Abstract
Background:
While neuropsychological deficits have been reported in healthy individuals who use street cannabis, data in patients with multiple sclerosis (MS) are lacking. Given that MS is associated with cognitive deterioration, the aim of this study was to determine the neuropsychological effects of cannabis use in this population.
Methods:
Two groups, each of 25 patients with MS (cannabis users and nonusers), were administered the Minimal Assessment of Cognitive Function in MS battery of neuropsychological tests, the Hospital Anxiety and Depression Scale (HADS), and the Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I). Group-matching and regression analysis were used to control for the effects of age, sex, education, premorbid intelligence, disability, and disease course and duration on cognitive function.
Results:
Cannabis users performed significantly more poorly than nonusers on measures of information processing speed, working memory, executive functions, and visuospatial perception. They were also twice as likely as nonusers to be classified as globally cognitively impaired. There were no between-group differences on the HADS measures of depression and anxiety or lifetime SCID-I psychiatric diagnoses.
Conclusion:
This cross-sectional study provides empirical evidence that prolonged use of inhaled or ingested street cannabis in patients with MS is associated with poorer performance on cognitive domains commonly affected in this population. Whatever subjective benefits patients may derive from using street cannabis (e.g., pain and spasticity relief) should be weighed against the associated cognitive side effects.
Cannabis research in patients with multiple sclerosis (MS) has largely focused on synthetic derivatives of the drug. The clinical trials literature is small and suggests treatment may have some mildly beneficial effects particularly in alleviating pain1 and bladder dysfunction,2 but equivocal benefits for spasticity.3 The only clinical trial specifically focused on cognition as the primary outcome measure failed to find any cognitive deficits associated with use of a cannabis-based extract.4
Even less attention has focused on inhaled “street” cannabis. Data show that 36%–43% of patients with MS have at some time smoked cannabis.5,6 The figure for current use, 14%–18%, is more modest, but indicates that a substantial minority of patients with MS find cannabis helpful for relief from pain, spasticity, insomnia, bladder problems, tremors, and emotional distress.5,6
The benefits reported above are, however, subjective, and whether they are offset by potentially adverse cognitive effects has yet to be determined. A single pilot study suggested that MS cannabis smokers had further compromise with respect to information processing speed compared to nonusers.7 Given that approximately 40%–60% of patients with MS are cognitively impaired to begin with,8,9 any drug that may add to this burden gives cause for concern. The purpose of our study, therefore, was to examine the neuropsychological effects of inhaled or ingested cannabis on cognition in patients with MS.
METHODS
Sample selection.
Patients between the ages of 18 and 65 with confirmed MS10 were recruited from tertiary care MS clinics affiliated with the University of Toronto. Exclusion criteria included history of traumatic brain injury, psychotic illness, concurrent neurologic diseases, and poor visual acuity (less than 20/70 corrected, both eyes). Those who had undergone neuropsychological testing within the last year were also excluded in order to avoid possible practice effects.
Cannabis sample.
Subjects who used cannabis recently and whose urine tested positive for cannabinoids only (i.e., no other illicit drugs were permissible) on the day of assessment were included. Subjects who reported cannabis use less than 12 hours prior to testing were excluded.
In addition, a history of cannabis use including age at onset, duration, and frequency of cannabis use was recorded for all subjects. The reasons for smoking cannabis were divided into 3 categories: medical, recreational, or a combination.
Control sample.
MS cannabis users were group-matched (on age, sex, Expanded Disability Status Scale [EDSS], disease course and duration, level of education, and premorbid IQ based on the American National Adult Reading Test [ANART]) to a control group of 25 noncannabis-using patients with MS derived from a larger control sample of 38 cannabis-naïve subjects with MS. The control group was made up of subjects with MS who reported no recent history of cannabis use and had urine that tested negative for cannabinoids and other illicit drugs. A remote history of occasional teenage use was not an exclusionary factor.
Urinalysis.
