Abstract
Infection with seasonal influenza viruses induces a certain extent of protective immunity against potentially pandemic viruses of novel subtypes, also known as heterosubtypic immunity. Here we demonstrate that infection with a recent influenza A/H3N2 virus strain induces robust protection in ferrets against infection with a highly pathogenic avian influenza virus of the H5N1 subtype. Prior H3N2 virus infection reduced H5N1 virus replication in the upper respiratory tract, as well as clinical signs, mortality, and histopathological changes associated with virus replication in the brain. This protective immunity correlated with the induction of T cells that cross-reacted with H5N1 viral antigen. We also demonstrated that prior vaccination against influenza A/H3N2 virus reduced the induction of heterosubtypic immunity otherwise induced by infection with the influenza A/H3N2 virus. The implications of these findings are discussed in the context of vaccination strategies and vaccine development aiming at the induction of immunity to pandemic influenza.
Since highly pathogenic influenza A viruses of the H5N1 subtype continue to circulate among domestic bird populations and occasionally cause fatal infections of humans, a pandemic outbreak with these viruses is still feared (47). To become pandemic, these viruses need to adapt to their new mammalian host and become transmissible from human to human. Apparently, the influenza A/H1N1(2009) viruses that originated in swine possessed these properties and were responsible for the first influenza pandemic in the 21st century.
Probably the most effective measure to reduce the impact of influenza pandemics is the development and use of vaccines. Several candidate vaccines have been developed against influenza A/H5N1 viruses, which in some cases contain adjuvants to make these vaccines more efficacious and to achieve dose sparing (2, 19, 24, 27). Vaccines were also developed against the influenza A/H1N1(2009) virus. Although these vaccines were efficacious (12, 20), they arrived late and after the peak of the pandemic (8), which precluded optimal profit from the protection these vaccines would have afforded. Therefore, there is interest in the development of more universal vaccines (13, 42) that could induce protective immunity to influenza A viruses of various subtypes, including influenza A/H5N1 virus. A full understanding of the heterosubtypic immunity that is induced by infection with influenza A viruses may help in developing such vaccines. Indeed, prior infection with an influenza A virus can reduce morbidity and mortality caused by infection with an influenza A virus of another subtype, including influenza A/H5N1 virus, as demonstrated with various animal models (15, 16, 21, 22, 39). Thus, individuals previously infected with seasonal influenza viruses may be less susceptible than immunogenically naïve subjects to developing severe disease after infection with (pandemic) viruses of a novel subtype (11). This might also explain, at least in part, the disproportionate age distribution of severe cases of infection with influenza A/H5N1 (41) and A/H1N1(2009) (23) viruses, with higher incidences in subjects of young age who had not yet developed robust heterosubtypic immunity after one or more infections with seasonal influenza viruses.
Elucidating the basis of heterosubtypic immunity has been the topic of numerous studies (for a review, see reference 15). It is generally accepted that arms of the immune system other than serum antibodies to hemagglutinin (HA) and neuraminidase contribute to heterosubtypic immunity, such as CD4+ and CD8+ T cells specific for conserved viral proteins, mucosal antibodies, and B cells (1, 15, 21, 25, 26).
So far, infection-induced heterosubtypic immunity to highly pathogenic influenza A/H5N1 viruses has been studied predominantly in mice (21, 28, 39). Since the pathogenesis of influenza virus infections of mice differs from that for humans and results obtained for mice are considered to be of limited predictive value for humans (7), we studied the induction of heterosubtypic immunity to H5N1 influenza viruses by using seasonal influenza virus in a ferret model, which resembles the human situation more closely.
In addition, we investigated if vaccination of ferrets against seasonal influenza A/H3N2 virus would interfere with the induction of heterosubtypic immunity to influenza A/H5N1 viruses otherwise induced by infection with influenza A/H3N2 virus, as was demonstrated recently for mice (3, 4).
To this end, ferrets were vaccinated with a subunit vaccine based on a recent influenza A/H3N2 virus vaccine strain or mock vaccinated and subsequently were inoculated (or not) with a matching influenza A/H3N2 virus strain. Four weeks after inoculation with the influenza A/H3N2 virus strain, all ferrets were infected with the highly pathogenic H5N1 strain A/Indonesia/5/2005 (A/Ind/5/05) to assess the presence of heterosubtypic immunity and the effect that H3N2 vaccination had on the development of this immunity. It was shown that vaccination against A/H3N2 virus infection affected the induction of heterosubtypic immunity to H5N1 influenza virus. This may have implications for the universal recommendation to vaccinate all healthy children in some countries and highlights the need for the development of broadly protective vaccines.
MATERIALS AND METHODS
Influenza A viruses.
Influenza A/Ind/5/05 virus (H5N1; clade 2.1) was propagated in confluent MDCK cells. After cytopathic changes were complete, culture supernatants were harvested, cleared by low-speed centrifugation, and stored at −80°C. The influenza A/Brisbane/010/2007 (H3N2) virus was propagated in the allantoic cavities of 11-day-old embryonated chicken eggs. Allantoic fluid was harvested at 2 days postinoculation (dpi), cleared by low-speed centrifugation, and stored at −80°C. Virus titers were determined in MDCK cells as described previously (32). All experiments with the influenza A/H5N1 virus were performed under biosafety level 3 (BSL-3) conditions.
Vaccine preparation.
Preparation and purity testing of egg-grown influenza A virus subunit antigen (SU) derived from influenza A/Uruquay/716/2007 (NYMC X-175-C; H3N2) virus were performed essentially as described previously (3, 9). Influenza virus strain NYMC X-175-C was the H3N2 vaccine strain for influenza seasons 2008 to 2009 and 2009 to 2010 and is closely related to the vaccine reference strain for these seasons, influenza A/Brisbane/010/07 (H3N2) virus. The viral nucleoprotein and matrix protein could not be detected in the vaccine preparation by SDS-PAGE and Coomassie brilliant blue staining, while small traces were detected on a Western blot and on a silver-stained SDS-PAGE gel (see Fig. S1 in the supplemental material).
Ferrets.
Healthy young adult outbred female ferrets (Mustela putorius furo; between 6 and 12 months of age) were purchased from a commercial breeder. Ferrets were tested for the presence of serum antibodies against recent influenza A/H1N1 and A/H3N2 viruses and for the presence of antibodies against influenza A/Indonesia/5/2005 (H5N1) virus by hemagglutination inhibition (HI) assay. Ferrets were also tested for the presence of antibodies against Aleutian disease virus, and when the absence of these antibodies was confirmed, ferrets were randomly assigned to one of four experimental groups (see below). During the experiment, ferrets were housed in groups and received food and water ad libitum. An independent animal ethics committee (DEC Consult) approved the experimental protocol before the start of the experiments.
Immunization and infection of ferrets.
