Abstract
Background. Individuals who acquire human immunodeficiency virus (HIV) may experience an immediate disruption of genital tract immunity, altering the ability to mount a local and effective immune response. This study examined the impact of early HIV infection on new detection of human papillomavirus (HPV).
Methods. One hundred fifty-five Zimbabwean women with observation periods before and after HIV acquisition and 486 HIV-uninfected women were selected from a cohort study evaluating hormonal contraceptive use and risk of HIV acquisition. Study visits occurred at 3-month intervals. Cervical swab samples available from up to 6 months before, at, and up to 6 months after the visit when HIV was first detected were typed for 37 HPV genotypes or subtypes.
Results. We observed ∼5-fold higher odds of multiple (≥2) new HPV detections only after HIV acquisition, relative to HIV-negative women after adjusting for sexual behavior and concurrent genital tract infections. We also observed ∼2.5-fold higher odds of single new HPV detections at visits before and after HIV acquisition, relative to HIV-uninfected women in multivariable models.
Conclusions. These findings suggest that HIV infection has an immediate impact on genital tract immunity, as evidenced by the high risk of multiple new HPV detections immediately after HIV acquisition.
Cervical human papillomavirus (HPV) infections are substantially more common among women infected with human immunodeficiency virus (HIV), compared with HIV-uninfected women with similar sexual histories [1–10]. During the chronic stage of HIV infection, the natural history of HPV is independently associated with peripheral markers of immune suppression (ie, CD4 cell count) and HIV load [11]. Notably, incident detection of HPV DNA from the lower genital tract of sexually abstinent women was reported to range from 5% in HIV-negative women to 22% in HIV-positive women with a CD4 cell count of <200 cells/μL, suggesting that a substantial fraction of new HPV detection reflects reactivation of controlled, undetectable DNA rather than new acquisition [11].
Recent studies suggest a compartmentalized HIV-mediated immune suppression, specifically CD4+ memory T cell depletion, within days of HIV acquisition. HIV and simian immunodeficiency virus (SIV), upon entry, target a subset of memory CD4+ T cells localized in the mucosal compartments, particularly the gut, to produce the peak viremia associated with acute HIV infection [12–19]. The targeted memory CD4+ T cells are activated and carry the CCR5 coreceptor, both of which are necessary for HIV entry and dissemination. One study of the endocervix, conducted in rhesus macaques, found that nearly 90% of the memory CD4+ T cells were infected and depleted during acute SIV infection to drive early viral production [20]. Since CD4+ T cell help appears to be critical for effective control of reactivation of chronic or latent infections such as cytomegalovirus and varicella-zoster virus [21–25], it is possible that the presumed reactivation of HPV infection observed in chronically HIV-infected, sexually abstinent women will increase immediately following HIV acquisition.
We therefore sought to investigate whether newly detected HPV infections would significantly increase immediately after HIV acquisition, when there is believed to be a compartmentalized immune suppression in the genital tract mucosa. To achieve this, we measured newly detected HPV type-specific infections among women who did and women who did not acquire HIV with 3-month interval sampling, in a subset of Zimbabwean women participating in the prospective Hormonal Contraception and the Risk of HIV Acquisition (HC-HIV) study.
MATERIALS AND METHODS
Study Population
The HC-HIV study cohort has been described in detail elsewhere [26]. Briefly, from November 1999 through January 2004, 6156 women from the general population seeking reproductive and general health care services were recruited from Uganda, Zimbabwe, and Thailand. Inclusion criteria included being 18–35 years of age, sexually active, and HIV-negative.
Women who enrolled were followed up every 3 months for 15–24 months. At enrollment and each follow-up visit, women answered a standardized questionnaire to capture demographic, behavioral, and health history information. Women also underwent a physical examination and provided blood, vaginal, and cervical samples for testing of HIV, herpes simplex virus type-2 (HSV-2), and concurrent sexually transmitted infections (STIs). All participants received HIV pretest and posttest counseling, risk reduction counseling, and access to hormonal contraception, condoms, and treatment for concurrent STIs during the course of the study.