A broad-spectrum analysis was conducted to determine the presence of the following substances: cannabis, cocaine, opiates, amphetamines, and phencyclidine. The cannabinoid assay detects 11-nor-Δ-9-tetrahydrocannabinol-9-carboxylic acid-B glucuronide (THC-COOH glucuronide) and 11-nor-Δ-9-tetrahydrocannabinol-9-carboxylic acid (THC-COOH) in urine. These levels are combined to provide a composite score.
Demographic and neurologic data.
Demographic and disease-related variables, namely age, sex, education, marital or partner status, employment status, disease course, disease duration, and current medications were collected from each patient and their medical charts. Neurologic disability according to the EDSS11 was recorded from patient files. Alcohol consumption referred to the total number of drinks (a glass of wine, shot of spirits, or standard bottle [330 mL] of beer) consumed weekly. Visual acuity was assessed using the Rosenbaum Pocket Screener.
Psychiatric assessment.
The presence of lifetime psychiatric disorders was established using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Mood and Anxiety Disorder sections.12 In addition, all subjects completed the Hospital Anxiety and Depression Scale (HADS), validated for use with patients with MS.13 Fatigue was measured using the Modified Fatigue Impact Scale (MFIS).14
Neuropsychological assessment.
Premorbid intellectual functioning was assessed with the ANART.15 Thereafter, patients were administered the Minimal Assessment of Cognitive Function in MS (MACFIMS), a comprehensive battery of 7 tests measuring 11 cognitive indices considered optimum for teasing out deficits in this population.16 The MACFIMS is regarded as the gold standard for cognitive assessment in MS and was put together by consensus following a meeting of leading neuropsychologists involved in MS research.17 The battery includes the following tests: verbal learning and memory: The California Verbal Learning Test–Revised (CVLT-II)18; visuospatial memory and learning: Brief Visuospatial Memory Test–Revised (BVMT-R)19; visual perception/spatial processing: the Judgment of Line Orientation (JLO)20; verbal fluency/word retrieval: the Controlled Oral Word Association Test (COWAT)21; executive functions: the Delis-Kaplan Executive Function System (D-KEFS) Sorting Test22; information processing speed and working memory: the Paced Auditory Serial Addition Test (PASAT)8,23 with 3.0- and 2.0-second interstimulus intervals; information processing speed: the Symbol Digit Modalities Test (SDMT).24
As specified in the MACFIMS validation data,17 global cognitive impairment represents failure on 2 or more of 11 cognitive indices. Impairment on a single test is defined as a z score of 1.5 or more below norms derived from age-, sex-, and education-matched healthy control subjects. These normative data are provided in the test manuals.
Statistical analyses.
Primary analysis included between-group comparisons with t tests and χ2 analyses. The Mann-Whitney U test was used for non-normally distributed variables.
Further cognitive comparisons between cannabis users and nonusers were performed with a series of linear regression analyses, with each of the 11 cognitive indices as the dependent variable and cannabis use as the independent variable. Age, sex, education, EDSS, alcohol consumption, depression, anxiety, and fatigue were entered sequentially into the analysis as covariates. These variables were selected due to their potential effects on cognition. Only covariates that changed the group coefficient by 10% or more were retained in the final model for each cognitive measure.
Similarly, binary logistic regression analysis was conducted to investigate the effect of cannabis use on global cognitive impairment after controlling for the potential confounds mentioned above. As before, only covariates that changed the group coefficient by 10% or more were retained in the final model.
Pearson correlations were conducted to determine the association between age at onset of cannabis use, duration of cannabis use, and urine cannabinoid levels on the one hand and global cognitive impairment on the other. χ2 Analysis was used to determine the association between duration of abstinence (12–24 hours vs greater than 24 hours) and global cognitive impairment.
Standard protocol approvals, registrations, and patient consents.
Ethics approval for the study was obtained from Research Ethics Boards at Sunnybrook Health Sciences Centre and St. Michael's Hospital, both affiliated with the University of Toronto. All participants provided written informed consent prior to participating in the study. Patients were clearly informed of the aim of the study, namely to examine the effect of cannabis use on neuropsychiatric functioning in MS.
RESULTS
Demographics and neurologic variables.
Entire sample.