Forty ferrets were divided into four groups of 10 animals each. Ferrets of groups 2 and 3 were immunized twice with 15 μg influenza virus subunit vaccine in combination with Titermax Gold adjuvant (Sigma-Aldrich, St. Louis, MO), with an interval of 4 weeks, while ferrets of groups 1 and 4 were mock vaccinated with phosphate-buffered saline (PBS). The experimental groups are listed in Table 1. All animals were tested simultaneously in a single experiment. Vaccinations were performed in the quadriceps muscles of the left hind leg in a total volume of 0.25 ml under anesthesia with ketamine. Four weeks after the second immunization, ferrets of groups 1 and 2 were inoculated intranasally with 1 × 106 50% tissue culture infective doses (TCID50) of influenza A/Brisbane/010/2007 (H3N2) virus in a total volume of 0.5 ml PBS, while ferrets of groups 3 and 4 were inoculated with PBS. Four weeks after inoculation with the influenza A/H3N2 virus, two ferrets of each group were euthanized and their lungs, nasal cavities, and tracheas taken out for histopathological evaluation, while the eight remaining ferrets of each group were inoculated intranasally with 5 × 106 TCID50 influenza A/Ind/5/05 virus. All inoculations were performed under anesthesia with ketamine-medetomidine (reversed with atipamezole). During infection experiments, ferrets were checked daily for the presence of clinical signs. Before infection and at 2, 4, and 7 dpi for the influenza A/H3N2 virus and before infection and at 2, 4, 6, and 7 dpi for the H5N1 influenza virus, ferrets were weighed and nasal and pharyngeal swabs were collected while the animals were anesthetized with ketamine. Seven days after inoculation with influenza A/H5N1 virus, or earlier when ferrets became moribund, animals were weighed and subsequently killed by exsanguination while under anesthesia with ketamine and medetomidine. Necropsies were performed according to standard procedures, and samples of the olfactory bulb, cerebrum, lungs (all lobes of the right lung and the accessory lobe), spleen, and duodenum were collected for determination of virus titers and evaluation of histopathological changes.
TABLE 1.
Experimental groupsc
| Group | Vaccinationa | Primary infectionb |
|---|---|---|
| 1 | Mock | A/H3N2 virus |
| 2 | H3N2 vaccine | A/H3N2 virus |
| 3 | H3N2 vaccine | Mock |
| 4 | Mock | Mock |
The H3N2 vaccine was a subunit vaccine derived from influenza A/Uruquay/716/2007 (NYMC X-175-C; H3N2) virus adjuvanted with Titermax Gold adjuvant.
A/H3N2 virus, influenza A/Brisbane/010/07 (H3N2) virus.
All groups were challenged with influenza A/Indonesia/5/05 (H5N1) virus.
Serology.
Serum samples were collected before and 28 days after the first and second vaccinations and 28 days after infection with influenza A/Brisbane/010/07 (H3N2) virus. Sera were stored at −20°C until use. Sera were tested for the presence of antibodies against influenza X-175-C (H3N2), A/Brisbane/010/07 (H3N2), and A/Indonesia/5/05 (H5N1) viruses by using an HI assay performed with four hemagglutinating units of virus and 1% turkey erythrocytes and a micro-virus neutralization assay (VN assay) performed with 100 TCID50 of the respective virus as described previously (29, 33).
Preparation of viral antigens for T-cell proliferation assay.
Influenza A viruses A/Brisbane/010/07 (H3N2) and A/Ind/5/2005 (H5N1) were propagated in MDCK cells and then purified and concentrated by isopycnic density centrifugation. Subsequently, virus was inactivated by dialysis against PBS containing 0.1% formaldehyde for 4 days with continuous stirring at room temperature. After inactivation, antigen was dialyzed against PBS. The purity of the antigens was tested by SDS-PAGE, and inactivation was confirmed by failure of passage on MDCK cells. The protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce).
T-cell proliferation assay.
Blood samples were collected from the jugular veins of ferrets 28 days after the second vaccination and 28 days after infection with influenza A/H3N2 virus, and isolation of peripheral blood mononuclear cells (PBMC) and the T-cell proliferation assay were performed as described previously (6). In brief, cryopreserved PBMC were thawed, washed, and labeled with 0.3 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) for 5 min in PBS (Invitrogen, Breda, Netherlands). After being washed, the cells were resuspended in RPMI 1640 (Cambrex) containing 10% (vol/vol) fetal calf serum, penicillin (100 μg/ml), streptomycin (100 U/ml), and l-glutamine (2 mM), seeded (5 × 105 cells per well) in a 96-well round-bottom plate in the presence or absence of 50 ng of inactivated influenza A/Brisbane/010/07 or A/Indonesia/5/2005 virus antigen, and incubated at 37°C and 5% CO2. After 2 days, the supernatant of concanavalin A-stimulated ferret lymph node cells was added (36). For each condition, duplicate samples were tested. After four more days, cells were incubated with a monoclonal antibody directed to human CD8 (OKT-8)-eFluor 450 (eBioscience, San Diego, CA) and with Live/Dead Aqua fixable dead cell stain (Invitrogen, Breda, Netherlands). Cells were subsequently fixed and permeabilized with Cytofix and Cytoperm (BD Pharmingen, Alphen a/d Rijn, Netherlands) and then incubated with an Alexa Fluor 647-labeled monoclonal antibody specific for human CD3 (PC3/188A) (Santa Cruz Biotechnology, Santa Cruz, CA). Fluorescence of cells was assessed by flow cytometry, using a FACSCanto-II flow cytometer, and analyzed with FACS Diva software (BD). The specific proliferation of CD3+ CD8− cells for each antigen was calculated by subtracting the mean number of CD3+ CD8− CFSElow cells for the medium controls from the mean number of CD3+ CD8− CFSElow cells stimulated with antigen derived from A/Brisbane/010/07 (H3N2) or A/Ind/5/05 (H5N1) virus. The same calculation was performed for CD3+ CD8+ cells.
Virus titers in tissue and in nasal and pharyngeal swabs.
Tissue samples from ferrets were collected, snap-frozen on dry ice with ethanol, and stored at −70°C until further processing. Tissue samples were weighed, homogenized with a FastPrep-24 homogenizer (MP Biomedicals, Eindhoven, Netherlands) in Hanks' balanced salt solution containing 0.5% lactalbumin, 10% glycerol, 200 U/ml penicillin, 200 μg/ml streptomycin, 100 U/ml polymyxin B sulfate, 250 μg/ml gentamicin, and 50 U/ml nystatin (ICN Pharmaceuticals, Zoetermeer, Netherlands), and centrifuged briefly.
After collection of nasal and pharyngeal swabs, swabs were stored at −70°C in the same medium as that used to homogenize lung samples. Quadruplicate 10-fold serial dilutions of swab and tissue samples were used to infect MDCK cells as described previously (32). HA activity of the culture supernatants collected at 5 days postinfection was used as an indicator of infection. The titers were calculated according to the Spearman-Karber method and are expressed as log10 TCID50 per gram (for tissue) or per milliliter (for swabs) (18).
Histopathology and immunohistochemistry.
