsv1) and PI 96983 (Rsv1), tissues derived from only PI 96983 (Rsv1) were targeted for analysis. If a molecularly cloned variant was virulent on all of the Rsv1 soybeans, total RNA from L78-379 (Rsv1) was utilized instead of RNA from any other Rsv1 cultivar. Regardless of the soybean genotype, the entire HC-Pro and P3 cistrons of the progeny viruses were subjected to RT-PCR and sequencing. The oligonucleotides used for RT-PCR and sequencing of the progeny viruses are listed in Table S1 in the supplemental material.
RESULTS AND DISCUSSION
Comparative genomic analysis between avirulent SMV-N and the naturally occurring virulent SMV-G7 identified three virulence determinants.
The genomes of the naturally occurring avirulent SMV-N (avrN) and virulent SMV-G7 (virG7) are similar in size, and each encodes a polyprotein of 3,066 amino acids, but the virG7 polyprotein has 89 amino acid substitutions relative to avrN (Fig. 1). By comparative genomic analyses between avrN and virG7, followed by construction and analysis of recombinants and site-directed mutants, the virulence determinants for SMV in L78-379 (Rsv1) were previously mapped to three amino acids residing in the viral polypeptide, one in the HC-Pro (R682M) and two in the P3 (R787I and A947T) (9) regions (Fig. 1). A variant derived from molecularly cloned avrN, but concurrently harboring the three mutations, virNR682M+R787I+A947T, was virulent on Rsv1 soybean L78-379 (9) as well as on L800 and PI 96983 soybeans when inoculated biolistically (Fig. 1; Table 1).
TABLE 1.
Systemic infection of rsv1 (susceptible) and Rsv1 (functionally immune) soybean genotypes following biolistic or mechanical inoculation with mutant virusesa
| Mutantb | Systemic infection rate by genotype, cultivar, and infection method (no. of plants infected/no. of plants inoculated)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
rsv1 |
Rsv1 |
|||||||||
| Williams 82 |
Lee 68 |
L800 |
PI 96983 |
L78-379 |
||||||
| Biolistic | Mechanical | Biolistic | Mechanical | Biolistic | Mechanical | Biolistic | Mechanical | Biolistic | Mechanical | |
| SMV-NG319S | 3/3 | 4/4 | 6/6 | 1*/4 | 0/4 | 0/6 | 0/6 | |||
| SMV-NG319S+K321E | 3/3 | 4/4 | 6/6 | 1*/4 | 0/9 | 0/13 | 0/7 | |||
| SMV-NK321E | 3/3 | 8/8 | 6/6 | 0/4 | 1*/8 | 0/6 | 0/4 | |||
| SMV-NT341I | 3*/3 | 4/4 | 6/6 | 0/3 | 0/6 | 0/9 | 0/8 | |||
| SMV-NK321E+T341I | 3*/3 | 6/7 | 6/6 | 0/4 | 0/4 | 0/12 | 0/6 | |||
| SMV-NR682M | 3*/3 | 3/3 | 6/6 | 0/4 | 0/4 | 0/8 | 0/7 | |||
| SMV-NR787I | 3/3 | 4/4 | 6/6 | 0/3 | 1*/6 | 0/6 | 0/12 | |||
| SMV-NR945G | 3/3 | 2/3 | 5/5 | 2*/3 | 4/5 | 0/7 | 0/9 | |||
| SMV-NA947T | 3/4 | 2/2 | 5/5 | 2*/3 | 2/2 | 0/4 | 0/4 | |||
| SMV-NA947V | 3/3 | 7/7 | 6/6 | 3*/3 | 8/9 | 0/5 | 0/6 | |||
| SMV-NP948L | 3/3 | 7/7 | 7/7 | 1*/4 | 4/5 | 0/6 | 0/6 | |||
| SMV-NK952E | 3/3 | 3/4 | 6/6 | 0/3 | 1*/7 | 0/6 | 0/6 | |||
| SMV-NV1045A | 3/3 | 3/3 | 6/6 | 1*/3 | 4/5 | 0/8 | 0/8 | |||
| SMV-NM1157I | 4*/4 | 6/6 | 6/6 | 0/3 | 0/5 | 0/6 | 0/6 | |||
| SMV-NR787I+A947T | 3/3 | 5/6 | 5/5 | 2*/3 | 6/6 | 0/12 | 0/22 | |||
| SMV-NK321E+R945G | 3/3 | 3/3 | 6/6 | 2*/4 | 3/5 | 0/16 | 0/7 | |||
| SMV-NK321E+A947V | 3/3 | 6/6 | 6/6 | 3*/4 | 4/4 | 0/6 | 0/6 | |||
