Abstract
Objective:
To compare interferon β-1b (IFNβ-1b) and glatiramer acetate (GA) on new lesion (NL) (gadolinium-enhancing, new T2) evolution into permanent black holes (PBH)—a marker of irreversible tissue damage—in patients with relapsing-remitting multiple sclerosis (RRMS).
Methods:
BEYOND was a large, phase III, clinical trial comparing IFNβ-1b 250 μg, IFNβ-1b 500 μg, and GA (2:2:1). Patient scans were reexamined post hoc for PBH in a rater-blinded manner. Two predefined coprimary endpoints compared IFNβ-1b 250 μg with GA: first, number of PBH per patient at year 2 evolving from year 1 NL, then proportion of year 1 NL evolving into PBH at year 2. IFNβ-1b 500 μg and GA were compared in an exploratory fashion.
Results:
Approximately 90% (1,957/2,244) of patients had NL at year 1 with follow-up at year 2. Mean numbers of PBH per patient at year 2 evolving from year 1 NL were lower for IFNβ-1b 250 μg than GA (0.30 vs 0.43; p = 0.0451). The proportion of NL evolving into PBH was similar (IFNβ-1b 250 μg vs GA: 21.6% vs 23.5%; p > 0.20). For IFNβ-1b 500 μg, both the mean PBH number per patient at year 2 evolving from year 1 NL (0.26 vs 0.43; p = 0.0037) and proportion of NL evolving into PBH (16.3% vs 23.5%; p = 0.0409) were lower relative to GA.
Conclusion:
IFNβ-1b affected PBH development to a similar or better extent than GA. IFNβ-1b favorably influences an MRI outcome indicative of permanent tissue destruction in the brains of patients with multiple sclerosis.
Classification of evidence:
This study provides Class III evidence that IFNβ-1b is associated with a reduction in MRI PBH formation and evolution compared with GA between years 1 and 2 of treatment.
Treatment-naive patients with relapsing-remitting multiple sclerosis (RRMS) were randomized (2:2:1) to receive interferon β-1b (IFNβ-1b) 250 μg (Betaseron®, Emeryville, CA), IFNβ-1b 500 μg, or glatiramer acetate (GA, Copaxone®, Teva-Marion Partners, Kansas City, MO) in the BEYOND study.1 No differences were found for relapse risk—the primary outcome variable—or other clinical outcomes. Differences in favor of IFNβ-1b were found for some MRI parameters: cumulative new T2 lesion number, relative change in T2 lesion volume from screening to last available scan (LAS), and cumulative volume of gadolinium-enhancing lesions. Cumulative gadolinium-enhancing lesion number and change in T1-hypointense lesion volume from screening to LAS, which captures all T1-hypointense lesions irrespective of age or permanence, were similar.
Permanent T1-hypointense lesions, permanent black holes (PBH), are considered to be markers of irreversible brain tissue damage in multiple sclerosis (MS),2–6 with a strong correlation between the degree of T1 hypointensity and percentage of residual axons.7 Monitoring of PBH formation requires assessment of new lesion (NL) evolution over time,4 with lesions remaining T1-hypointense for >6–12 months considered permanent.4,8,9
The BECOME study compared the ability of IFNβ-1b 250 μg and GA to suppress MRI disease activity in patients with RRMS or clinically isolated syndrome using monthly imaging.10 From month 1 to year 2, IFNβ-1b–treated patients had a significantly lower proportion of NL that became PBH than GA-treated patients.11 On the basis of hypotheses generated in BECOME, patient scans from BEYOND were reanalyzed to assess PBH evolution at year 2 from NL at year 1.
METHODS
Standard protocol approvals, registrations, and patient consents.
BEYOND (NCT00099502) was done according to good clinical practice and the International Conference on Harmonization guidelines. The institutional review boards of all participating centers approved the study protocol, and patients provided written informed consent before entering into the trial.
Patients.
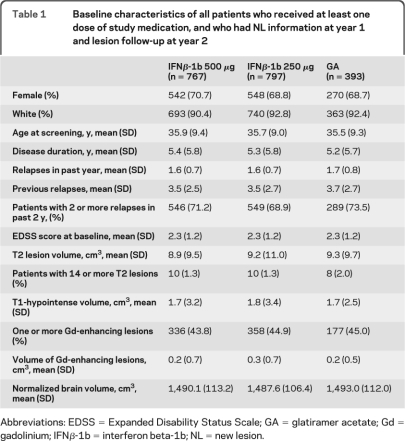
BEYOND was a large (n = 2,244), phase III, prospective, multicenter, randomized, parallel-group, blinded clinical study of treatment-naive patients with RRMS and baseline Expanded Disability Status Scale (EDSS) scores between 0 and 5 (table e-1 on the Neurology® Web site at www.neurology.org). Patients were randomized (2:2:1) to receive IFNβ-1b 250 μg subcutaneously every other day, IFNβ-1b 500 μg subcutaneously every other day, or GA 20 mg subcutaneously daily for a minimum of 2 years and up to 3.5 years. MRI scans were acquired at baseline and annually thereafter. The primary results have been published.1
All available MRI scans were reanalyzed post hoc to the BEYOND trial in a blinded manner by the Neuroimaging Research Unit in Milan, Italy. The image acquisition protocol is described elsewhere.1 To qualify, patients had to have NL information at year 1 and lesion follow-up at year 2. Using year 1 scans as the baseline allowed the assessment of only those lesions that formed while on treatment. NL included gadolinium-enhancing and new T2 lesions with or without associated black holes at year 1. A new T2 lesion was defined as an area of hyperintensity on dual-echo scans at year 1 arising from an area of previously normal-appearing white matter on baseline scans. PBH were defined as those lesions with signal intensity between that of gray matter and CSF on postcontrast T1-weighted images. Year 3 scans were not reanalyzed as only approximately one-quarter of patients (23.4%, 312/1,333) were followed until this time point.
