Abstract
Objective:
To characterize the effects of cerebrovascular (CV) risk factors on preclinical memory decline in cognitively normal individuals at 3 levels of genetic risk for Alzheimer disease (AD) based on APOE genotype.
Methods:
We performed longitudinal neuropsychological testing on an APOE ε4 enriched cohort, ages 21–97. The long-term memory (LTM) score of the Auditory Verbal Learning Test (AVLT) was the primary outcome measure. Any of 4 CV risk factors (CVany), including hypercholesterolemia (CHOL), prior cigarette use (CIG), diabetes mellitus (DM), and hypertension (HTN), was treated as a dichotomized variable. We estimated the longitudinal effect of age using statistical models that simultaneously modeled the cross-sectional and longitudinal effects of age on AVLT LTM by APOE genotype, CVany, and the interaction between the two.
Results:
A total of 74 APOE ε4 homozygotes (HMZ), 239 ε4 heterozygotes (HTZ), and 494 ε4 noncarriers were included. APOE ε4 carrier status showed a significant quadratic effect with age-related LTM decline in all models as previously reported. CVany was associated with further longitudinal AVLT LTM decline in APOE ε4 carriers (p = 0.02), but had no effect in noncarriers. When ε4 HTZ and HMZ were considered separately, there was a striking effect in HMZ (p < 0.001) but not in HTZ. In exploratory analyses, significant deleterious effects were found for CIG (p = 0.001), DM (p = 0.03), and HTN (p = 0.05) in APOE ε4 carriers only that remained significant only for CIG after correction for multiple comparisons.
Conclusion:
CV risk factors influence age-related memory decline in APOE ε4 HMZ.
Cerebrovascular (CV) risk factors including diabetes mellitus (DM),1 hypertension (HTN),2 hypercholesterolemia (CHOL),3 and cigarette smoking (CIG)4 have been associated with elevated risk for dementia and dementia severity. Large-vessel atherosclerosis, the result of CV risk factors, also has been correlated with memory decline5 and Alzheimer disease (AD),6 although whether CV risk factors increase AD risk specifically or vascular pathology alone remains controversial.2,3,7–10 In either case, better cardiovascular health has been correlated with better functional aging11,12 yet treatment of DM,13 HTN,14 and CHOL15 individually has failed to prevent AD. Combined treatment of multiple CV risk factors can slow the progression of MRI lesions,16 but cognitive outcomes have shown mixed results.17,18
APOE ε4 is a proven genetic risk factor for AD19 and memory declines more quickly in APOE ε4 carriers than noncarriers beginning around age 55–60 years as a preclinical harbinger of impending mild cognitive impairment (MCI) and dementia.20 CV risk factors further influence the effect of APOE ε4 carrier status on cognitive decline in elderly individuals,21 but their interaction with APOE ε4 gene dose might underlie inconsistent therapeutic outcomes. There is now good evidence that AD has an extended preclinical phase, so the interactions of APOE genotype and CV risk factors in younger individuals warrant further exploration to better understand potential treatment opportunities for the prevention or delay of AD onset.
We therefore sought to characterize the effects of any of 4 major CV risk factors (CVany) including HTN, DM, CHOL, and CIG on preclinical memory decline (an established sensitive marker of AD onset) in a longitudinal cohort of APOE ε4 homozygotes (HMZ), heterozygotes (HTZ), and noncarriers, as well as explore the effects of each risk factor individually.
METHODS
Study participants.
From January 1, 1994, through August 6, 2007, cognitively normal residents of Maricopa County age 21 years and older were recruited through local media advertisements and underwent APOE genotyping and longitudinal neuropsychological assessment.20 Demographic, family, and medical history data were obtained on each individual undergoing APOE genotyping, and identity was coded by a study assistant. Ancestral origin was self-reported. Genetic determination of APOE allelic status was performed using a PCR-based assay.
