Abstract
Although a lot of progress has been made in development of lentiviral vectors for gene therapy, the interactions of these vectors with cellular factors have not been explored adequately. Here we show that lentivirus infection phosphorylates JNK and that blocking the kinase activity of JNK decreases gene transfer in a dose-dependent manner, regardless of the viral envelope glycoprotein. Knockdown by small interfering RNA (siRNA) revealed that JNK1 but not JNK2 was required for productive gene transfer. The effect of JNK on gene transfer was not due to changes in the cell cycle, as JNK knockdown did not affect the cell cycle profile of target cells and even increased cell proliferation. In addition, confluent cell monolayers also exhibited JNK phosphorylation upon lentivirus infection and a dose-dependent decrease in gene transfer efficiency upon JNK inhibition. On the other hand, JNK activation was necessary for lentivirus internalization into the cell cytoplasm, while inhibition of JNK activity decreased virus entry without affecting binding to the cell surface. These experiments suggest that JNK is required for lentivirus entry into target cells and may have implications for gene transfer or for development of antiviral agents.
The c-Jun N-terminal kinases (JNKs), also known as stress-activated protein kinases (7, 46), were originally identified by their ability to phosphorylate c-Jun and ATF2, which regulate the transcription factor activating protein 1 (AP-1) (13). Other studies showed that JNK signaling may be important in wound healing (21, 38, 39), apoptosis (17, 41, 47), cell survival (22), and tumor development (18). Accumulating evidence indicates that JNK has diverse functions. Several proteins, including paxillin (16), microtubule-associated proteins, e.g., MAP2 and DCX (15), and the actin-binding protein Spir (34), were identified as substrates of JNK. The role of JNK in regulating its own scaffolding protein (JIP) in the kinesin complex has also been reported for Drosophila (14), implicating JNK in cargo transport along microtubules. Recent evidence suggested that a pool of phosphorylated JNK is localized in the cell periphery (2), where it may affect lamellipodium extension (3) and cell migration by phosphorylating the focal adhesion protein paxillin (16). Recently, JNK was found to bind to and phosphorylate β-catenin in the adherens junction complex (23) and to regulate α-catenin-β-catenin interaction and adherens junction formation (24). Blocking the kinase activity of JNK was shown to promote adherens junction formation, reorganize actin into bundles underneath the junctions, and prevent colony dispersion of epithelial cells (23). These studies improved our understanding of the role of JNK in cell migration, cell adhesion, and regulation of protein trafficking and raised the possibility that JNK may be involved in virus infection.
Viruses have been known to utilize various cellular signaling pathways to achieve successful infection and replication (33). Several studies reported JNK activation by a number of viruses, including human immunodeficiency virus type 1 (HIV-1) (31), herpes simplex virus (30), influenza virus (26), adenovirus (27), varicella-zoster virus (48), and Kaposi's sarcoma-associated herpesvirus (35). The activity of JNK has been linked to virus replication mainly through the effect of AP-1 on transcriptional regulation of viral genes (26-28, 42). Other observations linked JNK inactivation with a reduction in the number of herpesvirus genome copies, suggesting that JNK may play a role at a step before the start of viral replication (9, 35). In the case of lentiviruses (LV), HIV proteins such as Vpr (45) and Tat (12, 20) were shown to activate the JNK-AP-1 pathway in order to promote infection.
In this study, we implicate JNK in gene transfer by a 3rd-generation self-inactivating lentivirus lacking the vif, vpr, vpu, nef, and tat accessory genes (8). Our results demonstrate that lentiviral infection phosphorylates JNK and that blocking JNK with chemical inhibitors or small interfering RNA (siRNA) decreases gene transfer significantly. In addition, we found that JNK activation occurred after virus entry into the cytosol, regardless of the entry route. Conversely, blocking JNK phosphorylation decreased virus entry and gene transfer significantly.
MATERIALS AND METHODS
Cell culture.
Primary keratinocytes were isolated from neonatal human foreskins as described before (4). They were cultured and passaged routinely in keratinocyte serum-free medium supplemented with epidermal growth factor (EGF) and bovine pituitary extract (Invitrogen, Carlsbad, CA). A431 and 293T cells were cultured and passaged routinely in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
Lentivirus production and transduction.
We employed a 3rd-generation lentiviral vector, pCSCG (Addgene Inc., Cambridge, MA), carrying the indicated gene under the control of the cytomegalovirus (CMV) promoter. The internal ribosome entry site (IRES) was placed immediately downstream of the gene of interest and allowed expression of enhanced green fluorescent protein (EGFP) from the same promoter. For production of the 3rd-generation lentivirus, 293T/17 cells (ATCC) were transiently cotransfected with four plasmids by using the standard calcium phosphate precipitation method (43). Briefly, when cells reached 70 to 80% confluence in a T-150 flask, they were transfected with a mixture of the following plasmids: 33.6 μg lentiviral vector, 16 μg pMDL-gag/pol, 9.6 μg pSRV-rev, and 4.8 μg pMDG-VSVG. For amphotropic enveloped lentivirus, pMDG-VSVG was replaced with 50 μg of the pCAE plasmid, encoding the envelope glycoprotein (gp70)of amphotropic-type murine leukemia retrovirus. For nonenveloped virus-like particles (VLPs), the envelope-encoding plasmid pMDG-VSVG was omitted from the mixture of plasmids used for transfection. At 22 to 24 h posttransfection, the medium was replaced with fresh medium, and lentivirus-containing medium was harvested 24 h later. The virus supernatant was filtered using a 0.45-μm filter (Millipore, Bedford, MA) to remove cell debris, concentrated by ultracentrifugation (50,000 × g at 4°C for 2 h), resuspended in keratinocyte serum-free medium (Invitrogen), and stored at −80°C until use.