The sample consisted of 25 cannabis users (11 women) and 25 nonusers (12 women). The mean age of the entire sample was 43.60 (SD 10.7). Thirty-three patients (66.0%) were married or cohabitating and 21 (42.0%) were employed. Patients had received an average of 14.0 years (SD 2.8) of education. Average disease duration was 12.1 years (SD 9.4). The breakdown of disease course was as follows: relapsing-remitting 72.0%; secondary progressive 18.0%; primary progressive 10.0%. The median EDSS score was 3.0 (mean 3.32, SD 2.39, range 0–8.5). Twenty patients (40.0%) were taking disease-modifying drugs. Patients consumed a median of 2.0 alcoholic beverages per week (mean 3.0, SD 3.3, range 0–12). Four subjects had taken steroids within the last 3 months.
Comparison between cannabis users and nonusers.
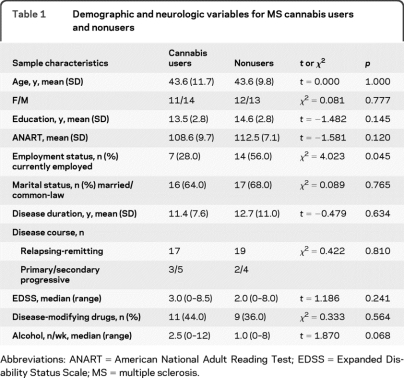
Comparisons between cannabis users and nonusers on demographic and disease-related variables are presented in table 1. There were no statistically significant group differences for age, sex, years of education, marital status, EDSS, disease course, duration of MS, and use of disease-modifying drugs. Cannabis users were significantly more likely to be unemployed. Cannabis users also reported slightly higher alcohol consumption compared to nonusers, although this difference did not reach statistical significance.
Table 1.
Demographic and neurologic variables for MS cannabis users and nonusers
Abbreviations: ANART = American National Adult Reading Test; EDSS = Expanded Disability Status Scale; MS = multiple sclerosis.
Cannabis use.
The average age at onset of cannabis use was 17.0 years (median 15.0, SD 6.6, range 13–47) and the average duration of cannabis use was 26.6 years (median 31.0, SD 12.1, range 1–41). Eighteen subjects (72.0%) used cannabis on a daily basis, 6 (24.0%) reported weekly use, and one reported biweekly use. Most cannabis users (n = 24) reported inhalation (smoking or vaporization) whereas one reported consumption of food products containing cannabis. Eight subjects (32.0%) reported using cannabis for medicinal reasons, 3 (12.0%) for recreational reasons, and 14 (56.0%) for a combination.
Mean level of urine cannabinoid metabolites was 174.4 μg/L (SD 40.8, range 61–≥200) and the broad-spectrum drug screen indicated that no subject had used any illicit drugs other than cannabis. The period of abstinence from cannabis use ranged from 12 hours to 14 days prior to testing with most patients (n = 18) reporting their last use on the evening prior to testing. The remaining 7 subjects had not used cannabis for more than 24 hours.
Urine drug screening indicated that none of the noncannabis users tested positive for cannabinoids or any other nonmedicinal substances.
Psychiatric assessment.
The lifetime prevalence of major depression for the entire sample was 56.0%. Lifetime prevalences for anxiety disorders were as follows: generalized anxiety disorder 26.0%; panic disorder 20.0%; phobia 4.0%; obsessive compulsive disorder 2.0%; and post-traumatic stress disorder 4.0%. Lifetime prevalence for any of the anxiety disorders was 36.0%.
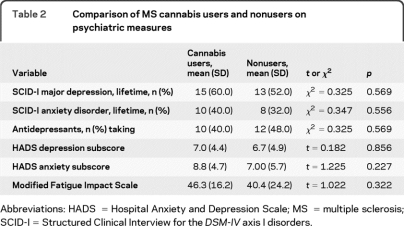
Psychiatric comparison between cannabis users and nonusers is presented in table 2. There were no significant differences between groups in the lifetime prevalences of psychiatric disorders and use of antidepressant medication. Similarly, scores on HADS Depression and Anxiety subscales and the MFIS showed no significant differences between cannabis users and nonusers (table 2).
