Abstract
Background. Elderly persons often experience a reduced immune response to influenza vaccination. We evaluated the usual dose of AS03A-adjuvanted H5N1 pandemic vaccine (3.75 μg hemagglutinin of A/Vietnam/1194/2004-like strain) compared with a double dose in an elderly population.
Methods. This phase 2, open-label study (NCT00397215; http://www.clinicaltrials.gov) randomized participants (age, ≥61 years) to receive, on days 0 and 21: (1) a single dose of AS03A-adjuvanted vaccine (n = 152), (2) a single dose of nonadjuvanted vaccine (n = 54), (3) a double dose of AS03A-adjuvanted vaccine (n = 145), or (4) a double dose of nonadjuvanted vaccine (n = 44). The primary end point was hemagglutination inhibition (HI) and neutralizing antibody response against vaccine antigen (according-to-protocol cohort).
Results. Day 42 geometric mean titers for HI antibodies were 126.8 and 237.3 for single and double doses of the AS03A-adjuvanted vaccine, respectively. Corresponding values for neutralizing antibodies were 447.3 and 595.8. Although the immune response was higher with the double dose, European Committee for Human Medicinal Products criteria for seroconversion and seroprotection rates were achieved in both AS03A-adjuvanted groups. Antigen-specific CD4 T cell responses were elicited. Immune response persistence at 6 months was high. Immune response in the non-adjuvanted groups was considerably less.
Conclusions. The AS03A-adjuvanted H5N1 vaccine can be administered elderly persons at the same dose and schedule as in younger adults.
The past decade has seen unprecedented preparation for a human influenza pandemic. Until the 2009 outbreak of human cases of (swine) influenza A (H1N1), the avian influenza H5N1 virus was considered a potential cause of a human influenza pandemic. The H5N1 virus remains a considerable threat to human health, and it is important that pandemic preparedness planning for an outbreak continues. Moreover, vaccine development strategies for a potential H5N1 pandemic can guide development of vaccines to combat the 2009/10 H1N1 pandemic and other future potential influenza pandemics.
Development of an influenza vaccine that induces a cross-reactive immune response is a key component of a pandemic preparedness strategy. Such a vaccine is likely to prime the immune system to mount a rapid response to vaccination with a drifted strain and/or to infection and may be used before the onset of a pandemic or in its early stages. Even partial cross-protection may have a considerable impact on infection rates during early pandemic stages [1, 2]. The vaccine must provide a high and long-lasting immune response at a relatively low antigen dose, because the need for a high dose would exhaust the limited global production capacity for influenza antigen. Formulation of pandemic vaccines using adjuvants to stimulate a robust immune response is an important approach to reducing the antigen dose and eliciting a cross-reactive response [3].
An H5N1 pandemic vaccine based on the A/Vietnam/1194/2004 clade 1 strain is licensed to be used in the event of an imminent H5N1 pandemic. The vaccine is adjuvanted with AS03A, a novel tocopherol oil-in-water emulsion-based adjuvant system (11.86 mg of tocopherol). It is licensed to be delivered via a schedule of 2 injections administered 3 weeks apart [4]. In a study in adults aged 18-60 years, the lowest dose investigated (3.75 μg hemagglutinin [HA]) elicited immune responses against the vaccine strain that met all US Center for Biologics Evaluation and Research and European Committee for Human Medicinal Products (CHMP) immunologic licensure criteria [5]. Much higher doses were needed to induce a moderate immune response with a nonadjuvanted H5N1 split-virus vaccine [6], indicating that adjuvantation with AS03A allows successful dose sparing. In addition, the vaccine has been shown to induce a cross-reactive immune response (hemagglutination inhibition [HI]) and neutralizing antibodies and CD4 T cell–mediated immune response) against drifted clade 2 H5N1 strains [5, 7–10] An acceptable safety and reactogenicity profile has been demonstrated [5, 8, 11].
Studies with seasonal influenza vaccines suggest that the reduced immune response to vaccination in elderly populations is due – at least in part – to immunosenescence [12]. It is possible that elderly people may therefore need additional or higher doses. This study aimed to assess the immune response in an elderly population to 2 single or 2 double doses of the AS03A-adjuvanted H5N1 pandemic vaccine, administered 21 days apart, compared with the same dosage and schedule without the adjuvant system.
METHODS
The primary objectives of the study were to evaluate the immunogenicity of the H5N1 vaccine 21 days after receipt of the first dose of vaccine (day 21) and 21 days after receipt of the second dose (day 42) and to assess persistence of the immune response for up to 2 years. Safety was also assessed. This article reports immunogenicity data up to 180 days; safety data are presented in a supplement.
The study was conducted in accordance with the current version of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The protocol was approved by the independent ethics committee or institutional review board of each study center, and written informed consent was obtained from each participant.