Study Design
We selected women from the Zimbabwe study site who acquired HIV (N = 155) and matched them to women who remained HIV-uninfected (N = 486) on time in study, age, and genital tract infections. Women were frequency matched on the following age categories: 18–19, 20–24, 25–29, and 30–35 years. A woman was positive for the composite STI variable if any genital tract infections (Chlamydia trachomatis infection, Neisseria gonorrhoeae infection, or bacterial vaginosis) were present at the visit when HIV was first detected or the prior visit. Up to 4 HIV-uninfected women were matched to each HIV-infected woman.
Up to 5 total cervical swab samples for each woman were selected for HPV testing, anchored by the first HIV polymerase chain reaction (PCR)–positive visit (index visit) among the seroconverters. The 5 visits included the intended visits 3 and 6 months prior to the index visit (t−2 and t−1, respectively), the index visit (tindex), and the visits 3 and 6 months following the index visit (t+1 and t+2, respectively). Because HIV-negative women were matched to case women on time in follow-up, the index visit among the HIV-negative women corresponds to the study visit number of the matching seroconverter.
Cervical samples were collected by inserting a small tipped swab into the endocervix and rotating it for 15–30 s before placing into 1 mL of Amplicor C. trachomatis and N. gonorrhoeae lysis buffer (Roche Diagnostics) for C. trachomatis and N. gonorrhoeae PCR. After agitation and removal of the swab, 1 mL of diluent was mixed with the sample. Aliquots of the residual processed cervical samples were shipped on ice to Johns Hopkins Bloomberg School of Public Health (Baltimore, MD) and stored at −80°C until testing.
The study was approved by the institutional review boards of the participating institutions in the United States and Zimbabwe. All women provided written informed consent prior to study participation.
Laboratory Methods
HIV Testing.
DNA PCR results from dried blood spots ultimately determined the visit when a participant was first positive for HIV (Amplicor HIV-1 DNA test; version 1.5; Roche Diagnostics). In brief, enzyme-linked immunoassays were performed on serum samples at each study visit. Any positive result was confirmed with (1) rapid testing and (2) Western blot assay or PCR. For women with confirmed HIV seroconversion, PCR testing was performed serially on stored blood spots from prior visits to determine the date of HIV acquisition [26].
HPV Detection and Genotyping.
DNA was extracted from a 250-μL aliquot of exfoliated cell samples using the QIAamp DNA blood MDx protocol (Qiagen). DNA was resuspended in 185 μL of Tris-EDTA buffer, and an 8-μL aliquot of the purified DNA was amplified using the Roche HPV Linear Array test [27], which co-amplifies with high efficiency >40 HPV genotypes and subtypes known to infect the genital tract. High-risk HPV types were defined as 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. Low-risk HPV types were defined as 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 67, 69, 70, 71, 72, 73, 81, 82, 82 subtype (IS39), 83, 84, and 89. Positive and negative controls were used to monitor extraction, amplification, and detection steps of each assay.
STI/Genital Tract Infection Testing.
In the parent study, PCR assays were conducted at each visit to detect C. trachomatis and N. gonorrhoeae (Amplicor; Roche Diagnostics). Amsel criteria were used to diagnose bacterial vaginosis [28]. Enzyme-linked immunosorbent assays were conducted on serum samples collected at baseline and during follow-up to determine prevalent and incident infections with HSV-2 (Focus Technologies) [26, 29].
Statistical Analyses
A total of 641 women were selected for the study. Eight women who did not have samples from either of the visits before the index visit (t−2 or t−1) and 1 woman who had insufficient cellular material at her only available visit before the index visit were excluded, leaving a total analysis population of 632 women. Baseline characteristics were assessed at the t−2 visit (or at the t−1 visit when the t−2 visit was missing). At each visit of a woman's follow-up (t−1, tindex, t+1, and t+2), the median duration of months and interquartile ranges were estimated for those who did and those who did not acquire HIV.
Time-varying composite variables were constructed to describe sexual behaviors of the participant and her primary partner [30]. In brief, a participant's behavior was dichotomized as high sexual risk if she reported having multiple sex partners, having a new sex partner, and/or engaging in commercial sex. A primary partner's behavior was dichotomized as having high sexual risk if the participant reported having a partner who had HIV infection, urethral discharge, significant weight loss, sex with a commercial sex worker, and/or spent nights away from home. Peripheral blood CD4+ T cell counts and plasma HIV RNA loads were available for 111 (72%) of 155 HIV-infected women. Baseline covariates were compared between those who remained HIV-negative and those who acquired HIV by use of the Pearson χ2 test and the Wilcoxon rank-sum test.