Tissues from ferrets euthanized 28 days after inoculation with influenza A/Brisbane/010/07 (H3N2) virus and 7 days after inoculation with influenza A/Ind/5/05 (H5N1) virus were examined macroscopically for the presence of lesions, and the lungs (after inflation with 10% neutral buffered formalin), liver, brain, spleen, and duodenum were fixed with 10% neutral buffered formalin. After being fixated and embedded in paraffin, tissues were sectioned at 4 μm, and tissue sections were examined by staining with hematoxylin and eosin (HE). Using an immunoperoxidase method, serial lung tissue sections were also stained with a monoclonal antibody directed against the nucleoprotein of the influenza A virus for the detection of virus-infected cells in the respective tissues (35).
Statistical analysis.
The presence of statistically significant differences in virus titers in nasal and pharyngeal swabs between groups after inoculation with influenza A/Brisbane/010/07 (H3N2) virus was assessed using the Mann-Whitney U test, while differences in the numbers of CD3+ CD8− CFSElow cells, differences in virus titers in nasal and pharyngeal swabs, and differences in weight loss after challenge with influenza A/H5N1 virus were calculated using the Games-Howell test. Differences were considered significant when P values were <0.05.
RESULTS
Antibody responses after vaccination.
Twenty-eight days after the first vaccination with the Titermax-adjuvanted X-175-C subunit preparation, 11 of 20 ferrets of groups 2 and 3 had developed HA-specific antibodies against influenza A/Brisbane/010/07 (H3N2) virus, as measured by HI assay (geometric mean titer [GMT], 28), while no antibodies were detected in mock-vaccinated ferrets of groups 1 and 4 (Fig. 1 A). Twenty-eight days after the second vaccination, virus-specific HI antibodies were detected in all ferrets of groups 2 and 3 (GMT, 192). In the virus neutralization assay, antibodies against influenza A/Brisbane/010/07 (H3N2) virus were detected in 12 of 20 ferrets of groups 2 and 3 (GMT, 15) 28 days after the first vaccination and in 16 of 20 ferrets of groups 2 and 3 (GMT, 52) 28 days after the second vaccination (data not shown). Of all ferrets tested, only a small number of ferrets showed a discrepancy in the presence of antibodies as measured in the VN and HI assays (VN− HI+), which most likely was caused by the low antibody levels in these ferrets. Four weeks after inoculation with influenza A/Brisbane/010/07 virus, antibodies against influenza A/Brisbane/010/07 (H3N2) virus only were detected in all vaccinated and/or infected animals. In addition, no antibodies against influenza A/Ind/5/05 virus were detected in any of the sera of the animals in the HI and VN assays (data not shown).
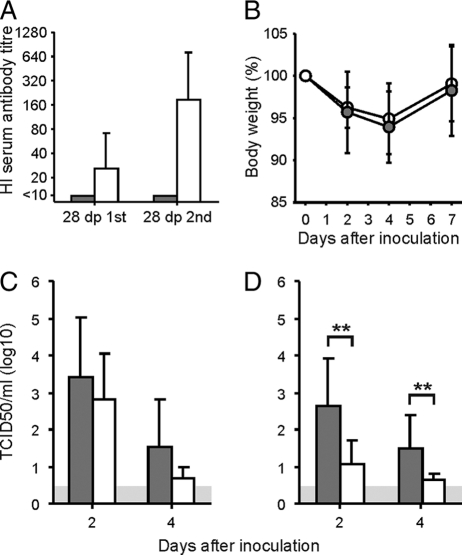
FIG. 1.
Antibody responses after vaccination and outcomes of infection with influenza A/H3N2 virus. (A) Mean virus-specific antibody responses ± standard deviations (SD), as measured with the hemagglutination inhibition assay 28 days after the first vaccination and 28 days after the second vaccination for unvaccinated animals (shaded bars) and vaccinated animals (open bars). (B) Mean weight loss of vaccinated (open circles) and unvaccinated (shaded circles) animals after inoculation with influenza A/H3N2 virus. (C and D) Virus titers of nasal (C) and pharyngeal (D) swabs collected at 2 and 4 dpi with influenza A/H3N2 virus. Shaded bars represent mean results ± SD for unvaccinated animals, and open bars represent mean results ± SD for vaccinated animals. **, P < 0.01 compared with unvaccinated animals. The gray area indicates the detection limit of the assay.
Outcome of inoculation with influenza A/H3N2 virus.
Following inoculation with influenza A/Brisbane/010/07 (H3N2) virus, unvaccinated ferrets of group 1 developed mild to moderate clinical signs, including sneezing, decreased appetite, and weight loss (mean percentage of weight loss at day 4, 6%). The ferrets of group 2 displayed similar weight losses (mean percentage of weight loss at day 4, 5%) (Fig. 1B), although their clinical signs were less severe than those observed in group 1. Observed clinical signs are listed in Table S1 in the supplemental material. The observed differences in clinical signs correlated with differences in virus titers in pharyngeal swabs collected 2 and 4 days after inoculation of ferrets of both groups. Mean virus titers in pharyngeal swabs collected at 2 and 4 dpi from unvaccinated ferrets of group 1 were 102.6 and 101.5 TCID50/ml, respectively, while significantly lower (P < 0.01 for both time points) virus titers were detected in pharyngeal swabs collected at 2 and 4 dpi from vaccinated ferrets of group 2 (101.1 and 100.6 TCID50/ml) (Fig. 1D). The mean virus titer in the nasal swabs of unvaccinated ferrets of group 1 was 103.4 TCID50/ml at 2 dpi and 101.5 TCID50/ml at 4 dpi, while somewhat lower mean virus titers were detected in nasal swabs collected from vaccinated ferrets of group 2 (102.8 and 100.7 TCID50/ml at 2 and 4 dpi, respectively), although these differences were not statistically significant (P = 0.32 and P = 0.14 at 2 and 4 dpi, respectively) (Fig. 1C). No virus was detected in nasal and pharyngeal swabs collected from H3N2 virus-inoculated ferrets at 7 dpi and in nasal and pharyngeal swabs collected from mock-infected ferrets of groups 3 and 4 (data not shown). In addition, at 28 dpi, no histopathological differences were observed in the brains and nasal and lung tissues of ferrets of each group (data not shown).
Virus-specific T-cell responses after inoculation with influenza A/H3N2 virus.
T-cell responses against both influenza A/Brisbane/010/07 (H3N2) and A/Indonesia/5/05 (H5N1) virus antigens were assessed using PBMC collected from ferrets of all groups 28 days after inoculation with influenza A/H3N2 virus or PBS. Influenza A/H3N2 virus antigen-specific proliferation of CD3+ CD8− cells was observed in only 1 of 10 mock-vaccinated and mock-infected ferrets of group 4, while influenza A/H3N2 virus antigen-specific proliferation was observed in all ferrets of groups 1 and 3 and in 9 of 10 ferrets of group 2 (although only small numbers of CFSElow cells were observed in 4 ferrets of group 3). In Fig. 2 A, the number of proliferating cells of each individual ferret and the mean number of proliferating cells of each group are indicated. Differences in influenza A/H3N2 virus antigen-specific proliferation of CD3+ CD8− cells were statistically significant between ferrets of groups 1 and 2 and ferrets of group 4 (P values of 0.01 and 0.02, respectively), while differences between groups 3 and 4 approached statistical significance (P = 0.05). No significant differences were observed between other groups. Furthermore, similar antigen-specific proliferation of CD3+ CD8− cells was observed 28 days and 56 days after the second vaccination for mock-infected, vaccinated ferrets of group 3 (data not shown). In addition, influenza A/H5N1 virus antigen-specific proliferation of CD3+ CD8− cells was observed in 9 of 10 ferrets of groups 1 and 2, while H5N1 viral antigen-specific proliferation was virtually absent in ferrets of group 3 and group 4. The number of proliferating cells of each individual ferret and the mean number of proliferating cells of each group are indicated in Fig. 2B. However, only differences between groups 2 and 4 were statistically significant (P = 0.02).