For transduction, 1 × 105 cells were plated in 24-well plates. The next day, the cells were incubated with purified lentivirus containing 8 μg/ml Polybrene for the indicated times. The virus was removed and replaced with fresh medium. Gene transfer efficiency was measured by flow cytometry 2 days later and reported as the fraction of GFP+ cells.
shRNA constructs.
For gene silencing, a short hairpin RNA (shRNA) sequence targeting JNK1 (GGGCCTACAGAGAGCTAGTTCTTAT), JNK2 (GCCAACTGTGAGGAATTATGTCGAA), or JNK1/2 (AAAGAATGTCCTACCTTCT) was cloned into the lentiviral vector pLVTHM (Addgene Inc., Cambridge, MA). For pLVTHM virus production, a 2nd-generation packaging system was used whereby 293T cells were cotransfected with three plasmids: the pLVTHM vector, the packaging plasmid pSPAX2, and the envelope-encoding plasmid pMDG-VSVG. Lentivirus was harvested and processed as described above.
Cell cycle analysis.
To determine cell cycle distribution, subconfluent and confluent A431 cells were collected from 24-well plates. Following trypsinization, 70% ethanol fixation (−20°C, 1 h), and phosphate-buffered saline (PBS) washes, the cells were resuspended in 1 ml of PBS containing RNase (1 mg/ml) and propidium iodide (50 μg/ml; Molecular Probes, Eugene, OR) and then incubated for 20 min in the dark at room temperature before flow cytometry. The data were analyzed using ModFit LT analysis software (Verity Software House, Inc.).
Western blot analysis.
Western blotting was performed as described previously (23). The following antibodies were used, with incubation overnight at 4°C: rabbit-anti human phospho-JNK (1:1,000 in 5% bovine serum albumin [BSA]; Cell Signaling Technology, Danvers, MA), rabbit anti-human total JNK1/2 (1:1,000 in 2% milk; Cell Signaling Technology), mouse anti-human JNK1 (1:1,000 in 2% milk; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human JNK2 (1:1,000 in 2% milk; Cell Signaling Technology), rabbit anti-phospho-c-Jun (Ser63) (1:1,000 in 5% BSA; Cell Signaling Technology), mouse anti-p24 (1:1,000 in 5% milk; NIH AIDS Research and Reference Reagent Program), and mouse anti-β-actin (1:5,000 in 5% milk; Sigma, St. Louis, MO).
Immunofluorescence.
Immunofluorescence analysis was performed as described previously (23). Briefly, cells were seeded on cover glass in their respective media. The next day, the cells were treated with lentivirus for 30 min and then fixed with 4% paraformaldehyde for 10 min and immunostained with the following antibodies at 4°C overnight: rabbit anti-phospho-c-Jun (Ser63) (1:100; Cell Signaling Technology) or mouse anti-p24 (1:100; NIH AIDS Research and Reference Reagent Program). Actin was stained with phalloidin (1:40; Invitrogen, Eugene, OR) at room temperature for 20 min. Images were acquired using a Zeiss Axio Observer inverted fluorescence microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) or a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss MicroImaging, Inc.) equipped with an Orca-ER charge-coupled device (CCD) digital camera (Hamamatsu, Bridgewater, NJ). For quantification of the percentage of p-c-Jun-positive cells and the intensity of p24 viral protein, the images were analyzed by ImageJ (version 1.37; National Institutes of Health, Bethesda, MD).
Statistical analysis.
Statistical analysis of the data was performed using two-tailed Student's t test (α = 0.05) in Microsoft Excel (Microsoft, Redwood, CA). Each experiment was repeated two or three times each with triplicate samples, unless indicated otherwise.
RESULTS
Lentiviral transduction activates the JNK pathway.
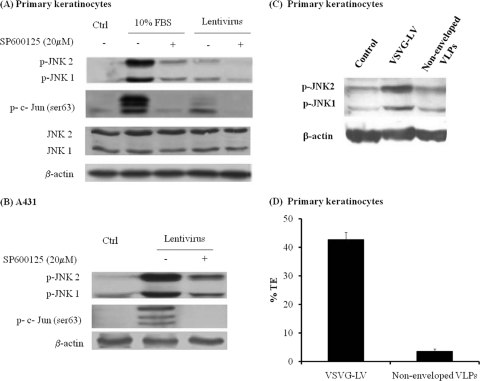
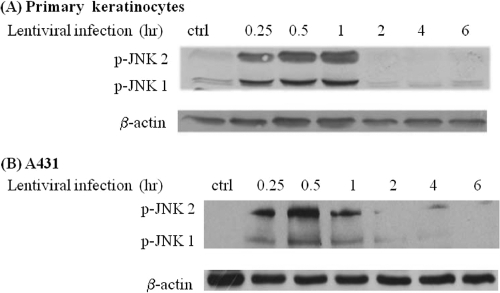
JNK is known to play a major role in coordinating cellular responses to stress. Since viral infection may be a cause of stress, we hypothesized that LV transduction may activate the JNK pathway. To test this hypothesis, human primary keratinocytes or epidermal carcinoma cells (A431) were exposed to recombinant LV for 30 min, and the levels of phospho-JNK and c-Jun were determined by Western blotting. Indeed, exposure to LV increased phosphorylation of JNK1, JNK2, and the downstream effector of JNK, c-Jun. In contrast, simultaneous treatment with the JNK inhibitor SP600125 decreased phosphorylation of both JNK1/2 and c-Jun (Fig. 1 A). The addition of serum (FBS), a known activator of the JNK pathway, was used as a positive control. Similar results were observed with A431 epidermoid carcinoma cells (Fig. 1B). In contrast, nonenveloped virus or VLPs failed to phosphorylate JNK (Fig. 1C) and exhibited significantly reduced transduction efficiencies (Fig. 1D), suggesting that JNK activation was the effect of an infectious lentivirus preparation and was not due to the presence of other factors that might be cocentrifuged with viral particles. Interestingly, phosphorylation of JNK was observed as early as 15 min after addition of the virus, remained at a high level until 1 h, and decreased to basal levels by 2 h of exposure to the virus (Fig. 2).
