Abstract
Many species have morphologically and genetically differentiated sex chromosomes, such as the XY pair of mammals. Y chromosomes are often highly degenerated and carry few functional genes, so that XY males have only one copy of most X-linked genes (whereas females have two). As a result, chromosome-wide mechanisms of dosage compensation, such as the mammalian X-inactivation, often evolve to reestablish expression balance. A similar phenomenon is expected in female-heterogametic species, where ZW females should suffer from imbalances due to W-chromosome degeneration. However, no global dosage compensation mechanisms have been detected in the two independent ZW systems that have been studied systematically (birds and silkworm), leading to the suggestion that lack of global dosage compensation may be a general feature of female-heterogametic species. However, analyses of other independently evolved ZW systems are required to test if this is the case. In this study, we use published genomic and expression data to test for the presence of global dosage compensation in Schistosoma mansoni, a trematode parasite that causes schistosomiasis in humans. We find that Z-linked expression is reduced relative to autosomal expression in females but not males, consistent with incomplete or localized dosage compensation. This gives further support to the theory that female-heterogametic species may not require global mechanisms of dosage compensation.
Keywords: dosage compensation, sex chromosome, evolution
Dosage Compensation in Male- and Female-Heterogametic Species
In dioecious species, a pair of autosomes often evolves into distinct sex chromosomes (X and Y or Z and W) that determine the sex of each individual. In male-heterogametic species, such as all mammalian species, females carry two copies of the X chromosome, whereas males carry one X chromosome and one Y chromosome. In female-heterogametic species, such as birds, snakes, and butterflies, the opposite is true: males are homogametic (ZZ), whereas females are heterogametic (ZW). The selective pressures shaping the evolution of Y and W chromosomes from regular autosomes are thought to be similar, and the two share many peculiarities: both are mostly heterochromatic, contain a high amount of repetitive DNA and carry few functional genes (Berlin and Ellegren 2005; Berlin et al. 2007; Vitkova et al. 2007). The loss of functional Y/W-linked genes during sex chromosome evolution leads to imbalances of X/Z-linked versus autosomal gene expression in the heterogametic sex. This is thought to favor the evolution of dosage compensation mechanisms that increase the expression of genes on the single X/Z chromosome of the heterogametic sex (Charlesworth 1978). Dosage compensation has indeed been observed in all XY species for which autosomal and sex-linked expression has systematically been studied (Gupta et al. 2006; Nguyen and Disteche 2006; Straub and Becker 2007; Prince et al. 2010; but it should be noted that a smaller scale study in platypus has not shown this pattern, Deakin et al. 2008).
Because genes on Z chromosomes often lack a functional homolog on the W, dosage compensation of the female Z chromosome is also expected. However, when female and male expression levels were compared in two birds species using microarray data, only a few Z-linked genes were found to be dosage compensated (Ellegren et al. 2007). Overall, bird Z-linked genes are expressed at significantly higher levels in males than females, suggesting that a chromosome-wide mechanism of dosage compensation is absent (Arnold et al. 2008; Mank and Ellegren 2009; Itoh et al. 2010). An expression study of the Bombyx mori (silkworm) Z chromosome came to similar conclusions (Zha et al. 2008). Thus, this has led to the suggestion that a lack of chromosome-wide dosage compensation may be a general feature of female-heterogametic species (Graves and Disteche 2007; Mank 2009; Vicoso and Bachtrog 2009). Establishing whether this is indeed the case or a mere coincidence in the two independently evolved ZW systems studied will require the analysis of other independently evolved systems with female heterogamety.