Study Vaccines
The H5N1 inactivated, split-virion recombinant vaccine was manufactured by GlaxoSmithKline (GSK) Biologicals, as described elsewhere [5, 7]. The vaccine contained 3.75 μg HA of the A/Vietnam/1194/2004-like NIBRG-14 clade 1 strain (National Institute for Biological Standards and Control). An adjuvanted and a nonadjuvanted vaccine were used in this study. The adjuvanted vaccine (Prepandrix a trademark of the GSK group of companies) contained AS03A, an oil-in-water emulsion-based adjuvant system containing DL-α-tocopherol (11.86 mg) [5]. The vaccines (0.5 mL per single dose) were administered intramuscularly into the deltoid region of the nondominant arm for the single dose and into the deltoid region of each arm for the double dose.
Study Design
This was a phase 2, randomized, open-label study (NCT00397215). The study was conducted in 2 phases, with the primary phase taking place between days 0 and 51, and the follow-up phase taking place between days 51 and the end of the study. The primary phase ended 30 days after the receipt of the last vaccine dose, the period during which participants recorded all adverse events. Participants were enrolled independently in each phase and did not have to complete the primary phase (ie, to comply with telephone contact at day 51) to enroll in the follow-up phase.
Male and female participants aged ≥61 years, who were in good health or who had well-controlled underlying disease, were enrolled in the study. Participants were randomized at a ratio of 3:1:3:1 to 1 of 4 vaccine study groups: (1) a single dose of the AS03A-adjuvanted vaccine (1 × H5N1-AS), (2) a single dose of the nonadjuvanted vaccine (1 × H5N1), (3) a double dose of the AS03A-adjuvanted vaccine (2 × H5N1-AS), or (4) a double dose of the nonadjuvanted vaccine (2 × H5N1). Participants in the single-dose groups received one vaccine dose on day 0 and another on day 21. Participants in the double-dose groups received 2 doses on day 0 and 2 doses on day 21. Participants who had not received an influenza vaccine for the 2006-2007 season were vaccinated with FluarixNorthern hemisphere 2006/2007 (GSK Biologicals) at least 3 weeks before administration of the H5N1 vaccine.
A randomization blocking scheme was used. The randomization list was generated at GSK Biologicals using standard SAS software. Participants in each group were stratified at a ratio of 1:1:1 by age group (61-65, 66-70, and >70 years).
Immunogenicity Evaluation
Blood samples were taken on days 0, 21, 42, and 180. Additional samples will be taken from Belgian participants at month 12 and month 24. The primary end points were humoral immune responses in terms of anti-HA and neutralizing antibodies against the vaccine antigen. The cell-mediated immune response was also evaluated.
Anti-HA antibody titers were measured using an HI assay which corresponded to international standards [13,14]. Each serum sample was tested in duplicate. The following parameters with 95% confidence intervals (CIs) were determined: (1) geometric mean titer (GMT) on days 0, 21, 42, and 180; (2) seroconversion rate (SCR) on days 21, 42, and 180; (3) and seroprotection rate (SPR) on days 0, 21, 42, and 180. SCR was defined as the percentage of participants with either (1) a prevaccination titer <1:10 and a postvaccination titer ≥1:40, or (2) a prevaccination titer ≥1:10 and a ≥4-fold increase in postvaccination titer. SPR was defined as the percentage of participants with an HI titer ≥1:40.
Virus neutralization by serum antibodies was determined by a microneutralization assay on thawed frozen serum samples that had been subjected to heat inactivation for 30 min at 56°C. Each serum sample was tested in triplicate. A standard amount of virus was mixed with serial dilutions of serum and incubated to allow antibody binding to the virus. A cell suspension containing a defined number of Madin-Darby canine kidney cells was then added to the mixture of virus and antiserum and incubated for 7 days at 37°C. After the incubation period, virus replication was visualized by hemagglutination of chicken red blood cells. The 50% neutralization titer of the serum was calculated according to the method of Reed and Muench [15]. Neutralizing antibodies were evaluated in a subset of participants in the AS03A-adjuvanted vaccine groups using the following parameters with 95% CIs: (1) GMT on days 0, 42, and 180 and (2) SCR on days 42 and 180. Seroconversion was defined as the percentage of participants with a ≥4-fold increase in postvaccination neutralizing antibody titer.
The cell-mediated immune response was assessed using an adaptation of the method described by Maecker et al [16]. Peripheral blood mononuclear cells were restimulated in vitro using the H5N1 split antigen from the A/Vietnam/1194/2004-like NIBRG-14 vaccine strain. The cells were labeled using conventional immunofluorescence labeling of cellular phenotype markers and intracellular cytokines and analyzed by flow cytometry. Results were expressed as frequency of CD4+ or CD8+ T cells responding to the antigen and expressing at least 2 markers (of CD40L, interferon-γ, interleukin-2, and tumor necrosis factor–α) per million CD4+ or CD8+ T cells in total which is deemed to give an adequate estimate of the CD4 T cell specific response against the A/influenza antigen, on the basis of previous published observations [17–19]. Response was measured on days 0, 21, 42, and 180.