Statistical analyses.
Patient scans were reexamined for PBH post hoc to the BEYOND trial in a rater-blinded manner. The 2 predefined coprimary endpoints compared IFNβ-1b 250 μg with GA via conditional, sequential testing: first, the number of PBH per patient at year 2 that evolved from NL present at year 1 was analyzed; if significant, then the proportion of NL at year 1 that became PBH at year 2 was assessed. p Values less than 0.05 were considered significant. Conditional, sequential testing allowed for control of the type I error. IFNβ-1b 500 μg was also compared with GA on the 2 coprimary endpoints in an exploratory fashion.
Lesion counts were analyzed by negative binomial regression (without consideration of the presence of gadolinium-enhancing lesions at screening) and patient- and lesion-based proportions by logistic regression. Statistical analyses comparing the proportion of NL at year 1 that evolved into PBH at year 2 were adjusted for intrapatient correlations using generalized estimating equations (GEE). Sensitivity analyses that stratified by NL type (i.e., gadolinium-enhancing lesions, new T2 lesions associated with black holes at year 1, and new T2 lesions not associated with black holes at year 1) were performed, with nominal p values reported. In additional lesion-based analyses, year 1 NL volume (categorized into quartiles) or location (discrete, periventricular, posterior fossa, juxtacortical) was considered as a covariate (GEE analysis adjusted for intrapatient lesion correlation), and number of PBH per patient at year 2 evolving from year 1 NL was analyzed considering baseline T1-hypointense lesion volume (categorized according to median value), gender, and baseline EDSS score (<3, ≥3) as covariates (negative binomial regression). All analyses were performed using SAS statistical software package version 9.1.
RESULTS
Patients.
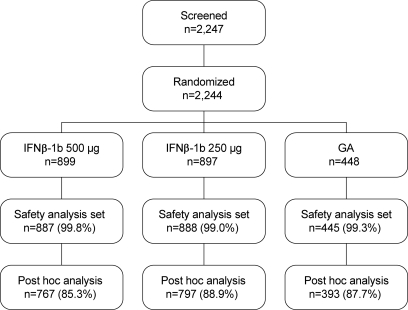
The patient flow diagram is depicted in figure 1. Demographic characteristics and clinical and MRI measures of disease at baseline were similar among the treatment arms (table 1). Of 1,957 patients, the proportions of patients in each treatment arm with at least one gadolinium-enhancing lesion at year 1 were as follows: IFNβ-1b 500 μg, 12.9% (99/767); IFNβ-1b 250 μg, 14.2% (113/797); and GA, 17.8% (70/393). The proportions of patients with at least one new T2 lesion at year 1 were as follows: IFNβ-1b 500 μg, 30.1% (231/767); IFNβ-1b 250 μg, 31.0% (247/797); and GA, 41.7% (164/393).
Figure 1. Patient flow diagram.
GA = glatiramer acetate; IFNβ-1b = interferon β-1b.
Table 1.
Baseline characteristics of all patients who received at least one dose of study medication, and who had NL information at year 1 and lesion follow-up at year 2
Abbreviations: EDSS = Expanded Disability Status Scale; GA = glatiramer acetate; Gd = gadolinium; IFNβ-1b = interferon beta-1b; NL = new lesion.
Number of PBH at year 2 that evolved from NL at year 1.
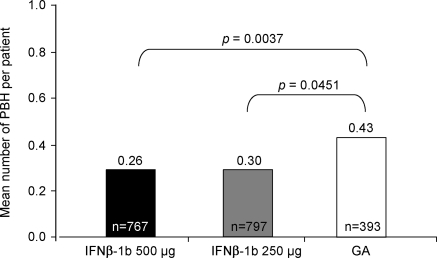
For the first coprimary endpoint, individuals treated with IFNβ-1b 250 μg had a lower mean number of PBH per patient at year 2 that evolved from NL at year 1 compared with GA-treated patients (0.30 vs 0.43; p = 0.0451). A reduced number of PBH at year 2 that evolved from NL at year 1 was observed when comparing IFNβ-1b 500 μg with GA (0.26 vs 0.43; p = 0.0037) (figure 2). These differences correspond to a relative risk reduction of 30% for IFNβ-1b 250 μg and 40% for IFNβ-1b 500 μg relative to GA.