Schistosoma mansoni Has an Independently Evolved ZW System
Schistosomes are trematode parasites that can infect mammals and birds and cause schistosomiasis, a chronic disease widespread throughout Africa (Steinmann et al. 2006; King and Dangerfield-Cha 2008). Their complex life cycle involves an intermediate snail host and a final vertebrate host. Unlike other trematodes, which are hermaphroditic, schistosomes have separate sexes. Sexual reproduction occurs in the vertebrate host, in which the large males recruit the much smaller, sexually immature, females to their ventral gynecophoral canal, where they are kept to mature, copulate, and lay eggs. Sex determination is genetic, with heterogametic females harboring a large Z chromosome and a highly heterochromatic W chromosome (Grossman et al. 1981). Because this sex chromosome system is exclusive to schistosomes and completely unrelated to any of the other sex chromosome systems that have been studied, it is ideal for an independent assessment of dosage compensation in a ZW species. Furthermore, a genome sequence has been published for S. mansoni, one of the trematodes that causes schistosomiasis in humans, and expression data for several tissues of this species are also available. By combining the genome and transcriptome data to compare Z-linked and autosomal expression in male and female tissues, we can test for the presence of dosage compensation in this organism.
The current S. mansoni genome assembly (version 3.1) consists of over 19,000 scaffolds (Berriman et al. 2009), for which no chromosomal information is available. It was therefore necessary to first identify Z-linked and autosomal genomic sequences. Although a linkage map has recently been published (Criscione et al. 2009), it only provides information for a subset of genomic scaffolds and disagrees substantially with the genome assembly map. We thus used an independent approach to identify autosomal and Z-linked regions based on genome coverage. We assigned sequence contigs to either the Z chromosome or to autosomes by comparing sequence read coverage from published male and female S. mansoni genomic libraries (SRR037058 and SRR037057 in the NCBI Sequence Reads Archive). Contigs derived from regions of the genome present in two copies in both males and females, such as autosomal and pseudo-autosomal regions, should have a female to male read ratio of about 1; Z-linked contigs, which are present in only one copy in females but two copies in males, should be close to 0.5. We therefore classified contigs that had a female to male ratio of 0.4–0.6 as Z-linked (1,256 contigs), whereas contigs with a female to male ratio of 0.9–1.1 were used as our autosomal sample (13,584 contigs; see supplementary fig. 1 and supplementary fig. 2, Supplementary Material online).
To verify that most contigs were mapped correctly, we selected all the genes probed in the microarray data set (see below) that matched our candidate Z-linked contigs (447 genes) and aligned them to scaffolds included in the linkage map. Of the 209 candidate Z-linked genes that aligned to scaffolds in the linkage map, 172 genes (82%) mapped preferentially to the Z-linked scaffolds. In comparison, only 14% of candidate autosomal genes mapped to Z-linked scaffold, confirming that our method is assigning sex linkage correctly for the majority of contigs.
Schistosoma mansoni Lacks Global Dosage Compensation
We combined the mapped genomic sequence with published microarray data from four tissues (whole body, microdissected controls, head, and hind; series GSE23942 on the NCBI Gene Expression Omnibus Web site), to compare Z-linked and autosomal expression in S. mansoni males and females. We mapped all the probes used in their study to our candidate autosomal and Z-linked genomic contigs, as well as to all S. mansoni gene sequences (available on the Sanger Institute Web site). The expression levels of all probes matching each gene were averaged, yielding expression estimates for 447 candidate Z-linked genes and 3,664 candidate autosomal genes.