Newly detected HPV infections were categorized as a multinomial outcome (0, 1, or ≥2 new HPV infections). At each follow-up visit (t−1, tindex, t+1, and t+2), the number of newly detected type-specific HPVs since the prior visit were summed prior to categorization. Because we tested for 37 HPV types at each visit, all women remained at risk for at least 1 new type at each pair-wise visit comparison. If there was an intercurrent negative detection for a type-specific infection (eg, positive, followed by negative, followed by positive), the follow-up detection of that type was counted as a newly detected infection. A sensitivity analysis was performed where type-specific infections detected after an intercurrent negative detection were excluded from the analysis.
We estimated the independent effect of HIV acquisition on new HPV DNA detection by use of multinomial logistic regression, adjusting for lifetime number of sex partners, primary partner risk, and genital tract infections (composite STI). The interpretation of the odds ratio is the likelihood of detecting a single or multiple new HPV types, as opposed to none, during the periods before and after HIV acquisition, independent of sexual behavior and genital tract infections. We incorporated a cluster variable to calculate 95% confidence intervals with robust standard errors to account for repeated measures within participants [31]. Covariates such as marital status, living with a partner, contraceptive method, and HSV-2 infection were found not to be informative and were not included in the final model. We used the Stata statistical package (version 9.2; StataCorp) for all analyses.
RESULTS
The median age at the index visit was 25 years. Women who acquired HIV were less likely to be in a monogamous marriage, living with their primary partner, and using a form of hormonal contraception (Table 1). They were also more likely to have >1 lifetime partner and have a high-risk primary partner. Women who acquired HIV had shorter intervals of follow-up time after HIV acquisition and were more likely to have gonorrhea, bacterial vaginosis, and HSV-2 infection. Baseline HPV prevalence differed between the 2 groups. Women who acquired HIV were more likely to have the following: (1) any HPV infection, (2) high-risk HPV infection, (3) low-risk HPV infection, and (4) multiple HPV type-specific infections at baseline, compared with HIV-uninfected women. There were no baseline differences in age, years of education, age at first sexual activity, number of lifetime pregnancies, coital frequency, participants’ sexual risk behavior, or co-infection with C. trachomatis.
Table 1.
Time-fixed and Time-varying Characteristics of Study Population by Human Immunodeficiency Virus Status Among the Baseline Visits
| Characteristic | No. (%) of HIV-infected women (n = 154) | No. (%) of HIV-uninfected women (n = 478) | Pa |
| Time fixed | |||
| Age, years | .98 | ||
| 18–19 | 14 (9) | 46 (10) | |
| 20–24 | 64 (42) | 205 (43) | |
| 25–29 | 47 (31) | 142 (30) | |
| 30–36 | 29 (19) | 85 (18) | |
| Marital status | <.01 | ||
| Monogamous | 113 (73) | 417 (87) | |
| Not marriedb | 25 (16) | 37 (8) | |
| Polygamous | 16 (10) | 24 (5) | |
| Living with partner | <.01 | ||
| Yes | 128 (83) | 441 (92) | |
| No | 26 (17) | 37 (8) | |
| Education level, years | .77 | ||
| 0–8 | 28 (18) | 92 (19) | |
| 9–17 | 126 (82) | 386 (81) | |
| Contraceptive method | .02 | ||
