Abstract
Objective:
This study investigates the interaction between brain lesion location and monoamine oxidase A (MAO-A) in the genesis of aggression in patients with penetrating traumatic brain injury (PTBI).
Methods:
We enrolled 155 patients with PTBI and 42 controls drawn from the Vietnam Head Injury Study registry. Patients with PTBI were divided according to lesion localization (prefrontal cortex [PFC] vs non-PFC) and were genotyped for the MAO-A polymorphism linked to low and high transcriptional activity. Aggression was assessed with the aggression/agitation subscale of the Neuropsychiatric Inventory (NPI-a).
Results:
Patients with the highest levels of aggression preferentially presented lesions in PFC territories. A significant interaction between MAO-A transcriptional activity and lesion localization on aggression was revealed. In the control group, carriers of the low-activity allele demonstrated higher aggression than high-activity allele carriers. In the PFC lesion group, no significant differences in aggression were observed between carriers of the 2 MAO-A alleles, whereas in the non-PFC lesion group higher aggression was observed in the high-activity allele than in the low-activity allele carriers. Higher NPI-a scores were linked to more severe childhood psychological traumatic experiences and posttraumatic stress disorder symptomatology in the control and non-PFC lesion groups but not in the PFC lesion group.
Conclusions:
Lesion location and MAO-A genotype interact in mediating aggression in PTBI. Importantly, PFC integrity is necessary for modulation of aggressive behaviors by genetic susceptibilities and traumatic experiences. Potentially, lesion localization and MAO-A genotype data could be combined to develop risk-stratification algorithms and individualized treatments for aggression in PTBI.
Aggressive behavior is a common problem following penetrating traumatic brain injury (PTBI), reported by at least a third of patients with PTBI.1 Aggression has been linked to different pathogenetic mechanisms, including brain alterations, psychological trauma, and genetic susceptibilities.2 The relative roles and the interactions between these factors, however, are only partially understood.
One of the genes that has been linked with aggression is the monoamine oxidase A (MAO-A) gene,3 which catalyzes oxidative deamination of amines. Compared to MAO-B, MAO-A presents with a higher affinity for serotonin, i.e., one of the neurotransmitters believed to be more involved in pathologic aggression.2,3 Among the polymorphic sites described in the MAO-A gene, a common variable number tandem repeat (VNTR) polymorphism is thought to be particularly relevant for aggression. This polymorphism encodes 2 distinct functional variants: 1) a high-activity (3.5 and 4 repeats) and 2) a low-activity allele (2, 3, and 5 repeats).4 The low-activity allele compared with the high-activity variant has been shown to present with relatively lower transcriptional activity4 and to be potentially related to aggressive behaviors.3,5,6 Pathologic aggression has been associated with prefrontal cortex (PFC) alterations in ventromedial areas7 but also in the orbitofrontal and anterior cingulate cortices,2,7–9 suggesting a pivotal role for a complex PFC-based network in aggression.
The goal of this study was to examine the effect of the interaction between location of brain damage and the MAO-A VNTR polymorphism on aggressive behaviors in subjects with PTBI and their impact on the relationship between psychological trauma and PTBI-related aggression.
METHODS
Subjects and behavioral evaluation.
Enrolled subjects were drawn from the Vietnam Head Injury Study (VHIS) phase 3 registry as described elsewhere.10 We conducted phase 3 between 2003 and 2006 at Bethesda National Naval Medical Center (36–39 years postinjury). Each subject underwent neurologic and psychiatric examinations and a noncontrast brain CT scan. Preinjury characteristics and clinical follow-up data of the participants were available from military and Veterans Administration records. Subjects with a diagnosis of alcohol or substance abuse disorder, dementia, or psychotic disorder as assessed by clinical data were not considered eligible.
We enrolled 155 male Vietnam-era veterans with PTBI due to low-velocity missile wounds. These subjects were divided into 2 groups according to the involvement of PFC territories (see below): the PFC lesion group (106 subjects) and the non-PFC lesion group (49 subjects). We also enrolled 42 healthy male subjects who served in Vietnam but did not sustain brain injuries. Demographic and clinical data (table) showed no differences in age (F2,194 = 2.8; p = 0.06), education (F2,194 = 2.5; p = 0.08), ethnicity (Pearson χ2 = 1.0; p = 0.81), preinjury IQ (F2,194 = 2.0; p = 0.08) as assessed with the Armed Forces Qualification Test (AFQT),10 or depression (F2,194 = 1.8; p = 0.16) assessed with the Beck Depression Inventory (BDI-II)11 among the 3 groups (PFC lesion, non-PFC lesion, controls).