FIG. 1.
Lentiviral transduction induces phosphorylation of JNK and c-Jun. Human primary keratinocytes (A) or A431 cells (B) were mock infected (Ctrl) or infected with lentivirus in the presence of dimethyl sulfoxide (DMSO) alone or the JNK inhibitor SP600125 (20 μM) for 30 min. (C) Primary keratinocytes were infected with VSV-G-enveloped viruses and nonenveloped VLPs. Cell lysates were analyzed by Western blotting, using antibodies against phosphorylated JNK (p-JNK) and phosphorylated c-Jun (at Ser63) (p-c-Jun Ser63). Blots were stripped and reprobed with antibodies to β-actin as a loading control. Treatment of cells with serum (FBS) was used as a positive control because serum is known to stimulate JNK. (D) Primary keratinocytes were transduced with enveloped viruses and nonenveloped VLPs for 2 h, and transduction efficiency (% EGFP+ cells) (TE) was measured at 2 days postinfection by using flow cytometry.
FIG. 2.
Kinetics of JNK activation following lentiviral transduction. Human primary keratinocytes (A) or A431 cells (B) were mock infected (Ctrl) or infected with lentivirus for the indicated times. Cell lysates were probed for phosphorylated JNK by Western blotting. The membranes were stripped and reprobed with antibody to β-actin as a loading control.
Blocking JNK kinase activity reduces lentiviral gene transfer.
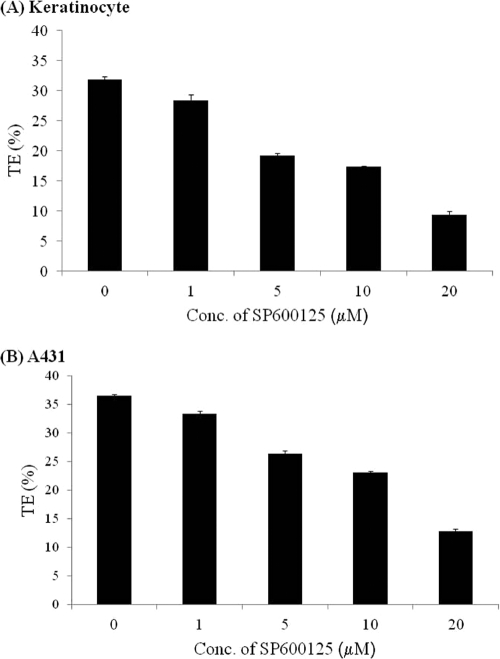
To examine whether JNK phosphorylation was required for productive lentiviral infection, we measured the efficiency of gene transfer in the presence of the JNK inhibitor SP600125. To this end, primary keratinocytes or A431 cells were pretreated with the indicated concentrations of SP600125 for 30 min prior to and during infection with a recombinant lentivirus encoding EGFP. After 30 min, the virus was removed, cells were incubated with fresh medium containing the same concentration of SP600125 for 24 h, and transduction efficiency was measured on day 2 postinfection, using flow cytometry. As shown in Fig. 3 A and B, SP600125 reduced lentiviral transduction of primary keratinocytes and A431 cells in a dose-dependent manner, reaching 30% of the control level at a concentration of 20 μM. SP600125 also inhibited lentiviral gene transfer to mouse 3T3 fibroblasts, suggesting that the effect of JNK was not limited to epithelial cells (see Fig. S1 in the supplemental material).
FIG. 3.
JNK inhibitor SP600125 reduces lentiviral transduction in a dose-dependent manner. Human primary keratinocytes (A) or A431 cells (B) were pretreated with the JNK inhibitor SP600125 at increasing concentrations (1, 5, 10, and 20 μM) or left untreated for 30 min and then transduced with EGFP-encoding lentivirus for 30 min. The cells were blocked with SP600125 during lentiviral infection and for 24 h thereafter. EGFP-positive cells were quantified at 2 days postinfection by flow cytometry.
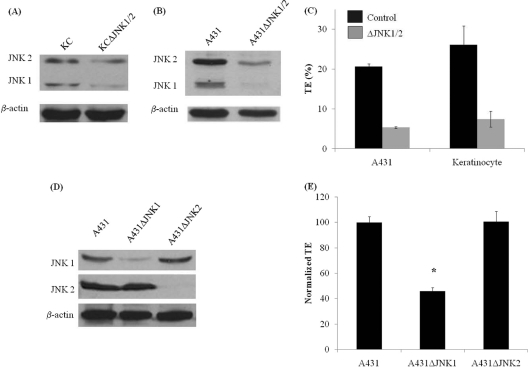
We also established primary keratinocytes and A431 cells lacking JNK1 and JNK2 (ΔJNK1/2) by using an shRNA-encoding lentivirus. Expression of siRNA significantly decreased the levels of both JNK1 and JNK2 (Fig. 4 A and B) and also significantly decreased lentiviral transduction efficiency (Fig. 4C), in agreement with the results of SP600125 treatment. To determine which JNK isoform affected lentiviral infection, we employed siRNA to knock down JNK1 or JNK2 in A431 cells (Fig. 4D). Interestingly, decreased transduction efficiency was observed only in A431ΔJNK1 cells, not in A431ΔJNK2 cells (Fig. 4E), implicating JNK1 in lentiviral infection.
FIG. 4.