Table 2.
Comparison of MS cannabis users and nonusers on psychiatric measures
Abbreviations: HADS = Hospital Anxiety and Depression Scale; MS = multiple sclerosis; SCID-I = Structured Clinical Interview for the DSM-IV axis I disorders.
Neuropsychological assessment.
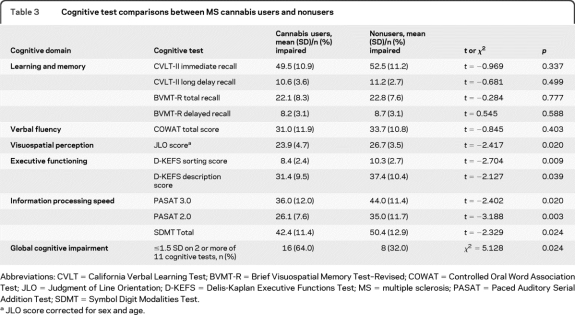
Neuropsychological comparisons between cannabis users and nonusers are presented in table 3. Cannabis users scored significantly lower on the PASAT-3, PASAT-2, JLO, SDMT, and D-KEFS Sorting Test and Description scores. There were no between-group differences on CVLT–immediate recall, CVLT–delayed recall, BVMT–immediate recall, BVMT–delayed recall, and COWAT.
Table 3.
Cognitive test comparisons between MS cannabis users and nonusers
Abbreviations: CVLT = California Verbal Learning Test; BVMT-R = Brief Visuospatial Memory Test–Revised; COWAT = Controlled Oral Word Association Test; JLO = Judgment of Line Orientation; D-KEFS = Delis-Kaplan Executive Functions Test; MS = multiple sclerosis; PASAT = Paced Auditory Serial Addition Test; SDMT = Symbol Digit Modalities Test.
JLO score corrected for sex and age.
Of all 50 patients, 24 (48.0%) were classified as cognitively impaired. Cannabis users were significantly more likely to be classified as globally impaired compared to nonusers (χ2 = 5.13, p = 0.024; table 3).
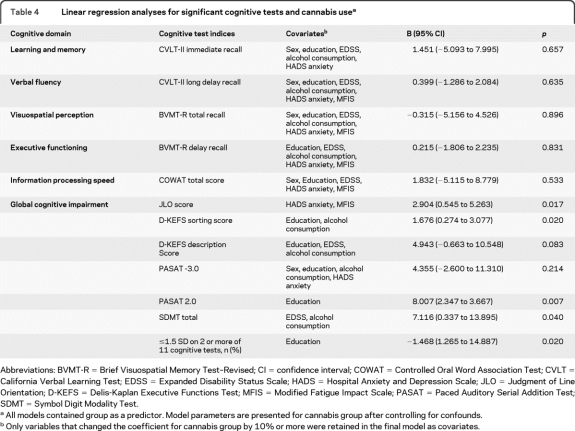
Although the cannabis users and nonusers were group-matched on demographic and disease-related variables, we further analyzed our data with the aim of exploring the effect of cannabis use on each cognitive measure independent of age, sex, education, alcohol consumption, EDSS, depression, anxiety, and fatigue. The final regression models reveal that cannabis use remained a significant independent predictor of performance on the PASAT-2, JLO, SDMT, D-KEFS Sorting Score, and global cognitive impairment, but not on CVLT–immediate recall, CVLT–delayed recall, BVMT–immediate recall, BVMT–delayed recall, COWAT, PASAT-3, and D-KEFS Description Score (table 4). Exclusion of the one subject who indicated only ingesting cannabis in food products did not significantly alter the findings.
Table 4.
Linear regression analyses for significant cognitive tests and cannabis usea
Abbreviations: BVMT-R = Brief Visuospatial Memory Test–Revised; CI = confidence interval; COWAT = Controlled Oral Word Association Test; CVLT = California Verbal Learning Test; EDSS = Expanded Disability Status Scale; HADS = Hospital Anxiety and Depression Scale; JLO = Judgment of Line Orientation; D-KEFS = Delis-Kaplan Executive Functions Test; MFIS = Modified Fatigue Impact Scale; PASAT = Paced Auditory Serial Addition Test; SDMT = Symbol Digit Modality Test.