FIG. 2.
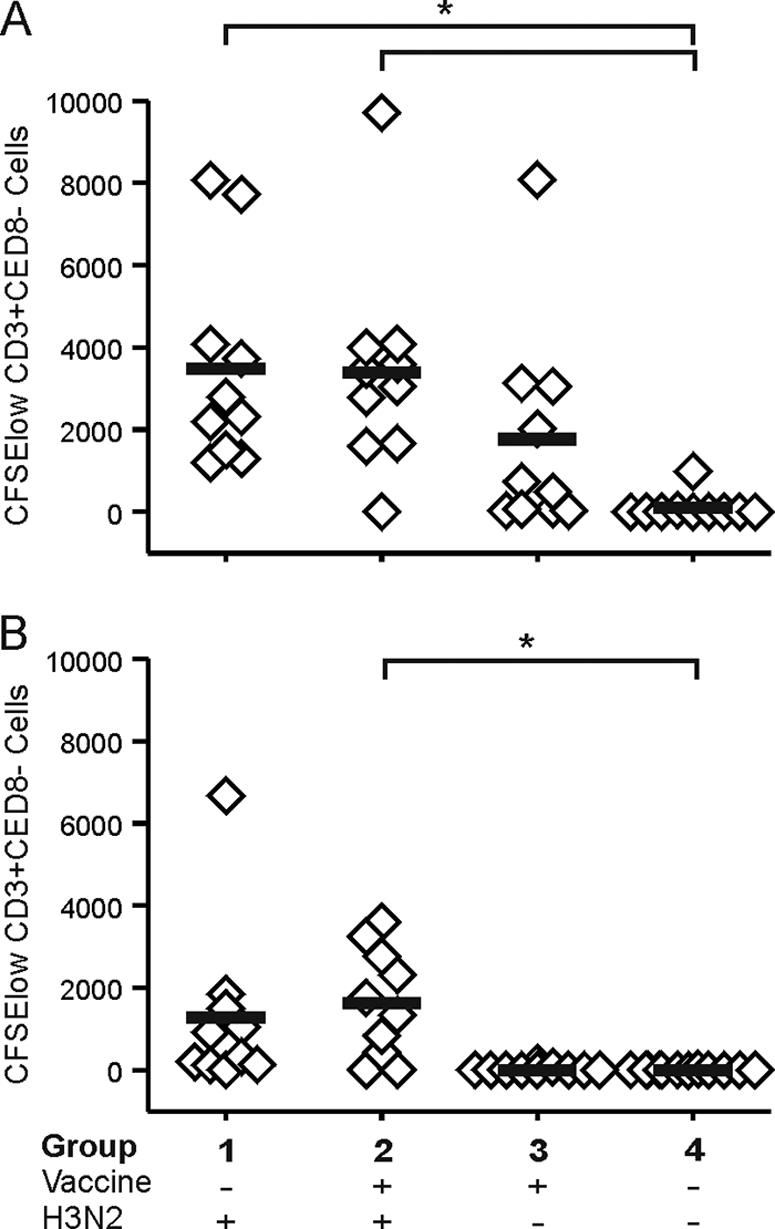
T-cell immune responses 28 days after infection with influenza A/H3N2 virus. Proliferation of CD3+ CD8− cells was measured upon stimulation with whole inactivated influenza A/Brisbane/010/07 (H3N2) virus antigen (A) and upon stimulation with whole inactivated influenza A/Indonesia/5/2005 (H5N1) virus antigen (B). *, P < 0.05 compared with unvaccinated, mock-infected animals.
Only very small amounts of viral antigen-specific proliferation of CD3+ CD8+ cells were observed in ferrets of all groups, and no significant differences were observed between groups (data not shown).
Outcome of inoculation with influenza A/H5N1 virus.
Twenty-eight days after inoculation with the A/H3N2 virus, ferrets were inoculated with influenza A/Ind/5/05 (H5N1) virus. Clinical signs were observed in ferrets from day 1 after inoculation onwards and included anorexia, weight loss, labored breathing, neurological disorders, and diarrhea. In Fig. 3, the percentages of weight loss of all individual ferrets are indicated, and in Table S1 in the supplemental material, observed clinical signs are listed per group. Severe clinical signs, including weight loss, were observed in all mock-vaccinated and mock-infected ferrets of group 4 and in the H3N2 virus-vaccinated and mock-infected ferrets of group 3. In contrast, only mild to moderate clinical signs were observed in the ferrets of group 1, who had experienced an H3N2 virus infection. These animals hardly lost body weight upon infection with influenza A/Ind/5/05 virus and developed less severe clinical signs. Prior vaccination against H3N2 virus infection partially prevented the protective effect of H3N2 virus infection, and 3 of 8 animals of group 2 developed severe clinical signs and displayed >15% loss of body weight. In total, 6 ferrets of groups 2, 3, and 4 died or had to be euthanized according to animal welfare regulations before all ferrets were euthanized at 7 dpi. No significant differences in weight loss were observed at 2 dpi between groups, while at 4 dpi, significantly more weight loss was observed in ferrets of group 4 than in those of groups 1 and 2 (P values of 0.001 and 0.01, respectively). In addition, on days 6 and 7, significantly more weight loss was observed in ferrets of group 4 than in those of group 1 (P values of 0.009 and 0.006, respectively). Differences in body weight loss on these days postinoculation between groups 2 and 4 were not statistically significant (P values of 0.07 and 0.08, respectively).
FIG. 3.
