Abstract
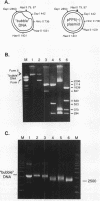
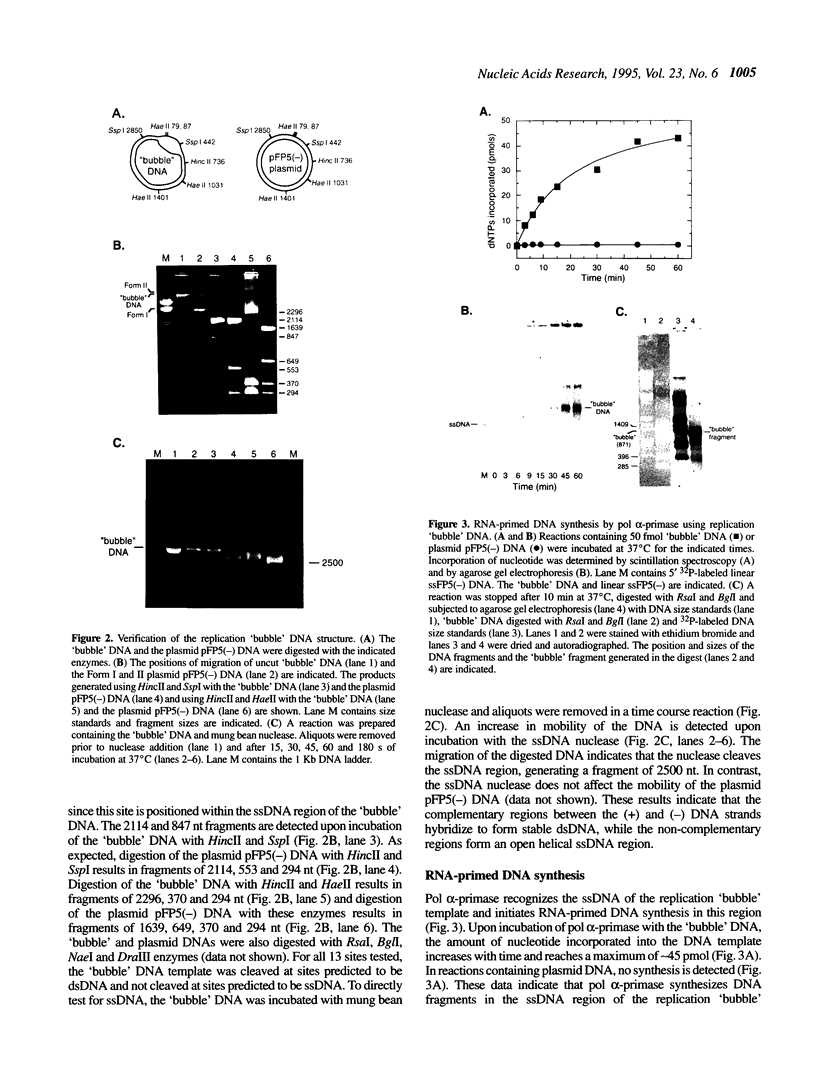
The initiation of new DNA strands at origins of replication in animal cells requires de novo synthesis of RNA primers by primase and subsequent elongation from RNA primers by DNA polymerase alpha. To study the specificity of primer site selection by the DNA polymerase alpha-primase complex (pol alpha-primase), a natural DNA template containing a site for replication initiation was constructed. Two single-stranded DNA (ssDNA) molecules were hybridized to each other generating a duplex DNA molecule with an open helix replication 'bubble' to serve as an initiation zone. Pol alpha-primase recognizes the open helix region and initiates RNA-primed DNA synthesis at four specific sites that are rich in pyrimidine nucleotides. The priming site positioned nearest the ssDNA-dsDNA junction in the replication 'bubble' template is the preferred site for initiation. Using a 40 base oligonucleotide template containing the sequence of the preferred priming site, primase synthesizes RNA primers of 9 and 10 nt in length with the sequence 5'-(G)GAAGAAAGC-3'. These studies demonstrate that pol alpha-primase selects specific nucleotide sequences for RNA primer formation and suggest that the open helix structure of the replication 'bubble' directs pol alpha-primase to initiate RNA primer synthesis near the ssDNA-dsDNA junction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burhans W. C., Huberman J. A. DNA replication origins in animal cells: a question of context? Science. 1994 Feb 4;263(5147):639–640. doi: 10.1126/science.8303270. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Rafter E., Augl C., Bollum F. J. Purification of a DNA polymerase-DNA primase complex from calf thymus glands. J Biol Chem. 1984 Dec 10;259(23):14679–14687. [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W. C., Wang T. S. Enzymatic characterization of the individual mammalian primase subunits reveals a biphasic mechanism for initiation of DNA replication. J Biol Chem. 1993 Dec 15;268(35):26179–26189. [PubMed] [Google Scholar]
- Davey S. K., Faust E. A. Murine DNA polymerase.alpha-primase initiates RNA-primed DNA synthesis preferentially upstream of a 3'-CC(C/A)-5' motif. J Biol Chem. 1990 Mar 5;265(7):3611–3614. [PubMed] [Google Scholar]
- DePamphilis M. L. Eukaryotic DNA replication: anatomy of an origin. Annu Rev Biochem. 1993;62:29–63. doi: 10.1146/annurev.bi.62.070193.000333. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L. Origins of DNA replication in metazoan chromosomes. J Biol Chem. 1993 Jan 5;268(1):1–4. [PubMed] [Google Scholar]