Silencing of JNK decreases lentiviral transduction. Primary keratinocytes and A431 cells were transduced with a recombinant lentivirus encoding shRNAs targeting the JNK1 and JNK2 mRNAs. The virus also encoded EGFP, which was used to sort transduced cells, termed KCΔJNK1/2 or A431ΔJNK1/2 cells. Cell lysates of KCΔJNK1/2 (A) and A431ΔJNK1/2 (B) cells were probed for total JNK by Western blotting. (C) Control and ΔJNK1/2 cells were infected with a lentivirus encoding DsRed-Express2, and transduction efficiency (TE; % DsRed+ cells) was quantified at 2 days postinfection by flow cytometry. (D) JNK1 or JNK2 was silenced individually in A431 cells (A431ΔJNK1 and A431ΔJNK2 cells) by use of a lentivirus encoding shRNA and EGFP. Cell lysates of A431ΔJNK1 and A431ΔJNK2 cells were probed with JNK1- or JNK2-specific antibodies in Western blots. (E) Transduction efficiencies were measured in A431, A431ΔJNK1, and A431ΔJNK2 cells as described for panel C and then normalized to that of control A431 cells.
JNK affects lentiviral transduction independent of the cell cycle.
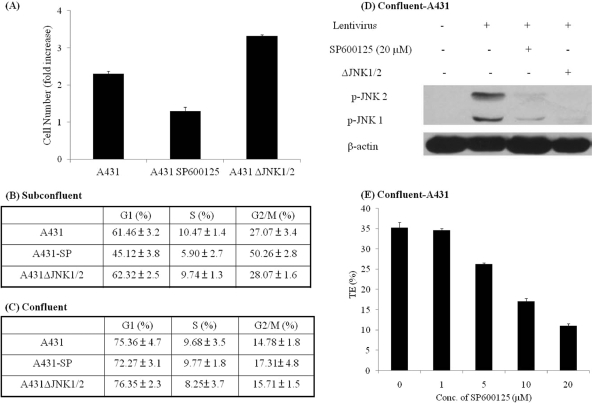
Since previous studies implicated SP600125 in reduced cell proliferation and cell cycle withdrawal (10), we examined whether reduced gene transfer upon JNK inhibition might be attributed to cell cycle effects. We found that exponentially growing A431 cells treated with SP600125 (20 μM) for 24 h exhibited reduced proliferation (Fig. 5 A), decreased accumulation of cells in the G1 and S phases of the cell cycle, and significantly increased accumulation in G2/M (Fig. 5B), indicating that SP600125 might arrest A431 cells in the G2/M phase of the cell cycle. In contrast, A431ΔJNK1/2 cells had a similar cell cycle profile to that of control A431 cells and showed increased proliferation (Fig. 5A and B), suggesting that the effect of JNK on lentiviral gene transfer was not mediated by growth arrest or changes in the cell cycle.
FIG. 5.
Effect of JNK on gene transfer is independent of the cell cycle. (A) Untreated A431 cells, SP600125-treated A431 cells (A431 SP600125), or A431ΔJNK1/2 cells were plated at 1 × 105 cells per cm2, and the number of cells was counted 3 days later and normalized to initial cell number. (B and C) Cells were seeded at a low cell density (1 × 105 cells per cm2), and the next day they were left untreated (A431JNK1/2 or A431) or treated with 20 μM SP600125 (A431-SP) for 24 h. The same cells were also seeded at a high cell density (2.5 × 105 cells per cm2) and formed a confluent monolayer the next day. The cells were treated with SP600125 (20 μM) on day 2 postconfluence. At the end of SP600125 treatment, subconfluent (B) or confluent (C) cells were stained with propidium iodide, and cell cycle analysis was done by flow cytometry. (D) Confluent A431 cells were mock infected or infected with lentivirus in the absence or presence of the JNK inhibitor SP600125 (20 μM) for 30 min. Cell lysates were analyzed by Western blotting, using antibodies against phosphorylated JNK (p-JNK). β-Actin served as a loading control. (E) Confluent A431 cells were pretreated with the JNK inhibitor SP600125 at increasing concentrations or left untreated for 30 min. SP600125 treatment continued during transduction with EGFP-encoding lentivirus (30 min) and for 24 h thereafter. EGFP+ cells were quantified on day 2 posttransduction, using flow cytometry.
In addition, we examined whether SP600125 treatment or JNK1/2 knockdown affected lentiviral gene transfer to confluent cell monolayers. As expected, confluence increased the fraction of G1-phase cells and decreased the fraction of G2/M-phase cells, but SP600125 treatment or JNK1/2 knockdown had no additional effect on the cell cycle distribution (Fig. 5C). Nevertheless, exposure to lentivirus induced JNK1/2 phosphorylation, which was significantly reduced by SP600125 or JNK1/2 siRNA (Fig. 5D). SP600125 also decreased gene transfer to confluent cell monolayers in a dose-dependent manner (Fig. 5E), suggesting that inhibition of JNK might decrease gene transfer through a mechanism that is independent of the cell cycle.
JNK is activated upon virus entry into the cytosol.
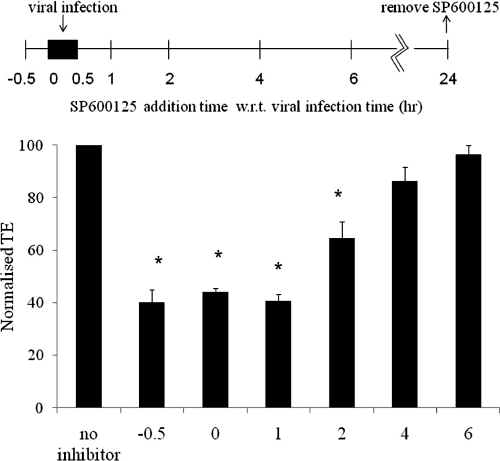
Next, we examined whether JNK was important during early or late steps of lentiviral transduction. To this end, A431 cells were infected for 30 min and SP600125 was added at the indicated times before, during, or after addition of the virus and then removed 24 h later. Interestingly, SP600125 reduced the efficiency of gene transfer only when it was added within 2 h after removal of the virus (Fig. 6). Treatment with SP600125 at later times did not affect the fraction of EGFP+ cells, suggesting that activation of JNK might be required during the early steps of lentiviral infection.