Figure 2. Mean number of permanent black holes (PBH) at year 2 evolving from new lesions present at year 1.
GA = glatiramer acetate; IFNβ-1b = interferon β-1b.
Proportion of NL at year 1 that became PBH at year 2.
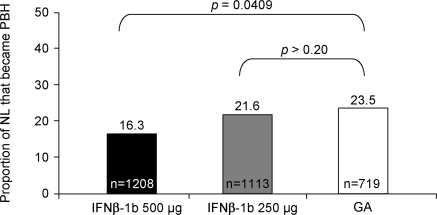
Among IFNβ-1b 250 μg-treated patients, 1,113 lesions (263 gadolinium-enhancing and 850 new T2 lesions) were found at year 1 that were not present at baseline; of these NL, 240 developed into PBH at year 2. Thus, the proportion of NL at year 1 that became PBH at year 2 was 21.6% (240/1,113) (figure 3). For the higher dose cohort, 1,208 NL (233 gadolinium-enhancing and 975 new T2 lesions) and 197 PBH developed, a proportion of 16.3%. A total of 719 NL at year 1 (180 gadolinium-enhancing and 539 new T2 lesions) and 169 PBH at year 2 were detected among GA-treated patients, corresponding to 23.5% (169/719). Although no difference between IFNβ-1b 250 μg and GA (p > 0.20) was found for the second coprimary endpoint, nominal difference in favor of IFNβ-1b 500 μg was detected when the higher dose group was compared with GA (p = 0.0409) (figure 3). This corresponds to a relative risk reduction of 31% in favor of IFNβ-1b 500 μg vs GA.
Figure 3. Proportion of year 1 new lesions (NL) that evolved into permanent black holes (PBH) at year 2 by treatment arm.
GA = glatiramer acetate; IFNβ-1b = interferon β-1b.
Sensitivity analyses.
Sensitivity analyses were performed to assess potential NL subtype contributions. A lower number of PBH at year 2 that evolved from NL at year 1 was observed for IFNβ-1b–treated patients relative to GA-treated patients. Sensitivity analyses attributed this finding predominantly to PBH at year 2 evolving from new T2 lesions associated with black holes at year 1 (IFNβ-1b 250 μg vs GA: 0.25 vs 0.36, p = 0.0467; IFNβ-1b 500 μg vs GA: 0.22 vs 0.36, p = 0.0084), while the number of PBH at year 2 that evolved from gadolinium-enhancing lesions had a similar, but nonsignificant, trend between IFNβ-1b and GA treatment arms (IFNβ-1b 250 μg vs GA: 0.04 vs 0.05, p = 0.7826; IFNβ-1b 500 μg vs GA: 0.03 vs 0.05, p = 0.2085).
Covariate analyses.
Number of PBH at year 2 that evolved from NL at year 1.
Adjusting for baseline T1-hypointense lesion volume, the findings on the numbers of PBH per patient at year 2 were lower for both IFNβ-1b doses than GA (IFNβ-1b 250 μg vs GA, p = 0.0856; IFNβ-1b 500 μg vs GA, p = 0.0038). Consistent patterns by treatment arms were observed when adjusting for gender (IFNβ-1b 250 μg vs GA, p = 0.0741; IFNβ-1b 500 μg vs GA, p = 0.0065), with more pronounced effects among men (IFNβ-1b 250 μg vs GA, 0.31 vs 0.68; p = 0.0062; IFNβ-1b 500 μg vs GA, 0.36 vs 0.68; p = 0.0198) than women (IFNβ-1b 250 μg vs GA, 0.30 vs 0.32; p = 0.8006; IFNβ-1b 500 μg vs GA, 0.21 vs 0.32; p = 0.0903). After adjusting for baseline EDSS, findings on the numbers of PBH per patient (IFNβ-1b 250 μg vs GA, p = 0.0463; IFNβ-1b 500 μg vs GA, p = 0.0027) were consistent with the primary outcome analysis, with potentially more pronounced effects among patients with baseline EDSS scores ≥3 (IFNβ-1b 250 μg vs GA, 0.27 vs 0.55; p = 0.0238; IFNβ-1b 500 μg vs GA, 0.21 vs 0.55; p = 0.0031) than <3 (IFNβ-1b 250 μg vs GA, 0.32 vs 0.38; p = 0.4074; IFNβ-1b 500 μg vs GA, 0.28 vs 0.38; p = 0.1194).
Proportion of NL at year 1 that became PBH at year 2.
As year 1 served as the baseline measurement in this analysis, the potential influence of year 1 NL location (discrete, periventricular, posterior fossa, juxtacortical) and volume on the proportion of PBH at year 2 was also assessed. Neither adjustment for location (IFNβ-1b 250 μg vs GA, p = 0.5861; IFNβ-1b 500 μg vs GA, p = 0.0471) nor for categorized lesion volume (IFNβ-1b 250 μg vs GA, p = 0.5310; IFNβ-1b 500 μg vs GA, p = 0.1316) altered the overall results. While categorized NL volume did not influence the treatment effects on PBH evolution, there was a potentially marginal contribution of the IFNβ-1b 500 μg dose on very large lesions (i.e., ≥185.6 mL) (proportion of PBH: IFNβ-1b 250 μg vs GA, 41.8% [119/285] vs 43.8% [89/203], p = 0.2741; IFNβ-1b 500 μg vs GA, 32.4% [88/272] vs 43.8%, p = 0.0397).