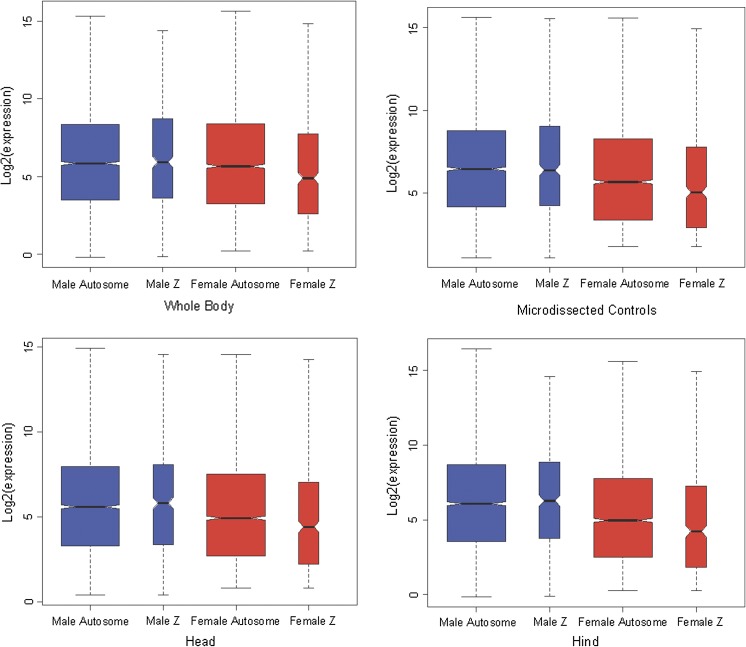
In order to test for dosage compensation, two strategies have commonly been used: one is to compare the male to female ratio of expression of X/Z-linked and autosomal genes; in species with incomplete dosage compensation, X/Z-linked genes will be overexpressed in the homogametic sex relative to the heterogametic, whereas autosomal genes will have similar expression in both sexes. The other consists of comparing X/Z expression to autosomal expression within each sex and see if the heterogametic sex, but not the homogametic sex, has reduced levels of expression on the Z/X relative to the autosomes. This second approach is a better reflection of the biology of dosage compensation, which is thought to arise primarily to readjust the Z/X to autosome expression ratio in the heterogametic sex. Following this approach, we compared the expression of Z-linked to autosomal genes within sexes, to see if we detected a female-specific reduction in Z-linked expression relative to autosomal expression. Consistent with the expectation of incomplete dosage compensation, Z-linked expression was significantly lower than autosomal expression level in all female tissues (fig. 1 and table 1; MA-plots are provided in supplementary fig. 3, Supplementary Material online). This was never the case for male tissues, where no significant difference was detected (fig. 1, table 1, and supplementary fig. 3, Supplementary Material online).
FIG. 1.—
Z-linked and autosomal expression in male and female tissues. The y axis represents the log2 of the expression.
Table 1.
Comparison of Z and Autosomal Expression Levels in Males and Females
| Whole Body | Head | Hind | Microdissected | |
| Male expression in different tissues | ||||
| Autosomal median | 57.30 | 47.84 | 67.34 | 88.48 |
| Z median | 60.71 | 55.69 | 77.34 | 83.88 |
| Z/autosomal | 105.9% | 116.4% | 114.8% | 94.8% |
| P value | 0.43 | 0.49 | 0.36 | 0.75 |
| Female expression in different tissues | ||||
| Autosomal median | 50.48 | 30.25 | 31.15 | 51.33 |
| Z median | 29.51 | 21.16 | 18.66 | 33.34 |
| Z/autosomal | 58.5% | 69.9% | 59.9% | 64.9% |
| P value | 0.001 | 0.007 | 0.0005 | 0.001 |
NOTE.—The expression values provided in the table correspond to processed probe intensity values in the microarray data. P values were obtained using an unpaired Wilcoxon test.
A Possible Association between Female Heterogamety and Incomplete Dosage Compensation
This analysis brings to seven the number of independently evolved systems where the presence of dosage compensation has been systematically assessed: four male-heterogametic and three female-heterogametic taxa. Although drawing conclusions from such a small number of datapoints is premature, our results are consistent with a possible association between female heterogamety and incomplete dosage compensation. Several possibilities have been put forward to account for such an association. First, dosage compensation may not always be necessary, and only some organisms with heteromorphic chromosomes will ever develop it (Graves and Disteche 2007). If Z/W sex chromosomes evolve from a pair of autosomes that carries very few genes, for instance, the dosage imbalance resulting from W degeneration may not be deleterious enough for selection to favor the evolution of chromosome-wide dosage compensation. Genomic data of the three female-heterogametic systems studied to date, however, do not suggest that Z chromosomes are particularly gene poor (Vicoso and Bachtrog 2009 and this study).