| SMV-NK321E+T341I+A947V | 3/3 | 6/6 | 6/6 | 2/4 | 5/6 | 4/4 | 5/6 | 3*/4 | 2/7 | |
| SMV-NK321E+T341I+A947VDsR | 3/3 | 3/3 | 4/4 | 4/4 | 3/4 | |||||
| SMV-NK321E+T341I+A947V+V1045A | 3/3 | 7/7 | 6/6 | 4/4 | 4/4 | 4/4 | 6/6 | 4*/4 | 14/14 | |
| SMV-NG319S+K321E+A947V | 3/3 | 3/3 | 7/7 | 4/4 | 2/6 | 4*/4 | 5/10 | 0/4 | 0/5 | |
| SMV-NG319S+K321E+A947V+K952E | 2/2 | 3/3 | 6/6 | 5/5 | 6/6 | 4/4 | 6/6 | 1*/4 | 7/7 | |
| SMV-NG319S+K321E+R682M+A947V+K952E+M1157I | 3/3 | 3/3 | 5/5 | 3/4 | 7/7 | 3/4 | 12/12 | 4*/4 | 4/5 | |
| SMV-NG319S+K321E+R682M+A947V+K952E+M1157IDsR | 3/3 | 3/3 | 4/4 | 4/4 | 4/4 | |||||
| SMV-NR682M+R787I+A947T | 3/3 | 2/2 | 11/11 | 3/4 | 2/2 | 4/4 | 5/7 | 4*/4 | 14/14 | |
| SMV-G7I788Rc | 3/3 | 6/8 | 13/15 | 3/4 | 2/5 | 3*/4 | 0/7 | |||
| SMV-G7dI788Rc | 3/3 | 8/8 | 6/6 | 4/4 | 5/5 | 6/6 | 4*/4 | |||
For biolistic inoculation, plasmids containing full-length infectious cDNA clones were delivered biolistically by a helium gene gun system into fully expanded primary leaves of 2-week-old soybean seedlings. For mechanical inoculations, infectious extract from biolistically inoculated Williams 82 (rsv1) was used as an inoculum and rub inoculated onto fully expanded primary leaves of 2-week old soybean seedlings. The inoculated plants were maintained in a growth chamber (22°C) until evaluated for infection based on symptom expression. Absence of virus in asymptomatic plants was confirmed by ELISA. The asterisk indicates that for each virus variant, total RNA was extracted from one infected plant of the indicated genotype and subjected to RT-PCR. The stability of the introduced mutation(s) and lack of any newly emerged substitution(s) in progeny viruses was confirmed by sequencing the entire HC-Pro and P3 cistrons. Infection of a single L800 (Rsv1) plant following direct biolistic inoculation with SMV-NG319S was associated with sequence polymorphism at nucleotide position 1114 where both C and T were present. Thus, codons CCT and CTT encoding proline and leucine, respectively, were both present at amino acid position 328. Infection of a single L800 (Rsv1) by SMV-NK321E upon mechanical inoculation was associated with a newly emerged silent mutation in HC-Pro cistron (A2174G). Infection of a single L800 (Rsv1) by SMV-NK952E upon mechanical inoculation was associated with a newly emerged silent mutation (A1271G) in HC-Pro and a sequence polymorphism in P3 at position 2709 where both TCA and ACA codons encoding serine and threonine, respectively, were present at polyprotein position 860. Infection of L78-379 (Rsv1) by SMV-G7dI788R was associated with a missense mutation in P3 (G2752T) resulting in a C874F substitution in the virus polyprotein. No newly emerged mutations in HC-Pro and P3 cistrons of the progeny derived from any of the other molecularly cloned mutant viruses described in this table and Fig. 2 were detected.
DsR, DsRedT4 expression was visualized with an Olympus fluorescence stereomicroscope.
Substitutions at position 788 in the genomes of SMV-G7 and SMV-G7d correspond to 787 in the genome of SMV-N. This is because of lack of a codon in P1 cistron of SMV-N, which is located upstream of the P3 cistron (14).