All identified ε4 HMZ were matched by age, gender, and education to one ε3/4 HTZ and 2 noncarriers. We identified more HTZ and noncarriers than HMZ (especially those persons over age 70 years, reflecting the greater number of ε4 HMZ developing MCI and AD by this age), who were also eligible for enrollment. Each participant had screening tests to establish their neuropsychiatrically normal state including a neurologic examination, the Folstein Mini-Mental State Examination (MMSE), the Hamilton Depression (Ham-D) Rating Scale, the Functional Activities Questionnaire (FAQ), Instrumental Activities of Daily Living (IADL), and Structured Psychiatric Interview for DSM-III-R. We excluded anyone with potentially confounding medical, neurologic, or psychiatric problems (including a history of stroke). None met the published criteria for MCI,22 AD,23 other forms of dementia, or major depressive disorder.24 Entry criteria for all participants included a score of at least 27 on the MMSE (and scoring at least 1 out of 3 on the recall subtest), a score of 10 or less on the Ham-D rating scale at the time of their first visit, and no indication of loss of function on the FAQ and IADL.
We also excluded from the analysis anyone who subsequently met published criteria for MCI, AD, or any other form of dementia during follow-up, and thus excluded 16 participants (4 noncarriers, 4 heterozygous persons, and 8 homozygous persons). Diagnostic status at entry and follow-up was determined by a consensus panel of behavioral neurologists (R.J.C., M.N.S., G.L.A., S.Z.R., J.S., and B.K.W.). The resulting study population was identical to that previously reported20 save for the omission of 8 individuals in whom CV risk data were missing.
Standard protocol approval and patient consent.
All individuals gave their written, informed consent to participate in the study and have the results of the APOE test withheld from them, approved by the Institutional Review Boards of all participating institutions.
Memory testing.
The Auditory Verbal Learning Test (AVLT) was repeated every 1 to 2 years as part of a standardized battery of neuropsychological tests. We found the long-term memory score (LTM) of the AVLT25 to be a sensitive marker of preclinical cognitive decline in APOE ε4 carriers,20 so LTM was again used as the primary endpoint in this study.
Cerebrovascular risk factors.
CHOL, CIG, DM, and HTN were the 4 considered CV risk factor categories. CVany refers to the presence of any one or more of these 4 categories. Diagnosis made by their primary physician was supported by published guidelines for existing treatment (CHOL, DM, and HTN),26–28 physical examination (blood pressure, height, and weight), and serum total cholesterol measurements (available in 172 members of the APOE cohort). CIG was considered present if greater than 1 pack-year of lifetime cigarette use was endorsed. (Because there is no objective cutoff for a positive smoking history, we chose to err on the more conservative side, realizing that inclusion of individuals with limited smoking exposure could weaken our ability to find a significant effect.) CVany, CHOL, CIG, DM, and HTN were each dichotomized as present or absent regardless of treatment. CHOL, DM, HTN, and CIG were recorded at each epoch and if present at any study epoch, were treated as present even if not endorsed at each epoch. (Most individuals reported the risk factor at entry. Those diagnosed at later epochs either did not have the risk factor at entry [incident cases] or else had it but were not yet diagnosed. Given the chronicity of these risk factors relative to the study's duration, we assumed the latter as more biologically plausible.)
Longitudinal growth modeling.
The longitudinal growth model we have previously used20 was modified to incorporate individual binary cardiovascular risk factors. The original mixed model approach isolated the longitudinal effect of age on the AVLT in a cross-sectional and longitudinal sample.29,30 The modified model employed here allows for comparison of the predicted annual change in AVLT within noncarriers between subjects with and without a given risk factor; the same comparison within carriers; and a comparison of the effect of the risk factor between noncarriers and carriers.
