SECTION 1
A 46-year-old right-handed African American man presented to the emergency department after a 10-minute episode of sudden-onset left-hand weakness and slurred speech. His medical history was significant for a left cerebellar stroke 6 years ago. That event occurred during a physical training exercise and was thought to be secondary to a vertebral artery dissection.1,2 He was placed on aspirin 81 mg daily. The patient had minimal residual deficits, with left face numbness and left hypoacusis. He did not smoke, drink, or use recreational drugs. There was no family history of stroke or vascular disease.
On initial examination, the patient was normotensive. His mental status was intact, with a Mini-Mental State Examination (MMSE) score of 30/30. There was no apraxia, aphasia, or visual field deficit. Cranial nerve examination had normal results with the exception of left hypoacusis and left facial numbness over the V1–V3 distribution to pinprick. Funduscopic examination revealed no papilledema or vasculopathy. Motor, sensory, and cerebellar examinations were normal. Deep tendon reflexes were 2+ throughout with bilateral plantar responses.
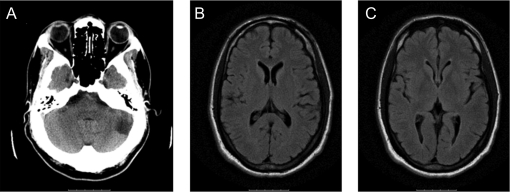
Laboratory testing including a complete blood count, serum chemistry, coagulation panel, and liver function tests were normal. EKG showed normal sinus rhythm and no evidence of left ventricular hypertrophy. MRI showed no evidence of acute intracranial abnormalities; a chronic left cerebellar infarct was seen (figure 1). CT angiography demonstrated severe bilateral middle cerebral artery (MCA) stenosis, worse on the right, as well as an absent left vertebral artery which was confirmed by conventional angiography (figure 2A).
Figure 1. Initial imaging.
Noncontrast head CT (A) on admission demonstrating chronic left cerebellar infarct. Fluid-attenuated inversion recovery (FLAIR) MRI on admission shows no evidence of ischemic changes in the middle cerebral artery territory (B). FLAIR was also negative (C).
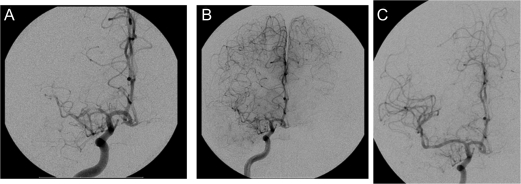
Figure 2. Initial cerebral angiogram.
Angiogram on admission (A), at time of cognitive decline (B), and poststent placement (C) documenting progressive stenosis of middle cerebral artery and restoration of flow with stent placement.
Questions for consideration:
What is the differential diagnosis of intracranial stenosis?
What diagnostic studies should be considered?
SECTION 2
The most common cause of intracranial large-vessel stenosis is intracranial atherosclerotic disease.3 Other less common causes are primary angiitis of the CNS, a rare and poorly understood form of vascular inflammatory disease restricted to the brain and spinal cord, and secondary vasculitis of the CNS. These are a heterogeneous group of disorders characterized by blood vessel inflammation, producing different but frequently overlapping clinical manifestations. Histologic changes allow classification according to the size of the vessel implicated. These include giant cell arteritis, polyarteritis, and systemic vasculitis secondary to Wegener granulomatosis, systemic lupus erythematosus, Sjögren syndrome, collagen diseases, and those associated with neurocutaneous syndromes or malignancy (leukocytoclastic vasculitis, intravascular lymphoma). Other noninflammatory vasculopathies to consider include vasospasm (cocaine-induced, reversible cerebral vasoconstriction syndrome), infections (Lyme disease, meningovascular syphilis, and HIV-associated infections), and hematologic conditions (i.e., sickle cell disease). Other vasculopathies associated with intracranial stenosis are radiation vasculopathy and fibromuscular dysplasia. Finally, moyamoya disease is a noninflammatory vasculopathy with progressive stenosis or occlusion of the distal internal carotid artery and proximal cerebral arteries.4
This patient's evaluation included a lipid panel, 24-hour Holter monitoring, and echocardiogram. Due to the patient's young age, a hypercoagulability panel was considered. Since magnetic resonance angiography does not have sufficient resolution to document changes in medium and small-vessel vasculitis, angiography was performed for better evaluation of the intracranial vasculature, as well as to look for evidence of dissection. CSF was analyzed for evidence of a vasculitic inflammatory process and importantly, to exclude an infectious process or malignancy. HIV testing and urine toxicology was also performed.