Experimental adaptation of avrN-derived progenies to Rsv1 genotype soybeans was associated with mutations in HC-Pro, P3, or both.
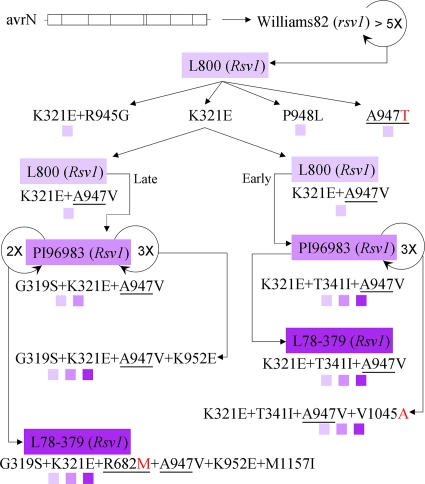
To test whether experimental conversion of avrN to virulence on Rsv1 soybeans leads to the same genetic determinants identified through the comparative genomic analyses (Fig. 1), an in vivo serial passage protocol was utilized. The molecularly cloned avrN was passaged multiple times through a susceptible soybean cultivar, Williams 82 (rsv1), to generate genetic diversity (7) (Fig. 2). This approach relies upon error-prone replication of RNA viruses (8), which in the absence of a positive selection pressure leads to accumulation of a diversified viral population (23). However, as three concurrent substitutions in HC-Pro and P3 are required for conversion of avrN to virulence on functionally immune PI 96983 (Rsv1) and L78-379 (Rsv1) (Fig. 1), it seemed unlikely that these beneficial mutations would occur simultaneously during experimental adaptation and under selection pressure. We therefore decided to increase stepwise the Rsv1-mediated selection pressure on the virus by adapting initially avrN-derived progenies passaged in Williams 82 (rsv1) to L800 (Rsv1) and subsequently to PI 96983 (Rsv1) and L78-379 (Rsv1). Unlike PI 96983 (Rsv1), L800 (Rsv1) contains only one of six candidate genes from the complex chromosomal region of PI 96983 (Rsv1). In a previous study, it was shown that L800 (Rsv1), unlike PI 96983 (Rsv1), was susceptible to isolates of SMV strains G3 and G5 (15). This susceptibility was attributed to lack of possession of all the resistance factors from the genetically complex Rsv1 locus (15). Nevertheless, L800 (Rsv1), similar to PI 96983 (Rsv1), is functionally immune to avrN (Fig. 1) as well as to other isolates of the G2 strain of SMV to which avrN belongs (15).
FIG. 2.
Experimental adaptation of progenies derived from molecularly cloned avrN to functionally immune Rsv1 genotype soybeans via passaging. Cloned avrN was biolistically inoculated to susceptible Williams 82 (rsv1) soybean. Progenies derived from multiple passages (circular arrow) in Williams 82 (rsv1) plants established infection only in recombinant soybean L800 (Rsv1) and not in PI 96983 (Rsv1) or L78-379 (Rsv1). Mutations in HC-Pro or P3 observed in consensus sequences of viral progenies derived from each of the infected Rsv1 genotype soybeans are indicated. One virus population with the K321E mutation was passaged once more in L800 (Rsv1), and progenies from two infected plants, one exhibiting symptoms at 7 days postinoculation (dpi) (early) and the other at 14 dpi (late), were individually adapted to other Rsv1 genotype soybeans through initial multiple passages in PI 96983 (Rsv1), followed by direct inoculation to L78-379 (Rsv1). Direct role(s) of the mutation(s) in adaptation was confirmed by reconstruction of substitutions identified in viral progenies into cloned avrN followed by biolistic inoculation to Rsv1 genotype soybeans. The small color-coded squares indicate virulence of molecularly cloned avrN-derived variants containing a mutation(s) on the indicated Rsv1 genotype soybeans. Purple shading of different Rsv1 soybean genotypes is as described in the legend of Fig. 1. The positions of amino acids identified by both the functional genomic approach (Fig. 1) and experimental adaptation are underlined, and residues identical to those of virulent SMV-G7 (virG7) are shown in red (compare with Fig. 1). Note that the amino acid residue of virG7 at the position corresponding to V1045A is also alanine.