All models contained group as a predictor. Model parameters are presented for cannabis group after controlling for confounds.
Only variables that changed the coefficient for cannabis group by 10% or more were retained in the final model as covariates.
Global cognitive impairment was not significantly correlated with urine cannabinoid levels (r = −0.321, p = 0.118), age at cannabis use onset (r = −0.321, p = 0.118), or duration of cannabis use (r = 0.158, p = 0.451). The period of abstinence from cannabis use was not associated with global cognitive impairment (χ2 = 0.198, p = 0.673).
DISCUSSION
The specific aim of this prospective study was to examine the effects of smoked or ingested cannabis on cognitive function in patients with MS. We found that cannabis users had greater deficits on information processing speed, working memory, executive function, and visuospatial perception compared to a sample of nonusers group-matched on age, sex, education, premorbid intelligence, EDSS, and disease course. Cannabis users were also twice as likely as nonusers to meet criteria for global cognitive impairment. Most of these between-group differences were retained after controlling for potential confounds.
Cognitive dysfunction affects approximately 40%–60% of patients with MS8,9 with detrimental effects on personal, social, and occupational functioning.8 Cognitive functioning is also a major determinant of quality of life.25 Given these adverse psychosocial effects, identifying risk factors associated with further cognitive impairment is important. Although not the focus of the present investigation, it is plausible that the additional cognitive deficits associated with chronic cannabis use have deleterious psychosocial ramifications. For example, our study found that cannabis users were twice as likely to be unemployed than nonusers. While the reasons for this are not clear, an association between impaired cognitive performance and unemployment in patients with MS has been reported,26 thereby suggesting a putative link with our cannabis findings.
To date, the clinical trials literature on the effects of cannabis on cognition in patients with MS is sparse, largely limited to synthetic cannabis derivatives or cannabis-based extracts, with measures of cognition confined to secondary analysis. Results are equivocal, with deficits in long-term memory storage reported by one study1 contrasting with an absence of deleterious cognitive problems associated with cannabinoids reported by others.4,27 The discrepancy between these negative findings and our results may be attributable to differences in pharmacokinetics between the various forms of cannabis and their routes of administration. Oral administration of cannabinoids has a slower onset of action, more erratic patterns of absorption, and lower peak concentration compared to inhaled cannabis, which allows for better absorption than oral THC.
The results of our study are consistent with data from an earlier pilot study that revealed that patients with MS who smoked cannabis performed significantly more poorly than cannabis-naïve patients on a test of information processing speed.7 While informative, the earlier study had a small sample size (10 cannabis users), a limited neuropsychological battery, and the absence of urinalysis confirming cannabis use. Our present study, by virtue of a more robust methodology, extends these earlier results and links smoked or ingested cannabis to more extensive cognitive deficits.
The paucity of cognitive data pertaining to the use of inhaled cannabis in patients with MS contrasts with a much larger literature obtained from general population studies. Results here have varied according to the timing of the neuropsychological inquiry. For example, there is a consistent body of evidence showing that individuals who are acutely intoxicated display impaired memory, slowed information processing speed, and poor attention.28,29 What defines acute intoxication in the literature is somewhat arbitrary, with studies using 4 to 24 hours as the cutoff period.28,29 Of note, however, is that pharmacokinetic studies have shown that the acute cognitive effects of cannabis attributable to the initial rapid rise in serum THC begin tapering off 3 to 5 hours after consumption.30,31 Given that we did not want to test cognition in patients who were acutely intoxicated, we set a time frame for psychometric testing as greater than 12 hours following the last inhalation or ingestion of cannabis. The literature from the general population suggests, with few exceptions,32 that there are residual, adverse cognitive difficulties extending beyond this period.33–35 Our finding in the cannabis users replicates this picture and points toward the detrimental effects of cannabis persisting beyond intoxication. While it is likely that these persistent deficits are due to the residual effects of the drug itself, whether and to what extent withdrawal effects following a short period of abstinence contribute as well cannot be ascertained from our data. Notably, we did not find an association between cognitive performance and duration of abstinence (12–24 hours vs greater than 24 hours) in this study.