Statistical Analysis
The primary immunogenicity analysis was performed on the according-to-protocol (ATP) cohort, including all participants who met all eligibility criteria, complied with protocol procedures, and had antibody assay results available. The immunogenicity analysis at day 180 was performed on the ATP persistence cohort. A subset of 90 participants in each AS03A-adjuvanted vaccine group enrolled in Belgium was randomly selected for measurement of neutralizing antibodies.
The HI antibody response was analyzed in terms of CHMP criteria for approval of an influenza vaccine in adults aged ≥60 years (ie, the SCR and SPR must be >30% and >60%, respectively). No correlate of protection for neutralizing antibodies has been established, but the conventional postvaccination 4-fold increase in titer seems a reasonable standard.
The target sample size was 480 participants—180 in each AS03A-adjuvanted vaccine group and 60 in each nonadjuvanted vaccine group—to achieve 456 evaluable participants, assuming a 5% drop-out rate. The co-primary GMT end point was used to estimate sample size. A sample size of 170 evaluable participants per group was estimated to have 86% power to detect a 1.7-fold difference between the 2 AS03A-adjuvanted vaccine groups using a 2 sample t test with a .05 2-sided significance level, assuming that the standard deviations were 0.738 and 0.656 (log10 unit) for the 2 × H5N1-AS group and the 1 × H5N1-AS group, respectively, as previously observed [5].
Antibody titers below the assay cut-off value were given an arbitrary value of one-half the cut-off for the GMT calculation. A seronegative participant was defined as having an antibody titer less than the cut-off value; a seropositive participant was defined as having a titer greater than or equal to the cut-off value. Proc StatXact 5.0 was used to calculate 95% CIs for GMT, SCR, and SPR. The single-dose and double-dose groups were compared for HI antibodies in terms of GMT ratios and difference in SCRs. The 95% CIs of the GMT ratios (double dose/single dose and AS03A-adjuvanted/nonadjuvanted) were computed using an analysis of covariance model (ANCOVA) with the vaccine group as a fixed effect and the pre-vaccination titer as regressor. For the difference in SCR (double-dose minus single-dose and AS03A-adjuvanted minus nonadjuvanted), the asymptotic standardized 95% CIs were computed. Results for neutralizing antibodies and cell-mediated immunity were reported descriptively.
RESULTS
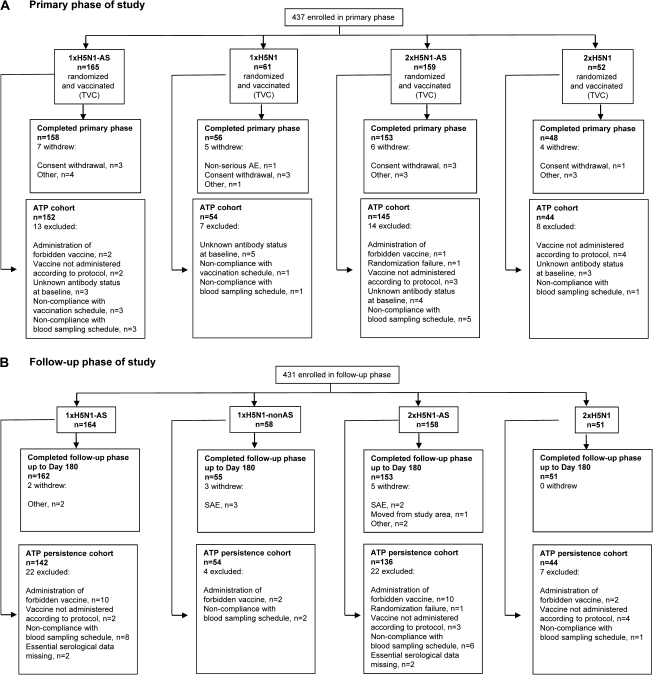
The study took place during the period from November 2006 and March 2008 (up to the point of the present analysis at Day 180) in seven centers in Belgium and five centers in Italy. A total of 437 participants were enrolled in the primary study phase, which 415 completed (Figure 1a). Although the target number of evaluable participants was not quite reached, satisfactory statistical power was achieved. A total of 431 participants continued in the follow-up phase, which 421 completed (Figure 1b).
Figure 1.
