Abstract
Background:
There is a paucity of therapies for gait impairment in Parkinson disease (PD). Open-label studies have suggested improved gait after treatment with methylphenidate (MPD).
Objective:
To evaluate the efficacy of MPD for the treatment of gait impairment in PD.
Methods:
Twenty-seven subjects with PD and moderate gait impairment were screened for this 6-month placebo-controlled, double-blind study. Subjects were randomly assigned to MPD (maximum, up to 80 mg/day) or placebo for 12 weeks and crossed over after a 3-week washout. The primary outcome measure was change in a gait composite score (stride length + velocity) between groups at 4 and 12 weeks. Secondary outcome measures included changes in motor function, as measured by the Unified Parkinson's Disease Rating Scale (UPDRS), Freezing of Gait Questionnaire (FOGQ), number of gait-diary freezing episodes, and measures of depression, sleepiness, and quality of life. Three-factor repeated-measures analysis of variance was used to measure changes between groups.
Results:
Twenty-three eligible subjects with PD were randomized and 17 completed the trial. There was no change in the gait composite score or treatment or time effect for any of the variables. Treatment effect was not modified by state or study visit. Although there was a trend for reduced frequency of freezing and shuffling per diary, the FOGQ and UPDRS scores worsened in the MPD group compared to placebo. There was a marginal improvement in some measures of depression.
Conclusions:
MPD did not improve gait and tended to worsen measures of motor function, sleepiness, and quality of life.
Classification of evidence:
This study provides Class III evidence for the lack of benefit of MPD on PD-associated gait impairment. Clinical trial registration: NCT00526630.
Gait impairment in the form of shuffling and freezing of gait often occurs during the course of Parkinson disease (PD), generating substantial disability.1 Dopaminergic treatments and currently available surgical therapies are often insufficiently effective for gait disorders in PD.2,3 Freezing of gait has been proposed to result in part from the noradrenaline reduction due to locus ceruleus degeneration and the subsequent dopamine-noradrenergic imbalance.4 Methylphenidate (MPD, Ritalin®), an amphetamine-like psychomotor stimulant, is a candidate to support this hypothesis given its inhibitory action on the striatal and cortical presynaptic transporter for dopamine5 and norepinephrine,6 thus enhancing synaptic levels of both neurotransmitters.
Three open-label studies have reported efficacy of MPD in PD-associated gait impairment. In the first, gait speed, stride time variability, and a timed gait test improved following a single 20-mg dose of MPD given to 21 patients with PD.7 In the second, total walking time, total freezing time, number of freezing episodes, and nonfreezing walking time improved after a single 10-mg dose of MPD given to 5 patients with PD.8 In the third, freezing episodes decreased and timed gait improved after a daily 50–80 mg dose of MPD given for 3 months to 17 subthalamic deep brain stimulation (STN DBS)–treated patients with PD.9 We sought to study in a double-blind, placebo-controlled manner the extent to which MPD improves gait in a population of patients with advanced PD in whom festination and freezing of gait have become a primary source of disability. We hypothesized that daily treatment with a maximally tolerable daily oral dose of MPD would improve gait velocity, stride length, and cadence, and decrease freezing of gait in adult patients without dementia with moderately advanced PD and moderately severe gait impairment (Hoehn & Yahr stage 2–3).
METHODS
Subjects and design.
We recruited consecutive consenting patients with PD, between the ages of 35 and 85 years, with mild to severe gait disturbance with score >1 on the motor subscale of the Unified Parkinson's Disease Rating Scale (UPDRS) item 29 in the “on” state but without need for a continuous ambulatory aid such as a walker or wheelchair (Hoehn & Yahr stage 3). Patients were on a stable dose of antiparkinsonian medications and were expected to require no medication adjustments during the course of the study. The following exclusion criteria were applied: 1) musculoskeletal disorders such as severe arthritis, post knee surgery, hip surgery, or any other condition that the investigators determine may impair assessment of gait; 2) previous treatment with DBS; 3) history of stroke; 4) cerebellar, vestibular, or sensory ataxia; 5) Mini-Mental State Examination score <25; 6) concurrent use of, or within 2 weeks from discontinuing, MAO inhibitor drugs (selegiline, rasagiline); and 7) women of childbearing potential. The study followed a double-blind crossover prospective experimental design. All subjects underwent a standardized preliminary assessment of their gait using validated rating scales (see Clinical interventions) as well as structured gait and balance assessments both before and after their randomization into one of the study arms. A 3-week washout was deemed sufficient to prevent bias related to carryover effects from the first treatment. All study-related assessments took place twice: first during the “practically defined off period,” at least 12 hours from the last dose of any antiparkinsonian medication,10 and then 30 to 60 minutes later, during the “on” state, once the subject's usual dopaminergic dose, administered as levodopa equivalents,11 led to maximum clinical benefits.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the University of Cincinnati Institutional Review Board and all subjects provided informed consent. The clinical trial identifier number assigned by clinicaltrials.gov was NCT00526630.