Cognitive dysfunction in our sample was not associated with the level of cannabinoid metabolites detected in the urine and the age at onset, duration of cannabis use, or abstinence. It is, however, possible that the lack of association may be an artifact of our sample selection where the overwhelming majority of our cannabis users began using the drug in adolescence and in whom urinary levels of metabolites clustered tightly at the upper limits of the range of detection. Our data also diverged from the general population finding of higher rates of psychopathology in cannabis users.36 This pertained both to the lifetime prevalence of these disorders and current indices of emotional distress as captured by the HADS. This result may, in part, be attributed to the already high prevalence of depressive and anxiety disorders associated with MS itself.37,38
A notable cognitive finding from our study was that twice as many cannabis users were rated as globally impaired when compared with the noncannabis users. While our methodology did not address etiologic constructs, fMRI data from patients with MS have consistently shown that in response to a cognitive challenge, ancillary brain activation occurs as a compensatory response to the presence of cerebral pathology.39,40 It is therefore tempting to speculate that the deleterious effects of cannabis may be linked to an inhibition of these compensatory responses. In addition, functional imaging findings from the general psychiatry literature have demonstrated lower global and prefrontal blood flow in cannabis users even before they are challenged with a cognitive task.40 These resting state data suggest that a degree of “background” cerebral compromise may further complicate cognitive functioning.
Our study has limitations. The cross-sectional design limits our ability to establish a cause and effect relationship between cannabis use and greater cognitive dysfunction. It is also important to emphasize that our results are derived from patients who have smoked cannabis on a regular basis, as much as several times per day, for more than 2 decades. These results do not necessarily extend to occasional cannabis use or frequent use for a brief period of time. Indeed, studies of cannabis use in healthy individuals have shown that the cognitive effects of cannabis are dose-dependent, with deficits in cognition primarily observed in heavy cannabis users34,35 and those who use cannabis over a long period of time.35 Our study also does not address the reversibility of these cognitive deficits following long-term abstinence from the drug. Finally, our modest sample size introduces a cautionary note.
Our study demonstrates that inhaled or ingested cannabis is associated with adverse effects on cognition following prolonged use. Given the prevalence of cannabis use in patients with MS, further research is needed to replicate these findings in a larger sample and to explore the cerebral underpinnings of how these changes may come about.
ACKNOWLEDGMENT
The authors thank Alex Kiss, PhD, Institute for Clinical and Evaluative Sciences and Department of Research Design and Biostatistics, University of Toronto, for providing statistical support; Warren Walsh, BSc, Senior Resource Technologist at the Hospital for Sick Children, Department of Pediatric Laboratory Medicine, for supervising all toxicology testing; and Liesly Lee, MD, and Kathleen Carr, RN, for assisting with recruitment.