Disposition of study participants. Vaccination groups are as follows: 1 × H5N1-AS, single dose of the AS03A-adjuvanted vaccine; 1 × H5N1, single dose of the nonadjuvanted vaccine; 2 × H5N1-AS, double dose of the AS03A-adjuvanted vaccine; 2 × H5N1, double dose of the nonadjuvanted vaccine. AE, adverse event; ATP, according-to-protocol; SAE, serious adverse event; TVC, total vaccinated cohort.
The ATP cohort for immunogenicity (primary phase) and ATP persistence cohort (follow-up phase) included 395 and 376 participants, respectively (Figure 1a and b). Demographic characteristics were similar across the study groups. The mean age (± standard deviation) in the ATP cohort for immunogenicity was 69.7 ± 6.5 years (range, 61–89 years); 45% of subjects were women, and 97% were of Caucasian/European heritage. The number of participants in different age groups is shown in Table 1. Characteristics were similar in the ATP persistence cohort (data not shown) and the total vaccinated cohort (data shown in safety supplement).
Table 1.
Age Range of Participants (According to Protocol Cohort)
| No. of participants in each age group |
||||
| Age, years | 1×H5N1-AS | 1×H5N1 | 2×H5N1-AS | 2×H5N1 |
| 61-65 | 45 | 15 | 46 | 12 |
| 66-70 | 46 | 19 | 46 | 14 |
| 71-75 | 30 | 9 | 27 | 7 |
| 76-80 | 18 | 6 | 16 | 5 |
| ≥81 | 13 | 5 | 10 | 6 |
NOTE. Vaccination groups are as follows: 1 × H5N1-AS, single dose of the AS03A-adjuvanted vaccine; 1 × H5N1, single dose of the nonadjuvanted vaccine; 2 × H5N1-AS, double dose of the AS03A-adjuvanted vaccine; 2 × H5N1, double dose of the nonadjuvanted vaccine.
HI Antibody Response
In both AS03A-adjuvanted vaccine groups, GMTs rose substantially ≤3 weeks after receipt of the first dose of vaccine (day 21) (Table 2; total ATP cohort). Values increased further within 3 weeks after receipt of the second dose of vaccine (day 42) (Table 2). Although there was a good immune response in the 1 × H5N1-AS group, it was greater in the 2 ×x H5N1-AS group, with a GMT ratio (double dose/single dose) on day 42 of 1.87 (95% CI, 1.36–2.59; P =< .001).
Table 2.
Geometric Mean Titer (GMT), Seroconversion Rate (SCR), and Seroprotection Rate (SPR) for H5N1 HI Antibodies Against A/Vietnam/1194/2004
| Parameter | ATP cohort (total) |
ATP cohort (seronegative at baseline) |
|||||||
| 1×H5N1-AS | 1×H5N1 | 2×H5N1-AS | 2×H5N1 | 1×H5N1-AS | 1×H5N1 | 2×H5N1-AS | 2×H5N1 | ||
| No. of subjects | Time | 152 | 54 | 145 | 44 | 90 | 33 | 93 | 28 |
| GMT (95% CI) | Day 0 | 11.3 (9.2–13.9) | 9.7 (7.3–13.0) | 10.2 (8.4–12.5) | 8.8 (6.6–11.8) | 5.0 (5.0–5.0) | 5.0 (5.0–5.0) | 5.0 (5.0–5.0) | 5.0 (5.0–5.0) |
| Day 21 | 50.0 (38.1–65.6) | 16.8 (11.7–24.0) | 69.4 (52.1–92.3) | 20.8 (13.0–33.3) | 25.3 (18.5–34.6) | 9.7 (6.4–14.7) | 40.2 (29.5–54.8) | 10.5 (6.5–16.9) | |
| Day 42 | 126.8 (99.4–161.7) | 22.7 (15.1–34.1) | 237.3 (191.9–293.6) | 25.3 (16.0–40.1) | 80.0 (58.3–109.8) | 13.4 (8.0–22.5) | 198.5 (157.5–250.2) | 13.1 (8.1–21.3) | |
| SCR, % (95% CI) | Day 21 | 45.4 (37.3–53.7) | 14.8 (6.6–27.1) | 52.4 (44.0–60.8) | 18.2 (8.2–32.7) | 44.4 (34.0–55.3) | 18.2 (7.0–35.5) | 50.5 (40.0–61.1) | 14.3 (4.0–32.7) |
| Day 42 | 72.4 (64.5–79.3) | 22.2 (12.0–35.6) | 88.3 (81.9–93.0) | 22.7 (11.5–37.8) | 73.3 (63.0–82.1) | 21.2 (9.0–38.9) | 94.6 (87.9–98.2) | 17.9 (6.1–36.9) | |
| SPR, % (95% CI) | Day 0 | 18.4 (12.6–25.5) | 13.0 (5.4–24.9) | 15.9 (10.3–22.8) | 4.5 (0.6–15.5) | 0.0 (0.0–4.0) | 0.0 (0.0–10.6) | 0.0 (0.0–3.9) | 0.0 (0.0–12.3) |
| Day 21 | 61.2 (53.0–69.0) | 27.8 (16.5–41.6) | 62.1 (53.6–70.0) | 34.1 (20.5–49.9) | 44.4 (34.0–55.3) | 18.2 (7.0–35.5) | 50.5 (40.0–61.1) | 14.3 (4.0–32.7) | |