FIG. 6.
JNK affects an early step(s) of lentiviral transduction. A431 cells were transduced with EGFP-encoding lentivirus for 30 min, and SP600125 (20 μM) was added at different times, including 30 min before exposure to the virus (pretreatment; −0.5), at the time of infection (0 h), and at different times posttransduction, as indicated. SP600125 was removed at 24 h postinfection, and EGFP+ cells were measured 2 days later, using flow cytometry.
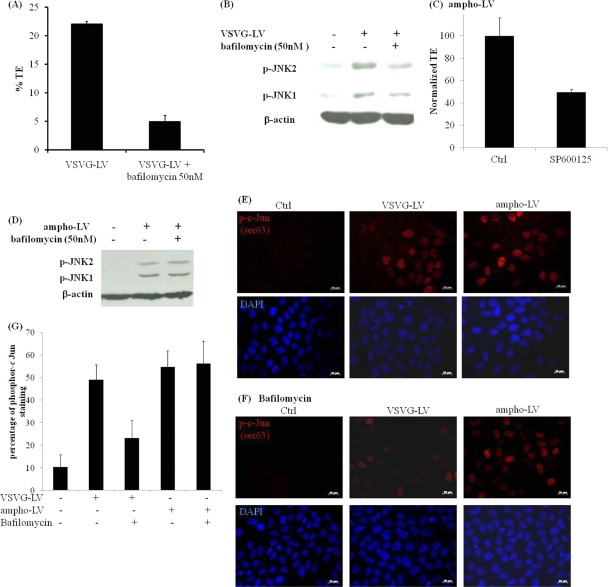
This result prompted us to examine whether JNK activation was induced by lentivirus binding to its cellular receptor or upon release of viral proteins into the cell cytoplasm. Since vesicular stomatitis virus glycoprotein-pseudotyped lentivirus (VSV-G-LV) is known to enter cells via the endocytic route (1), we studied the level of JNK upregulation upon inhibition of endosomal acidification by using bafilomycin A1 (6). Treatment with bafilomycin A1 (50 nM) successfully restricted endosomal release of lentivirus, resulting in a 4- to 5-fold reduction in transduction efficiency (Fig. 7 A). Most notably, bafilomycin A1 prevented JNK phosphorylation, suggesting that lentivirus release into the cytoplasm was required for JNK activation (Fig. 7B).
FIG. 7.
Activation of JNK requires virus entry into the cytosol but is not specific to the viral envelope. (A) Primary keratinocyte cells were pretreated with bafilomycin A1 (50 nM) for 30 min prior to transduction with EGFP-encoding, VSV-G-enveloped lentivirus in the presence of bafilomycin A1 (50 nM) for 2 h. Control cells were transduced for 2 h without bafilomycin A1 either prior to or during transduction. EGFP+ cells were measured 2 days later, using flow cytometry. (B) Primary keratinocyte cells were pretreated with bafilomycin A1 (50 nM) for 30 min prior to transduction with EGFP-encoding, VSV-G-enveloped lentivirus in the presence of bafilomycin A1 (50 nM) for 30 min. Control cells were transduced with the virus for 30 min without bafilomycin A1. Cell lysates were probed for phosphorylated JNK in Western blots. The membranes were stripped and reprobed with antibody to β-actin as a loading control. EGFP-encoding lentivirus was pseudotyped with VSV-G (VSVG-LV) or the amphotropic envelope of murine leukemia retrovirus (ampho-LV). (C) A431 cells were treated with SP600125 (20 μM) for 30 min prior to transduction with ampho-LV (6 h), and treatment was continued during and for 24 h after infection. The transduction efficiency was measured by flow cytometry 2 days later and normalized to that of untreated cells (Ctrl). (D) Same as in panel B, except that ampho-LV was used. (E) A431 cells were mock infected (Ctrl) or infected for 30 min with VSV-G-LV or ampho-LV and then fixed and stained for phospho-c-Jun (p-c-Jun Ser63). Cell nuclei were visualized with Hoechst stain (blue). (F) Same as in panel E, but cells were treated with bafilomycin A1 before (1 h) and during exposure to the virus (30 min). Magnification, ×40. Bar = 20 μm. (G) The percentage of p-c-Jun-positive cells under the indicated conditions was quantified by ImageJ software. The results are plotted as means ± standard errors for three independent experiments.
Furthermore, we investigated whether the effects of JNK on lentiviral transduction depended on the route of entry, that is, membrane fusion versus endocytosis. To this end, we employed a lentivirus that was pseudotyped with the amphotropic envelope (gp70) of murine leukemia retrovirus (ampho-LV), which follows a pH-independent membrane fusion entry mechanism (5, 25). Similar to the case with VSV-G-LV, SP600125 (20 μM) treatment decreased ampho-LV gene transfer >2-fold (P < 0.05; n = 6) (Fig. 7C). In addition, ampho-LV also induced JNK phosphorylation (Fig. 7D), suggesting that JNK activation occurred in a manner that was not specific to the viral envelope glycoprotein. In contrast to the case with VSV-G-LV, inhibition of endosomal acidification by bafilomycin A1 did not decrease JNK phosphorylation by the endocytosis-independent ampho-LV (Fig. 7D). This result was further verified by immunostaining for phospho-c-Jun, a downstream target of JNK. As shown in Fig. 7E, a similar fraction of target cells displayed c-Jun phosphorylation when cells were exposed to either VSV-G-LV or ampho-LV, but bafilomycin A1 decreased c-Jun phosphorylation only by VSV-G-LV, not by ampho-LV (Fig. 7F and G). Taken together, these results suggested that JNK may be activated after internalization of the virus into the cytosol, regardless of the entry route.