Dosage schedule.
Patients received a maximum dose of 1 mg/kg of MPD divided in 3 doses (at 8 am, noon, and 4 pm). A 4-week titration period was used, with 0.25-mg/kg increments per week until achieving the weight-adjusted target dosage, which ranged from 5 to 8 10-mg tablets per day. The maximum daily dose was 80 mg/day, similar to the target used in the French open-label trial.9 Tolerability of MPD was monitored for each patient throughout the treatment period and, if necessary, dose reduction was permitted. Compliance with medication schedules was determined through pill counts.
Gait measurements.
We used a valid and reliable gait analysis system (GAITRite, CIR Systems, Inc., Havertown, PA),12,13 consisting of an electronic walkway measuring 24 inches (61 cm) wide and 144 inches (366 cm) long, with a total of 13,824 embedded sensors. Accompanying software controlled the functionality of the walkway, processed the raw data into footfall patterns, and computed the temporal (timing) and spatial (distance) gait variables, including, among others, stride length, cadence, velocity, single and double support, and stance and swing phases. Data were collected in the “off” and “on” state after subjects walked on a predefined 10-m path, beginning and ending at the same point over a roundtrip path. The path included the 3.66-meter electronic walkway, which recorded initiation of gait, straight walk, and turns.
Clinical interventions.
For each of the 6 assessments, participants' degree of motor impairment was rated by the primary investigator (A.J.E.) using the UPDRS14 and Hoehn & Yahr15 scales during the “off” and “on” states. Gait function at home was ascertained with the self-administered Freezing of Gait Questionnaire (FOGQ)16 and the gait diary, detailing the incidence and time spent freezing, tripping, and falling, on an hourly basis and according to medication state (“off” and “on”). From these data the hours spent freezing and falling were collated for analysis. To examine the effect of MPD on mood, we included the clinician-administered Montgomery-Åsberg Depression Rating Scale (MADRS), considered the most widely used depression-measuring instrument in treatment trials.17,18 This scale is validated, reliable, and as sensitive to depression as the Hamilton Depression Rating Scale, devised primarily for use on patients already diagnosed with affective disorders.19 Two self-administered scales complemented the MADRS: the 15-item Geriatric Depression Scale (GDS-15) and the 20-item Zung Self-Rating Depression scale.20 The Zung scale has been validated both as a screening instrument and as one sensitive to change. To assess quality of life and activities of daily living, we used the EQ-5D Health Questionnaire21 and the activities of daily living subcomponent of the UPDRS (part II). Finally, Epworth Sleepiness Scale (ESS)22 was applied to document any wake-enhancing effects associated with MPD.
Outcome measures.
The primary outcome measure was the change in the gait composite score of GAITRite-measured velocity and stride length between the MPD and placebo groups, at week 12 after completion of each study arm. Cadence was not incorporated into the gait composite score given its poor sensitivity to capturing changes in gait.23 Secondary outcome measures included changes in gait diary (number of freezing episodes and falls), FOGQ (total score), MADRS, EQ-5D, and ESS between days 1 and 84; that is, at the end of week 12 for each study arm.
Statistical analyses.
Assuming an effect size of 20% as reported by the timed gait test outcome from the French study,9 and a SD of 0.30, we had 80% power, allowing for a type I error of 5%, of detecting significant differences with 10 subjects randomized into each treatment arm (total of 20 patients), in the paired design of this crossover trial. To account for anticipated dropouts, the recruitment goal was a total sample of 24 patients.