| None | 54 (35) | 115 (24) | |
| Depot-medroxyprogesterone acetate | 52 (34) | 173 (36) | |
| Combined oral contraceptives | 48 (31) | 190 (40) | |
| Age at first sexual activity, years | .14 | ||
| 12–17 | 52 (34) | 193 (40) | |
| 18–27 | 102 (66) | 285 (60) | |
| Lifetime no. of pregnancies | .67 | ||
| 0–1 | 51 (33) | 157 (33) | |
| 2 | 62 (40) | 177 (37) | |
| ≥3 | 41 (27) | 144 (30) | |
| Lifetime no. of sex partners | <.01 | ||
| 1 | 92 (60) | 364 (76) | |
| 2 | 36 (23) | 72 (15) | |
| ≥3 | 26 (17) | 42 (9) | |
| Time varying | |||
| Median duration at each follow-up visit, months (IQR) | |||
| t−1 | 2.7 (2.5–2.8) | 2.6 (2.5–2.8) | .54 |
| tindex | 2.7 (2.5–3.9) | 2.7 (2.5–3.3) | .53 |
| t+1 | 2.5 (1.1–5.0) | 2.7 (2.6–2.8) | .01 |
| t+2 | 1.2 (.5–2.8) | 2.7 (2.6–2.9) | <.01 |
| Coital frequency, months | .51 | ||
| 0–14 | 85 (55) | 245 (51) | |
| 15–29 | 50 (33) | 180 (38) | |
| ≥30 | 19 (12) | 53 (11) | |
| Participant's sexual risk behaviorc | .25 | ||
| Low | 151 (98) | 474 (99) | |
| High | 3 (2) | 4 (1) | |
| No. of nights primary partner spent away from home per monthd | .57 | ||
| 0 | 94 (61) | 313 (66) | |
| 1–15 | 40 (26) | 114 (24) | |
| 16–30 | 20 (13) | 49 (10) | |
| Primary partner with CSW since last visitd | .04 | ||
| No | 138 (90) | 447 (94) | |
| Yes | 13 (8) | 30 (6) | |
| Primary partner with another woman since last visit | .02 | ||
| No | 66 (43) | 260 (54) | |
| Yes | 28 (18) | 53 (11) | |
| Do not know | 60 (39) | 165 (35) | |
| Primary partner sexual risk behaviore | .03 | ||
| Low | 48 (31) | 196 (41) | |
| High | 106 (69) | 282 (59) | |
| Chlamydiad | .16 | ||
| Negative | 146 (95) | 466 (98) | |
| Positive | 8 (5) | 11 (2) | |
| Gonorrhead | <.01 | ||
| Negative | 141 (92) | 470 (98) | |
| Positive | 13 (8) | 7 (2) | |
| Bacterial vaginosis | <.01 | ||
| Negative | 105 (68) | 381 (80) | |
| Positive | 49 (32) | 97 (20) | |
| Composite STIdf | .77 | ||
| Negative | 50 (33) | 157 (33) | |
| Positive | 100 (65) | 313 (66) | |
| HSV-2 serologyd | <.01 | ||
| Negative | 27 (18) | 227 (48) | |
| Positive | 126 (82) | 250 (52) | |
| Any HPV | <.01 | ||
| Negative | 55 (36) | 251 (53) | |
| Positive | 99 (64) | 227 (48) | |
| Any high-risk HPV type | <.01 | ||
| Negative | 73 (47) | 313 (66) | |
| Positive | 81 (53) | 165 (35) | |
| Any low-risk HPV type | <.01 | ||
| Negative | 80 (52) | 348 (73) | |
| Positive | 74 (48) | 130 (27) | |
| No. of HPV types | <.01 | ||
| 0 | 55 (36) | 251 (53) | |
| 1 | 24 (16) | 120 (25) | |
| 2 | 21 (14) | 54 (11) | |
| 3 | 24 (16) | 22 (5) | |
| 4–15 | 30 (20) | 31 (7) | |
NOTE. CSW, commercial sex worker; HPV, human papillomavirus; HSV-2, herpes simplex virus type 2; IQR, interquartile range; STI, sexually transmitted infection; t−1, last visit before that when human immunodeficiency virus (HIV) was detected; tindex, index visit when HIV was detected; t+1, first visit after that when HIV was detected; t+2, second visit after that when HIV was detected.
Pearson χ2 and Wilcoxon rank-sum tests.
Not married includes never married, separated, divorced, or widowed.
High risk was defined as having multiple partners, a new partner, commercial sex, and/or sex with another man in the past 3 months.
Some samples are missing.
High risk was defined as a partner who was HIV-positive, had abnormal discharge, had weight loss, had sex with another women, or spent 1 or more nights away from home in a typical month since the last visit.