Footnotes
- ANART
- American National Adult Reading Test
- BVMT-R
- Brief Visuospatial Memory Test–Revised
- COWAT
- Controlled Oral Word Association Test
- CVLT-II
- California Verbal Learning Test–Revised
- D-KEFS
- Delis-Kaplan Executive Function System
- EDSS
- Expanded Disability Status Scale
- HADS
- Hospital Anxiety and Depression Scale
- JLO
- Judgment of Line Orientation
- MACFIMS
- Minimal Assessment of Cognitive Function in MS
- MFIS
- Modified Fatigue Impact Scale
- MS
- multiple sclerosis
- PASAT
- Paced Auditory Serial Addition Test
- SDMT
- Symbol Digit Modalities Test
- SCID-I
- Structured Clinical Interview for the DSM-IV Axis I Disorders
DISCLOSURE
K. Honarmand has received speaker honoraria from EMD Serono, Inc.; and receives/has received support from an Ontario Graduate Scholarship and an MS Society of Canada Research Studentship grant. Dr. Tierney reports no disclosures. Dr. O'Connor serves on scientific advisory boards for Novartis, Sanofi-Aventis, Bayer Schering Pharma, Genentech, Inc., and Roche; has received speaker honoraria from Biogen Idec, Teva Pharmaceutical Industries Ltd., Novartis, and Sanofi-Aventis; has served as a consultant for Biogen Idec, Actelion Pharmaceuticals Ltd, Bayer Schering Pharma, EMD Serono, Inc., Teva Pharmaceutical Industries Ltd., Genentech Inc., and Warburg Pincus; has received research support from Abbott, Bayer Schering Pharma, Novartis, BioMS Medical, Sanofi-Aventis, CIS Pharma, Genmab A/S, Cognosci, Inc., Wyeth, Daiichi Sankyo, and Roche; and serves as the National Scientific and Clinical Advisor to the MS Society of Canada. Dr. Feinstein has served on scientific advisory boards for Merck Serono and Avanir Pharmaceuticals; has received speaker honoraria from Merck Serono, Teva Pharmaceutical Industries Ltd., Bayer Schering Pharma, and Biogen Idec; serves on the editorial boards of Multiple Sclerosis and the African Journal of Psychiatry; receives publishing royalties for The Clinical Neuropsychiatry of Multiple Sclerosis (Cambridge University Press, 2007); serves on the Medical Advisory Committee for the Multiple Sclerosis Society of Canada; conducts neuropsychiatric evaluation, cognitive testing, brain imaging in neuropsychiatry in his clinical practice (20% effort); and receives research support from Teva Pharmaceutical Industries Ltd., Merck Serono, Canadian Institute of Health Research, and the Multiple Sclerosis Society of Canada.
REFERENCES
- 1. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812–819 [DOI] [PubMed] [Google Scholar]
- 2. Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE, Wright D, Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomized placebo-controlled trial (CAMS-LUTS). Int Urogynecol J 2006;17:636–641 [DOI] [PubMed] [Google Scholar]
- 3. UK MS Research Group. Zajicek J, Fox P, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo-controlled trial. Lancet 2003;362:1517–1526 [DOI] [PubMed] [Google Scholar]
- 4. Aragona M, Onesti E, Tomassini V, et al. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clin Neuropharmacol 2009;32:41–47 [DOI] [PubMed] [Google Scholar]
- 5. Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology 2004;62:2098–2100 [DOI] [PubMed] [Google Scholar]
- 6. Martinez-Rodriguez JE, Munteis E, Carreno M, et al. Cannabis use in Spanish patients with multiple sclerosis: Fulfillment of patient expectations? J Neurol Sci 2008;273:103–107 [DOI] [PubMed] [Google Scholar]
- 7. Ghaffar O, Feinstein A. Multiple sclerosis and cannabis: a cognitive and psychiatric study. Neurology 2008;71:164–169 [DOI] [PubMed] [Google Scholar]
- 8. Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis: I: frequency, patterns, and prediction. Neurology 1991;41:685–691 [DOI] [PubMed] [Google Scholar]
- 9. Lyon-Caen O, Jouvent R, Hauser S, et al. Cognitive function in recent-onset demyelinating diseases. Arch Neurol 1986;43:1138–1141 [DOI] [PubMed] [Google Scholar]
- 10. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001;50:121–127 [DOI] [PubMed] [Google Scholar]
- 11. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33:1444–1452 [DOI] [PubMed] [Google Scholar]
- 12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 13. Honarmand K, Feinstein A. Validation of the Hospital Anxiety and Depression Scale for use with multiple sclerosis patients. Mult Scler 2009;15:1518–1524 [DOI] [PubMed] [Google Scholar]