| Day 42 | 83.6 (76.7–89.1) | 35.2 (22.7–49.4) | 95.9 (91.2–98.5) | 38.6 (24.4–54.5) | 73.3 (63.0–82.1) | 21.2 (9.0–38.9) | 94.6 (87.9–98.2) | 17.9 (6.1–36.9) | |
| ATP persistence cohort | |||||||||
| No. of subjects | 142 | 54 | 136 | 44 | |||||
| GMT (95% CI) | Day 180 | 38.5 (30.0–49.5) | 16.3 (11.0–24.3) | 53.5 (41.9–68.4) | 13.1 (8.9–19.2) | ND | ND | ND | ND |
| SCR, % (95% CI) | Day 180 | 37.1 (29.1–45.7) | 12.5 (4.7–25.2) | 53.8 (44.9–62.6) | 14.3 (5.4–28.5) | ND | ND | ND | ND |
| SPR, % (95% CI) | Day 180 | 52.9 (44.2–61.3) | 26.0 (14.6–40.3) | 69.5 (60.8–77.2) | 20.5 (9.8–35.3) | ND | ND | ND | ND |
NOTE. Vaccination groups are as follows: 1 × H5N1-AS, single dose of the AS03A-adjuvanted vaccine; 1 × H5N1, single dose of the nonadjuvanted vaccine; 2 × H5N1-AS, double dose of the AS03A-adjuvanted vaccine; 2 × H5N1, double dose of the nonadjuvanted vaccine. European Committee for Human Medicinal Products (CHMP) criteria in adults aged ≥60 years were an SCR >30% and an SPR >60%.
SCR was defined as the percentage of participants with either (1) a prevaccination titer <1:10 and a postvaccination titer ≥1:40 or (2) a prevaccination titer ≥1:10 and a ≥4-fold increase in postvaccination titer; only (1) applies to initially seronegative participants. SPR: was defined as the percentage of participants with a hemagglutination inhibition titer ≥1:40 ATP, according to protocol; CI, confidence interval; HI,; ND, not done.
GMTs were considerably lower in the nonadjuvanted vaccine groups (Table 2). The GMT ratio (AS03A-adjuvanted/nonadjuvanted) was 5.58 (95% CI, 3.48–8.95; P < .001) for the single-dose groups and 9.38 (95% CI, 5.93–14.83; P < .001) for the double-dose groups.
Results for SCR and SPR mirrored those for GMT (Table 2). For SCR and SPR, CHMP criteria were met for both AS03A-adjuvanted vaccine dose groups (1 × H5N1-AS and 2 × H5N1-AS) at days 21 and 42 but were not met at all for the nonadjuvanted groups (1 × H5N1 and 2 × H5N1). All CHMP requirements were met for both adjuvanted vaccine dose groups in the different age strata (61–65, 66–70, and >70 years; data not shown). Comparing the double dose of the AS03A-adjuvanted vaccine with the single dose, the difference in SCR (double dose minus single dose) was 15.91% (95% CI, 7.00%–24.79%; P < .001), although a high SCR was achieved with the single dose. Comparing the AS03A-adjuvanted and nonadjuvanted vaccines, the difference in SCR (AS03A-adjuvanted minus nonadjuvanted) was 50.15% (95% CI, 35.59%–61.73%; P < .001) for the single-dose groups and 65.55% (95% CI, 50.30%–76.76%; P < .001) for the double-dose groups.
Remarkably, 38% of participants were seropositive for HI antibodies against the vaccine antigen before vaccination, and the proportion of seropositive subjects tended to increase with age. An exploratory analysis was therefore undertaken in participants who were seronegative at baseline. There was a high immune response in this population, although GMTs were somewhat lower than in the ATP cohort as a whole (Table 2). However, all CHMP criteria were met in both the single-dose and double-dose AS03A-adjuvanted vaccine groups in seronegative participants, except for SPR at day 21 (Table 2).
There was a decrease in GMT levels between days 42 and 180; levels remained considerably higher in the AS03A-adjuvanted vaccine groups (Table 2). SCR was above the postvaccination licensure criterion in both AS03A-adjuvanted vaccine groups at day 180, and SPR was above the criterion in the double-dose group (Table 2).