Three-factor repeated-measures analysis of variance was used to evaluate for statistically significant changes in the gait composite scores between groups (MPD, placebo), while also accounting for variation due to state (“off”/“on”) and time (baseline, 4-week visit, 12-week visit) and their interactions with group. The combined effect of treatment at the second visit (after dose titration) and the third visit (after 8 weeks at the stable target dose) was explored. For outcome variables of interest, the average value at the second and third assessments was determined. The relative change was computed as (average at second and third visit − baseline assessment value)/baseline assessment value. The relative change of the variables was compared between groups using paired t test. Fisher exact test was performed to compare the proportions of patients withdrawn between groups before and after crossover. All statistical analyses were calculated using SAS 9.0. Predetermined significance level was for p values less than 5%.
RESULTS
Patient disposition and characteristics.
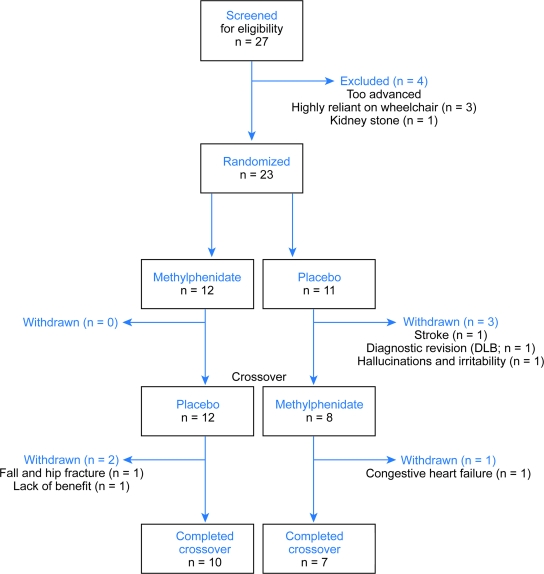
A total of 27 patients (22 male) were screened during the study period (figure 1). Twenty-three subjects were randomized and 17 completed the entire 6-month study evaluations (figure 2). The mean dose of MPD was 64.4 ± 18.7 mg/day. There was a greater dropout rate than anticipated, likely due to complications inherent to a moderately advanced PD population. The withdrawal rate was comparable between groups before (p = 0.09) and after crossover (p = 1.00), though it was clearly greater in the placebo arm. Of the 6 subjects who withdrew from the study, only one was in the MPD arm (figure 1).
Figure 1. CONSORT diagram.
DLB = dementia with Lewy bodies.
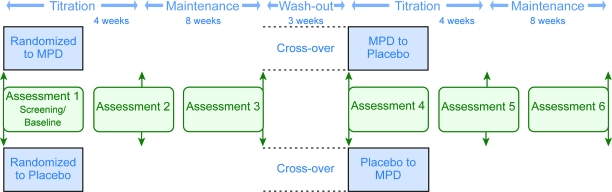
Figure 2. Study flow and timeline for acquisition of data.
MPD = methylphenidate.
Outcome measures.
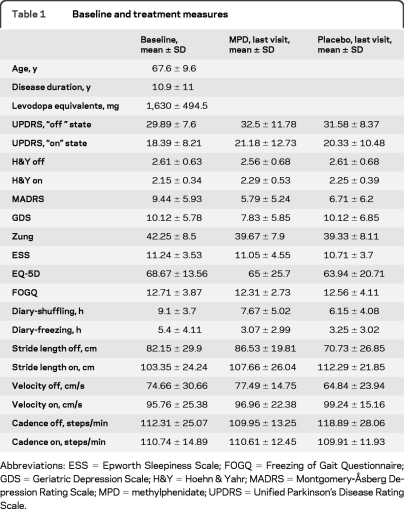
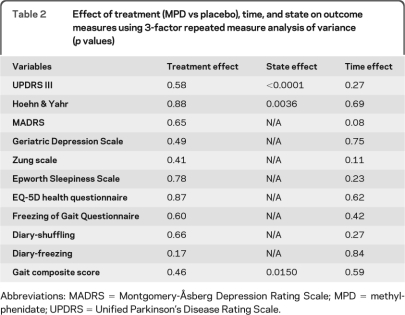
Little change was noted between baseline and last visit (table 1). There was no treatment or time effect for any of the variables. The expected state effect (improvements in the “on” vs “off” state) was observed in all the variables (table 2). The effect of treatment (group) was not affected by state or study visit (time). About a third of patients or their caregivers reported subjective gait benefits within the first 3 weeks of titration, with a loss of such benefit after increasing doses as stipulated in the protocol. These patients were allowed to return to the dose they believed was effective but a restoration of their perceived benefit was rarely regained as measured by the blinded primary investigator.