Table.
Demographic and neuropsychological data for the experimental and control groups
Abbreviations: BDI = Beck Depression Inventory; ETI = Early Trauma Inventory; MAO-A = monoamine oxidase A; NPI-a = aggression/agitation subscale of the Neuropsychiatric Inventory; PFC = prefrontal cortex; PTSD = posttraumatic stress disorder.
Aggression levels were measured with the agitation/aggression subscale of the Neuropsychiatric Inventory (NPI-a),12 a widely used tool for assessment of behavioral disturbances in neurologic patients. The NPI is based on a structured interview with a caregiver and evaluates the severity and frequency of psychopathology along different dimensions. For each dimension, frequency is rated 1–4 and severity is scored 1–3, while their product represents the total score. The NPI-a subscale has been used to quantify aggressive behaviors in patients with cognitive impairment13 and as a treatment outcome measure in pharmaceutical intervention studies for aggression control in patients with TBI.14
Because childhood psychological trauma and a diagnosis of posttraumatic stress disorder (PTSD) have been shown to potentially impact aggression levels,5,15 we evaluated early psychological trauma by administering the Early Trauma Inventory (ETI)16 (a validated 56-item interview designed for the assessment of traumatic experiences in childhood), while current PTSD symptomatology was assessed using the Clinician-Administered PTSD Scale (CAPS)17,18 (a tool designed to assess PTSD symptoms according to the DSM-IV-TR).19
Standard protocol approvals, registrations, and patient consents.
All subjects gave informed written consent before enrollment in the study. The National Naval Medical Center and the NIH Institutional Review Boards approved all the study procedures.
CT imaging and lesion identification.
Axial noncontrast CT scans were acquired and analyzed as described in e-Methods on the Neurology® Web site at www.neurology.org and in Raymont et al.10 PFC territories were identified in each individual scan as described in e-Methods. Note that if the patient's lesion overlapped or partially overlapped with PFC territories, he was then included in the PFC lesion group; otherwise, he was included in the non-PFC lesion group.
Genotyping protocol.
Genotyping for the MAO-A VNTR was performed according to Ducci and collaborators.20 Subjects were divided into 2 subgroups according to the VNTR MAO-A alleles (MAO-A high-activity group [3.5 and 4 repeats] and MAO-A low activity group [2, 3, and 5 repeats]) as reported in the table. The observed high-activity allele/low-activity allele ratio was 1.78, in line with published data.4 MAO-A high-activity and low-activity groups demographic and clinical data were matched on age (t = −0.2; p = 0.85), education (t = −1.0; p = 0.29), ethnic background (Pearson χ2 = 0.3; p = 0.62), preinjury IQ assessed with the AFQT10 (t = 1.4; p = 0.15), and depression levels (t = 0.76; p = 0.45)11 (table).
Lesion maps.
First, the relationship between lesion location and aggressive behavior was explored using a lesion overlap approach.18 Subjects were divided into 2 groups according to NPI-a scores: a nonaggressive group (n = 60) showing no pathologically aggressive behaviors (NPI-a = 0) vs an aggressive group (n = 58) showing pathologically aggressive behaviors (NPI-a higher than the PTBI whole group mean: 1.2 ± 1.3). Both groups were matched on preinjury AFQT percentile score (aggressive vs nonaggressive subjects: 59.9 ± 1.2 vs 62.6 ± 1.3, t = 1.0, p = 0.2), age (58.2 ± 0.3 vs 58.4 ± 0.6, t = 0.4, p = 0.7), education (14.8 ± 0.2 vs 14.6 ± 0.3 years, t = 0.8, p = 0.6), or BDI-II scores (10.3 ± 0.9 vs. 8.9 ± 0.4, t = 1.2, p = 0.1). Overlap lesion maps were then generated for both groups; moreover, subtraction lesion maps were generated to show which areas were relatively more lesioned in the aggressive compared to the nonaggressive group and vice versa. To assess the significance of the prefrontal lesions distribution difference, a χ2 test was applied comparing the frequency of subjects with prefrontal vs nonprefrontal lesions in the 2 groups.
Analysis of polymorphism/lesion interactions.