Positive for Chlamydia trachomatis, Neisseria gonorrhoeae, or bacterial vaginosis at index visit or last negative visit.
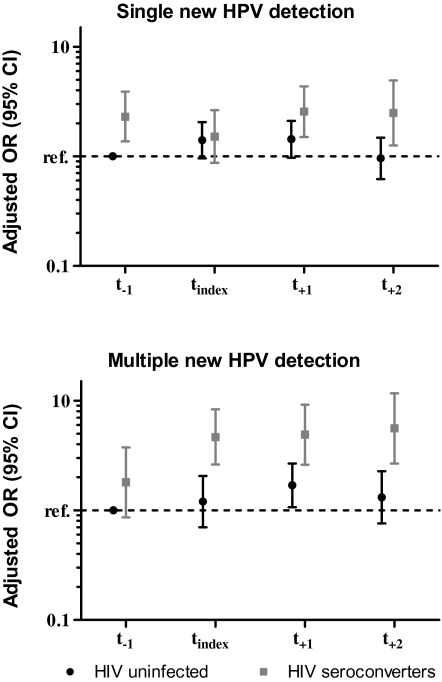
The crude proportions of single or multiple new HPV types varied for the women who acquired HIV over time (Table 2). HIV-infected women had a 2-fold increased odds ratio for a single detection of HPV both before and after HIV acquisition relative to HIV-negative women (Figure 1). In contrast, the risk of detection of multiple new HPV types was only increased after HIV acquisition. Specifically, women who acquired HIV had no increased risk of detection of multiple new HPV types when they remained HIV-negative, but they did have a ∼5-fold increased odds ratio of new detection of multiple type-specific HPV infections starting at the first HIV-positive visit through the next 2 sampling intervals compared with women who remained HIV-uninfected (Figure 1). Similar results were obtained after excluding new type-specific detections following intercurrent negative infections (163 intercurrent negative infections of 1033 newly detected infections [16%]).
Table 2.
Crude Proportions of Newly Detected Human Papillomavirus Types Stratified by Human Immunodeficiency Virus Status and Visit
| No. of newly detected HPV types | No. (%) of HIV-uninfected women | No. (%) of HIV-infected women | ||||||
| t−1(n = 388) | tindex(n = 455) | t+1(n = 394) | t+2(n = 334) | t−1(n = 119) | tindex(n = 150) | t+1(n = 127) | t+2(n = 120) | |
| 0 | 310 (80) | 340 (75) | 286 (73) | 262 (78) | 77 (65) | 89 (59) | 66 (52) | 59 (49) |
| 1 | 53 (14) | 81 (18) | 69 (18) | 43 (13) | 30 (25) | 24 (16) | 31 (24) | 33 (28) |
| ≥2 | 25 (6) | 34 (8) | 39 (10) | 29 (9) | 12 (10) | 37 (25) | 30 (24) | 28 (23) |
NOTE. HPV, human papillomavirus; t−1, last visit before that when human immunodeficiency virus (HIV) was detected; tindex, index visit when HIV was detected; t+1, first visit after that when HIV was detected; t+2, second visit after that when HIV was detected.
Figure 1.
Newly detected human papillomavirus (HPV) infection by follow-up time for human immunodeficiency virus (HIV)–uninfected and HIV-infected participants. The last visit before that when HIV was detected (pre-index visit; t−1) serves as the reference category; estimates were adjusted for lifetime number of sex partners, primar partner risk, and genital tract infections (composite sexually transmitted infection). Adjusted for lifetime number sex partners, primary partner risk and composite STI. CI, confidence interval; HPV, human papillomavirus; OR, odds ratio; ref, reference group; tindex, index visit; t+1, first visit after that when HIV was detected; t+2, second visit after that when HIV was detected.
CD4+ T cell counts and HIV RNA loads at the visit after HIV acquisition (t+1) did not significantly differ by HPV status, as defined by any prevalent HPV infection, any single new HPV detection, or detection of multiple new HPV types (data not shown). Adjustment for markers of sexual risk, including lifetime number of partners, partner's sexual risk behavior, and genital tract infections, did not substantially attenuate the estimates associated with HIV acquisition (Tables 3 and 4) but were independently associated with new HPV detection.