JNK inhibition decreases lentivirus internalization.
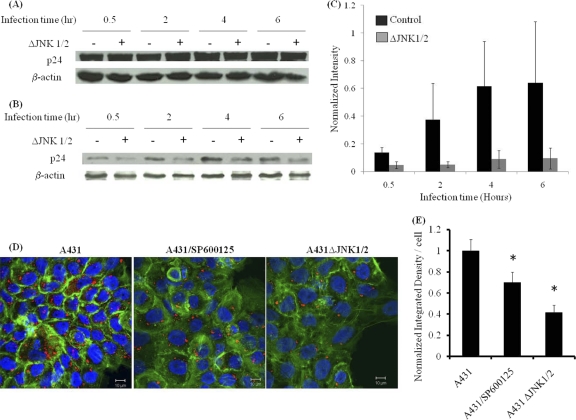
Next, we examined whether JNK activation is necessary for lentivirus entry into target cells. To investigate this hypothesis, we measured the amounts of internalized and total lentiviral particles (bound plus internalized) associated with target cells. To this end, A431 or A431ΔJNK1/2 cells were exposed to lentivirus at the indicated times, and cell-associated virus was measured in cell lysates by Western blotting for the viral matrix protein p24. To measure internalized virus, target cells were first trypsinized to remove surface-bound viral particles and then resuspended in lysis buffer. Interestingly, while the amounts of total cell-associated virus were similar between A431 and A431ΔJNK1/2 cells at all time points (Fig. 8 A), the amount of internalized virus was significantly reduced in A431ΔJNK1/2 cells (Fig. 8B and C). To ensure that this result was independent of Polybrene—a polycation that promotes virus binding by screening electrostatic interactions between viral particles and target cells—the experiment was repeated in the absence of Polybrene and yielded similar results (see Fig. S2 in the supplemental material). Additional evidence was provided by confocal microscopy of lentivirus-infected cells that were immunostained for p24 after 30 min of exposure to the virus (Fig. 8D). Measurements of fluorescence intensity from sequential half-micrometer confocal z stacks showed significantly decreased p24 signals in SP600125-treated and A431ΔJNK1/2 cells (Fig. 8E), indicating that inhibition of JNK decreased lentivirus entry significantly.
FIG. 8.
JNK is required for efficient lentivirus entry. A431 or A431ΔJNK1/2 cells were exposed to lentivirus for the indicated times, and the amounts of total cell-associated virus (A) and internalized virus (B) were quantified by Western blotting for the viral capsid protein p24. (C) The band intensities in panel B were determined using Kodak gel documentation software, and for each sample, the p24 intensity was normalized to that of the corresponding β-actin control. The data represent the means ± standard errors for three independent experiments. (D) Confocal images of A431 cells, SP600125-treated A431 cells, and A431ΔJNK1/2 cells stained with p24 (red) and actin phalloidin (green). The nuclei were visualized with Hoechst stain (blue). Magnification, ×63. Bar = 10 μm. (E) The intensity of p24 staining was quantified using ImageJ software. The data represent the means ± standard errors for three independent experiments.
DISCUSSION
We report that the JNK pathway is involved in lentivirus gene transfer. Specifically, we found that exposure to lentivirus phosphorylated JNK and that blocking JNK with a chemical inhibitor or siRNA decreased gene transfer significantly, indicating that activation of the JNK pathway is necessary for efficient lentiviral transduction. Cell cycle experiments with exponentially growing or confluent cells lacking JNK1/2 suggested that the effects of JNK were not mediated by changes in the cell cycle. Interestingly, while not necessary for virus binding to the cell surface, JNK activation was required for efficient lentivirus entry into target cells. These results shed light into the role of JNK in early steps of the lentivirus life cycle and may suggest strategies for antiretroviral therapies or for promoting gene transfer for gene therapy applications.
Notably, activation of JNK by lentivirus was sustained for only 1 h and decreased sharply by 2 h, despite continued exposure of cells to the virus. Interestingly, the kinetic profile of JNK phosphorylation by lentivirus was similar to that induced by tumor necrosis factor alpha (TNF-α), which was shown to suppress JNK signaling through NF-κB activation (36, 37). Several viruses, including HIV-1, influenza virus, hepatitis B and C viruses, and herpesvirus, have been shown to activate NF-κB (40), raising the possibility that lentivirus may also activate NF-κB, which in turn suppresses JNK to prevent apoptosis of infected cells. If this is true, it may be a cellular protective mechanism that evolved to prevent more viral particles from entering an infected cell.
Although lentivirus infection does not require cell division (32), the growth rate of target cells was shown to affect the transduction efficiency (11). Since the JNK inhibitor SP600125 changed the cell cycle profile and lowered the growth rate of target cells, we examined whether the effects of JNK inhibition on gene transfer were due to indirect effects on cell proliferation. Surprisingly, JNK knockdown by siRNA had no effect on the cell cycle and even increased target cell proliferation. In addition, JNK inhibition decreased gene transfer to confluent cell monolayers, where most cells were already arrested in G1 phase, and neither SP600125 nor JNK1/2 siRNA had additional effects on cell cycle distribution. Taken together, these experiments suggested that the effect of JNK on gene transfer was not due to indirect effects on the cell cycle. Furthermore, while siRNA knockdown of JNK1 decreased gene transfer, JNK2 knockdown had no effect, clearly implicating only the JNK1 isoform in lentivirus-cell interactions. Interestingly, JNK1 was reported to be localized in focal adhesions, where it promotes cell migration by phosphorylating paxillin (16), and more recently, JNK1 was shown to be part of the adherens junction complex, phosphorylate β-catenin (23), control α-catenin-β-catenin binding (24), and regulate cell-cell adhesion.