Second, the interactions among MAO-A expression, lesion location, and trauma on aggression levels were explored. A 2 × 3 analysis of covariance (ANCOVA) on NPI-a scores was conducted with genotype (MAO-A high-activity, MAO-A low-activity) and group (PFC lesion, non-PFC lesion, control) as between-subjects factors and ETI scores and PTSD symptomatology as covariates. In a planned secondary analysis, t tests were then used to explore differences on NPI-a aggression scores between carriers of MAO-A high-activity and MAO-A low-activity alleles separately for each group (PFC, non-PFC, control). These analyses were performed using NPI-a scores adjusted for the ANCOVA covariates (NPI-a least square means).
Traumatic experiences and aggression.
Third, the effects of PTBI and MAO-A expression on the relationship between aggressive behavior and early traumatic experiences as well as PTSD were explored. Spearman correlations were used to correlate NPI-a scores with ETI and PTSD symptoms separately for the 3 lesion groups (PFC, non-PFC, control).
Lesion volume and aggression.
Finally, using Spearman correlations the percentage of PFC volume loss due to lesion was correlated with NPI-a scores separately for MAO-A high and MAO-A low allele carriers in the PFC and non-PFC groups. The subgroups were matched in volume loss (PFC: 3.4% ± 0.3 vs 3.6% ± 0.6, p = 0.61; non-PFC: 2.4% ± 0.3 vs 2.7% ± 0.5, p = 0.61).
Significance level for all analyses was set at p = 0.05 (2-tailed). All results are reported as means ± standard errors.
RESULTS
Lesion maps.
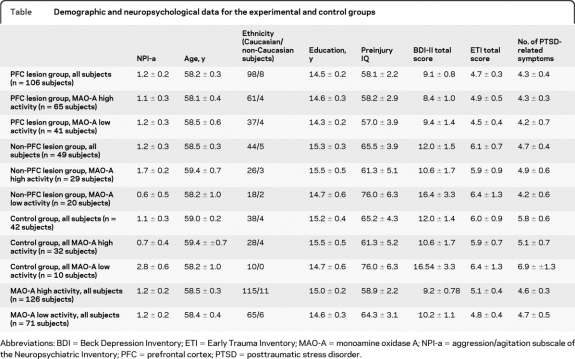
Lesion maps of the aggressive and nonaggressive groups are shown in figure 1. Subtraction lesion maps showing those brain regions that were more likely to contain a lesion in the aggressive group compared to the nonaggressive group and vice versa are shown in figure 2. Note the more focal involvement of PFC areas in the aggressive group compared to a more widespread lesion pattern in the nonaggressive group. In the aggressive group (58 subjects), 46 subjects presented with PFC lesions and 12 subjects presented with non-PFC lesions while in the nonaggressive group (60 subjects) 28 subjects presented with PFC lesions and 32 subjects presented with non-PFC lesions. χ2 statistics revealed that PFC lesions were more represented in the aggressive compared to the nonaggressive group (p = 0.001).
Figure 1. Lesion overlap map for the aggressive (A, red areas) and nonaggressive subjects (B, blue areas).
Results are overlaid on Montreal Neurological Institute T1 brain standard template. Images in radiologic convention (left side of the brain is represented on the right side of the pictures).
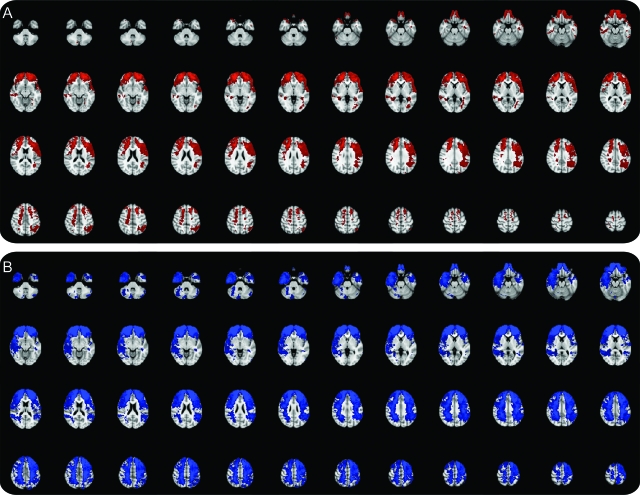
Figure 2. Lesion subtraction map between the aggressive and nonaggressive subjects.