Neutralizing Antibody Response
Most participants (>90%) were seropositive for neutralizing antibodies before vaccination. Nevertheless, there was a substantial increase in GMT levels after vaccination and a high SCR in both AS03A-adjuvanted vaccine groups (Table 3). The immune response was somewhat higher in the double-dose group (2 × H5N1-AS), compared with the singe-dose group (1 × H5N1-AS). As seen with HI antibodies, GMTs and SCRs decreased between day 42 and Day 180, although GMTs remained above pre-vaccination level (Table 3).
Table 3.
Geometric Mean Titer (GMT) and Seroconversion Rate (SCR) for Neutralizing Antibodies Against A/Vietnam/1194/2004 in the AS03A-Adjuvanted Groups
| Parameter | |||
| Subset of ATP cohort | Time | 1×H5N1-AS | 2×H5N1-AS |
| No. of subjects | 87 | 82 | |
| GMT (95% CI) | Day 0 | 121.1 (94.6–154.9) | 112.5 (90.7–139.5) |
| Day 42 | 447.3 (359.3–557.0) | 595.8 (487.7–727.8) | |
| SCR, % (95% CI) | Day 42 | 44.8 (34.1–55.9) | 56.1 (44.7–67.0) |
| Subset of ATP persistence cohort | |||
| No. of subjects | 76 | 73 | |
| GMT (95% CI) | Day 180 | 218.2 (172.4–276.2) | 260.9 (207.7–327.8) |
| SCR, % (95% CI) | Day 180 | 21.1 (12.5–31.9) | 28.8 (18.8–40.6) |
NOTE. Vaccine groups are as follows: 1×H5N1-AS, single dose of the AS03A-adjuvanted vaccine; 2 × H5N1-AS, double dose of the AS03A-adjuvanted vaccine. ATP: according to protocol; CI, confidence interval.
Cell-Mediated Immunity
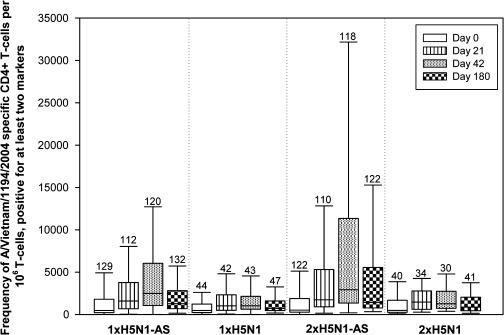
Antigen-specific CD4 T cell responses were elicited in all study groups but were markedly higher in the AS03A-adjuvanted vaccine groups, particularly the double-dose group (Figure 2). As with humoral immunity, there was a good response after the first vaccination, with a further increase after the second vaccination. Responses decreased somewhat by day 180, although persistence was higher in the AS03A-adjuvanted vaccine groups than in the nonadjuvanted groups (Figure 2). No CD8 T cell responses were observed.
Figure 2.
CD4 T cells specific for A/Vietnam/1194/2004 split-virus antigen, positive for ≥2 immunological markers. *Antigen-specific cells expressing ≥2 of the following immunological markers: CD40L, interleukin-2, tumor necrosis factor–α, or interferon-γ. Line in box, median value; top line of box, thirrd quartile; bottom line of box, first quartile; lower limit of error bar, minimum value; upper limit of error bar, maximum value; values above the bars, number of participants evaluated. Vaccination groups are as follows: 1 × H5N1-AS, single dose of the AS03A-adjuvanted vaccine; 1 × H5N1, single dose of the nonadjuvanted vaccine; 2 × H5N1-AS, double dose of the AS03A-adjuvanted vaccine; 2 × H5N1, double dose of the nonadjuvanted vaccine.
Safety
Safety and reactogenicity data are presented in the safety supplement. No vaccine-related serious adverse events were reported and no safety concerns were identified.
DISCUSSION
A high immune response in terms of HI antibodies, neutralizing antibodies, and cell-mediated immunity was induced by 2 injections of a single dose of 3.75 μg HA AS03A-adjuvanted vaccine given 3 weeks apart to participants aged >60 years. All CHMP requirements for influenza vaccines in the elderly population were met for this regimen. The more stringent CHMP criteria for vaccination of younger adults (>40% for SCR and >70% for SPR) were also achieved. The benefit of the AS03A adjuvant system was demonstrated since the nonadjuvanted vaccine groups showed markedly lower immune responses that failed to meet regulatory acceptance criteria. Persistence of the immune response was also high 6 months after vaccination. The SCR was still above the postvaccination licensure criterion with both adjuvanted formulations, and the SPR was above the criterion for the double-dose adjuvanted formulation. Although the specified sample size was not reached, adequate statistical power was achieved. Originally, a sample size of 170 subjects per group was estimated to have 86% power to detect a 1.7-fold difference between GMTs in the 2 AS03-adjuvanted groups. In fact, there was a 1.87-fold difference between GMTs in the 2 groups, and the reestimated power based on this difference was calculated to be 94.1%.