DISCUSSION
Assessment into the evolution of NL into PBH allows for monitoring of brain tissue destruction4,12,13 and may be used as a biomarker for neuroprotection.6 MRI evidence supporting a neuroprotective role for GA stems from the reduction in the number of NL that evolved into PBH relative to placebo.4 Similar results have been reported for natalizumab.14 In addition, the proportion of PBH that evolved from NL over 3 years of IFNβ-1b use was lower than that in the 3 years before treatment initiation.15 The BECOME study, which compared IFNβ-1b 250 μg with GA, revealed that a lower proportion of NL evolved into PBH in IFNβ-1b–treated than in GA-treated patients.10 This outcome served as the basis for the hypotheses tested in this analysis.
In this PBH analysis of the BEYOND study, the first coprimary endpoint—the number of PBH per patient at year 2 that evolved from NL at year 1—reflects the net number of such lesions that formed while patients were on treatment and can be considered a measure of the overall extent of irreversible tissue damage occurring under treatment. The mean numbers of PBH per patient at year 2 that evolved from NL present at year 1 were lower for both doses of IFNβ-1b than GA. The second coprimary endpoint, the proportion of NL converting into PBH, provides insight into the developmental fate of lesions that occur on treatment. While the proportion of NL at year 1 that evolved into PBH at year 2 was similar between IFNβ-1b 250 μg and GA, treatment with IFNβ-1b 500 μg resulted in fewer NL that became PBH than GA.
As the original BEYOND study design used infrequent, annual MRI scans, acute NL presence at year 1 served as the gating measurement of new, on-treatment disease activity. This analysis excluded baseline lesions because such lesions would not yet have been influenced by treatment during their subacute evolution. In this study, NL included both gadolinium-enhancing and new T2 lesions. Though only infrequent MRI scans were available from the BEYOND dataset, studies with frequent imaging have established that T2 lesions evolve from gadolinium-enhancing lesions in more than 95% of instances.4,11,16 This thus permits use of new T2 lesions as a marker of cumulative NL activity. To examine PBH origin by NL type, sensitivity analyses were performed. These analyses indicated that the observed differences between IFNβ-1b 250 μg and GA for the 2 coprimary endpoints were mostly attributable to PBH at year 2 evolving from new T2 lesions at year 1, and to a lesser degree to gadolinium-enhancing lesions. This is likely due to the frequency of scanning: gadolinium-enhancing lesions are transient with the vast majority inevitably lost when performing annual scans, but new T2 lesions can be seen on the last scan independently of the time of their formation during the interval between 2 consecutive scans. Adjusting for the covariates NL location, NL volume, baseline EDSS, and gender suggested that men and patients with higher baseline EDSS scores or higher lesion volume may in part contribute to the formation of a greater number of PBH. However, these adjustments did not alter the interpretation of the overall analysis.
The previous BEYOND MRI analysis on the change in volume of all T1 hypointensities showed no differences between the treatment groups.1 The partial differences between the primary BEYOND MRI analysis and this lesion-based assessment may be explained by the following: first, the primary MRI analysis measured T1-hypointense lesion volume change from screening to last available scan, while the post hoc analysis examined individual lesion evolution. Second, this post hoc analysis used only those lesions that formed while patients were on treatment. This controlled for the variable time disease-modifying therapies may need to exert their effects.17 Third, unlike the previous analysis, the design of this analysis distinguishes between acute and permanent T1 hypointensities. Fourth, new black holes that develop on treatment represent only a very small proportion of the overall burden of existing T1-hypointense lesions found at baseline,1 likely reducing the sensitivity of this measure.
The primary analysis of the BEYOND study also examined another potential marker of permanent tissue destruction, whole brain volume. No differences were found among the treatment arms for the change in brain volume from screening to LAS. However, from screening to year 1, patients treated with either IFNβ-1b dose experienced greater decreases in brain volume than did those assigned to GA. This effect was not seen from years 1 to 2 or from years 2 to 3,1 suggesting that IFNβ-1b may have more pronounced effects on whole brain inflammation soon after treatment initiation than GA. As with the change in T1-hypointense lesion volume from screening to LAS, whole brain volume is a coarse measurement. We believe that lesion-by-lesion analyses, such as those reported here, provide greater sensitivity and insight into the effects of disease-modifying treatments on MS course.