When avrN-derived progenies were successively passaged at least five times in Williams 82 (rsv1) and subsequently inoculated to L800 (Rsv1), only 1 out of 10 plants became infected and showed systemic necrosis on aerial parts of the plant (data not shown). RT-PCR amplification and sequencing of the entire HC-Pro and P3 cistrons of progenies derived from the infected tissues, harvested at 16 and 25 dpi, showed only a single amino acid substitution (K321E) in the HC-Pro region (Fig. 2). However, in progenies collected at 33 dpi, a sequence polymorphism was also present at nucleotide position 3057, where both A and G were present. Thus, codons AAT and GAT encoding asparagine and aspartic acid, respectively, were both present at amino acid 976 (data not shown).
Additional serial passages of avrN-derived progenies in Williams 82 (rsv1) (5 to 10 times) resulted in a higher rate of adaptation to L800 (Rsv1), suggesting that a more diversified progeny population had developed. When L800 (Rsv1) was challenged with one such population, three out of five inoculated plants became infected, but each exhibited a distinct phenotype (Fig. 3). RT-PCR and sequencing of HC-Pro and P3 derived from each of these virulent progenies showed different single mutations in P3 (either A947T or P948L) in populations derived from two plants, while progenies from the third plant had mutations in both HC-Pro (K321E) and P3 (R945G) (Fig. 3). Virus from each of these virulent populations was passaged once more in L800 (Rsv1), but no additional mutation was selected in the HC-Pro or the P3 cistron of any of these progenies, and their phenotypes remained unaltered (Fig. 3). The A947T population induced widespread severe systemic necrosis and stunting, whereas the population with both the K321E and R945G mutations (here, the K321E+R945G population) and the P948L population caused only limited systemic necrosis.
FIG. 3.
Phenotypic differences in responses of soybean L800 (Rsv1) to inoculation with avrN-derived virulent progenies A947T, K321E+R945G, and P948L. Infectious extract derived from soybean L800 (Rsv1) plants infected individually with each of the three progeny viruses served as an inoculum and was mechanically inoculated to primary leaves. Plants were photographed at 16 days postinoculation. Note distinct phenotype of each of the virulent progenies.
In a previous study, we experimentally adapted progenies from avrN-derived chimeras with a modified P3 to PI 96983 (Rsv1) and L78-379 (Rsv1) (14). Therefore, in this study we attempted to adapt avirulent progenies with a modified HC-Pro rather than P3. The K321E viral population collected from L800 (Rsv1) soybean at 23 dpi, which appeared by the consensus sequence to have a single mutation in HC-Pro, was selected for further diversification in L800 (Rsv1), followed by adaptation to PI 96983 (Rsv1) and L78-379 (Rsv1) (Fig. 2). Interestingly, the K321E mutation was stable and was retained in progenies even after three successive passages in susceptible Williams 82 (rsv1) plants where Rsv1-mediated selection pressure was absent (data not shown).
Infectious extract from L800 (Rsv1) plants containing progenies with the K321E mutation was again inoculated to L800 (Rsv1), and four out of five plants became infected. One of the infected plants showed symptoms at 7 dpi, whereas the remaining plants exhibited symptoms at about 14 dpi. The RT-PCR amplification and sequencing of the entire HC-Pro and P3 cistrons of viral progenies derived from each of the infected plants showed no difference in the consensus sequences. All contained, in addition to K321E, an additional mutation in P3, A947V. The selections of K321E and A947V mutations in independent L800 (Rsv1) plants (Fig. 2) indicate the presence of strong parallel selection pressures on these positions for change. As the A947V mutation was selected in all of four L800 (Rsv1)-infected plants (data not shown), this mutation may have been present in the initial K321E viral population used as the inoculum, but its presence was below the level of detection in the consensus sequence. Progenies from the plant exhibiting early symptoms as well as a representative population from the second group with delays in symptom appearance were selected for individual adaptation to PI 96983 (Rsv1) and L78-379 (Rsv1). These viral progenies will be referred to here as K321E+A947V (early) and K321E+A947V (late), respectively. Interestingly, four successive passages of the K321E+A947V (early) population in L800 (Rsv1) did not lead to selection of any additional mutations in either the HC-Pro or P3 cistron (data not shown).