If W chromosomes degenerate much more slowly on average than Y chromosomes, there may not be a general selective pressure to evolve a chromosome-wide dosage compensation system; instead, dosage-sensitive genes on the Z may individually adjust their sex-specific expression level. This could be due to the different biology of Y and W chromosomes, which are either male limited or female limited. For instance, males often have higher mutation rates in their germline, so that chromosomes that spend more time in males, such as Y and Z chromosomes, are often subject to higher mutation rates (Vicoso and Bachtrog 2009; Naurin et al. 2010). Similarly, sexual selection often reduces the male effective population size relative to the female effective population size. This will decrease the effective population size of Z and Y chromosomes, which are often/always in males, to a larger extent than the effective population size of the W and Z chromosome (Mank et al. 2010). Both of those processes could speed up the rate of degeneration of a male-limited Y chromosome relative to the W, but how these differences affect the evolution of global versus local mechanisms of dosage compensation has yet to be investigated.
Furthermore, if the Z/W systems that have been studied are very young, there may have not been enough time to evolve dosage compensation. However, the bird ZW system is about as old as the mammalian ZW pair (Bergero and Charlesworth 2009) and older than the Drosophila sex chromosomes, which argues against a recent origin as the cause for the observed lack of chromosome-wide dosage compensation in this clade. Similarly, the S. mansoni sex chromosomes are shared by all schistosomes (Grossman et al. 1981), some of which have diverged by 25% on the DNA level (Lockyer et al. 2003), making it unlikely that young age alone could account for the lack of dosage compensation.
Finally, Z chromosomes may accumulate genes with male-biased functions and consequent male-biased expression, which could be mistaken for, or could result in, a lack of dosage compensation. Sex chromosomes are known to carry a nonrandom distribution of genes with sex-biased expression (reviewed in Ellegren and Parsch 2007), and theoretical work has shown that Z chromosomes are particularly prone to accumulate male-beneficial genes (Reeve and Pfennig 2003; Albert and Otto 2005). If Z-linked genes that are well adapted for males increase their expression in males relative to females, this could be mistaken as a lack of dosage compensation; however, this would also increase expression of Z-linked genes in males relative to autosomes, inconsistent with our observations. On the other hand, male-beneficial Z-linked genes may be under reduced selection to evolve dosage compensation in females.
Currently, it is unclear what evolutionary processes are underlying the different levels of chromosome-wide dosage compensation in different organisms, and if those differences are indeed related to male- versus female-heterogamety. Our analysis of the S. mansoni genome provides yet another example of a ZW system lacking a general dosage compensation mechanism. Theoretical work combined with analysis of gene expression in further ZW and XY species with a variety of life histories should help to clarify these issues.
Materials and Methods
Genomic Data
Genomic contigs and scaffolds (v3.1 and 3.0) and gene sequences (v4.0) were downloaded from the Sanger Institute Web site: http://www.sanger.ac.uk/resources/downloads/helminths/schistosoma-mansoni.html.
Contig Mapping
Version 3.1 of the S. mansoni genome consists of over 50,000 contigs, grouped into about 19,000 scaffolds. A linkage map has been published (Criscione et al. 2009), and we initially used it to select autosomal and Z-linked scaffolds for our analysis. We selected scaffolds (from version 3.0 of the genome, which was used in this study) that contained markers mapped to each autosome (supplementary file 1 of Criscione et al. 2009), as well as scaffolds containing Z-specific markers (a large fraction of the Z-chromosomeis pseudoautosomal; in these regions, the W and Z chromosome are similar in gene content, and therefore, dosage compensation is not required). To check for Z-specificity, we downloaded published Solexa genomic reads obtained from male and female S. mansoni (SRR037058 and SRR037057 from the NCBI Sequence Reads Archive) and aligned them to the genomic scaffolds using SoapAligner. We then compared the number of female and male reads that aligned to each chromosomal scaffold. In the case of autosomal regions, we expected a 1:1 ratio of female to male number of reads (F/M) that align to each contig (the median F/M for the whole sample is 1.07, in agreement with this). In the case of Z-linked contigs, we expect a 1:2 ratio of female to male number of reads, as females have only one copy of the Z, and males have two copies.
As expected, coverage in females was lower for Z-linked scaffolds (fig. S1). However, this was less pronounced than the expected 2-fold reduction, suggesting that a subset of the regions that were classified as Z-specific based on linkage information are not completely degenerated on the W chromosome, which could interfere with our analysis. Also, only a subset of genomic scaffold was placed onto the linkage map, and the assembly and linkage map disagree substantially (Criscione et al. 2009). We thus used an independent approach to identify autosomal and Z-linked regions based on genome coverage.