While its precise mechanism of action remains unknown, IFNβ-1b may indirectly promote neuronal preservation by altering lesion destructiveness through a downregulation of matrix metalloproteinases, which are known to degrade extracellular matrices.18 Second, relative to healthy controls, immune cells harvested from patients with RRMS secrete reduced levels of brain-derived neurotrophic factor (BDNF), a protein that prevents neuronal cell death and promotes remyelination.19 Treatment with IFNβ has been shown to upregulate expression of BDNF.20,21 Also, nerve growth factor is induced in a dose-dependent manner by IFNβ22 and is inversely correlated to MRI measures of axonal loss.23 Third, IFNβ-1b induces the expression of proteins associated with the inhibition of oxidative stress.24–26 These potential pleiotropic effects may allow IFNβ-1b to confer neuroprotection via a variety of synergistic mechanisms.
The reasons for the different effects of the 2 IFNβ-1b doses on the proportion of NL that evolved into PBH at year 2 remain to be established. Two potential, not necessarily mutually exclusive, explanations might be considered. Either there is a dose response, meaning the higher, experimental dose (IFNβ-1b 500 μg) is more neuroprotective than IFNβ-1b 250 μg, or the extent of destructiveness of the lesions that have already formed is systematically reduced by a more anti-inflammatory effect exerted by IFNβ-1b 500 μg relative to IFNβ-1b 250 μg. However, in the absence of MRI sequences with an increased specificity toward the most destructive aspects of MS pathology (i.e., irreversible demyelination and axonal loss) and of a more frequent MRI scanning schedule (e.g., monthly, as was the case for BECOME), it is not possible to determine on which aspect of lesion evolution—formation or resolution— IFNβ-1b 500 μg is exerting its effects.
This study was not without limitations. First, it was based on a post hoc reanalysis of MRI scans obtained from the BEYOND study. Second, it was methodologically limited by the neutral outcomes on the primary and secondary endpoints of the BEYOND study.1 Third, our dataset captured only infrequent MRI time points, thus allowing for only a partial assessment of lesion evolution and limiting our ability to define the actual time when PBH occurred. Despite these limitations, we believe in the internal validity of our data, since we achieved high ascertainment of a large study population, with resulting treatment cohorts that were well-matched on both disease and demographic characteristics. Furthermore, the proportion of NL that developed into PBH was similar to that measured in other studies.4,10 Sensitivity and covariate analyses did not alter the overall interpretation. Finally, the outcomes achieved upon comparison of IFNβ-1b 250 μg with GA were corroborated by the IFNβ-1b 500 μg dataset. Overall, these data show that IFNβ-1b 250 μg favorably influences an MRI outcome indicative of permanent tissue destruction in the brains of patients with MS—to at least a similar degree as GA. The more pronounced effect of the higher, experimental dose is intriguing, and warrants further investigation into its potential mechanisms of action.
Supplementary Material
ACKNOWLEDGMENT
This article was drafted by Massimo Filippi, MD, and Volker Knappertz, MD, with medical writing support (assisted with drafting the manuscript and responding to reviewer comments under the direction of the authors) by Tiffany DeSimone, PhD, of PAREXEL, who was funded by Bayer HealthCare Pharmaceuticals. Maria Bell of PAREXEL, who was funded by Bayer HealthCare Pharmaceuticals, copyedited (reviewed grammar, consistency, and spelling) and stylized the manuscript.
Supplemental data at www.neurology.org
- BDNF
- brain-derived neurotrophic factor
- EDSS
- Expanded Disability Status Scale
- GA
- glatiramer acetate
- GEE
- generalized estimating equation
- IFNβ-1b
- interferon β-1b
- LAS
- last available scan
- MS
- multiple sclerosis
- NL
- new lesion
- PBH
- permanent black hole
- RRMS
- relapsing-remitting multiple sclerosis
DISCLOSURE