Red blobs represent those areas relatively more lesioned in the aggressive than in the nonaggressive group while the blue blobs represent those areas relatively more lesioned in the nonaggressive than in the aggressive group. Results are overlaid on Montreal Neurological Institute T1 brain standard template. Images in radiologic convention (left side of the brain is represented on the right side of the pictures). A greater likelihood of brain damage in prefrontal areas (especially ventral, medial, and frontopolar regions) can be seen for the aggressive vs nonaggressive group subtraction map.
Analysis of polymorphism/lesion interactions.
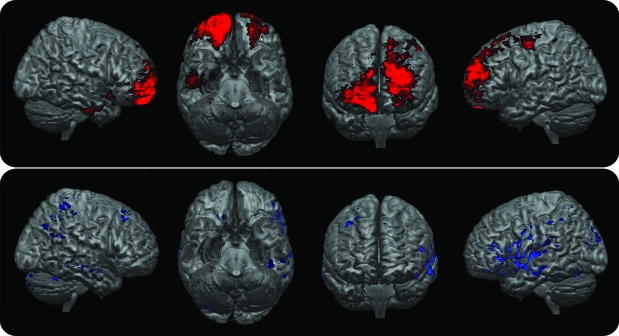
Second, the interactions among MAO-A expression, lesion location, and trauma on aggression were explored. The 2 × 3 ANCOVA on NPI-a scores revealed no main effects for genotype (F1,184 = 0.5; p = 0.46) and group (F2,184 = 0.2; p = 0.85), but an interaction effect for genotype × group (F2,184 = 5.4; p = 0.005) and an effect for both the covariates studied, i.e., the ETI score (F1,184 = 6.5; p = 0.01) and number of PTSD symptoms (F1,184 = 7.4; p = 0.007). All the other interactions were not significant. The planned follow-up analyses revealed lower NPI-a scores for the MAO-A high-activity compared to the MAO-A low-activity allele carriers in the control group (MAO-A high vs MAO-A low least squares means: 0.5 ± 0.1 vs 2.4 ± 0.5, t = 3.3; p = 0.005). Although MAO-A activity did not modulate aggression levels in patients with PFC lesions (MAO-A high vs MAO-A low least squares means: 1.1 ± 0.3 vs 1.2 ± 0.3; p = 0.967), higher NPI-a scores for MAO-A high activity compared to the MAO-A low activity carriers were found in patients with non-PFC lesions (MAO-A high vs MAO-A low least squares means: 2.1 ± 0.2 vs 0.6 ± 0.2, t = 3.0; p = 0.007). Raw NPI-a scores divided according to MAO-A activity and lesion location are reported in the table. Least squares means according to MAO-A activity and lesion location are reported in figure 3.
Figure 3. Least squares means aggression/agitation subscale of the Neuropsychiatric Inventory (NPI-a) scores (mean ± SEM) corrected for Early Trauma Inventory scores and posttraumatic stress disorder symptoms for all experimental subjects divided according to monoamine oxidase A activity and lesion location.
(A) Prefrontal cortex (PFC) lesion group; (B) non-PFC lesion group; (C) controls.
Traumatic experiences and aggression.
Third, the effects of PTBI and MAO-A expression on the relationship between aggressive behavior and early traumatic experiences as well as PTSD were explored. Statistics for early traumatic experiences (ETI scores) and PTSD (CAPS scores) are reported in the table. Analyzing separately the PFC, non-PFC, and control groups, we showed positive correlations between NPI-a and ETI and CAPS scores only for the control group (ETI: rho = 0.35, p = 0.02; PTSD: rho = 0.30, p = 0.02) and non-PFC lesion group (ETI: rho = 0.28, p = 0.04; PTSD: rho = 0.43, p = 0.01) while we did not find any correlation between NPI-a and ETI and CAPS scores in the PFC lesion group (ETI: rho = 0.1, p = 0.15; PTSD: rho = 0.09, p = 0.15).
Lesion volume and aggression.
Finally, we found a positive correlation between volume loss and NPI-a scores in MAO-A high carriers of the non-PFC group (rho = 0.52, p = 0.002) while NPI-a scores did not correlate with volume loss in all the other subgroups (non-PFC: MAO-A low [rho = 0.2, p = 0.6]; PFC: MAO-A high [rho = 0.1, p = 0.5]; MAO-A low [rho = 0.2, p = 0.4]).