Table 1.
Baseline and treatment measures
Abbreviations: ESS = Epworth Sleepiness Scale; FOGQ = Freezing of Gait Questionnaire; GDS = Geriatric Depression Scale; H&Y = Hoehn & Yahr; MADRS = Montgomery-Åsberg Depression Rating Scale; MPD = methylphenidate; UPDRS = Unified Parkinson's Disease Rating Scale.
Table 2.
Effect of treatment (MPD vs placebo), time, and state on outcome measures using 3-factor repeated measure analysis of variance (p values)
Abbreviations: MADRS = Montgomery-Åsberg Depression Rating Scale; MPD = methylphenidate; UPDRS = Unified Parkinson's Disease Rating Scale.
Comparison of relative change variables.
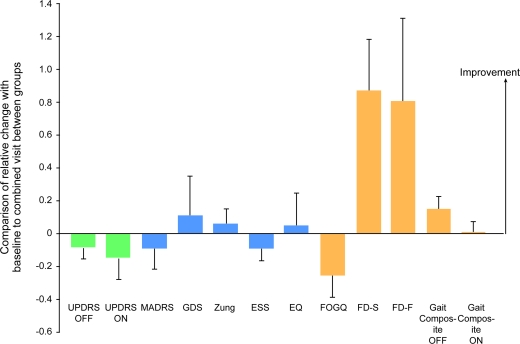
Given similar outcomes at 1 and 3 months, we also explored the combined outcome of these study visits (table e-1 on the Neurology® Web site at www.neurology.org; figure 3). Although there was a lower frequency of freezing and shuffling in the MPD group as measured per diary, the gait composite was not improved (p = 0.08 in the “off” state, p = 0.91 in the “on” state) and the FOGQ marginally favored placebo (p = 0.11). Further, overall motor function, as measured by UPDRS, tended to worsen with MPD. Post hoc analysis showed neither clinical nor statistical effect of MPD on axial UPDRS items (neck rigidity, posture, and postural stability), singly or in isolation. There was no clear benefit for depression as MADRS worsened in the MPD group even though the self-reported GDS and Zung scores improved. There were no improvements in sleepiness or quality of life.
Figure 3. Post hoc comparison of the relative change between combined visits (4 and 12 weeks) and baseline visit in all variables between MPD and placebo groups.
Values above 0 indicate improvement for the MPD group compared to placebo. ESS = Epworth Sleepiness Scale; EQ = EQ-5D Health Questionnaire; FD-S and FD-F = freezing diary for shuffling and freezing; FOGQ = Freezing of Gait Questionnaire; GDS = Geriatric Depression Scale; MADRS = Montgomery-Åsberg Depression Rating Scale; UPDRS = Unified Parkinson's Disease Rating Scale.
Tolerability.
Peak-dose dyskinesias, hypersexuality, mania, irritability, and sweating were among the changes noted more commonly among those on MPD (table e-2). Surprisingly, lack of energy or decreased strength was reported by 25% in this group. Nevertheless, 10 of 19 patients requested MPD as part of their poststudy medication regimen.