Dr. Filippi serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd. and Genmab A/S; has received funding for travel from Bayer Schering Pharma, Biogen-Dompè AG, Genmab A/S, Merck Serono, and Teva Pharmaceutical Industries Ltd.; serves on editorial boards of the American Journal of Neuroradiology, BMC Musculoskeletal Disorders, Clinical Neurology and Neurosurgery, Erciyes Medical Journal, Journal of Neuroimaging, Journal of Neurovirology, Lancet Neurology, Magnetic Resonance Imaging, Multiple Sclerosis, and Neurological Sciences; serves as a consultant to Bayer Schering Pharma, Biogen-Dompè AG, Genmab A/S, Merck Serono, Pepgen Corporation, and Teva Pharmaceutical Industries Ltd.; serves on speakers' bureaus for Bayer Schering Pharma, Biogen-Dompè AG, Genmab A/S, Merck Serono, and Teva Pharmaceutical Industries Ltd.; and receives research support from Bayer Schering Pharma, Biogen-Dompè AG, Genmab A/S, Merck Serono, Teva Pharmaceutical Industries Ltd., Fondazione Italiana Sclerosi Multipla, and Fondazione Mariani. Dr. Rocca serves as consultant to Bayer Schering Pharma; and served on the speakers' bureau for Biogen-Dompé AG. Dr. Camesasca reports no disclosures. Dr. Cook serves on scientific advisory boards and as a consultant for Bayer Schering Pharma, Merck Serono, Biogen Idec, Sanofi-Aventis, Novartis, Neurology Reviews, Genmab A/S, and Actinobac Biomed Inc.; has received speaker honoraria from Bayer Schering Pharma and Merck Serono; serves on the editorial board of Neurology Reviews; receives publishing royalties for Editor Handbook of Multiple Sclerosis (Taylor and Francis, 2004); serves on the speakers' bureau for Bayer Schering Pharma; and receives research support from Bayer Schering Pharma and Merck Serono. Dr. O'Connor serves on scientific advisory boards for Novartis, Bayer Schering Pharma, Sanofi-Aventis, Genentech, Inc., and Roche; has received funding for travel from Biogen Idec and Teva Pharmaceutical Industries Ltd.; has received speaker honoraria from Biogen Idec, Novartis, and Sanofi-Aventis; serves/has served as a consultant for Biogen Idec, Actelion Pharmaceuticals Ltd., Bayer Schering Pharma, EMD Serono, Inc., Teva Pharmaceutical Industries Ltd., Genentech, Inc., and Warburg Pincus; and receives research support from Bayer Schering Pharma, Novartis, BioMS Medical, Sanofi-Aventis, CIS Pharma, Roche, and the MS Society of Canada. Dr. Arnason serves on scientific advisory boards for Bayer Schering Pharma, Sanofi-Aventis, Acorda Therapeutics Inc., and Novartis; serves on the editorial board of the International MS Journal; is listed as inventor on a patent re: Recombinant molecule that binds to Fc gamma receptors; has received honoraria from Sanofi-Aventis; serves as a consultant for Bayer Schering Pharma; and receives research support from Questcor Pharmaceuticals, Inc. Dr. Kappos has received research support through the University Hospital Basel from Acorda Therapeutics Inc., Actelion Pharmaceuticals Ltd, Advancell, Allozyne, Barofold, Bayer HealthCare Pharmaceuticals, Bayer Schering Pharma, Bayhill, Biogen Idec, BioMarin, Boehringer Ingelheim, CSL Behring, Geneuro, Genmab, GlaxoSmithKline, Glenmark, Merck Serono, MediciNova, Novartis, Sanofi-Aventis, Santhera Pharmaceuticals, Shire Plc, Roche, Teva, UCB, and Wyeth; also from the Swiss MS Society, the Swiss National Research Foundation, European Union, Gianni Rubato, Roche and Novartis Foundations. He serves on the editorial board of Multiple Sclerosis Journal, International Journal of Multiple Sclerosis, and Swiss Archives of Neurology and Psychiatry. Dr. Goodin has served on scientific advisory boards for Bayer Schering Pharma and Merck Serono; has received funding for travel and honoraria for speaking and consulting from Bayer Schering Pharma, Teva Pharmaceutical Industries Ltd., and Merck Serono; serves as Editor-in-Chief for the International MS Journal; has received speaker honoraria from Bayer Schering Pharma; has received research support from Bayer Schering Pharma and Novartis; and has served as an expert witness in medico-legal cases. Dr. Jeffery has received honoraria for speaking and consulting from Biogen Idec, Bayer Schering Pharma, Teva Pharmaceutical Industries Ltd., Merck Serono, Pfizer Inc., Novartis, Acorda Therapeutics Inc., and GlaxoSmithKline; serves on the editorial board of the Journal of Neurological Sciences; serves on speakers' bureaus for Biogen Idec, Bayer Schering Pharma, Teva Pharmaceutical Industries Ltd., Novartis, and Acorda Therapeutics Inc.; and has received research support from Biogen Idec, Bayer Schering Pharma, Teva Pharmaceutical Industries Ltd., Merck Serono, Pfizer Inc., and Novartis. Dr. Hartung serves on scientific advisory boards for Octapharma AG, Merck Serono, Teva Pharmaceutical Industries Ltd., Biogen Idec, and Eli Lilly and Company; and has