- 14. Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the Fatigue Impact Scale. Clin Infect Dis 1994;18:S79–S83 [DOI] [PubMed] [Google Scholar]
- 15. Nelson HE. National Adult Reading Test (NART): test manual. Windsor, UK: NFER Nelson; 1982 [Google Scholar]
- 16. Benedict RHB, Fischer JS, Archibald CJ, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol 2004;16:381–397 [DOI] [PubMed] [Google Scholar]
- 17. Benedict RHB, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2006;12:549–558 [DOI] [PubMed] [Google Scholar]
- 18. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual, Adult Version, 2nd ed San Antonio, TX: Psychological Corporation; 2000 [Google Scholar]
- 19. Benedict RHB. Brief Visuospatial Memory Test–Revised: Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc.; 1997 [Google Scholar]
- 20. Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to Neuropsychological Assessment, 2nd ed. New York: Oxford University Press; 1994 [Google Scholar]
- 21. Benton AL, Hamsher K. Multilingual Aphasia Examination Manual. Iowa City: University of Iowa; 1989 [Google Scholar]
- 22. Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation; 2001 [Google Scholar]
- 23. Gronwall DMA. Paced Auditory Serial Addition Test: a measure of recovery from concussion. Percept Mot Skills 1977;44:367–373 [DOI] [PubMed] [Google Scholar]
- 24. Smith A. Symbol Digit Modalities Test Manual. Los Angeles: Western Psychological Services; 1973 [Google Scholar]
- 25. Benito-Leon J, Morales JM, Rivera-Navarro J. Health-related quality of life and its relationship to cognitive and emotional functioning in multiple sclerosis patients. Eur J Neurol 2002;9:497–502 [DOI] [PubMed] [Google Scholar]
- 26. Beatty WW, Blanco CR, Wilbanks SI, Paul RH. Demographic, clinical, and cognitive characteristics of multiple sclerosis patients who continue to work. Neurorehabil Neural Repair 1995;9:167–173 [Google Scholar]
- 27. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler 2004;10:434–441 [DOI] [PubMed] [Google Scholar]
- 28. Leirer VO, Yesavage JA, Morrow DG. Marijuana carry-over effects on aircraft pilot performance. Aviat Space Environ Med 1991;62:221–227 [PubMed] [Google Scholar]
- 29. Barnett G, Licko V, Thompson T. Behavioral pharmacokinetics of marijuana. Psychopharmacology 1985;85:51–56 [DOI] [PubMed] [Google Scholar]
- 30. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinetics 2003;42:327–360 [DOI] [PubMed] [Google Scholar]
- 31. Berghaus G, Krüger HP, Vollrath M. Beeinträchtigung fahrrelevanter Leistungen nach Rauchen von Cannabis und nach Alkoholkonsum: Eine vergleichende Metaanalyse experimenteller Studien [Impairment of driving-related performance after smoking of cannabis and of alcohol use: a comparative meta-analysis of experimental studies]. In: Berghaus G, Krüger HP. eds. Cannabis im Straßenverkehr [Cannabis and Driving]. Stuttgart: Gustav Fisher Verlag; 1998:99–112 [Google Scholar]
- 32. Carlin AS, Trupin EW. Effect of long-term chronic marihuana use on neuropsychological functioning. Int J Addict 1977;12:617–624 [DOI] [PubMed] [Google Scholar]
- 33. Pope HG, Gruber AJ, Yurgelun-Todd D. Residual neuropsychological effects of cannabis. Curr Psychiatry Rep 2001;3:507–512 [DOI] [PubMed] [Google Scholar]
- 34. Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana: a comparison with pre-drug performance. Neurotoxicol Teratol 2005;27:231–239 [DOI] [PubMed] [Google Scholar]
- 35. Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry 1995b;37:731–739 [DOI] [PubMed] [Google Scholar]
- 36. Cheung JTW, Mann RE, Lalomiteanu A, et al. Anxiety and mood disorders and cannabis use. Am J Drug Alcohol Abuse 2010;36:118–122 [DOI] [PubMed] [Google Scholar]
- 37. Minden SL, Schiffer RB. Affective disorders in multiple sclerosis: review and recommendations for clinical research. Arch Neurol 1990;47:98–104 [DOI] [PubMed] [Google Scholar]
- 38. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler 2007;13:67–72 [DOI] [PubMed] [Google Scholar]
- 39. Forn C, Barros-Loscertales A, Escudero J, et al. Compensatory activations in patients with multiple sclerosis during preserved performance on the auditory N-back task. Hum Brain Mapp 2007;28:424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin-Santos R, Fagundo AB, Crippa JA, et al. Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med 2010;40:383–398 [DOI] [PubMed] [Google Scholar]