DISCUSSION
This is the first double-blind placebo-controlled trial to explore a possible benefit of MPD on freezing of gait in patients with PD. Contrary to open-label data, MPD did not improve gait as assessed by FOG-Q, the freezing diary, and kinematic variables except for a slight improvement of the gait composite score in the “off” medication state. Further, motor function, as measured by UPDRS, and depression, as measured by MADRS, tended to deteriorate in patients treated with MPD compared to placebo. Unexpectedly, there were no benefits in sleepiness or quality of life measures. The diary-only trend for improvement of freezing and shuffling in the MPD group is of questionable value, as most subjects correctly identified their study drug allocation when queried after all study assessments were completed. This may have induced a logging of their freezing and shuffling that did not correspond with more objective measures, including the FOGQ. Our results are also tempered by a dropout larger than anticipated in the placebo arm (5/6 withdrawn patients were on placebo), which may be explained by the fragility of the advanced PD population. The observed effect size for the primary outcome measure of change in gait composite score was rather small (15% in the “off” state and 1% in the “on” state). While the sample size of the study became smaller than expected due to the large dropout rate, it is unlikely that an increase of sample size would have given us statistically significant differences, particularly in the “on” state, when the benefits for gait would have been most relevant.
Of interest, over half of participating subjects requested MPD for poststudy routine treatment, suggesting a potential benefit of the drug in unmeasured nonmotor domains, perhaps attention or executive function. Studies showing the enhanced attention among subjects given MPD in a single dose7 or over a longer period (in STN DBS patients, simple reaction task among subjects with PD without dementia)9 support the hypothesis that MPD's effect on attention may have justified the subjects' choice to continue this drug after the completion of a negative trial. It is possible that this mechanism or a “halo effect” (the perception that any gait benefits of MPD are influenced by the purported wake- or attention-enhancing properties of this drug) may have contributed to the modest diary-only improvements in gait as well as the choice of many subjects to continue on the drug after the trial. Unfortunately, most studies (including ours) have not incorporated measures of attention or executive function, important shortcomings when considering the growing body of evidence linking gait to higher cognitive functions.24,25
Many of our subjects and caregivers reported a subjective benefit within the first 3 weeks of their study participation, but this change was not sustained and certainly not documented after 4 or 8 weeks. As such, the immediate benefit noted in patients participating in the single-dose open-label studies that supported the rationale for the current trial7,8 may have been largely driven by a placebo effect. Alternatively, lower MPD doses (10–20 mg/day) used in these single-dose studies may be more effective than higher ones (50–80 mg/day) used in the 3-month study on STN DBS-treated subjects with PD reporting freezing9 and in our clinical trial (∼65 mg/day). This explanation could account for the transient benefit noted in this study during the early titration phase, when the MPD dose would have been closer to 10–20 mg/day. A third possibility is that the higher levodopa doses taken by our subjects compared to the previous open-label high-dose MPD study (1,630 mg vs 675 mg)9 may have limited MPD benefits because a ceiling effect was reached in presence of higher exogenous dopamine concentrations. Finally, high and low MPD doses could arguably exert distinct pharmacodynamic actions, explaining the apparently paradoxical worsening in sleepiness, quality of life, and even overall motor function as target doses were reached. It must be pointed out, however, that the presumed positive effects of MPD on sleepiness and quality of life, although plausible, have never been confirmed in a controlled clinical trial.26 MPD has only been carefully examined in PD-associated fatigue.27
Current treatment strategies, particularly manipulations of dopaminergic drugs, have failed to provide meaningful improvement in, and attenuate the disability derived from, PD-associated gait impairment. Ascertaining benefits in a controlled study using nondopaminergic approaches to the complex problem of gait festination and freezing is a major unmet need in enhancing the quality of life of those with moderate and advanced PD. Despite promising open-label data for the readily available (and generally safe) MPD, our randomized controlled clinical trial failed to ascertain benefits. The data obtained here argue against the use of MPD for the gait impairment in PD but do not negate a potential effect on nonmotor areas, such as attention or executive function. If a link between gait and higher cognitive functions were to be confirmed, efforts at augmenting gait function in PD may need to move from dopamine- and norepinephrine-based therapies to enhancing acetylcholine concentrations in the striatum, cerebellum, and thalamus, where cholinergic deficits correlate with disturbances of balance and gait.28 Besides the currently available acetylcholinesterase inhibitors, an attractive option is varenicline, an α-4 β-2 nicotinic acetylcholine receptor agonist, for which there is preliminary evidence of efficacy in patients with spinocerebellar ataxia.29 Further research in nondopaminergic, possibly cholinergic therapies is warranted as a strategy to mitigate gait impairment in patients with PD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Shanna Savage, research coordinator, for her assistance in data collection, and Martha Headworth, medical illustrator at the University of Cincinnati Neuroscience Institute, for figure designs for this manuscript.