We aligned these same male and female genomic reads to all contigs of version 3.1 of the S. mansoni genome using Soapalign and compared, for each contig, the number of aligned female and male reads. We then selected contigs with a female to male coverage ratio of 0.9 to 1.1 and at least 50 reads in female and used them as our autosomal sample. Contigs with a female to male coverage ratio of 0.4 to 0.6 and at least 50 female reads were classified as Z-linked (1,256 contigs). We used only contigs that had a read count of at least 50 in females (and about 100 in males) to make sure that this biased F/M ratio reflected differences in the amount of DNA present and not noise in the data.
In order to check if this method is appropriate for retrieving Z-linked genes, we used blat to align all the candidate Z-linked genes from the microarray data set (447 genes) to scaffolds that were classified as autosomal or Z-linked in the linkage map. Using a minimum blat score of 100, 209 genes aligned to scaffolds located in the linkage map: 74 genes mapped preferentially to the Z-specific scaffolds, 98 to Z/W scaffolds, and 37 aligned to autosomal scaffolds, so that about 82% of the Z-candidate genes that had a match mapped back to known Z-linked scaffolds. When we performed the same analysis for our candidate autosomal genes (3,664 genes), 2,111 aligned to autosomal scaffolds and only 337 (14% of aligned genes) mapped to the Z-linked scaffolds.
Microarray Analysis
We downloaded expression data from the Geo Web site (series GSE23942) that had been processed by extracting data from scanned images with Feature Extraction Software 9.1 (Agilent) using default parameters (protocol GE1-v5_95 and Grid: 024207_D_F_20090609) to obtain background subtracted and spatially detrended Processed Signal intensities. One biological replicate was available per tissue. All probe sequences were provided in the platform. We used blat to map them to genomic contigs and kept only those whose strongest hit matched our candidate Z-linked or autosomal contigs. We also used blat to assign probes to known gene sequences downloaded from the Sanger institute Web site; when different probes corresponded to one gene, their expression values were averaged. Male and female expression of Z-linked versus autosomal genes was then compared in four tissues (whole body, head, hind, and microdissected male and female controls) using Wilcoxon unpaired tests.
Supplementary Material
Supplementary figures S1–S3 are available at Genome Biology and Evolutiononline (http://www.gbe.oxfordjournals.org/).
Acknowledgments
This research is supported by National Institutes of Health grants (R01GM093182 and R01GM076007) and a David and Lucile Packard fellowship (to D.B.). We are grateful to J.J. Emerson for normalizing the microarray data and plotting the MA-plots. We also thank J.J. Emerson, Vera Kaiser, Nick Toda, and Qi Zhou for discussion and comments on the manuscript and two anonymous referees for helpful suggestions.