Our previous effort in adapting avirulent avrN-derived chimeras with a modified P3 to PI 96983 (Rsv1) and L78-379 (Rsv1) showed that gain of virulence on L78-379 (Rsv1) required more mutations in HC-Pro or P3 than on PI 96983 (Rsv1) (14). Hence, in the current study we decided to initially adapt each of the early and late K321E+A947V viral progenies from L800 (Rsv1) to the intermediate-resistant host PI 96983 (Rsv1) and subsequently to more resistant L78-379 (Rsv1). Adaptation of the K321E+A947V (early) progenies to PI 96983 (Rsv1) was associated with accumulation of an additional substitution in HC-Pro (T341I) (Fig. 2). Progenies with K321E+T341I+A947V from PI 96983 (Rsv1) were also virulent on L78-379 (Rsv1) without selection of any additional mutations (Fig. 2 and 4; Table 1). Interestingly, when the K321E+A947V (early) viral progenies with three passages in L800 (Rsv1), instead of two, were used as an inoculum, the virulent variant on PI 96983 (Rsv1) isolated from the third and fourth upper leaves of an infected plant separately, both had a T341I substitution in the HC-Pro cistron (data not shown). Selection of the T341I mutation in independent plants is indicative of a strong parallel selection pressure at this position.
FIG. 4.
Infection of soybean genotypes with DsRed (DsR)-tagged avirulent SMV-N (avrNDsR) or its experimentally evolved virulent derivative, SMV-NK321E+T341I+A947V (virNDsR) (Fig. 2). Primary leaves of susceptible (rsv1) or functionally immune (Rsv1) genotype soybeans were inoculated biolistically with molecularly cloned avrNDsR or virNDsR. Leaflets from the secondary leaves of the inoculated plants were visualized under white or UV lights for the expression of DsRed at 14 to 28 days postinoculation. Note that both viruses were capable of expressing DsRed in the secondary leaves from susceptible soybean Williams 82 (rsv1). Only the experimentally evolved virNDsR, but not its progenitor avrNDsR, expressed DsRed in noninoculated secondary leaves from all functionally immune Rsv1 genotype soybeans. Purple shading of different Rsv1 genotype soybeans is as described in the legend of Fig. 1.
Passage of the K321E+T341I+A947V viral progenies twice in PI 96983 (Rsv1) did not result in selection of any new mutation in either HC-Pro or P3 cistrons (data not shown). However, one more passage for a total of three in PI 96983 (Rsv1) resulted in selection of an additional substitution in P3, V1045A (Fig. 2). The K321E+T341I+A947V+V1045A viral progenies, similar to the progenitor virus, K321E+T341I+A947V, were virulent on L78-379 (Rsv1) (Table 1). However, the K321E+T341I+A947V+V1045A progeny-induced symptoms appeared faster and were more uniformly spread to all secondary leaves of the infected Rsv1 soybeans (data not shown).
In contrast to the K321E+A947V (early) population, the K321E+A947V (late) population gained virulence on PI 96983 (Rsv1) following selection of the G319S mutation in HC-Pro (Fig. 2). However, the G319S+K321E+A947V population remained avirulent on L78-379 (Rsv1) (Fig. 2; Table 1). Nevertheless, after two additional passages in PI 96983 (Rsv1), the progenies gained virulence on L78-379 (Rsv1) (Fig. 2). This virulence was associated with selection of three additional mutations, one in HC-Pro (R682M) and two in P3 (K952E+M1157I) (Fig. 2). Three successive passages of the G319S+K321E+A947V population in PI 96983 (Rsv1) also resulted in selection of an additional mutation in P3 (K952E) (Fig. 2). This population was virulent on L78-379 (Rsv1) (Fig. 2; Table 1); however, unlike the G319S+K321E+R682M+A947V+K952E+M1157I viral population, it did not spread efficiently to all secondary leaves of the infected L78-379 (Rsv1) plants (data not shown).
In general, virulence of avrN-derived progenies on L800 (Rsv1) was associated with fewer mutations, while more were required on both PI 96983 (Rsv1) and L78-379 (Rsv1) (Fig. 2; Table 1). This finding is in agreement with similar observations made during experimental adaptation of avrN-derived P3 chimeras to Rsv1 soybeans (14) and reflects the complex nature of the Rsv1 locus (15).