Supplemental data at www.neurology.org
- ESS
- Epworth Sleepiness Scale
- FOGQ
- Freezing of Gait Questionnaire
- GDS
- Geriatric Depression Scale
- MADRS
- Montgomery-Åsberg Depression Rating Scale
- MPD
- methylphenidate
- PD
- Parkinson disease
- STN DBS
- subthalamic deep brain stimulation
- UPDRS
- Unified Parkinson's Disease Rating Scale
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. A.K. Dwivedi and Dr. R. Shukla.
DISCLOSURE
Dr. Espay is supported by the KL2 Research Scholars mentored career development award through the NIH Institutional Clinical and Translational Science Award (RR026315-02); has served on scientific advisory boards for Boehringer Ingelheim and Solvay Pharmaceuticals, Inc.; serves on the editorial advisory board for The European Neurological Journal; has served on speakers' bureaus for UCB, Medtronic, Inc., and Novartis; and receives research support from Medtronic, Inc., Allergan, Inc., Cleveland Medical Devices Inc., the Davis Phinney Foundation, and the Michael J. Fox Foundation. Dr. Dwivedi, M. Payne, L. Gaines, and J.E. Vaughan report no disclosures. Dr. Maddux serves on speakers' bureaus for Boehringer Ingelheim, Teva Pharmaceutical Industries Ltd., and Lundbeck Inc.; and has received research support from Teva Pharmaceutical Industries Ltd. and Cleveland Medical Devices Inc. Dr. Slevin serves/has served on speakers' bureaus for Boehringer Ingelheim, Novartis, and Teva Pharmaceutical Industries Ltd.; and receives research support from Solvay Pharmaceuticals, Inc./Abbott, the US Department of Veterans Affairs, and the NIH. M. Gartner reports no disclosures. Dr. Sahay has received funding for travel or speaker honoraria from Amarin Corporation, Boehringer Ingelheim, GlaxoSmithKline, Pfizer Inc., Merck Serono, Novartis, Teva Pharmaceutical Industries Ltd., UCB, and Curry Rockefeller Group; and receives research support from Teva Pharmaceutical Industries Ltd., Merck Serono, Cephalon, Inc., GlaxoSmithKline, Boehringer Ingelheim, Merz Pharmaceuticals, LLC, the NIH, the Michael J. Fox Foundation for Parkinson Research, and the Parkinson Study Group. Dr. Revilla serves as a consultant for Lundbeck Inc.; has received speaker honoraria from Medtronic, Inc.; and receives research support from the NIH, the Davis Phinney Foundation, and the Gardner Family Center for Parkinson's Disease and Movement Disorders. Dr. Duker serves on the speakers' bureau for Allergan, Inc. Dr. Shukla has received research support from the NIH.