Other than elevated total cholesterol (190 mg/dL) and low-density lipoprotein (130 mg/dL) levels, the evaluation was negative, including negative results for transesophageal echocardiogram, hypercoagulability panel, CSF analysis, serologic testing, HIV testing, urine toxicology, peripheral smear, and sickle cell screening. No arrhythmias were identified on 24-hour Holter monitoring. Cerebral angiography showed moderate to severe right MCA stenosis and moderate left MCA stenosis (figure 2A). There was no acute evidence of dissection or underlying vasculopathy or vasospasm. The decision was made to treat the patient with medical therapy. Aspirin was discontinued and the patient was placed on clopidogrel, simvastatin, and low-dose amlodipine with no recurrence of symptoms.
Two months later, the patient returned to the clinic with the complaint of intermittent numbness and weakness on his left side. He had experienced a significant decline in his memory and functional status. He was unable to return to work. His wife noted that he appeared “slow” and irritable. He had begun dragging his left leg when walking and had gotten lost while driving. He was noted to have a mild hemiparesis on the left. His MMSE had declined to 26/30.
Questions for consideration:
What additional test would you consider?
Would you recommend changes in his medical regimen?
SECTION 3
The patient is being treated medically for intracranial stenosis and now presents with worsening symptoms, with progressing left-sided deficits as well as cognitive decline. There are no randomized data to support the practice of switching antiplatelet agents or the use of anticoagulation for intracranial stenosis.5 Brain MRI is indicated to rule out the presence of acute or subacute infarction. CT angiography should be considered to evaluate for worsening intracranial stenosis and hypoperfusion. Neuropsychological testing was performed to better define areas of cognitive impairment.
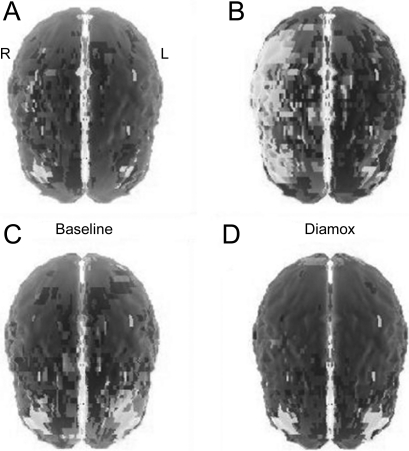
A SPECT scan with acetazolamide revealed decreased perfusion in the cerebral hemispheres, worse on the right, consistent with decreased vascular reserve in the MCA territories (figure 3A). Neuropsychological evaluation (which provides an additional degree of sensitivity in terms of detecting cerebral dysfunction) strongly suggested deficits in right hemisphere functioning (impaired visuospatial judgment, constructional apraxia, and reduced insight and self-monitoring) with no evidence of impersistence (motor or otherwise). His visual perception skills fell in the impaired range and his copy of a complex figure was notable for omission of elements as well as inaccuracies. He was slow to acquire verbal information when presented in list format and recalled only 5 of 10 items despite multiple trials. There was evidence of executive dysfunction with impairments on clock drawing (number repetitions, hands incorrectly placed). There was no evidence of anxiety or depression. These isolated deficits in learning and memory, executive function, and visual spatial functioning were consistent with decreased perfusion to cerebral structures supplied by the MCA, including the temporal lobe.
Figure 3. Assessment of cerebral perfusion.
SPECT scan (A, B) with acetazolamide (C, D) revealed decreased perfusion in the cerebral hemispheres, worse on the right, consistent with decreased vascular reserve in the middle cerebral artery territories (A, B) prestent, with a return of cerebral blood flow reserve (C, D) after stent placement.
Repeat MRI did not show evidence of new infarction. Repeat angiogram (figure 2B) demonstrated worsening right MCA stenosis.
Questions for consideration:
What is the cause of this patient's rapidly progressive cognitive decline?
What treatment would you consider at this stage?
SECTION 4
This patient has a rapidly progressive dementia. The differential for this is broad but with his known MCA stenosis and deficits on SPECT scan, the most likely etiology is hypoperfusion.
As the patient now had progressive symptoms of hypoperfusion, referable to symptomatic intracranial stenosis, despite optimal medical management, interventional management was considered. The patient underwent placement of a self-expandable Wingspan stent in the right MCA (figure 2C) 2 weeks after SPECT and cognitive testing. Diamox brain SPECT repeated 3 days poststent revealed improved cortical perfusion with no new areas of impaired vascular reserve (figure 3B). Four weeks later, repeat neuropsychological testing revealed intact cognitive function (average or better) including normal visual spatial skills, verbal learning, and memory and executive function, with exception of mild deficiencies in complex visual memory retrieval with benefit from recognition cues. The underlying etiology for this patient's intracranial disease remains unknown.