The model for Yij (the jth response for the ith individual) is as follows:
E(Yijb1i) = β1 + β2 Carrieri + β3 RiskFactori + β4 Ageci1 + β5 Carrieri × Ageci1 + β6 RiskFactori × Ageci1 + β7 Carrieri × RiskFactori + β8 Carrieri × RiskFactori × Ageci1 + β9 Ageci12 + β10 Carrieri × Ageci12 + β11 Agecij + β12 Carrieri × Agecij + β13 RiskFactori × Agecij + β14 Carrieri × RiskFactori × Agecij + β15 Agecij2 + β16 Carrieri × Agecij2 + b1i where Carrieri is the carrier status for the ith individual (1 = carrier; 0 = noncarrier), RiskFactori is the presence of a given risk factor for the ith individual (1 = risk factor reported at any time point; 0 = risk factor not reported at any time point), Agecij is the age − 60 (i.e., centered age) of the ith individual at the time of the jth response, and b1i is an individual specific random effect allowing each subject to have a different intercept. Age is centered to reduce the correlation between the age and age-squared terms. From this, the longitudinal models for noncarriers without and with a given risk factor are given by:
Without risk factor: E(Yij −Yi1) = β11 (Agecij −Ageci1) + β15 (Agecij2 − Ageci12)
With risk factor: E(Yij −Yi1) = (β11 + β13) (Agecij − Ageci1) + β15 (Agecij2 − Ageci12)
and the longitudinal models for carriers without and with a given risk factor are given by:
Without risk factor: E(Yij −Yi1) = (β11 + β12) (Agecij − Ageci1) + (β15 + β16) (Agecij2 − Ageci12)
With risk factor: E(Yij −Yi1) = (β11 + β12 + β13 + β14) (Agecij − Ageci1) + (β15 + β16) (Agecij2 − Ageci12)
We previously reported on the difference between carriers and noncarriers in the quadratic longitudinal effect of aging on AVLT20 and thus focus this analysis on the difference between subjects with and without individual cardiovascular risk factors and whether this difference varies between carriers and noncarriers. From these longitudinal models, the estimate of β13 with confidence interval (CI) was used to assess the difference in predicted annual change in AVLT between subjects with and without a given risk factor within noncarriers, and the estimate of β13 + β14 with CI was used to assess the difference in predicted annual change in AVLT between subjects with and without a given risk factor within carriers. A test of significance of β14 was used to assess whether the impact of the given risk factor differed between carriers and noncarriers. Modeling was carried out using SAS PROC MIXED (SAS version 9).
In exploratory analyses, the model was modified to replace the RiskFactori variable with a continuous variable equal to the number of risk factors (ranging from 0 to 4) present in the subject used to test for linear trend associated with risk-dose. In an additional analysis of CVany, the Carrieri variable was replaced with 2 indicator variables to assess differences between noncarriers and HTZ and between noncarriers and HMZ. Baseline characteristics and those recorded during follow-up were compared among groups by using the 2-sample t test or Pearson χ2 test.
RESULTS
A total of 74 ε4 HMZ, 239 HTZ, and 494 ε4 noncarriers were included. A total of 58.1% of the cohort members had at least one CV risk factor (CVany) that did not differ between genetic subgroups (p = 0.27). APOE ε4 HMZ were generally younger (mean age 56.1 ± 9.3 years) with a greater frequency of first-degree relatives with dementia and had slightly longer follow-up than the other genetic subgroups, but did not appear less healthy overall (table 1). Systolic blood pressure was 138.2 ± 18.8 mm Hg in 211 treated hypertensive subjects, and 123.5 ± 15.8 mm Hg in 301 nonhypertensive subjects (p < 0.001). Serum total cholesterol was 206.9 ± 44.4 mg/dL in 38 treated hyperlipidemic and 183.5 ± 31.7 in 134 normolipidemic individuals (p < 0.001). Regarding race and ethnic background, 81% of subjects identified themselves as white non-Latino, 12% as Latino, and 7% as other.
Table 1.
Demographics of the study cohorta
Abbreviations: BMI = body mass index; CHOL = hypercholesterolemia; CIG = cigarette smoking; DM = diabetes mellitus; HTN = hypertension; MI = myocardial infarction.
Values are mean (SD) or %.