Mutations in HC-Pro and P3 alone were sufficient for conversion of molecularly cloned avrN to virN on Rsv1 soybeans.
To demonstrate the direct role for each of the mutations associated with the adaptation of avrN-derived progenies to Rsv1 soybeans (Fig. 2), each was reconstructed, individually or in combination, into the molecularly cloned avrN. The molecularly cloned avrN-derived variants were then biolistically inoculated directly to primary leaves of various soybean genotypes. Also, virulence of derivative progenies was examined by mechanical inoculation. Data in Fig. 2 and 4 as well as Table 1 confirm that mutations in P3 combined with HC-Pro were sufficient for avrN conversion to virN on all Rsv1 genotype soybeans. Moreover, the data confirmed that gain of virulence by avrN on PI 96983 (Rsv1) and L78-379 (Rsv1) requires at least three mutations in both HC-Pro and P3. This is in complete agreement with the previous finding via the comparative genomic approach (Fig. 1).
However, not all mutations conferred virulence to avrN on L800 (Rsv1) (Table 1). None of the mutations in HC-Pro, including R682M, individually or in combination, converted avrN to virN on L800 (Rsv1) in a consistent manner. Thus, it is likely that HC-Pro mutations are involved in interactions with Rsv1-derived resistance factor(s) that are absent in L800 (Rsv1) but are present in PI 96983 (Rsv1) and L78-379 (Rsv1). This possibility is supported by our previous finding that conversion of avrN-derived chimeras containing P3 from virG7 or virG7d to virulence on PI 96983 (Rsv1) and L78-379 (Rsv1) required mutation in HC-Pro (14). All these observations point to the genetic complexity of the Rsv1 locus (15).
A few of the mutations in P3, such as R787I, K952E, or M1157I, also failed individually to convert avrN to virN on L800 (Rsv1), which has the least resistance among the Rsv1 soybeans (Table 1). Interestingly, reciprocal substitution at the corresponding position to R787 in both virG7 and virG7d (i.e., Ile to Arg at position 788) did not compromise virulence of these viruses on Rsv1 soybeans (Table 1). Furthermore, a Lys-to-Glu substitution at position 953 of virG7 and virG7d genomes, corresponding to codon 952 in SMV-N, did not compromise virulence of the two viruses on PI 96983 (Rsv1) (12, 13). Hence, it is likely that the role of this group of mutations is secondary in nature. Possibly, they alleviate the deleterious effect of the earlier mutations. Alternatively, they improve the fitness of the mutant viruses in Rsv1 soybeans (3, 7, 20).
Several virulence determinants selected through experimental adaptation were identical to those that evolved naturally.
A number of virulence determinants selected experimentally were identical to those that evolved naturally in virG7. These include R682M in HC-Pro as well as A947T in P3 (compare mutations shown in Fig. 2 with those in Fig. 1). The substitution at position 1045 (Val to Ala) in virNK321E+T341I+A947V+V1045A (Fig. 2) is identical to the amino acid residue at the corresponding polyprotein position of virG7 (data not shown).
Mutations at position 947 exhibited instances of both parallel and directional evolution as they occurred naturally as well as experimentally in independent plants, and the substitutions involved two different amino acids (Val or Thr for Ala) (Fig. 2). A mutation at position 319 (Gly to Ser) is another example of directional evolution as it was selected previously during experimental adaptation of two different avrN-derived P3 chimeras to PI 96983 (Rsv1); however, these substitutions involved Gly to Asp or Gly to Ser (14). A mutation at position 341 (Thr to Ile) identified in this study also exhibited another instance of parallel mutation as it was selected in independent plants. Moreover, it was also selected previously during experimental adaptation of an avrN-derived P3 chimera to PI 96983 (Rsv1) (14). A mutation at position 682 (Arg to Met) also was selected previously during experimental adaptation of two different avrN-derived P3 chimeras to PI 96983 (Rsv1) and L78-379 (Rsv1) (14). However, these substitutions involved Arg to Gly and Arg to Lys, respectively. Thus, the mutation at position 682 exhibits an instance of parallel as well as directional evolution. A Lys-to-Glu substitution at position 952 was also selected previously at the corresponding position of virG7, leading to the emergence of VirG7d (12). Directional and parallel mutations are features of convergent evolution (4, 5).