Assessment of a double-dose vaccine regimen was motivated by the well-recognized lower immune response in elderly persons [12]. Accordingly, the single-dose regimen induced lower immune responses after 2 vaccinations than usually observed in adults aged 18-60 years [5, 8, 9]. However, although the double dose of the AS03A-adjuvanted vaccine produced a higher immune response, licensing criteria were easily met with the single vaccine dose following a 2-vaccination administration schedule. This supports the use of the single dose in both the elderly population and among adults aged 18-60 years, as has been recognized by regulatory authorities with the recent extension of the vaccine licence to all adults aged >18 years [4].
When the overall study population was considered, only 1 dose of AS03A-adjuvanted vaccine (either single or double) seemed sufficient to reach both CHMP SCR and SPR criteria. However, further exploratory analysis showed that this effect was mainly driven by the HI immune response in initially seropositive individuals, who represented 38% of the ATP cohort for immunogenicity. In initially seronegative individuals, the response was lower, and all CHMP criteria were only met after 2 vaccinations with both the single- and double-dose regimens, indicating that a majority of elderly subjects will benefit from a 2-vaccination administration schedule, with doses given 21 days apart. The proportion of initially seropositive participants was higher than previously observed in younger adult populations [5, 8, 9] and may have occurred as a result of previous seasonal influenza vaccination or natural infection, as several studies have demonstrated cross-reactivity between avian and human strains for cell-mediated immunity and neutralizing antibody response [20–22]. More recently, cross-reactivity for an HA-specific antibody response was demonstrated in a study in which a seasonal influenza vaccine containing H1 and H3 antigens produced antibodies that cross-reacted with H5 HA [23]. Alternatively, the high seropositivity may reflect false-positive assay results, and it is possible that the microneutralization assay may have captured some N1 response. However, the HI assay used corresponds to international standards [14], and we are confident that the antibody activity measured is specific to H5N1. Thus, we believe that cross-reactivity between seasonal and avian influenza strains is a more likely explanation of the high levels of prevaccination seropositivity. Antibody titers may have limitations as the sole indicator of influenza vaccine efficacy in elderly persons, and it has been suggested that measures of the cell-mediated immune response should be included in evaluations of vaccines in this population [24–27]. Measurement of cell-mediated immunity was therefore included in the present study to gain a better understanding of the overall immune response induced by the vaccine and, thus, a better evaluation of its protective potential. The study showed that the pattern of the immune response to the AS03A-adjuvanted H5N1 vaccine was similar in terms of HI antibodies, neutralizing antibodies, and cell-mediated immunity (CD4 T cell responses). No CD8 T cell responses were observed. This may be due to the assay method or the protein content of the vaccine, although the possibility that the vaccine does not induce CD8 T cell responses cannot be excluded.
It is interesting to note that the kinetics of the immune response to the AS03A-adjuvanted H5N1 vaccine in elderly subjects seemed different from those observed in younger adults: 3 weeks after the first dose of vaccine was administered, HI antibody titers against the vaccine strain in the present study were higher than those in younger adults [5, 8, 9]. In contrast, 3 weeks after the second dose of vaccine was administered, titers were higher in the younger individuals. Although there was some decline, the immune response persisted up to 6 months after first vaccination, consistent with results seen in younger adults [8].
The safety and reactogenicity profile of the AS03A-adjuvanted H5N1 vaccine in elderly subjects was similar to that in younger adults (data shown in safety supplement), and no safety concerns were raised. Although there were more local and general symptoms reported with the adjuvanted vaccine than the nonadjuvanted vaccine, they were mainly mild-to-moderate and transient in nature, and reactogenicity was clinically acceptable.
In conclusion, 2 single 3.75-μg HA doses of the AS03A-adjuvanted H5N1 vaccine administered 21 days apart induced a high immune response in an elderly population. The study indicates that elderly persons do not require a higher vaccine dose than younger adults and the vaccine can be administered according to the same schedule. This will support a dose-sparing strategy, because elderly persons are an important target for vaccination in a pandemic situation.
Acknowledgments
We are indebted to the participating study volunteers, clinicians, nurses, and laboratory technicians at the study site and the sponsor's project staff for their support and contributions throughout the study, in particular to Adriano Lazzarin, Giuseppe Ferrera, and Rosa Cristina Coppola as investigators, to Koen Ceulemans and Beata De Vos (GSK Biologicals, Belgium), Maria-Primula Leone and Miriam Vighini (GSK Biologicals, Italy) for study coordination, to Erick Rosas for preparation of the study protocol and related study documentation and to Joëlle Thonnard, safety physician. We are grateful to Pascal Gérard and his team and to Elizabeth Neumeier, who performed the serological laboratory work, and to Philippe Moris and his team for performing the CMI analysis. We are also grateful to the National Institute for Biological Standards and Control for providing the vaccine virus strain for the assays and reference standards and also to the Centers for Disease Control and Prevention (Centers for Disease Control and Prevention) for supplying the recombinant A/Indonesia/5/2005 strain. Finally we thank Mary Greenacre (An Sgriobhadair) who provided medical writing services and Isabelle Gautherot (GSK Biologicals, Belgium) and Geraldine Verplancke (Keyrus Biopharma, on behalf of GSK Biologicals Belgium) for coordination, funded by GSK Biologicals.