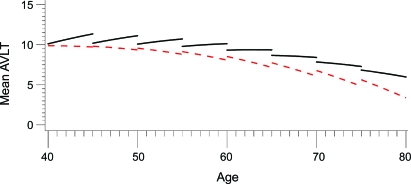
Table 2 shows the distribution of the number of CV risk factor categories (ranging from 0 to 4). Overall, 41.9% had none, 25.3% had 1, 20% had 2, 11% had 3, and 1.8% had 4. This distribution did not differ significantly between ε4 carriers and noncarriers (p = 0.10), but noncarriers had a greater proportion of individuals with 2 or more risk factors than carriers (36.3% vs 27.3%, p = 0.03). In all models, APOE ε4 had a significant longitudinal quadratic effect on AVLT LTM, as previously reported.30 CVany was associated with additional longitudinal AVLT LTM decline in APOE ε4 carriers (95% CI −0.195 to −0.018, p = 0.02), but the effect in noncarriers was not statistically significant. When ε4 HTZ and HMZ were classified as distinct groups, there was a striking effect found in HMZ (difference in predicted annual decline in LTM between subjects with and without CVany of 0.28 points, p < 0.001) but not in HTZ (difference in predicted annual decline in LTM between subjects with and without CVany annual decline in LTM of 0.012 points, p = 0.82) or noncarriers (difference in predicted annual decline in LTM between subjects with and without CVany of 0.000 points, p = 0.99) (figure).
Table 2.
Cerebrovascular risk factors including hypercholesterolemia, cigarette smoking, diabetes mellitus, and hypertensiona
Abbreviations: CVRF = cerebrovascular risk factors.
Values are percentages.
Figure. Cerebrovascular risk factors and APOE ε4 homozygotes.
Longitudinal performance in Auditory Verbal Learning Test (AVLT)–long-term memory scores improves in younger ε4 homozygotes without CV risk factors, but shows little improvement to frank decline even in the youngest age deciles of homozygotes with cerebrovascular risk factors. Solid black lines = without any of 4 cerebrovascular risk factors (CVany); dashed red lines = with CVany.
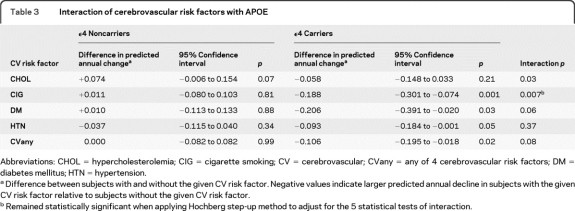
When each risk factor was considered separately, but without controlling for the presence of the remaining risk factors, there were significant effects of CIG (95% CI −0.301 to −0.074, p = 0.001), DM (95% CI −0.391 to −0.020, p = 0.03), and HTN (95% CI −0.184 to −0.001, p = 0.05) in ε4 carriers only (table 3 and table e-1 on the Neurology® Web site at www.neurology.org). There were insufficient numbers of HMZ with each risk factor to assess individual effects in this subgroup (table e-2). When APOE ε4 carriers and noncarriers were combined, there were significant interactions with APOE ε4 for CIG (p = 0.007), and trends for CHOL (p = 0.03) and DM (p = 0.06) and CVany (p = 0.08; table 3) when applying a multiple testing correction for 5 interaction tests. For the entire cohort of carriers and noncarriers, the greater the number of CV risk factor categories, the greater was the deleterious impact on AVLT LTM decline (linear trend, p = 0.01). Finally, adjusting for family history as well as using continuous systolic and diastolic blood pressure and lipid measures did not alter any of the results.
Table 3.
Interaction of cerebrovascular risk factors with APOE
Abbreviations: CHOL = hypercholesterolemia; CIG = cigarette smoking; CV = cerebrovascular; CVany = any of 4 cerebrovascular risk factors; DM = diabetes mellitus; HTN = hypertension.
Difference between subjects with and without the given CV risk factor. Negative values indicate larger predicted annual decline in subjects with the given CV risk factor relative to subjects without the given CV risk factor.
Remained statistically significant when applying Hochberg step-up method to adjust for the 5 statistical tests of interaction.
DISCUSSION
CV risk factors were associated with greater longitudinal memory decline in preclinical APOE ε4 carriers, especially in HMZ who are at the highest risk for AD, while the effect appeared limited in ε4 noncarriers. Further, the greater the number of CV risk factor categories, the greater the effect on memory decline. This relatively selective effect on ε4 carriers echoes the differentially worse outcomes previously reported to result from head trauma,31 stroke,32 carotid endarterectomy,33 subarachnoid and intracerebral hemorrhage,34 and carbon monoxide poisoning.35 The causes for these effects remain speculative, but collectively suggest that APOE ε4 carriers are more vulnerable to a variety of pathophysiologic insults.