DISCUSSION
The goals of this study were to examine the effect of the interaction between location of brain damage and MAO-A VNTR polymorphism on aggressive behaviors in subjects with PTBI, and to evaluate the effect of brain lesions and MAO-A activity on the relationship between psychological trauma and PTBI-related aggression. First, we demonstrated that PFC lesions are overrepresented in aggressive patients with PTBI compared to nonaggressive subjects with PTBI. Second, we showed an interaction between lesion localization and MAO-A activity on PTBI-related aggressive behavior. Although MAO-A low-activity allele carriers showed higher aggression than MAO-A high-activity allele carriers in the control group, the reverse effect was found in the non-PFC group, and no effect was found in the PFC group. Finally, we showed a significant association between negative childhood experiences and PTSD symptomatology and aggression levels only for the control and non-PFC groups, but not for the PFC group. Our findings seem thus to suggest that PFC structural integrity is necessary to allow other factors such as MAO-A genotype, childhood experiences, and PTSD to modulate PTBI-related aggression.
Our results in the normal group, showing a role for low-activity MAO-A allele on aggression levels, are consistent with previous studies.5,6,9,21 Absence of MAO-A activity has been shown to be related with aggressive antisocial behaviors in a Dutch kindred showing a MAO-A null point mutation6 and in MAO-A knockout animal models.21 Moreover, a seminal study of Caspi and collaborators5 showed a significant relationship between MAO-A low-activity allele and aggression subjects with negative childhood experiences. Finally, MAO-A represents one of the main modulators of serotonin levels, which are thought to play a key role in aggressive behaviors.9
Regarding the role of specific brain areas in TPBI-related aggression, our results reaffirm a key role for PFC territories in modulating aggression. Evidence of PFC involvement in aggressive behaviors has been shown in both functional neuroimaging and lesion studies.7,8,22–25 In healthy controls, functional neuroimaging studies showed both a reduction of ventral PFC activity during mental imagery of violent acts8,22 and an increase of ventral PFC activity during inhibition of impulsive behaviors.23 Moreover, focal brain lesion studies revealed a relationship between ventral PFC structural alterations and verbal aggression in subjects with PTBI.7 However, while ventromedial PFC is thought to play a pivotal role in aggression, other PFC regions including ventrolateral PFC and cingulate cortex24,25 are thought to play an important role as well.
Aside from PTBI, our results showed that other factors such as MAO-A genotype and psychologically traumatic experiences have a crucial influence on aggressive behavior. MAO-A alleles that alter expression levels have been shown to impact prefrontal structural and functional anatomy in previous neuroimaging studies.23,25 For example, during an emotional arousal task, carriers of MAO-A low-activity alleles showed reduced orbitofrontal activity compared to subjects with high-activity alleles.25 Reduced prefrontal activity in MAO-A low-activity allele compared to high-activity allele carriers was also shown during a working memory task and during an impulse inhibition task in healthy subjects.23
As both aggressive behaviors and reduced PFC activity have been associated with reduced MAO-A activity, and as low PFC activity has been linked with aggressive behaviors, MAO-A effects on aggression should be mediated through a modulation of PFC functions. This hypothesis is to be consistent with our observation of a lack of effect of MAO genotype on aggression levels in subjects with significant prefrontal PTBI.
Consistent with this observation, we also showed both a significant correlation between aggression and early traumatic experiences and PTSD in those subjects with PTBI without PFC lesions and in controls. Like MAO-A activity, negative experiences have also been related to frontal lobe structural and functional abnormalities. Reduced frontal volume has been shown in subjects who suffered childhood sexual abuse compared to controls in a volumetric MRI study,26 while retrieval of traumatic childhood memories has been linked to reduced activations of orbitofrontal and medial frontal territories in subjects with borderline personality disorder.27 These results suggest that factors such as MAO-A genotype, early traumatic experiences, and PTSD symptoms require an intact PFC in order to have a significant influence on aggressive behavior.