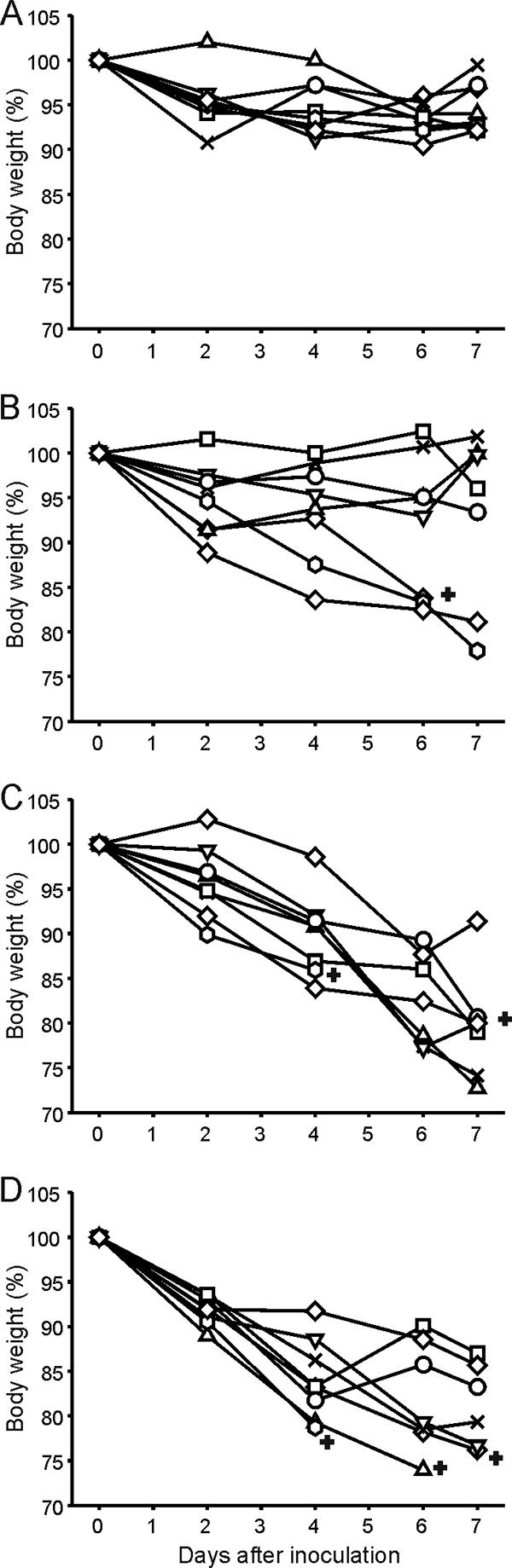
Weight loss after inoculation with influenza A/Indonesia/5/05 virus. After inoculation with influenza A/H5N1 virus, ferrets were weighed, and the relative weight loss compared to the body weight at the day of inoculation was calculated. The data shown are relative weight losses for all individual ferrets for unvaccinated, H3N2 virus-primed animals of group 1 (A), vaccinated, H3N2 virus-primed animals of group 2 (B), vaccinated, unprimed animals of group 3 (C), and unvaccinated and unprimed animals of group 4 (D). +, ferrets that died or had to be euthanized due to the presence of severe clinical symptoms.
Virus replication in the upper respiratory tract after inoculation with influenza A/H5N1 virus.
Nasal and pharyngeal swabs collected at 2, 4, 6, and 7 dpi with influenza A/Ind/5/05 virus were tested for the presence of infectious virus. Except for two ferrets of group 1, virus was detected in all nasal swabs collected at 2 and 4 dpi, with mean virus titers of 102.7, 104.3, 105.1, and 104.5 TCID50/ml at 2 dpi and 101.6, 103.6, 104.3, and 104.7 TCID50/ml at 4 dpi for groups 1, 2, 3, and 4, respectively. The average virus titer in nasal swabs for mock-vaccinated, H3N2 virus-infected ferrets of group 1 was significantly lower (P = 0.04) than that for mock-infected ferrets of group 4 at 2 dpi. At 4 dpi, differences between virus titers of group 1 were significantly lower than those for groups 2, 3, and 4 (P values of <0.01 for all comparisons). At 6 dpi, virus was undetectable in nasal swabs collected from ferrets of group 2 and in those from 7 of 8 ferrets of group 1. In contrast, virus was detected in all except one of the nasal swabs of ferrets of groups 3 and 4, which thus had significantly higher titers than those for groups 1 and 2 (P < 0.01 for both groups). No significant differences in virus titers were observed in nasal swabs collected at 7 dpi. In Fig. 4 A, the mean virus titers in nasal swabs collected from ferrets of each group are indicated.
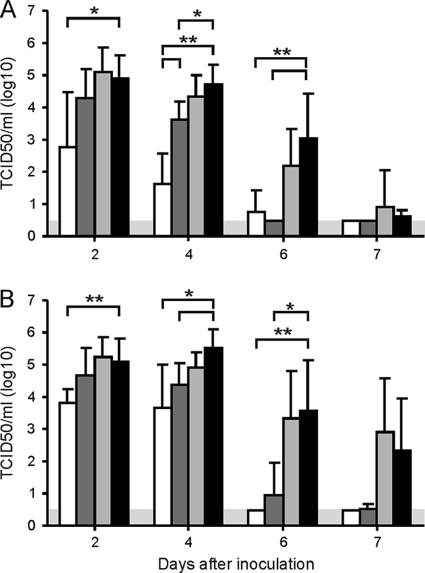
FIG. 4.
Virus titers in nasal and pharyngeal swabs collected after inoculation with influenza A/H5N1 virus. Nasal (A) and pharyngeal (B) swabs were collected at 2, 4, 6, and 7 dpi with influenza A/H5N1 virus, and virus titers were determined. The data shown are mean virus titers ± SD for unvaccinated, H3N2 virus-infected ferrets of group 1 (white bars), vaccinated, H3N2 virus-infected ferrets of group 2 (dark shaded bars), vaccinated, mock-infected ferrets of group 3 (light shaded bars), and unvaccinated, mock-infected ferrets of group 4 (black bars). *, P < 0.05 between groups; **, P < 0.01 between groups. The gray area indicates the detection limit of the assay.
Similar results were obtained for virus titers in the pharyngeal swabs. Virus was detected in all pharyngeal swabs collected from H5N1 virus-infected ferrets, with mean virus titers of 103.8, 104.7, 105.2, and 105.1 TCID50/ml at 2 dpi and 103.7, 104.4, 104.9, and 105.5 TCID50/ml at 4 dpi for ferrets of groups 1, 2, 3, and 4, respectively. Differences between virus titers in pharyngeal swabs collected at 2 dpi from ferrets of groups 1 and 4 were statistically significant (P < 0.01), while differences between ferrets of groups 1 and 4 (P = 0.01) and 2 and 4 (P = 0.02) were also statistically significant. At 6 and 7 dpi, no virus was detected in ferrets of group 1 and in two and one, respectively, of the ferrets of group 2, while virus was detected in all ferrets except one and two, respectively, for groups 3 and 4. Thus, significantly lower virus titers were detected in groups 1 (P = 0.004) and 2 (P = 0.01) than in group 4, while differences between these groups were not significant at 7 dpi. In Fig. 4B, the mean virus titers in pharyngeal swabs collected from ferrets of each group are indicated.
Pathology and immunohistochemistry after A/H5N1 virus infection.