received speaker honoraria from Biogen Idec, Teva Pharmaceutical Industries Ltd., Sanofi-Aventis, Merck Serono, Novartis, and Bayer Schering Pharma. Dr. Comi serves on speakers' bureaus for Teva Pharmaceutical Industries Ltd., Sanofi-Aventis, Merck Serono, Bayer Schering Pharma, Biogen-Dompé AG, Boehringer Ingelheim, and Novartis; and has received speaker honoraria from Teva Pharmaceutical Industries Ltd., Sanofi-Aventis, Merck Serono, Serono Symposia International Foundation, Bayer Schering Pharma, Novartis, Biogen-Dompè AG, and Merz Pharmaceuticals GmbH. Dr. Wolinsky has served on scientific advisory boards for Eli Lilly and Company and Bayer Schering Pharma; serves as an Associate Editor for ACPMedicine and on the editorial board of Multiple Sclerosis; has served as a consultant for Acorda Therapeutics Inc., Actelion Pharmaceuticals Ltd., Antisense Therapeutics Limited, Bayer Schering Pharma, EMD Serono, Inc., Facet Biotech Corporation, Genentech, Inc., Novartis, Peptimmune, Sanofi-Aventis, and Teva Pharmaceutical Industries Ltd.; serves on the speakers' bureau for Merck Serono; receives research support from Sanofi-Aventis, the NIH, the Clayton Foundation for Research, and the National Multiple Sclerosis Society; and receives royalties for monoclonal antibodies out-licensed through the University of Texas Health Science Center at Houston to Millipore (Chemicon International) Corporation. Dr. Bogumil is a full-time employee of Bayer HealthCare Pharmaceuticals. Dr. Pohl is a full-time employee of Bayer HealthCare Pharmaceuticals. K. Beckmann is a full-time employee of Bayer HealthCare Pharmaceuticals. Dr. Sandbrink is a full-time employee of Bayer HealthCare Pharmaceuticals. Dr. Croze is a full-time employee of Bayer HealthCare Pharmaceuticals. C. Brown was a full-time employee of Bayer HealthCare Pharmaceuticals at time of research conduct and is currently an employee of Novartis. Dr. DeSimone is an employee of PAREXEL MMS, which was funded by Bayer HealthCare Pharmaceuticals. Dr. Arnold serves on scientific advisory boards for Biogen Idec, Genentech, Inc., Roche, and Teva Pharmaceutical Industries Ltd.; has received speaker honoraria from Biogen Idec, Bayer Schering Pharma, CD-Pharma Interactive Medical Production, Genentech, Inc., EMD Serono, Inc., MS Forum, Sanofi-Aventis, GlaxoSmithKline, Merck Serono, and Teva Pharmaceutical Industries Ltd.; holds a patent re: Method of evaluating the efficacy of drug on brain nerve cells; has served as a consultant for Bayer Schering Pharma, Eisai Inc., Biogen Idec, NeuroRx Research Inc., Elan Corporation, Genentech, Inc., Genzyme Corporation, Eli Lilly and Company, GlaxoSmithKline, and Roche; has received research support from Biogen Idec, Bayer Schering Pharma, the Canadian Institutes of Health Research, and the Multiple Sclerosis Society of Canada; and holds stock in NeuroRx Research Inc. Dr. Cutter has served on scientific advisory boards for and received funding for travel from Millenium Pharmaceuticals, Inc., Klein Buendel, Inc., Alexion Pharmaceuticals, Inc., Androclus Therapeutics, Inc., University of Illinois, Amgen, New York University, and Somnus Therapeutics, Inc.; receives royalties from the publication of Evaluation of Health Promotion and Disease Prevention (The McGraw Hill Companies, 1984); has received honoraria from GlaxoSmithKline, Biogen Idec, Novartis, Advanced Health Media Inc., EMD Serono, Inc., EDJ Associates, Inc., the National Heart, Lung, and Blood Institute, National Institute of Neurological Diseases and Stroke, National Marrow Donor Program, Consortium of Multiple Sclerosis Centers; serves as a consultant to Peptimmune Inc., Aegis Creative Marketing, Novartis, National Industrial Sand Association, Bayer Pharmaceuticals, and Teva Pharmaceuticals Industries Ltd.; has served on independent data and safety monitoring committees for Antisense Therapeutics Limited, Sanofi-Aventis, Bayhill Pharmaceuticals, BioMS Medical Corp, Daiichi-Sankyo Co. Inc., GlaxoSmithKline, Genmab A/S, Medivation Inc., PTC Therapeutics Inc., Teva Pharmaceutical Industries Ltd., Vivus Inc., NHLBI, NINDS, NMSS; has received research support from ApopLogic Pharmaceuticals, LLC; receives research support from the NIH (NINDS/NIAID/NHLBI/NIDR/NIDDK), the Consortium of Multiple Sclerosis Centers, and the National Multiple Sclerosis Society; and serves as President of Pythagoras, Inc. Dr. Knappertz is a full-time employee of Bayer HealthCare Pharmaceuticals.