In general, the mutational patterns of avrN toward virulence on Rsv1 soybeans, observed here as well as previously (9, 14), revealed several identical and independent substitutions. This suggests that avrN has limited mutational pathways toward virulence on Rsv1 soybeans. A small number of mutational pathways also was reported for adaptation of rice yellow mottle virus (RYMV) to rymv1-resistant rice and was attributed to high specificity of interactions between the virus avirulent gene and the host resistance factor (21, 25).
Concluding remarks.
The goals of this study were to first identify the determinants of virulence for conversion of avrN to virN on functionally immune Rsv1 soybeans via an in vivo serial passage experiment and, secondly, to compare such determinants with those identified previously through comparative genomic analysis between avrN and the naturally occurring virG7. Thus, the purpose was to answer the question of whether experimental adaptation reveals virulence determinants that are similar to those evolved under natural conditions. Consequently, this study did not focus on understanding the function(s) of the mutations in virulence or revealing the underlying mechanism(s) by which Rsv1-mediated functional immunity operates against avrN.
The experimental evolution of virulence of avrN on Rsv1 soybeans confirmed that HC-Pro and P3 carry the essential determinants for defeating Rsv1-mediated resistance, in full agreement with comparative genomic analysis. Several of the virulence determinants identified via experimental adaptation of avrN were identical to those that evolved naturally in virG7. Thus, our observation vindicates an experimental evolutionary approach via serial passage to identify relevant virulence determinants of an RNA virus.
Supplementary Material
Acknowledgments
We are grateful to G. Kurath (Western Fisheries Research Center, Seattle, WA), W. L. Schneider (USDA-ARS, Fort Detrick, MD), D. A. Brian, and M. Mazarei (University of Tennessee, Knoxville, TN) for critical reviews of the manuscript and numerous invaluable comments.
This project was funded by the University of Tennessee College of Agricultural Sciences and Natural Resources, the Tennessee Agricultural Experiment Station, the Iowa State University College of Agriculture, and North Central Soybean Research Programs.
Footnotes
Published ahead of print on 29 December 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Adams, M. J., J. F. Antoniw, and C. M. Fauquet. 2005. Molecular criteria for genus and species discrimination within the family Potyviridae. Arch. Virol. 150:459-479. [DOI] [PubMed] [Google Scholar]
- 2.Bernard, R. L., R. L. Nelson, and C. R. Creemens. 1991. USDA soybean genetic collection: isoline collection. Soybean Genet. Newsl. 18:27-57. [Google Scholar]
- 3.Bloom, J. D., L. I. Gong, and D. Baltimore. 2010. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328:1272-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, E. G., H. Liu, L. C. Kit, S. Baird, and M. Nesrallah. 2001. Pattern of mutation in the genome of influenza A virus on adaptation to increased virulence in the mouse lung: identification of functional themes. Proc. Natl. Acad. Sci. U. S. A. 98:6883-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bull, J. J., et al. 1997. Exceptional convergent evolution in a virus. Genetics 147:1497-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevillotte, M., et al. 2010. A new tool linking human cytomegalovirus drug resistance mutations to resistance phenotypes. Antiviral Res. 85:318-327. [DOI] [PubMed] [Google Scholar]
- 7.Domingo, E., and J. J. Holland. 1997. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 51:151-178. [DOI] [PubMed] [Google Scholar]
- 8.Drake, J. W., and J. J. Holland. 1999. Mutation rate among RNA viruses. Proc. Natl. Acad. Sci. U. S. A. 96:13910-13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggenberger, A. L., M. R. Hajimorad, and J. H. Hill. 2008. Gain of virulence on Rsv1-genotype soybean by an avirulent Soybean mosaic virus requires concurrent mutations in both P3 and HC-Pro. Mol. Plant Microbe Interact. 21:931-936. [DOI] [PubMed] [Google Scholar]