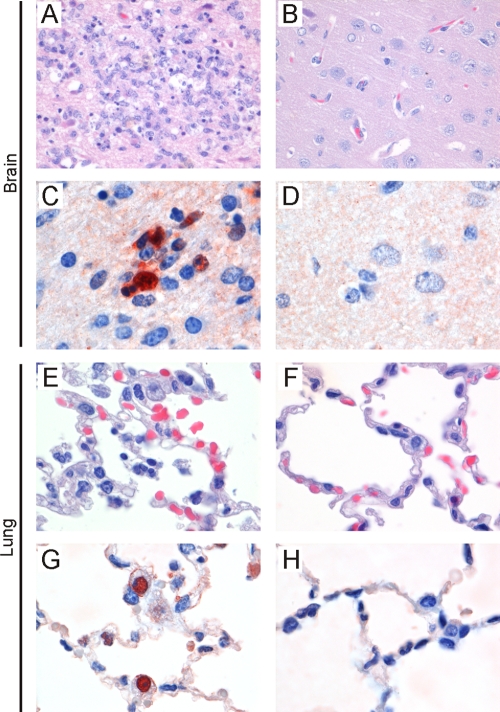
Macroscopically, only focally extensive pulmonary lesions were observed in a number of lungs of ferrets of groups 2, 3, and 4, while no lesions were observed in lungs of ferrets of group 1. Brains, lungs, spleens, livers, and duodenums were tested for the presence of histopathological changes caused by infection with influenza A/H5N1 virus (A/Ind/5/05). For three of eight ferrets of group 2 and all ferrets of groups 3 and 4, moderate to severe lesions were observed in the brain (Fig. 5 A), which colocalized with the abundant expression of influenza virus antigen (Fig. 5C). In contrast, in the brains of ferrets of group 1 and five ferrets of group 2, no or only mild lesions were observed (Fig. 5B), and influenza virus expression was absent or scarce (Fig. 5D).
FIG. 5.
Examples of histologic findings in tissues of ferrets inoculated with influenza A/H5N1 virus. Seven days after inoculation, histopathologic changes associated with the expression of influenza virus antigen were observed in brains and lungs of ferrets without heterosubtypic immunity, i.e., in three ferrets of group 2 (vaccinated, H3N2 virus-infected group) and in all ferrets of groups 3 (vaccinated, mock-infected group) and 4 (mock-vaccinated, mock-infected group) (A, C, E, and G). In the cerebrum, there is necrosis of neurons and infiltration with inflammatory cells (A) associated with expression of influenza virus antigen in neurons (C). (E) In the lungs, cell debris and erythrocytes are present in the alveolar lumens and neutrophils are present in the alveolar walls. (G) This is associated with the expression of influenza virus antigen in type II pneumocytes. Neither histopathological changes (B and F) nor influenza virus expression (D and H) were present in lungs and brains of five ferrets of group 2 (vaccinated, H3N2 virus-infected group) and all ferrets of group 1 (mock-vaccinated, H3N2 virus-infected group).
In lungs of some ferrets of groups 2, 3, and 4, a mild to moderate broncho-interstitial pneumonia was observed (Fig. 5E), which colocalized with expression of viral antigen (Fig. 5G). These lesions were not observed in the lungs of ferrets of group 1 (Fig. 5F), and no influenza virus antigen expression was detected (Fig. 5H).
In livers of a number of ferrets of group 2 and all ferrets of groups 3 and 4, there was a diffuse vacuolization of the hepatocellular cytoplasm, consistent with fat, which was not observed in livers of ferrets of group 1 and a number of ferrets of group 2 with heterosubtypic immunity against influenza A/H5N1 virus. These changes were considered to be hepatic lipidosis resulting from anorexia. No lesions were observed in spleens and duodenums of ferrets of all groups.
Presence of virus in tissues 7 days after inoculation.
For all ferrets on which necropsy was performed at 7 dpi, olfactory bulbs, brains, lungs, spleens, and duodenums were tested for the presence of infectious virus by virus isolation in MDCK cells. Virus was undetectable in spleens of all ferrets and in all tissues of ferrets of group 1. For all other groups, infectious virus was detected in tissues of one or more ferrets. In general, low virus titers were detected. In addition to the virus titrations, the presence of virus-infected cells in organs of ferrets collected at 7 dpi was tested by immunohistochemistry. For the lungs, spleens, and duodenums, the results obtained with immunohistochemistry correlated with those for virus isolation on MDCK cells. However, by immunohistochemistry, virus was detected in brains of almost all ferrets of groups 2, 3, and 4, while virus was not detected in brains of animals of group 1 (Table 2).
TABLE 2.
Influenza A/Indonesia/5/05 (H5N1) virus detection in tissues by virus isolation and immunohistochemistry at 7 dpi
| Group | Treatment |
No. of positive animals/total no. of animalsa |
||||||
|---|---|---|---|---|---|---|---|---|
| Brain |
Lung |
Duodenum |
||||||
| H3N2 vaccination | H3N2 infection | Virus isolation | IHC | Virus isolation | IHC | Virus isolation | IHC | |
| 1 | − | + | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| 2 | + | + | 0/7 | 2/7 | 0/7 | 1/7 | 0/7 | 0/7 |
| 3 | + | − | 3/7 | 7/7 | 3/7 | 4/7 | 2/7 | 0/7 |
| 4 | − | − | 0/6 | 5/6 | 1/6 | 2/6 | 0/6 | 0/6 |
IHC, immunohistochemistry. The number of tested tissues was smaller than 8 in some groups due to mortality before day 7.
DISCUSSION
In the present study, we demonstrated that infection with a seasonal influenza A/H3N2 virus induced robust heterosubtypic immunity against influenza A/H5N1 virus infection in ferrets. Prior infection with influenza A/Brisbane/010/07 (H3N2) virus reduced replication of influenza A/Ind/5/05 (H5N1) virus in the upper respiratory tract and the brain and prevented clinical signs and histopathological changes associated with infection.
The induction of heterosubtypic immunity by infection with influenza A viruses was demonstrated more than 40 years ago in animal models, and there is evidence for the presence of heterosubtypic immunity in humans (11, 15). In addition, it was demonstrated recently that infection with a seasonal influenza virus afforded protection against the pandemic influenza A/H1N1(2009) virus in the ferret model (10, 22). However, the immunological basis of heterosubtypic immunity has not been elucidated completely yet, and mucosal immunity, B cells, and both CD4+ and CD8+ T cells may contribute to this type of immunity (15). In concordance with our previous findings in the mouse model, we were unable to detect antibodies that were cross-reactive with the H5N1 virus strain after infection with influenza A/H3N2 virus by HI and virus neutralization assays, indicating that serum antibodies did not play a major role in the protection against influenza A/H5N1 virus. In contrast, infection with an influenza A/H3N2 virus induced strong T-cell responses that cross-reacted with influenza A/H5N1 viral antigen. To our knowledge, this is the first time that the presence of cross-reactive T-cell responses has been demonstrated in the ferret model. The cross-reactive in vitro proliferative response of CD3+ CD8− cells, most likely CD4+ T helper cells, obtained from A/H3N2 virus-infected ferrets correlated to some extent with the presence of heterosubtypic immunity. However, the three ferrets that displayed weight loss after infection with influenza A/H5N1 virus also had T-cell responses against the H5N1 viral antigen, indicating that other arms of the immune system also contribute to the development of heterosubtypic immunity. The serological data, the virological data after H3N2 virus infection, the virus-specific proliferation of CD3+ CD8− cells, and the weight loss after inoculation with influenza A/H5N1 virus are listed for all individual ferrets in Table S2 in the supplemental material. In addition, we were unable to assess CD8+ T-cell responses because the use of inactivated viral antigens probably precluded proper antigen processing and presentation to CD8+ T cells. Thus, we cannot exclude or confirm that virus-specific CD8+ T lymphocytes contributed to the heterosubtypic immunity observed after infection with the A/H3N2 influenza virus. Furthermore, the trace amounts of nucleoprotein and matrix protein present in the subunit vaccine used in this study could have induced immunodominant CD8+ T-cell responses that skewed subsequent CD8+ T-cell responses after infection with the influenza A/H3N2 virus, as observed previously in a mouse model for a whole inactivated influenza A/H3N2 virus vaccine (4).