DISCUSSION
Approximately 100,000 patients every year have ischemic events related to intracranial atherosclerotic disease, the majority of which are related to atherosclerotic disease.3 Intracranial stenosis can lead to chronic cerebral hypoperfusion and cerebral dysfunction, although these effects are not well understood.6 Cognitive improvement after revascularization of extracranial carotid disease by carotid endarterectomy, carotid artery stenting, or extracranial/intracranial (EC/IC) bypass has been reported.7 Over the past decade, angioplasty and stenting have emerged as therapeutic options for symptomatic intracranial arterial stenosis.3
In clinical settings, the relationship between chronic cerebral hypoperfusion and cognitive function is often difficult to confirm as factors such as aging, systemic disease, or educational background can influence cognitive performance. Neurons can exist at a hypofunctional but viable state for a prolonged period of time in the setting of decreased cerebral blood flow (CBF) and metabolism after experimental MCA occlusions, suggesting that these cells may be salvageable with restoration of CBF.8 EC/IC bypass surgery was used commonly during the 1970s and early 1980s to treat patients who had total or near-total occlusion of carotid arteries but was abandoned due to lack of efficacy.9 However, improvement in both cognitive function and SPECT perfusion have been well-documented following EC/IC bypass, suggesting that a group of carefully selected patients may benefit. Sasoh et al.10 documented a series of patients who underwent EC/IC bypass with reversal of cognitive impairment, some of whom presented with hemiparesis, cognitive dysfunction, and normal MRIs, similar to our patient. Ours is a report of objective cognitive improvement as documented by neuropsychological assessment after correction of a reversible SPECT perfusion deficit independent of structural changes on MRI in the MCA. This illustrates the importance of carefully performing sequential cognitive performance in patients with intracranial vascular disease, because despite acute changes on MRI, cerebral hypoperfusion can lead to significant progressive functional deficits. As safety of stents improves, this could represent an important therapeutic option for patients with intracranial stenosis.
ACKNOWLEDGMENT
The authors thank Jefferson Radiology for preparing the SPECT images.
DISCLOSURE
Dr. Rosario, Dr. Spiegel, and Dr. Tartar report no disclosures. Dr. McCullough serves on the speakers' bureau for Boehringer Ingelheim and receives research support from the NIH/NINDS.
REFERENCES
- 1. Lee H, Sohn SI, Jung DK, et al. Sudden deafness and anterior inferior cerebellar artery infarction. Stroke 2002;33:2807–2812 [DOI] [PubMed] [Google Scholar]
- 2. Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2008;79:1122–1127 [DOI] [PubMed] [Google Scholar]
- 3. Qureshi AI, Feldmann E, Gomez CR, et al. Intracranial atherosclerotic disease: an update. Ann Neurol 2009;66:730–738 [DOI] [PubMed] [Google Scholar]
- 4. Younger DS. Vasculitis of the nervous system. Curr Opin Neurol 2004;17:317–336 [DOI] [PubMed] [Google Scholar]
- 5. Turan TN, Maidan L, Cotsonis G, et al. Warfarin-Aspirin Symptomatic Intracranial Disease Investigators Failure of antithrombotic therapy and risk of stroke in patients with symptomatic intracranial stenosis. Stroke 2009:40:505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarti C, Pantoni L, Bartolini L, Inzitari D. Cognitive impairment and chronic cerebral hypoperfusion: what can be learned from experimental models. J Neurol Sci 2002;203–204:263–266. [DOI] [PubMed] [Google Scholar]
- 7. Komotar RJ, Starke RM, Otten ML, et al. The role of indirect extracranial-intracranial bypass in the treatment of symptomatic intracranial atheroocclusive disease. J Neurosurg 2009;110:896–904 [DOI] [PubMed] [Google Scholar]
- 8. Odano I, Miyashita K, Minoshima S, et al. A potential use of a 123I-labelled benzodiazepine receptor antagonist as a predictor of neuronal cell viability: comparisons with 14C-labelled 2-deoxyglucose autoradiography and histopathological examination. Nucl Med Commun 1995;16:443–446 [DOI] [PubMed] [Google Scholar]
- 9. The EC/IC Bypass Study Group Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med 1985;313:1191–1200 [DOI] [PubMed] [Google Scholar]
- 10. Sasoh M, Ogasawara K, Kuroda K, et al. Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol 2003;59:455–463 [DOI] [PubMed] [Google Scholar]