- Dornreiter I., Erdile L. F., Gilbert I. U., von Winkler D., Kelly T. J., Fanning E. Interaction of DNA polymerase alpha-primase with cellular replication protein A and SV40 T antigen. EMBO J. 1992 Feb;11(2):769–776. doi: 10.1002/j.1460-2075.1992.tb05110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornreiter I., Höss A., Arthur A. K., Fanning E. SV40 T antigen binds directly to the large subunit of purified DNA polymerase alpha. EMBO J. 1990 Oct;9(10):3329–3336. doi: 10.1002/j.1460-2075.1990.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L., Brewer B. J. Activation of replication origins within yeast chromosomes. Annu Rev Cell Biol. 1991;7:375–402. doi: 10.1146/annurev.cb.07.110191.002111. [DOI] [PubMed] [Google Scholar]
- Foiani M., Lindner A. J., Hartmann G. R., Lucchini G., Plevani P. Affinity labeling of the active center and ribonucleoside triphosphate binding site of yeast DNA primase. J Biol Chem. 1989 Feb 5;264(4):2189–2194. [PubMed] [Google Scholar]
- Hamlin J. L. Mammalian origins of replication. Bioessays. 1992 Oct;14(10):651–659. doi: 10.1002/bies.950141002. [DOI] [PubMed] [Google Scholar]
- Kitsberg D., Selig S., Keshet I., Cedar H. Replication structure of the human beta-globin gene domain. Nature. 1993 Dec 9;366(6455):588–590. doi: 10.1038/366588a0. [DOI] [PubMed] [Google Scholar]
- Kuchta R. D., Reid B., Chang L. M. DNA primase. Processivity and the primase to polymerase alpha activity switch. J Biol Chem. 1990 Sep 25;265(27):16158–16165. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S. How many pols does it take to replicate nuclear DNA? Cell. 1991 Jul 26;66(2):185–187. doi: 10.1016/0092-8674(91)90608-2. [DOI] [PubMed] [Google Scholar]
- Nasheuer H. P., Grosse F. DNA polymerase alpha-primase from calf thymus. Determination of the polypeptide responsible for primase activity. J Biol Chem. 1988 Jun 25;263(18):8981–8988. [PubMed] [Google Scholar]
- Perrino F. W., Loeb L. A. Differential extension of 3' mispairs is a major contribution to the high fidelity of calf thymus DNA polymerase-alpha. J Biol Chem. 1989 Feb 15;264(5):2898–2905. [PubMed] [Google Scholar]
- Roth Y. F. Eucaryotic primase. Eur J Biochem. 1987 Jun 15;165(3):473–481. doi: 10.1111/j.1432-1033.1987.tb11463.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenGupta D. J., Borowiec J. A. Strand-specific recognition of a synthetic DNA replication fork by the SV40 large tumor antigen. Science. 1992 Jun 19;256(5064):1656–1661. doi: 10.1126/science.256.5064.1656. [DOI] [PubMed] [Google Scholar]
- Sheaff R. J., Kuchta R. D., Ilsley D. Calf thymus DNA polymerase alpha-primase: "communication" and primer-template movement between the two active sites. Biochemistry. 1994 Mar 1;33(8):2247–2254. doi: 10.1021/bi00174a035. [DOI] [PubMed] [Google Scholar]
- Sheaff R. J., Kuchta R. D. Mechanism of calf thymus DNA primase: slow initiation, rapid polymerization, and intelligent termination. Biochemistry. 1993 Mar 30;32(12):3027–3037. doi: 10.1021/bi00063a014. [DOI] [PubMed] [Google Scholar]