Despite individual differences, all CV risk factors share important downstream pathogenic effects including endothelial dysfunction, the promotion of atherosclerosis, and stroke36 that frequently impairs cognition. Atherosclerosis-mediated cerebral hypoperfusion in the absence of frank infarction accounts for much of the lesion burden observed on the MRI scans of aging individuals,37 and is increased in the setting of CV risk factors.38 Clinical effects vary and led to past controversy, but most now agree that increasing lesion burden generally correlates with declining cognitive skills (especially in the executive domain) contributing to vascular cognitive impairment and frank dementia.39 However, although AD and vascular pathology frequently co-occur,40 the role of CV risk factors in the cause and progression of AD itself has not been established.
Memory performance differences between APOE ε4 carriers with and without CV risk factors were clinically modest, but evident in HMZ even before age 60 years. Repeated test administration normally results in improved performance over time, but even in the youngest age ranges of our study, no such test-retest benefit was observed in the ε4 HMZ with CV risk factors (figure). APOE ε4 HMZ comprise roughly 2% of the US population, and many have CV risk factors. Though they are only a subset of all those at risk, they are a particularly vulnerable group due to their very high rate of AD conversion and the younger age at which AD onset occurs.19 Early and aggressive intervention for CV risk factors in this select group might realistically offer the chance to shift their memory trajectory to at least the level of ε4 HMZ without CV risk factors, and thereby delay the decline that occurs with increasing age possibly delaying the onset of symptomatic memory loss.
There are several limitations that must be considered in the interpretation of our findings. First, while CV risk factors have been shown to correlate more strongly with measures of executive function,40 we instead chose to specifically focus on memory. This was done because of the sensitivity of the AVLT-LTM to preclinical decline previously demonstrated in APOE ε4 carriers.20 Because we did not observe a similar difference in age-related performance between ε4 carriers and noncarriers on an executive measure, the Controlled Oral Word Association Test, we chose instead to use the AVLT as a sensitive discriminator of AD-mediated preclinical decline. We cannot exclude the possibility that other executive measures might show a different pattern of change, or that CV risk factors might affect executive measures differently than memory. Nonetheless, we have no reason to believe that executive measures would be more sensitive indicators of preclinical AD, and so were less relevant for the aims of this study.
Because we relied upon existing diagnoses, we may have underestimated the number of individuals with more subtle degrees of CV risk, and this could have reduced our power to detect an effect of CV risk on LTM. However, the overall percentages of affected individuals in our cohort were similar to those reported in population-based studies (cited in 26–28) and there is no reason to believe it would selectively affect noncarriers to account for the differences we observed between groups. Also, the sample size of this study likely limited our ability to detect small effects. These 2 limitations may have contributed to the lack of statistically significant effects in the noncarrier and HTZ groups and the results for these groups should not therefore be interpreted that CV risk factors do not influence memory but rather that the effect was not detectable within the study's limitations.
We were unable to address treatment of CV risk factors since essentially all individuals were receiving treatment, and were unable to isolate each individual CV risk factor as this would have required a much larger number of participants thus limiting our main findings to CVany and the “dose” effect of multiple risk factor categories rather than continuous dose effects. Our study cohort is designed to be genetically enriched for APOE ε4 rather than a random community sample. Our approach capitalizes upon this known genetic risk factor, has proven sensitivity to age-related memory decline,20 and despite the many advantages of community sampling, allowed us to avoid its potential pitfalls of including unhealthy individuals, inadequate or incomplete testing, and inadequate numbers of ε4 HMZ, the subgroup in which we found the strongest effects.
With these limitations in mind, we found that CV risk factors had a deleterious effect on age-related memory decline in APOE ε4 HMZ evident even in the youngest members of our cohort, and the greater the number of CV risk factors, the greater the deleterious effect. Our findings support the general rationale that CV risk factors are therapeutically modifiable targets that contribute to memory decline in those at greatest risk for AD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Sandra Yee-Benedetto, Bruce Henslin, Jessie Jacobsen, Anita Prouty, Stephanie Reeder, Andrea Schmitt, and Richard Crook for technical assistance.