In contrast to our findings in the mouse model (3), none of the ferrets euthanized 28 days after influenza A/H3N2 virus inoculation developed inducible bronchus-associated lymphoid tissue (iBALT) or other histopathological changes. The infection of ferrets with influenza A/H3N2 virus was probably restricted to the upper respiratory tract, while infection of mice also involves the lower respiratory tract.
In the present study, we also demonstrated that effective vaccination against infection with the seasonal influenza A/Brisbane/010/07 (H3N2) virus hampered the induction of heterosubtypic immunity. The vaccinated ferrets suffered more from the subsequent infection with the highly pathogenic H5N1 influenza virus A/Ind/5/05 than did their unvaccinated counterparts. These findings in the ferret model are in concordance with those we obtained recently with the mouse model (3-5).
Since subunit preparations are poorly immunogenic in mice (3) and ferrets (10), we used an adjuvant (Titermax) to increase the immunogenicity of the A/H3N2 virus subunit vaccine. Although the use of adjuvanted subunit preparations induced potent antibody responses to the influenza A/H3N2 virus, it failed to induce heterosubtypic immunity and did not afford any protection against infection with the influenza A virus A/Ind/5/05 (H5N1), as expected. Thus, the use of a potent adjuvant did not induce T-cell responses against the trace amounts of NP and M1 present in the subunit H3N2 virus preparation that were strong enough to protect against the influenza A/H5N1 virus infection. Likewise, adjuvants have been used to increase the immunogenicity of seasonal and pandemic influenza A/H1N1(2009) virus vaccines (44, 45). Furthermore, the vaccine used in this study contained only influenza A/H3N2 virus antigen, while the currently used seasonal vaccine also contains influenza A/H1N1 virus and influenza B virus antigens. The addition of the influenza A/H1N1 virus antigen especially might have changed the results, as it has been described for mice that immunization with a seasonal influenza A/H1N1 virus vaccine induces cross-reactive antibodies against the neuraminidase of the influenza A/H5N1 virus (37). In the present study, the ferrets were inoculated with influenza A/Ind/5/05 (H5N1) virus by the intranasal route. Although unprotected ferrets developed severe clinical signs, most of them did not develop severe pneumonia, typically seen after intratracheal inoculation (43), that could account for the loss of body weight.
In contrast, these animals developed a moderate to severe meningoencephalitis, sometimes with a fatal outcome, before the end of the experiment on day 7 postinoculation, which was chosen for ethical reasons and to allow a meaningful comparison of the experimental groups at the same time point. The occurrence of meningoencephalitis also explains the neurological signs that we and others have observed after intranasal inoculation of ferrets with highly pathogenic avian influenza viruses of the H5N1 subtype (14, 48). The occurrence of severe encephalitis in the absence of severe pneumonia suggests that the brains of these ferrets were infected directly from the nasal cavity, as has been shown for influenza A/H5N1 virus infection in mice (30). The possible occurrence of this phenomenon will be explored further. Prior infection with a seasonal influenza virus of the H3N2 subtype prevented dissemination of the H5N1 virus and the clinical signs associated with virus replication in the brain, severe weight loss, and the development of moderate to severe meningoencephalitis. This protective effect correlated with a reduction of H5N1 virus replication in the upper respiratory tract and was prevented by vaccination against H3N2 virus infection in three of eight ferrets (38%). This scenario resembles observations made in Canada, where subjects vaccinated against seasonal influenza in previous seasons were more likely to develop severe disease caused by infection with pandemic influenza A/H1N1(2009) viruses (17, 40). However, the lack of heterosubtypic immunity in these patients was not confirmed.
Annual vaccination against influenza is beneficial, especially for patients with underlying disease and for the elderly, who are at high risk for developing complications caused by influenza virus infection. In some countries, annual vaccination is also recommended for all healthy children >6 months of age. Although annual vaccination can reduce the burden of disease caused by seasonal influenza, in the long term it may affect the development of heterosubtypic immunity in this age group. Our findings emphasize the need for vaccines that can induce more broadly protective immune responses. In this respect, the induction of cross-reactive virus-specific CD8+ T cells against conserved viral proteins such as NP and M1 holds promise (31, 34). Also, the induction of antibody responses to the M2 protein and the conserved stalk region of the HA molecule may be an interesting avenue for the development of more universal vaccines (38, 46).
Supplementary Material
Acknowledgments
We thank Peter van Run and the animal caretakers of the Dutch Vaccine Institute for excellent technical assistance.
This work was supported in part by TI Pharma grant T4-214.