REFERENCES
- 1. O'Connor P, Filippi M, Arnason B, et al. BEYOND Study Group 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol 2009;8:889–997 [DOI] [PubMed] [Google Scholar]
- 2. Barkhof F, Karas GB, van Walderveen MA. T1 hypointensities and axonal loss. Neuroimaging Clin N Am 2000;10:739–752 [PubMed] [Google Scholar]
- 3. Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 2009;5:256–266 [DOI] [PubMed] [Google Scholar]
- 4. Filippi M, Rovaris M, Rocca MA, et al. European/Canadian Glatiramer Acetate Study Group Glatiramer acetate reduces the proportion of new MS lesions evolving into “black holes.” Neurology 2001;57:731–733 [DOI] [PubMed] [Google Scholar]
- 5. Sahraian MA, Radue EW, Haller S, Kappos L. Black holes in multiple sclerosis: definition, evolution, and clinical correlations. Acta Neurol Scand 2010;122:1–8 [DOI] [PubMed] [Google Scholar]
- 6. Fox RJ. Primary neuroprotection: the Holy Grail of multiple sclerosis therapy. Neurology 2010;74:1018–1019 [DOI] [PubMed] [Google Scholar]
- 7. van Walderveen MA, Kamphorst W, Scheltens P, et al. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology 1998;50:1282–1288 [DOI] [PubMed] [Google Scholar]
- 8. van Waesberghe JH, van Walderveen MA, Castelijns JA, et al. Patterns of lesion development in multiple sclerosis: longitudinal observations with T1-weighted spin-echo and magnetization transfer MR. Am J Neuroradiol 1998;19:675–683 [PMC free article] [PubMed] [Google Scholar]
- 9. van den Elskamp IJ, Lembcke J, Dattola V, et al. Persistent T1 hypointensity as an MRI marker for treatment efficacy in multiple sclerosis. Mult Scler 2008;14:764–769 [DOI] [PubMed] [Google Scholar]
- 10. Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNβeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009;72:1976–1983 [DOI] [PubMed] [Google Scholar]
- 11. Cadavid D, Cheriyan J, Skurnick J, et al. New acute and chronic black holes in patients with multiple sclerosis randomised to interferon beta-1b or glatiramer acetate. J Neurol Neurosurg Psychiatry 2009;80:1337–1343 [DOI] [PubMed] [Google Scholar]
- 12. Brück W, Bitsch A, Kolenda H, et al. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol 1997;42:783–793 [DOI] [PubMed] [Google Scholar]
- 13. van Walderveen MAA, Barkhof F, Hommes OR, et al. Correlating MRI and clinical disease activity in multiple sclerosis: relevance of hypointense lesions on short TR/short TE (T1-weighted) spin-echo images. Neurology 1995;45:1684–1690 [DOI] [PubMed] [Google Scholar]
- 14. Dalton CM, Miszkiel KA, Barker GJ, et al. Effect of natalizumab on conversion of gadolinium enhancing lesions to T1 hypointense lesions in relapsing multiple sclerosis. J Neurol 2004;251:407–413 [DOI] [PubMed] [Google Scholar]
- 15. Bagnato F, Gupta S, Richert ND, et al. Effects of interferon beta-1b on black holes in multiple sclerosis over a 6-year period with monthly evaluations. Arch Neurol 2005;62:1684–1688 [DOI] [PubMed] [Google Scholar]
- 16. Bonzano L, Roccatagliata L, Mancardi GL, Sormani MP. Gadolinium-enhancing or active T2 magnetic resonance imaging lesions in multiple sclerosis clinical trials? Mult Scler 2009;15:1043–1047 [DOI] [PubMed] [Google Scholar]
- 17. Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis: European/Canadian Glatiramer Acetate Study Group. Ann Neurol 2001;49:290–297 [PubMed] [Google Scholar]
- 18. Bernal F, Elias B, Hartung HP, Kieseier BC. Regulation of matrix metalloproteinases and their inhibitors by interferon-beta: a longitudinal study in multiple sclerosis patients. Mult Scler 2009;15:721–727 [DOI] [PubMed] [Google Scholar]
- 19. Aharoni R, Kayhan B, Eilam R, et al. Glatiramer acetate-specific T cells in the brain express T helper 2/3 cytokines and brain-derived neurotrophic factor in situ. Proc Natl Acad Sci USA 2003;100:14157–14162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Azoulay D, Mausner-Fainberg K, Urshansky N, et al. Interferon-beta therapy up-regulates BDNF secretion from PBMCs of MS patients through a CD40-dependent mechanism. J Neuroimmunol 2009;211:114–119 [DOI] [PubMed] [Google Scholar]
- 21. Lalive PH, Kantengwa S, Benkhoucha M, et al. Interferon-beta induces brain-derived neurotrophic factor in peripheral blood mononuclear cells of multiple sclerosis patients. J Neuroimmunol 2008;197:147–151 [DOI] [PubMed] [Google Scholar]
- 22. Boutros T, Croze E, Yong VW. Interferon-beta is a potent promoter of nerve growth factor production by astrocytes. J Neurochem 1997;69:939–946 [DOI] [PubMed] [Google Scholar]
- 23. Biernacki K, Antel JP, Blain M, et al. Interferon beta promotes nerve growth factor secretion early in the course of multiple sclerosis. Arch Neurol 2005;62:563–568 [DOI] [PubMed] [Google Scholar]
- 24. Croze E, Velichko S, Tran T, et al. Betaseron induces a novel alternate start transcript in cells obtained from relapsing-remitting multiple sclerosis patients and human brain that is associated with control of oxidative resistance. Mult Scler 2009;15:S138 [Google Scholar]
- 25. Croze E, Beekman J, Knappertz V, et al. Betaseron increases expression of metallothioneins in relapsing-remitting multiple sclerosis patients suggesting a role for Betaseron in neuroprotection. Mult Scler 2009;15:S138–S139 [Google Scholar]
- 26. Shkolnik K, Ben-Dor S, Galiani D, et al. Molecular characterization and bioinformatics analysis of Ncoa7B, a novel ovulation-associated and reproduction system-specific Ncoa7 isoform. Reproduction 2008;135:321–333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.