Our experiments showed that JNK activation was not specific to the viral envelope and that JNK inhibition decreased gene transfer with a lentivirus that was pseudotyped with either VSV-G or the amphotropic gp70 envelope. On the other hand, bafilomycin A1 treatment reduced JNK and c-Jun phosphorylation by VSV-G-LV, which enters cells by endocytosis (1), but not that by ampho-LV, which enters via pH-independent membrane fusion (5, 25), indicating that JNK might be activated by internalized virions, regardless of the entry route. Although several HIV proteins, such as Tat, Nef, and Vpr, have been shown to induce activation of JNK (12, 20, 45), none of these proteins are present in the 3rd-generation lentiviral vector, suggesting that another viral protein—perhaps the capsid protein p24 and/or the viral RNA—may be responsible for JNK activation.
An interesting question then arises: if JNK activation is triggered by entry of the viral nucleocapsid into the cytosol, how do the cell-bound or endosome-resident viruses initially promote JNK phosphorylation? An intriguing possibility may be that a “positive cooperativity” mechanism is at work, whereupon the first viral particles that enter the cytosol accelerate uptake of additional viruses through JNK activation. Such positive feedback generated by a small initial number of invading viruses may also explain why SP600125 or JNK siRNA significantly reduced but did not eliminate lentivirus entry and gene transfer. Regardless, the mechanism through which JNK activation may affect virus entry remains elusive. Previous studies have also shown that filopodium and lamellipodium extensions in the cell leading edge promote virus surfing to entry sites, where branched actin facilitates entry (19, 29). Coincidently, JNK was shown to phosphorylate the actin-binding protein Spir, which interacts with Wiskott-Aldrich syndrome protein (WASP) and actin-related protein 2/3 (Arp2/3) to promote actin polymerization and branching (44). Indeed, actin stress fiber formation was shown to require activation of JNK through the RhoA-Rock-MEKK1 pathway (49). Taking these data together with our findings, we propose that JNK1 phosphorylation by lentivirus may facilitate virus entry by promoting branched actin organization. This intriguing hypothesis warrants further investigation.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 EB000876) and the National Science Foundation (BES-0354626) to S.T.A.
Footnotes
Published ahead of print on 29 December 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida, E. A., et al. 2000. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J. Cell Biol. 149:741-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altan, Z. M., and G. Fenteany. 2004. c-Jun N-terminal kinase regulates lamellipodial protrusion and cell sheet migration during epithelial wound closure by a gene expression-independent mechanism. Biochem. Biophys. Res. Commun. 322:56-67. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj, B. G., P. Lei, and S. T. Andreadis. 2005. Efficient gene transfer to human epidermal keratinocytes on fibronectin: in vitro evidence for transduction of epidermal stem cells. Mol. Ther. 11:969-979. [DOI] [PubMed] [Google Scholar]
- 5.Barnett, A. L., and J. M. Cunningham. 2001. Receptor binding transforms the surface subunit of the mammalian C-type retrovirus envelope protein from an inhibitor to an activator of fusion. J. Virol. 75:9096-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayer, N., et al. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 8.Dull, T., et al. 1998. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72:8463-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, S. J., J. H. Deng, and F. C. Zhou. 2003. Productive lytic replication of a recombinant Kaposi's sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 77:9738-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazel, A., T. Banno, R. Walsh, and M. Blumenberg. 2006. Inhibition of JNK promotes differentiation of epidermal keratinocytes. J. Biol. Chem. 281:20530-20541. [DOI] [PubMed] [Google Scholar]
- 11.Groschel, B., and F. Bushman. 2005. Cell cycle arrest in G2/M promotes early steps of infection by human immunodeficiency virus. J. Virol. 79:5695-5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu, Y., R. F. Wu, Y. C. Xu, S. C. Flores, and L. S. Terada. 2001. HIV Tat activates c-Jun amino-terminal kinase through an oxidant-dependent mechanism. Virology 286:62-71. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, S., et al. 1996. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 15:2760-2770. [PMC free article] [PubMed] [Google Scholar]
- 14.Horiuchi, D., et al. 2007. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr. Biol. 17:1313-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, C., K. Jacobson, and M. D. Schaller. 2004. MAP kinases and cell migration. J. Cell Sci. 117:4619-4628. [DOI] [PubMed] [Google Scholar]
- 16.Huang, C., Z. Rajfur, C. Borchers, M. D. Schaller, and K. Jacobson. 2003. JNK phosphorylates paxillin and regulates cell migration. Nature 424:219-223. [DOI] [PubMed] [Google Scholar]
- 17.Iordanov, M. S., et al. 2000. Differential requirement for the stress-activated protein kinase/c-Jun NH(2)-terminal kinase in RNA damage-induced apoptosis in primary and in immortalized fibroblasts. Mol. Cell. Biol. Res. Commun. 4:122-128. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy, N. J., and R. J. Davis. 2003. Role of JNK in tumor development. Cell Cycle 2:199-201. [PubMed] [Google Scholar]
- 19.Komano, J., K. Miyauchi, Z. Matsuda, and N. Yamamoto. 2004. Inhibiting the Arp2/3 complex limits infection of both intracellular mature vaccinia virus and primate lentiviruses. Mol. Biol. Cell 15:5197-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar, A., S. K. Manna, S. Dhawan, and B. B. Aggarwal. 1998. HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J. Immunol. 161:776-781. [PubMed] [Google Scholar]