Footnotes
Published ahead of print on 12 January 2011.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Benton, K. A., et al. 2001. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J. Immunol. 166:7437-7445. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein, D. I., et al. 2008. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J. Infect. Dis. 197:667-675. [DOI] [PubMed] [Google Scholar]
- 3.Bodewes, R., et al. 2009. Vaccination against human influenza A/H3N2 virus prevents the induction of heterosubtypic immunity against lethal infection with avian influenza A/H5N1 virus. PLoS One 4:e5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodewes, R., et al. 2010. Vaccination with whole inactivated virus vaccine affects the induction of heterosubtypic immunity against influenza virus A/H5N1 and immunodominance of virus-specific CD8+ T-cell responses in mice. J. Gen. Virol. 91:1743-1753. [DOI] [PubMed] [Google Scholar]
- 5.Bodewes, R., J. H. Kreijtz, and G. F. Rimmelzwaan. 2009. Yearly influenza vaccinations: a double-edged sword? Lancet Infect. Dis. 9:784-788. [DOI] [PubMed] [Google Scholar]
- 6.Bodewes, R., et al. 2010. A single immunization with CoVaccine HT-adjuvanted H5N1 influenza virus vaccine induces protective cellular and humoral immune responses in ferrets. J. Virol. 84:7943-7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodewes, R., G. F. Rimmelzwaan, and A. D. Osterhaus. 2010. Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Rev. Vaccines 9:59-72. [DOI] [PubMed] [Google Scholar]
- 8.Butler, D. 2010. Portrait of a year-old pandemic. Nature 464:1112-1113. [DOI] [PubMed] [Google Scholar]
- 9.de Wit, E., et al. 2005. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J. Virol. 79:12401-12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellebedy, A. H., et al. 15 September 2010. Impact of prior seasonal influenza vaccination and infection on pandemic A(H1N1) influenza virus replication in ferrets. Vaccine [Epub ahead of print.] doi: 10.1016/j.vaccine.2010.08.067. [DOI] [PMC free article] [PubMed]
- 11.Epstein, S. L. 2006. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J. Infect. Dis. 193:49-53. [DOI] [PubMed] [Google Scholar]
- 12.Girard, M. P., J. Katz, Y. Pervikov, L. Palkonyay, and M. P. Kieny. 2010. Report of the 6th meeting on the evaluation of pandemic influenza vaccines in clinical trials, World Health Organization, Geneva, Switzerland, 17-18 February 2010. Vaccine 28:6811-6820. [DOI] [PubMed] [Google Scholar]
- 13.Girard, M. P., A. Osterhaus, Y. Pervikov, L. Palkonyay, and M. P. Kieny. 2008. Report of the third meeting on “influenza vaccines that induce broad spectrum and long-lasting immune responses,” World Health Organization, Geneva, Switzerland, 3-4 December 2007. Vaccine 26:2443-2450. [DOI] [PubMed] [Google Scholar]
- 14.Govorkova, E. A., et al. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 79:2191-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grebe, K. M., J. W. Yewdell, and J. R. Bennink. 2008. Heterosubtypic immunity to influenza A virus: where do we stand? Microbes Infect. 10:1024-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinen, P. P., E. A. de Boer-Luijtze, and A. T. Bianchi. 2001. Respiratory and systemic humoral and cellular immune responses of pigs to a heterosubtypic influenza A virus infection. J. Gen. Virol. 82:2697-2707. [DOI] [PubMed] [Google Scholar]
- 17.Janjua, N. Z., et al. 2010. Seasonal influenza vaccine and increased risk of pandemic A/H1N1-related illness: first detection of the association in British Columbia, Canada. Clin. Infect. Dis. 51:1017-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karber, G. 1931. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 19.Keitel, W. A., et al. 2008. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I-II randomized clinical trial. J. Infect. Dis. 198:1309-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly, H., and I. Barr. 2010. Large trials confirm immunogenicity of H1N1 vaccines. Lancet 375:6-9. [DOI] [PubMed] [Google Scholar]
- 21.Kreijtz, J. H., et al. 2009. Infection of mice with a human influenza A/H3N2 virus induces protective immunity against lethal infection with influenza A/H5N1 virus. Vaccine 27:4983-4989. [DOI] [PubMed] [Google Scholar]
- 22.Laurie, K. L., et al. 2010. Multiple infections with seasonal influenza A virus induce cross-protective immunity against A(H1N1) pandemic influenza virus in a ferret model. J. Infect. Dis. 202:1011-1020. [DOI] [PubMed] [Google Scholar]
- 23.Lemaitre, M., and F. Carrat. 2010. Comparative age distribution of influenza morbidity and mortality during seasonal influenza epidemics and the 2009 H1N1 pandemic. BMC Infect. Dis. 10:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leroux-Roels, I., et al. 2007. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 370:580-589. [DOI] [PubMed] [Google Scholar]
- 25.Liang, S., K. Mozdzanowska, G. Palladino, and W. Gerhard. 1994. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 152:1653-1661. [PubMed] [Google Scholar]
- 26.Nguyen, H. H., F. W. van Ginkel, H. L. Vu, J. R. McGhee, and J. Mestecky. 2001. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J. Infect. Dis. 183:368-376. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson, K. G., et al. 2001. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357:1937-1943. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill, E., S. L. Krauss, J. M. Riberdy, R. G. Webster, and D. L. Woodland. 2000. Heterologous protection against lethal A/HongKong/156/97 (H5N1) influenza virus infection in C57BL/6 mice. J. Gen. Virol. 81:2689-2696. [DOI] [PubMed] [Google Scholar]
- 29.Palmer, D., W. Dowle, M. Coleman, and G. Schild. 1975. Haemagglutination inhibition test, p. 25-62. In Advances in laboratory techniques for influenza diagnosis: procedural guide. U.S. Department of Health, Education and Welfare, Atlanta, GA.
- 30.Park, C. H., et al. 2002. The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch. Virol. 147:1425-1436. [DOI] [PubMed] [Google Scholar]
- 31.Price, G. E., et al. 2010. Single-dose mucosal immunization with a candidate universal influenza vaccine provides rapid protection from virulent H5N1, H3N2 and H1N1 viruses. PLoS One 5:e13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimmelzwaan, G. F., M. Baars, E. C. Claas, and A. D. Osterhaus. 1998. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J. Virol. Methods 74:57-66. [DOI] [PubMed] [Google Scholar]
- 33.Rimmelzwaan, G. F., et al. 1999. Influenza virus subtype cross-reactivities of haemagglutination inhibiting and virus neutralising serum antibodies induced by infection or vaccination with an ISCOM-based vaccine. Vaccine 17:2512-2516. [DOI] [PubMed] [Google Scholar]
- 34.Rimmelzwaan, G. F., R. A. Fouchier, and A. D. Osterhaus. 2007. Influenza virus-specific cytotoxic T lymphocytes: a correlate of protection and a basis for vaccine development. Curr. Opin. Biotechnol. 18:529-536. [DOI] [PubMed] [Google Scholar]
- 35.Rimmelzwaan, G. F., et al. 2001. Pathogenesis of influenza A (H5N1) virus infection in a primate model. J. Virol. 75:6687-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimmelzwaan, G. F., et al. 1990. Establishment and characterization of canine parvovirus-specific murine CD4+ T cell clones and their use for the delineation of T cell epitopes. J. Gen. Virol. 71:1095-1102. [DOI] [PubMed] [Google Scholar]
- 37.Sandbulte, M. R., et al. 2007. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 4:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schotsaert, M., M. De Filette, W. Fiers, and X. Saelens. 2009. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert Rev. Vaccines 8:499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seo, S. H., and R. G. Webster. 2001. Cross-reactive, cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. J. Virol. 75:2516-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skowronski, D. M., et al. 2010. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: four observational studies from Canada. PLoS Med. 7:e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smallman-Raynor, M., and A. D. Cliff. 2007. Avian influenza A (H5N1) age distribution in humans. Emerg. Infect. Dis. 13:510-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stohr, K. 2010. Vaccinate before the next pandemic? Nature 465:161. [DOI] [PubMed] [Google Scholar]
- 43.van den Brand, J. M., et al. 2010. Severity of pneumonia due to new H1N1 influenza virus in ferrets is intermediate between that due to seasonal H1N1 virus and highly pathogenic avian influenza H5N1 virus. J. Infect. Dis. 201:993-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vesikari, T., N. Groth, A. Karvonen, A. Borkowski, and M. Pellegrini. 2009. MF59-adjuvanted influenza vaccine (FLUAD) in children: safety and immunogenicity following a second year seasonal vaccination. Vaccine 27:6291-6295. [DOI] [PubMed] [Google Scholar]
- 45.Waddington, C. S., et al. 2010. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ 340:c2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, T. T., et al. 2010. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc. Natl. Acad. Sci. U. S. A. 107:18979-18984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization (WHO). 9 December 2010, posting date. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. WHO, Geneva, Switzerland. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2010_12_09/en/index.html.
- 48.Zitzow, L. A., et al. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.