References
- Albert AYK, Otto SP. Sexual selection can resolve sex-linked sexual antagonism. Science. 2005;310:119–121. doi: 10.1126/science.1115328. [DOI] [PubMed] [Google Scholar]
- Arnold A, Itoh Y, Melamed E. A bird’s-eye view of sex chromosome dosage compensation. Annu Rev Genomics Hum Genet. 2008;9:109–127. doi: 10.1146/annurev.genom.9.081307.164220. [DOI] [PubMed] [Google Scholar]
- Bergero R, Charlesworth D. The evolution of restricted recombination in sex chromosomes. Trends Ecol Evol. 2009;24:94–102. doi: 10.1016/j.tree.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Berlin S, Ellegren H. Fast accumulation of nonsynonymous mutations on the female-specific W chromosome in birds. J Mol Evol. 2005;62:66–72. doi: 10.1007/s00239-005-0067-6. [DOI] [PubMed] [Google Scholar]
- Berlin S, Tomaras D, Charlesworth B. Low mitochondrial variability in birds may indicate Hill–Robertson effects on the W chromosome. Heredity. 2007;99:389–396. doi: 10.1038/sj.hdy.6801014. [DOI] [PubMed] [Google Scholar]
- Berriman M, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B. Model for evolution of Y chromosomes and dosage compensation. Proc Natl Acad Sci U S A. 1978;75:5618–5622. doi: 10.1073/pnas.75.11.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscione C, Valentim C, Hirai H, Loverde P, Anderson T. Genomic linkage map of the human blood fluke Schistosoma mansoni. Genome Biol. 2009;10:R71. doi: 10.1186/gb-2009-10-6-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin J, Hore T, Koina E, Marshall Graves J. The status of dosage compensation in the multiple X chromosomes of the platypus. PLoS Genet. 2008;4:e1000140. doi: 10.1371/journal.pgen.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H, et al. Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes. BMC Biol. 2007;5:40. doi: 10.1186/1741-7007-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet. 2007;8:689–698. doi: 10.1038/nrg2167. [DOI] [PubMed] [Google Scholar]
- Graves JAM, Disteche CM. Does gene dosage really matter? J Biol. 2007;6:1. doi: 10.1186/jbiol55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A, Short R, Cain G. Karyotype evolution and sex chromosome differentiation in Schistosomes (Trematoda, Schistosomatidae) Chromosoma. 1981;84:413–430. doi: 10.1007/BF00286030. [DOI] [PubMed] [Google Scholar]
- Gupta V, et al. Global analysis of X-chromosome dosage compensation. J Biol. 2006;5:3.1–3.10. doi: 10.1186/jbiol30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, et al. Sex bias and dosage compensation in the zebra finch versus chicken genomes: general and specialized patterns among birds. Genome Res. 2010;20:512–518. doi: 10.1101/gr.102343.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- Lockyer AE, et al. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology. 2003;126:203–224. doi: 10.1017/s0031182002002792. [DOI] [PubMed] [Google Scholar]
- Mank J. The W, X, Y and Z of sex-chromosome dosage compensation. Trends Genet. 2009;25:226–233. doi: 10.1016/j.tig.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank J, Ellegren H. All dosage compensation is local: gene-by-gene regulation of sex-biased expression on the chicken Z chromosome. Heredity. 2009;102:312–320. doi: 10.1038/hdy.2008.116. [DOI] [PubMed] [Google Scholar]
- Mank J, Vicoso B, Berlin S, Charlesworth B. Effective population size and the Faster-X effect: empirical results and their interpretation. Evolution. 2010;64:663–674. doi: 10.1111/j.1558-5646.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- Naurin S, Hansson B, Bensch S, Hasselquist D. Why does dosage compensation differ between XY and ZW taxa? Trends Genet. 2010;26:15–20. doi: 10.1016/j.tig.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nat Genet. 2006;38:47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]
- Prince EG, Kirkland D, Demuth JP. Hyperexpression of the X Chromosome in both sexes results in extensive female bias of X-linked genes in the flour beetle. Genome Biol Evol. 2010;2:336–346. doi: 10.1093/gbe/evq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve HK, Pfennig DW. Genetic biases for showy males: are some genetic systems especially conducive to sexual selection? PNAS. 2003;100:1089–1094. doi: 10.1073/pnas.0337427100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Straub T, Becker PB. Dosage compensation: the beginning and end of generalization. Nat Rev Genet. 2007;8:47–57. doi: 10.1038/nrg2013. [DOI] [PubMed] [Google Scholar]
- Vicoso B, Bachtrog D. Progress and prospects toward our understanding of the evolution of dosage compensation. Chromosome Res. 2009;17:585–602. doi: 10.1007/s10577-009-9053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkova M, Fukova I, Kubickova S, Marec F. Molecular divergence of the W chromosomes in pyralid moths (Lepidoptera) Chromosome Res. 2007;15:917–930. doi: 10.1007/s10577-007-1173-7. [DOI] [PubMed] [Google Scholar]
- Zha X, et al. Dosage analysis of Z chromosome genes using microarray in silkworm, Bombyx mori. Insect Biochem Mol Biol. 2008;39:315–321. doi: 10.1016/j.ibmb.2008.12.003. [DOI] [PubMed] [Google Scholar]