REFERENCES
- 1. Giladi N, McDermott MP, Fahn S, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology 2001;56:1712–1721 [DOI] [PubMed] [Google Scholar]
- 2. Garcia Ruiz PJ, Meseguer E, Del Val J, Vazquez A, Sanchez BV, Vazquez A. Motor complications in Parkinson disease: a prospective follow-up study. Clin Neuropharmacol 2004;27:49–52 [DOI] [PubMed] [Google Scholar]
- 3. Krystkowiak P, Blatt JL, Bourriez JL, et al. Effects of subthalamic nucleus stimulation and levodopa treatment on gait abnormalities in Parkinson disease. Arch Neurol 2003;60:80–84 [DOI] [PubMed] [Google Scholar]
- 4. Mizuno Y, Kondo T, Mori H. Various aspects of motor fluctuations and their management in Parkinson's disease. Neurology 1994;44:S29–S34 [PubMed] [Google Scholar]
- 5. Nutt JG, Carter JH, Sexton GJ. The dopamine transporter: importance in Parkinson's disease. Ann Neurol 2004;55:766–773 [DOI] [PubMed] [Google Scholar]
- 6. Keating GM, McClellan K, Jarvis B. Methylphenidate (OROS formulation). CNS Drugs 2001;15:495–500 [DOI] [PubMed] [Google Scholar]
- 7. Auriel E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson's disease: a pilot study. Clin Neuropharmacol 2006;29:15–17 [DOI] [PubMed] [Google Scholar]
- 8. Pollak L, Dobronevsky Y, Prohorov T, Bahunker S, Rabey JM. Low dose methylphenidate improves freezing in advanced Parkinson's disease during off-state. J Neural Transm Suppl 2007;145–148 [DOI] [PubMed] [Google Scholar]
- 9. Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 1992;7:2–13 [DOI] [PubMed] [Google Scholar]
- 11. Fine J, Duff J, Chen R, et al. Long-term follow-up of unilateral pallidotomy in advanced Parkinson's disease. N Engl J Med 2000;342:1708–1714 [DOI] [PubMed] [Google Scholar]
- 12. Webster KE, Wittwer JE, Feller JA. Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture 2005;22:317–321 [DOI] [PubMed] [Google Scholar]
- 13. Menz HB, Latt MD, Tiedemann A, Mun SK, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture 2004;20:20–25 [DOI] [PubMed] [Google Scholar]
- 14. Fahn S, Elton RL. Unified Parkinson's Disease Rating Scale. In: Fahn S, Goldstein M, Marsden D, Calne DB, eds. Recent Developments in Parkinson's Disease. Florham Park, NJ: MacMillan; 1987:153–163 [Google Scholar]
- 15. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442 [DOI] [PubMed] [Google Scholar]
- 16. Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord 2000;6:165–170 [DOI] [PubMed] [Google Scholar]
- 17. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389 [DOI] [PubMed] [Google Scholar]
- 18. Burns A, Lawlor B, Craig S. Assessment Scales in Old Age Psychiatry. London: Martin Dunitz; 1999 [Google Scholar]
- 19. Khan A, Khan SR, Shankles EB, Polissar NL. Relative sensitivity of the Montgomery-Asberg Depression Rating Scale, the Hamilton Depression rating scale and the Clinical Global Impressions rating scale in antidepressant clinical trials. Int Clin Psychopharmacol 2002;17:281–285 [DOI] [PubMed] [Google Scholar]
- 20. ZUNG WW. A self-rating depression scale. Arch Gen Psychiatry 1965;12:63–70 [DOI] [PubMed] [Google Scholar]
- 21. Schrag A, Selai C, Jahanshahi M, Quinn NP. The EQ-5D: a generic quality of life measure-is a useful instrument to measure quality of life in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2000;69:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar S, Bhatia M, Behari M. Excessive daytime sleepiness in Parkinson's disease as assessed by Epworth Sleepiness Scale (ESS). Sleep Med 2003;4:339–342 [DOI] [PubMed] [Google Scholar]
- 23. Espay AJ, Baram Y, Dwivedi AK, et al. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J Rehabil Res Dev 2010;47:573–581 [DOI] [PubMed] [Google Scholar]
- 24. Muslimovic D, Post B, Speelman JD, de Haan RJ, Schmand B. Cognitive decline in Parkinson's disease: a prospective longitudinal study. J Int Neuropsychol Soc 2009;15:426–437 [DOI] [PubMed] [Google Scholar]
- 25. Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 2007;130:1787–1798 [DOI] [PubMed] [Google Scholar]
- 26. Lou JS. Physical and mental fatigue in Parkinson's disease: epidemiology, pathophysiology and treatment. Drugs Aging 2009;26:195–208 [DOI] [PubMed] [Google Scholar]
- 27. Mendonca DA, Menezes K, Jog MS. Methylphenidate improves fatigue scores in Parkinson disease: a randomized controlled trial. Mov Disord 2007;22:2070–2076 [DOI] [PubMed] [Google Scholar]
- 28. Gilman S, Koeppe RA, Nan B, et al. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology 2010;74:1416–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zesiewicz TA, Sullivan KL. Treatment of ataxia and imbalance with varenicline (chantix): report of 2 patients with spinocerebellar ataxia (types 3 and 14). Clin Neuropharmacol 2008;31:363–365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.