Finally, we observed increased levels of aggression in those subjects with non-PFC lesions and MAO-A high-activity allele. While the role of MAO-A low activity as a susceptibility factor for aggressive behaviors has been confirmed in a recent meta-analysis,28 other studies have also shown a relationship between high MAO-A activity and clinical entities such as borderline personality disorder,29 attention-deficit/hyperactivity disorder,30 antisocial conduct,31,32 and childhood externalizing behaviors.28 Although to date the neurobiological basis of these conditions is not completely understood, they can be collectively characterized as impulsivity and behavioral dyscontrol problems. Consistent with our results, the relationship between behavioral dyscontrol (and thus inappropriate aggression) and the MAO-A high-activity alleles could be mediated by nonfrontal areas as suggested by a functional neuroimaging study of impulse inhibition.23 In that study, the presence of the high-activity MAO-A allele correlated with reduced activation in posterior cortices, thus suggesting an effect of the MAO-A high-activity allele on posterior cortical functional anatomy.23 In our non-PFC population, the MAO-A high-activity group had a higher NPI-a score than the MAO-A low-activity group. We argue that a cumulative effect of reduced posterior cortex activity due to the MAO-A high allele23 and lesion presence in the posterior cortex leads to a lower functioning impulse inhibition network and thus to inappropriate aggression. Our hypothesis is supported by the observation that for the non-PFC group increased lesion volume was associated with higher NPI-a scores in MAO-A high-activity allele carriers. However, further studies are needed to better clarify the effects of posterior cortices activity by TPBI and MAO-A polymorphisms on the key area of aggression control, i.e., the PFC.
Our data and the evidence reported in the literature suggest that several factors including genetic polymorphisms and brain damage may modulate aggressive behaviors via altered cognitive and social processes mediated by the PFC. However, we also showed that aggression levels in subjects with prefrontal PTBI are not modulated by MAO-A activity, early life experiences, or PTSD symptomatology, even though these factors are related to aggressive behaviors in subjects with posterior brain damage (but an intact PFC) and healthy controls.
One limitation of the current study is its reliance on combat veterans, i.e., a population trained to be aggressive and then exposed to experiences of physical aggression and bodily injuries. While this common background makes our subjects more homogeneous regarding previous exposure to aggression, studies on subjects without war-related experiences are needed to validate these findings in the general population.
Moreover, given the multifaceted nature of aggression, larger studies are needed to better quantify the relative importance of environmental, genetic, and brain structural factors in aggressive behaviors in different neurologic populations.
The differences in the effects of the MAO-A VNTR polymorphism on aggression between individuals without structural brain damage and those presenting with prefrontal or nonprefrontal PTBI seems to suggest that 1) different treatment protocols might be necessary for managing inappropriate aggressive behavior in patients with prefrontal or posterior lesions and 2) other factors ranging from the severity and frequency of previous traumatic experiences to genetic polymorphisms need to be taken into consideration when determining the effects of brain damage on aggression. Future studies are warranted to evaluate the clinical relevance of our observations regarding lesion location/polymorphism-guided pharmacologic (given the possible role of MAO-A in drug responses) and behavioral approaches (given the observed differences in negative experiences/aggression relationships we observed between the lesion groups) for PTBI-related aggression.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Vietnam veterans who participated in this study. Without their long-term commitment to improving the health care of veterans, this study could not have been completed. The authors thank the National Naval Medical Center for their support and provision of their facilities and S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding, and G. Tasick for their help with the testing of participants and organization of this study.
- AFQT
- Armed Forces Qualification Test
- ANCOVA
- analysis of covariance
- BDI
- Beck Depression Inventory
- CAPS
- Clinician-Administered PTSD Scale
- DSM-IV-TR
- Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision
- ETI
- Early Trauma Inventory
- MAO-A
- monoamine oxidase A
- NPI-a
- aggression/agitation subscale of the Neuropsychiatric Inventory
- PFC
- prefrontal cortex
- PTBI
- penetrating traumatic brain injury
- PTSD
- posttraumatic stress disorder
- VHIS
- Vietnam Head Injury Study
- VNTR
- variable number tandem repeat.
Editorial, page 1032
Supplemental data at www.neurology.org
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the US Government.
DISCLOSURE
Dr. Pardini and Dr. Krueger report no disclosures. Dr. Hodgkinson receives intramural research support from the NIH. Dr. Raymont and C. Ferrier report no disclosures. Dr. Goldman serves on the editorial boards of Biological Psychiatry and Addictions Biology. Dr. Strenziok and Dr. Guida report no disclosures. Dr. Grafman serves as Co-editor of Cortex and receives research support from the Intramural Research Program NIH/NINDS and the Henry M. Jackson Foundation.