- 21.Kwon, Y. C., S. H. Baek, H. Lee, and K. M. Choe. 2010. Nonmuscle myosin II localization is regulated by JNK during Drosophila larval wound healing. Biochem. Biophys. Res. Commun. 393:656-661. [DOI] [PubMed] [Google Scholar]
- 22.Lamb, J. A., J. J. Ventura, P. Hess, R. A. Flavell, and R. J. Davis. 2003. JunD mediates survival signaling by the JNK signal transduction pathway. Mol. Cell 11:1479-1489. [DOI] [PubMed] [Google Scholar]
- 23.Lee, M. H., P. Koria, J. Qu, and S. T. Andreadis. 2009. JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB J. 23:3874-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, M. H., R. Padmashali, P. Koria, and S. T. Andreadis. 4 November 2010. JNK regulates binding of alpha-catenin to adherens junctions and cell-cell adhesion. FASEB J. [Epub ahead of print.] doi:. [DOI] [PMC free article] [PubMed]
- 25.Lee, S., Y. Zhao, and W. F. Anderson. 1999. Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated-pit-mediated endocytic pathway. J. Virol. 73:5994-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludwig, S., et al. 2001. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J. Biol. Chem. 276:10990-10998. [PubMed] [Google Scholar]
- 27.Maguire, K., et al. 1991. Interactions between adenovirus E1A and members of the AP-1 family of cellular transcription factors. Oncogene 6:1417-1422. [PubMed] [Google Scholar]
- 28.Marini, E., et al. 2008. HIV-1 matrix protein p17 binds to monocytes and selectively stimulates MCP-1 secretion: role of transcriptional factor AP-1. Cell. Microbiol. 10:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald, D., et al. 2002. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 159:441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLean, T. I., and S. L. Bachenheimer. 1999. Activation of c-Jun N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 73:8415-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthumani, K., et al. 2004. Suppression of HIV-1 viral replication and cellular pathogenesis by a novel p38/JNK kinase inhibitor. AIDS 18:739-748. [DOI] [PubMed] [Google Scholar]
- 32.Naldini, L., et al. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen, D. G., et al. 2007. Identification of novel therapeutic targets for HIV infection through functional genomic cDNA screening. Virology 362:16-25. [DOI] [PubMed] [Google Scholar]
- 34.Otto, I. M., et al. 2000. The p150-Spir protein provides a link between c-Jun N-terminal kinase function and actin reorganization. Curr. Biol. 10:345-348. [DOI] [PubMed] [Google Scholar]
- 35.Pan, H., J. Xie, F. Ye, and S. J. Gao. 2006. Modulation of Kaposi's sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J. Virol. 80:5371-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papa, S., et al. 2004. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat. Cell Biol. 6:146-153. [DOI] [PubMed] [Google Scholar]
- 37.Papa, S., F. Zazzeroni, C. G. Pham, C. Bubici, and G. Franzoso. 2004. Linking JNK signaling to NF-kappaB: a key to survival. J. Cell Sci. 117:5197-5208. [DOI] [PubMed] [Google Scholar]
- 38.Ramet, M., R. Lanot, D. Zachary, and P. Manfruelli. 2002. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol. 241:145-156. [DOI] [PubMed] [Google Scholar]
- 39.Riesgo-Escovar, J. R., M. Jenni, A. Fritz, and E. Hafen. 1996. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 10:2759-2768. [DOI] [PubMed] [Google Scholar]
- 40.Santoro, M. G., A. Rossi, and C. Amici. 2003. NF-kappaB and virus infection: who controls whom. EMBO J. 22:2552-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang, G., et al. 2001. Inhibition of JNK activation through NF-kappaB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 42.Tardif, M. R., and M. J. Tremblay. 2005. Tetraspanin CD81 provides a costimulatory signal resulting in increased human immunodeficiency virus type 1 gene expression in primary CD4+ T lymphocytes through NF-kappaB, NFAT, and AP-1 transduction pathways. J. Virol. 79:4316-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian, J., and S. T. Andreadis. 2009. Independent and high-level dual-gene expression in adult stem-progenitor cells from a single lentiviral vector. Gene Ther. 16:874-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uruno, T., J. Liu, Y. Li, N. Smith, and X. Zhan. 2003. Sequential interaction of actin-related proteins 2 and 3 (Arp2/3) complex with neural Wiscott-Aldrich syndrome protein (N-WASP) and cortactin during branched actin filament network formation. J. Biol. Chem. 278:26086-26093. [DOI] [PubMed] [Google Scholar]
- 45.Varin, A., et al. 2005. Synthetic Vpr protein activates activator protein-1, c-Jun N-terminal kinase, and NF-kappaB and stimulates HIV-1 transcription in promonocytic cells and primary macrophages. J. Biol. Chem. 280:42557-42567. [DOI] [PubMed] [Google Scholar]
- 46.Weston, C. R., and R. J. Davis. 2002. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12:14-21. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida, K., T. Yamaguchi, T. Natsume, D. Kufe, and Y. Miki. 2005. JNK phosphorylation of 14-3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nat. Cell Biol. 7:278-285. [DOI] [PubMed] [Google Scholar]
- 48.Zapata, H. J., M. Nakatsugawa, and J. F. Moffat. 2007. Varicella-zoster virus infection of human fibroblast cells activates the c-Jun N-terminal kinase pathway. J. Virol. 81:977-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, L., et al. 2005. MEKK1 transduces activin signals in keratinocytes to induce actin stress fiber formation and migration. Mol. Cell. Biol. 25:60-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.