- 10.Gabriel, G., et al. 2005. The viral polymerase mediates adaptation of an avian influenza virus to a mammalian host. Proc. Natl. Acad. Sci. U. S. A. 102:18590-18595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajimorad, M. R., and J. H. Hill. 2001. Rsv1-mediated resistance against Soybean mosaic virus-N is hypersensitive response-independent at inoculation site, but has the potential to initiate a hypersensitive response-like mechanism. Mol. Plant Microbe Interact. 14:587-598. [DOI] [PubMed] [Google Scholar]
- 12.Hajimorad, M. R., A. L. Eggenberger, and J. H. Hill. 2003. Evolution of Soybean mosaic virus-G7 molecularly cloned genome in Rsv1-genotype soybean results in emergence of a mutant capable of evading Rsv1-mediated recognition. Virology 314:497-509. [DOI] [PubMed] [Google Scholar]
- 13.Hajimorad, M. R., A. L. Eggenberger, and J. H. Hill. 2005. Loss and gain of elicitor function of Soybean mosaic virus G7 provoking Rsv1-mediated lethal systemic hypersensitive response maps to P3. J. Virol. 79:1215-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajimorad, M. R., A. L. Eggenberger, and J. H. Hill. 2008. Adaptation of Soybean mosaic virus avirulent chimeras containing P3 sequences from virulent strains to Rsv1-genotype soybeans is mediated by mutations in HC-Pro. Mol. Plant Microbe Interact. 21:937-946. [DOI] [PubMed] [Google Scholar]
- 15.Hayes, A. J., et al. 2004. Recombination within a nucleotide-binding-site/leucine-rich-repeat gene cluster produces new variants conditioning resistance to soybean mosaic virus in soybeans. Genetics 166:493-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janzac, B., F. Fabre, A. Palloix, and B. Moury. 2009. Constraints on evolution of virus avirulence factors predict the durability of corresponding plant resistance. Mol. Plant Pathol. 10:599-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keleta, L., A. Ibricevic, N. V. Bovin, S. L. Brody, and E. G. Brown. 2008. Experimental evolution of human influenza virus H3 hemagglutinin in the mouse lung identifies adaptive regions in HA1 and HA2. J. Virol. 82:11599-11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi, M., et al. 2008. Selection of diverse and clinically relevant integrase inhibitor-resistant human immunodeficiency virus type 1 mutants. Antiviral Res. 80:213-222. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Picado, J., et al. 2000. Antiretroviral resistance during successful therapy of HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 97:10948-10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijhuis, M., et al. 1999. Increased fitness of drug resistant HIV-I protease as a result of acquisition of compensatory mutations during sub-optimal therapy. AIDS 13:2349-2359. [DOI] [PubMed] [Google Scholar]
- 21.Pinel-Galzi, A., et al. 2007. Theme and variations in the evolutionary pathways to virulence of an RNA plant virus species. PLoS Pathog. 3:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 23.Schneider, W. L., and M. J. Roossinck. 2001. Genetic diversity in RNA virus quasispecies is controlled by host-virus interactions. J. Virol. 75:6566-6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toews, M. W., et al. 2004. Establishment of mRFP1 as a fluorescent marker in Aspergillus nidulans and construction of expression vectors for high-throughput protein tagging using recombination in vitro (Gateway). Curr. Genet. 45:383-389. [DOI] [PubMed] [Google Scholar]
- 25.Traoré, O., et al. 2010. The adaptation of Rice yellow mottle virus to the elf(iso)4G-mediated rice resistance. Virology 408:103-108. [DOI] [PubMed] [Google Scholar]
- 26.Tumpey, T. M., et al. 2007. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 315:655-659. [DOI] [PubMed] [Google Scholar]
- 27.Urcuqui-Inchima, S., A.-L. Haenni, and F. Bernardi. 2001. Potyvirus proteins: a wealth of functions. Virus Res. 74:157-175. [DOI] [PubMed] [Google Scholar]
- 28.Wang, L., A. Eggenberger, J. Hill, and A. J. Bogdanove. 2006. Pseudomonas syringae effector avrB confers soybean cultivar-specific avirulence on Soybean mosaic virus adapted for transgene expression but effector avrPto does not. Mol. Plant Microbe Interact. 19:304-312. [DOI] [PubMed] [Google Scholar]
- 29.Wang, R. Y., and S. A. Ghabrial. 2002. Effect of aphid behavior on efficiency of transmission of Soybean mosaic virus by the soybean-colonizing aphid, Aphis glycines. Plant Dis. 86:1260-1264. [DOI] [PubMed] [Google Scholar]
- 30.Wen, R.-H., and M. R. Hajimorad. 2010. Mutational analysis of the putative pipo of soybean mosaic virus suggests disruption of PIPO protein impedes movement. Virology 400:1-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.