- AD
- Alzheimer disease
- AVLT
- Auditory Verbal Learning Test
- CHOL
- hypercholesterolemia
- CI
- confidence interval
- CIG
- cigarette use
- CV
- cerebrovascular
- CVany
- any of 4 cerebrovascular risk factors
- DM
- diabetes mellitus
- DSM-III-R
- Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- FAQ
- Functional Activities Questionnaire
- Ham-D
- Hamilton Depression Rating Scale
- HMZ
- homozygotes
- HTN
- hypertension
- HTZ
- heterozygote
- IADL
- Instrumental Activities of Daily Living
- LTM
- long-term memory
- MCI
- mild cognitive impairment
- MMSE
- Mini-Mental State Examination
Supplemental data at www.neurology.org
DISCLOSURE
Dr. Caselli serves as Medical Editor for Clinical Neurology News and receives research support from the NIH/NIA and The Arizona Alzheimer's Research Consortium. Dr. Dueck and Dr. Locke report no disclosures. Dr. Sabbagh serves on scientific advisory boards for AmeriSciences and Allon Therapeutics, Inc.; serves on the editorial boards of Clinical Neurology News, the Journal of Alzheimer's Disease, and BMC Neurology; receives publishing royalties for The Alzheimer's Answer (Wiley, 2008); serves as a consultant for Elan Corporation, GlaxoSmithKline, Pfizer Inc., Eisai Inc., and AmeriSciences; receives research support from Elan Corporation, Wyeth, Medivation, Inc., Avid Radiopharmaceuticals, Inc., Baxter International Inc., Eli Lilly and Company, GE Healthcare, Bayer Schering Pharma, Eisai Inc., and the NIH/NIA; and receives royalties from AmeriSciences for the commercial product Cognivite. Dr. Ahern reports no disclosures. Dr. Rapcsak receives research support from the NIH (NIA, NIDCD). Dr. Baxter and Dr. Yaari report no disclosures. Dr. Woodruff receives research support from the NIH/NIA. C. Hoffman-Snyder reports no disclosures. Dr. Rademakers holds patents re: Methods and materials for detecting and treating dementia and receives research support from the NIH, the Pacific Alzheimer Research Foundation (Canada), the Association for Frontotemporal Dementia, the Amyotrophic Lateral Sclerosis Association, and CurePSP. S. Findley reports no disclosures. Dr. Reiman serves on scientific advisory boards for Accera, Inc., Bayer Schering Pharma, Elan Corporation, Eli Lilly and Company, AstraZeneca, GlaxoSmithKline, Siemens, Takeda Pharmaceutical Company Limited, and Eisai Inc.; serves as Deputy Editor for the Journal of Clinical Psychiatry; holds a patent re: Methods for tracking the progression of Alzheimer's disease identifying treatment using transgenic mice; serves as a consultant for Amnestix/Sygnis; receives research support from Kronos Life Sciences, GlaxoSmithKline, AstraZeneca, Avid Radiopharmaceuticals, Inc., the NIH (NIA, NIMH), and the state of Arizona; and serves as Executive Director of the Banner Alzheimer's Institute and Director of the Arizona Alzheimer's Consortium, 501C3 organizations which have emphasized the use of brain imaging and other biomarker measurements in the evaluation of putative pre-symptomatic treatments for Alzheimer's disease.