REFERENCES
- 1. Tateno A, Jorge RE, Robinson RG. Clinical correlates of aggressive behavior after traumatic brain injury. J Neuropsychiatry Clin Neurosci 2003;15:155–160 [DOI] [PubMed] [Google Scholar]
- 2. Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci 2007;8:536–546 [DOI] [PubMed] [Google Scholar]
- 3. Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci 1999;22:197–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 1998;103:273–279 [DOI] [PubMed] [Google Scholar]
- 5. Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science 2002;297:851–854 [DOI] [PubMed] [Google Scholar]
- 6. Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 1993;262:578–580 [DOI] [PubMed] [Google Scholar]
- 7. Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology 1996;46:1231–1238 [DOI] [PubMed] [Google Scholar]
- 8. Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am J Psychiatry 2000;157:1772–1781 [DOI] [PubMed] [Google Scholar]
- 9. Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry 2008;165:429–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raymont V, Greathouse A, Reding K, Lipsky R, Salazar A, Grafman J. Demographic, structural and genetic predictors of late cognitive decline after penetrating head injury. Brain 2008;131:543–558 [DOI] [PubMed] [Google Scholar]
- 11. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories IA and II in psychiatric outpatients. J Pers Assess 1996;67:588–597 [DOI] [PubMed] [Google Scholar]
- 12. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314 [DOI] [PubMed] [Google Scholar]
- 13. Pritchard AL, Ratcliffe L, Sorour E, et al. Investigation of dopamine receptors in susceptibility to behavioural and psychological symptoms in Alzheimer's disease. Int J Geriatr Psychiatry 2009;24:1020–1025 [DOI] [PubMed] [Google Scholar]
- 14. Masanic CA, Bayley MT, VanReekum R, Simard M. Open-label study of donepezil in traumatic brain injury. Arch Phys Med Rehabil 2001;82:896–901 [DOI] [PubMed] [Google Scholar]
- 15. Jakupcak M, Conybeare D, Phelps L, et al. Anger, hostility, and aggression among Iraq and Afghanistan War veterans reporting PTSD and subthreshold PTSD. J Trauma Stress 2007;20:945–954 [DOI] [PubMed] [Google Scholar]
- 16. Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress Anxiety 2000;12:1–12 [DOI] [PubMed] [Google Scholar]
- 17. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress 1995;8:75–90 [DOI] [PubMed] [Google Scholar]
- 18. Koenigs M, Huey ED, Raymont V, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci 2008;11:232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 20. Ducci F, Enoch MA, Hodgkinson C, et al. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry 2008;13:334–347 [DOI] [PubMed] [Google Scholar]
- 21. Cases O, Seif I, Grimsby J, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 1995;268:1763–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strenziok M, Krueger F, Heinecke A, et al. Developmental effects of aggressive behavior in male adolescents assessed with structural and functional brain imaging. Soc Cogn Affect Neurosci Epub 2009 Sep 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Passamonti L, Fera F, Magariello A, et al. Monoamine oxidase-a genetic variations influence brain activity associated with inhibitory control: new insight into the neural correlates of impulsivity. Biol Psychiatry 2006;59:334–340 [DOI] [PubMed] [Google Scholar]
- 24. Raine A, Buchsbaum MS, Stanley J, Lottenberg S, Abel L, Stoddard J. Selective reductions in prefrontal glucose metabolism in murderers. Biol Psychiatry 1994;36:365–373 [DOI] [PubMed] [Google Scholar]
- 25. Meyer-Lindenberg A, Buckholtz JW, Kolachana B, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 2006;103:6269–6274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 2008;20:292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biol Psychiatry 2004;55:759–765 [DOI] [PubMed] [Google Scholar]
- 28. Kim-Cohen J, Caspi A, Taylor A, et al. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol Psychiatry 2006;11:903–913 [DOI] [PubMed] [Google Scholar]
- 29. Ni X, Sicard T, Bulgin N, et al. Monoamine oxidase a gene is associated with borderline personality disorder. Psychiatr Genet 2007;17:153–157 [DOI] [PubMed] [Google Scholar]
- 30. Manor I, Tyano S, Mel E, et al. Family-based and association studies of monoamine oxidase A and attention deficit hyperactivity disorder (ADHD): preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA). Mol Psychiatry 2002;7:626–632 [DOI] [PubMed] [Google Scholar]
- 31. Vollm BA, Zhao L, Richardson P, et al. A voxel-based morphometric MRI study in men with borderline personality disorder: preliminary findings. Crim Behav Ment Health 2009;19:64–72 [DOI] [PubMed] [Google Scholar]
- 32. Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by positron emission tomography. Biol Psychiatry 1997;42:495–508 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.