Supplementary Data
Supplementary data are available at http://www.oxfordjournals.org/our_journals/jid/online.
Funding
The study was funded by GSK Biologicals. Funding to pay the Open Access publication charges for this article was provided by GlaxoSmithKline Biologicals.
References
- 1.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Germann TC, Kadau K, Longini IM, Jr., Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci USA. 2006;103:5935–40. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennings LC, Monto AS, Chan PK, Szucs TD, Nicholson KG. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis. 2008;8:650–8. doi: 10.1016/S1473-3099(08)70232-9. [DOI] [PubMed] [Google Scholar]
- 4.European Committee for Proprietary Medicinal Products. Committee for Medicinal Products for Human Use post-authorisation summary of positive opinion for Prepandrix (EMEA/CHMP/334055/2009). Published London, May 2009. Available at: http://www.emea.europa.eu/pdfs/human/opinion/Prepandrix_33405509en.pdf. Accessed 1 September 2009. [Google Scholar]
- 5.Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine. Lancet. 2007;370:580–9. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 6.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1345–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 7.Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One. 2008;3:e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz TF, Horacek T, Knuf N, et al. Single dose vaccination with AS03A-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine. 2009;27:6284–90. doi: 10.1016/j.vaccine.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Chu DW, Hwang SJ, Lim FS, et al. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine. 2009;27:7428–35. doi: 10.1016/j.vaccine.2009.07.102. [DOI] [PubMed] [Google Scholar]
- 10.Diez-Domingo J, Garcés-Sanchez M, Baldó J-M, et al. Immunogenicity and safety of H5N1 A/Vietnam/1194/2004 (Clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase II, randomized, open, controlled study. Pediatr Infect Dis J. 2010;29 doi: 10.1097/INF.0b013e3181daf921. DOI:10.1097/INF.0b013e3181daf921. [DOI] [PubMed] [Google Scholar]
- 11.Rümke HC, Bayasb JM, de Juanesc JR, et al. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine. 2008;26:2378–88. doi: 10.1016/j.vaccine.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 12.Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–84. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- 13.Hehme NW, Künzel W, Petschke F, et al. Ten years of experience with the trivalent split-influenza vaccine. Fluarix Clin Drug Invest. 2002;22:751–69. [Google Scholar]
- 14.Stephenson I, Heath A, Major D, et al. Reproducibility of serologic assays for influenza virus A (H5N1) Emerg Infect Dis. 2009;15:252–9. doi: 10.3201/eid1508.081754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–7. [Google Scholar]
- 16.Maecker HT, Maino VC, Picker LJ. Immunofluorescence analysis of T-cell responses in health and disease. J Clin Immun. 2000;20:391–9. doi: 10.1023/a:1026403724413. [DOI] [PubMed] [Google Scholar]
- 17.Hammarlund E, Lewis MW, Hanson SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 18.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–7. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 19.Frentsch M, Arbach O, Kirchoff D, et al. Direct access to CD4+T cells specific for defined antigens according to CD154 expresion. Nat Med. 2005;11:1118–24. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 20.Gioia C, Castilletti C, Tempestilli M, et al. Cross-subtype immunity against avian influenza in persons recently vaccinated for influenza. Emerg Infect Dis. 2008;14:121–8. doi: 10.3201/eid1401.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee LYH, Ha DLA, Simmons C, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–90. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roti M, Yang J, Berger DA, Huston L, James EA, Kwok WK. Healthy human subjects have CD4+ T cells directed against H5N1 virus. J Immunol. 2008;180:1758–68. doi: 10.4049/jimmunol.180.3.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corti D, Suguitan AL, Jr., Pinna D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–73. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a Q1 combination of serologic assays. J Clin Microbiol. 1999;37:937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElhaney JE, Xie D, Hager WD, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–9. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 26.McElhaney JE, Dutz JP. Better influenza vaccines for older people: what will it take? J Infect Dis. 2008;198:632–4. doi: 10.1086/590435. [DOI] [PubMed] [Google Scholar]
- 27.McElhaney JE, Ewen C, Zhou X, et al. Granzyme B: correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009;27:2418–25. doi: 10.1016/j.vaccine.2009.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]