REFERENCES
- 1. Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology 2004;63:1187–1192 [DOI] [PubMed] [Google Scholar]
- 2. Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology 2002;58:1175–1181 [DOI] [PubMed] [Google Scholar]
- 3. Helzner EP, Luchsinger JA, Scarmeas N, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol 2009;66:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer's disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis 2010;19:465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wendell CR, Zonderman AB, Metter EJ, Najjar SS, Waldstein SR. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke 2009;40:3180–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer's Coordinating Center. Neurology 2005;64:494–500 [DOI] [PubMed] [Google Scholar]
- 7. Arvanitakis Z, Schneider JA, Wilson RS, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology 2006;67:1960–1965 [DOI] [PubMed] [Google Scholar]
- 8. Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology 2002;21:123–130 [DOI] [PubMed] [Google Scholar]
- 9. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: The Nun Study. JAMA 1997;277:813–817 [PubMed] [Google Scholar]
- 10. Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer's disease pathology. Neurology 2004;62:1148–1155 [DOI] [PubMed] [Google Scholar]
- 11. Llewellyn DJ, Lang IA, Xie J, Huppert FA, Melzer D, Langa KM. Framingham stroke risk profile and poor cognitive function: a population-based study. BMC Neurol 2008;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort-the cardiovascular health all stars study. J Am Geriatr Soc 2009;57:432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Areosa SA, Grimley EV. Effect of treatment of type II diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev 2002;4:CD003804. [DOI] [PubMed] [Google Scholar]
- 14. McGuiness B, Todd S, Passmore, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev 2009;4:CD004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev 2009;15:CD003160. [DOI] [PubMed] [Google Scholar]
- 16. Richard E, Gouw AA, Scheltens P, van Gool WA. Vascular care in patients with Alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI. Stroke Epub 2010. [DOI] [PubMed] [Google Scholar]
- 17. Richard E, Kuiper R, Dijkgraaf MG, Van Gool WA. Vascular care in patients with Alzheimer's disease with cerebrovascular lesions-a randomized clinical trial. J Am Geriatr Soc 2009;57:797–805 [DOI] [PubMed] [Google Scholar]
- 18. Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer's disease. Neurology 2009;73:674–680 [DOI] [PubMed] [Google Scholar]
- 19. Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele ε4 with late onset familial and sporadic Alzheimer's disease. Neurology 1993;43:1467–1472 [DOI] [PubMed] [Google Scholar]
- 20. Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med 2009;361:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE ε4 in modulating the effects of other risk factors for cognitive decline in elderly persons. JAMA 1999;281:40–46 [DOI] [PubMed] [Google Scholar]
- 22. Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1133–1142 [DOI] [PubMed] [Google Scholar]
- 23. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer's disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 24. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, fourth edition. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 25. Rey A. l'Examen Clinique en Psychologie. Paris: Presses Universitaires; 1964 [Google Scholar]
- 26. Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 27. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 28. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(suppl 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 29. Ware JH, Dockery DW, Louis TA, XU X, Ferris BG, Speizer FE. Longitudinal and cross-sectional estimates of pulmonary function decline in never-smoking adults. Am J Epidemiology 1990;132:685–700 [DOI] [PubMed] [Google Scholar]
- 30. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis (Section 15.4). Hoboken: John Wiley & Sons, Inc.; 2004 [Google Scholar]
- 31. Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA 1997;278:136–140 [PubMed] [Google Scholar]
- 32. Gromadzka G, Baranska-Gieruszczak M, Sarzynska-Dlugosz I, Cielielska A, Czlonkowska A. The APOE polymorphism and 1-year outcome in ischemic stroke: genotype-gender interaction. Acta Neurol Scand 2007;116:392–398 [DOI] [PubMed] [Google Scholar]
- 33. Heyer EJ, Wilson DA, Sahlein DH, et al. APOE-epsilon4 predisposes to cognitive dysfunction following uncomplicated carotid endarterectomy. Neurology 2010;65:1759–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martinez-Gonzalez NA, Sudlow CL. Effects of apolipoprotein E genotype on outcome after ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2006;77:1329–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hopkins RO, Weaver LK, Valentine KJ, Mower C, Churchill S, Carlquist J. Apolipoprotein E genotype and response of carbon monoxide poisoning to hyperbaric oxygen treatment. Am J Respir Crit Care Med 2007;176:1001–1006 [DOI] [PubMed] [Google Scholar]
- 36. Olsson Y, Brun A, Englund E. Fundamental pathological lesions in vascular dementia. Acta Neurol Scand Suppl 1996;168:31–38 [DOI] [PubMed] [Google Scholar]
- 37. O'Sullivan M, Lythgoe DJ, Pereira AC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 2002;59:321–326 [DOI] [PubMed] [Google Scholar]
- 38. DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHBLI twin study. Stroke 1999;30:529–536 [DOI] [PubMed] [Google Scholar]
- 39. Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurology 2008;7:246–255 [DOI] [PubMed] [Google Scholar]
- 40. Schneider JA, Arvatanakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009;66:200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.