- Shioda M., Nelson E. M., Bayne M. L., Benbow R. M. DNA primase activity associated with DNA polymerase alpha from Xenopus laevis ovaries. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7209–7213. doi: 10.1073/pnas.79.23.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Savoysky E., Izuta S., Tatebe M., Okajima T., Yoshida S. RNA priming coupled with DNA synthesis on natural template by calf thymus DNA polymerase alpha-primase. Biochemistry. 1993 Nov 30;32(47):12782–12792. doi: 10.1021/bi00210a030. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Erickson J. M., Goulian M. Initiator RNA of nascent DNA from animal cells. J Mol Biol. 1979 Apr 25;129(4):531–545. doi: 10.1016/0022-2836(79)90467-4. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Prussak C. E. Sequence and structural requirements for primase initiation in the SV40 origin of replication. Nucleic Acids Res. 1989 Mar 11;17(5):1953–1963. doi: 10.1093/nar/17.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Fairman M. P., Stillman B. Simian virus 40 DNA replication in vitro: identification of multiple stages of initiation. Mol Cell Biol. 1989 Sep;9(9):3839–3849. doi: 10.1128/mcb.9.9.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vishwanatha J. K., Yamaguchi M., DePamphilis M. L., Baril E. F. Selection of template initiation sites and the lengths of RNA primers synthesized by DNA primase are strongly affected by its organization in a multiprotein DNA polymerase alpha complex. Nucleic Acids Res. 1986 Sep 25;14(18):7305–7323. doi: 10.1093/nar/14.18.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. S. Eukaryotic DNA polymerases. Annu Rev Biochem. 1991;60:513–552. doi: 10.1146/annurev.bi.60.070191.002501. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Weissbach L., Borowiec J. A., Dean F. B., Murakami Y., Bullock P., Hurwitz J. Replication of simian virus 40 origin-containing DNA in vitro with purified proteins. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1834–1838. doi: 10.1073/pnas.84.7.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Kelly T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2523–2527. doi: 10.1073/pnas.85.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Weinberg D. H., Virshup D. M., Li J. J., Kelly T. J. Identification of cellular proteins required for simian virus 40 DNA replication. J Biol Chem. 1989 Feb 15;264(5):2801–2809. [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA replicase. DNA polymerase associated with a novel RNA polymerase activity to synthesize initiator RNA of strict size. J Biol Chem. 1982 Sep 25;257(18):11121–11127. [PubMed] [Google Scholar]
- Yamaguchi M., Hendrickson E. A., DePamphilis M. L. DNA primase-DNA polymerase alpha from simian cells: sequence specificity of initiation sites on simian virus 40 DNA. Mol Cell Biol. 1985 May;5(5):1170–1183. doi: 10.1128/mcb.5.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]