Table 3.
Unadjusted and Adjusted Associations of Newly Detected Human Papillomavirus Infections Before and After Human Immunodeficiency Virus Acquisition Relative to the Pre-index Visit of the Human Immunodeficiency Virus-uninfected Women
| Any new HPV type | Single new HPV type |
Multiple new HPV types |
|||
| Characteristic | Adjustedab RR (95% CI) | Unadjustedc OR (95% CI) | Adjustedac OR (95% CI) | Unadjustedc OR (95% CI) | Adjustedac OR (95% CI) |
| Women stratified by visit HIV uninfected | |||||
| t−1d | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| tindex | 1.2 (1.0–1.6) | 1.4 (1.0–2.0) | 1.4 (1.0–2.1) | 1.2 (.7–2.0) | 1.2 (.7–2.1) |
| t+1 | 1.4 (1.1–1.7) | 1.4 (1.0–2.1) | 1.4 (1.0–2.1) | 1.7 (1.1–2.6) | 1.7 (1.1–2.7) |
| t+2 | 1.1 (.8–1.4) | 1.0 (.6–1.5) | 1.0 (.6–1.5) | 1.4 (.8–2.3) | 1.3 (.8–2.3) |
| HIV infected | |||||
| t−1 | 1.7 (1.2–2.3) | 2.4 (1.4–4.1) | 2.3 (1.4–3.9) | 2.0 (1.0–4.2) | 1.8 (.9–3.7) |
| tindex | 1.9 (1.4–2.5) | 1.6 (.9–2.7) | 1.5 (.9–2.6) | 5.1 (2.9–8.9) | 4.7 (2.6–8.3) |
| t+1 | 2.2 (1.6–2.9) | 2.8 (1.6–4.6) | 2.6 (1.5–4.3) | 5.6 (3.1–10.1) | 4.9 (2.6–9.2) |
| t+2 | 2.2 (1.6–3.1) | 2.7 (1.4–5.3) | 2.5 (1.3–4.9) | 6.3 (3.1–12.9) | 5.6 (2.7–11.7) |
| Lifetime no. of sex partners | |||||
| 1 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 1.0 (.8–1.2) | 1.2 (.9–1.7) | 1.0 (.7–1.5) | 1.3 (.8–2.0) | 1.0 (.6–1.6) |
| ≥3 | 1.4 (1.2–1.8) | 2.0 (1.4–2.9) | 1.8 (1.2–2.6) | 2.5 (1.6–3.9) | 1.9 (1.2–3.1) |
| Primary partner riske | |||||
| Low | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| High | 1.4 (1.2–1.6) | 1.4 (1.1–1.8) | 1.2 (1.0–1.6) | 2.6 (1.9–3.7) | 2.3 (1.6–3.2) |
| Composite STIf | |||||
| Negative | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Positive | 1.4 (1.2–1.7) | 1.7 (1.3–2.2) | 1.7 (1.3–2.2) | 1.7 (1.1–2.4) | 1.5 (1.0–2.2) |
NOTE. CI, confidence interval; HPV, human papillomavirus; OR, odds ratio; RR, relative risk; STI, sexually transmitted infection; t−1, last visit before that when Human Immunodeficiency Virus (HIV) was detected; tindex, index visit when HIV was detected; t+1, first visit after that when HIV was detected; t+2, second visit after that when HIV was detected.
Adjusted for lifetime number of sex partners, primary partner risk, and composite STI. Marital status, living with partner, contraceptive method, and co-infection with herpes simplex virus type 2 were evaluated and not included in the final model.
Poisson regression.
Multinomial regression.
Pre-index visit (t−1) of HIV-uninfected women is the reference group.
High risk was defined as a partner who was HIV-positive, had abnormal discharge, had weight loss, had sex with another woman, or spent 1 or more nights away from home in a typical month since the last visit.
Positive for Chlamydia trachomatis, Neisseria gonorrhoeae, or bacterial vaginosis at index visit or prior visit.
Table 4.
Unadjusted and Adjusted Associations of Newly Detected Human Papillomavirus Infections for the Women Who Became Human Immunodeficiency Virus Infected During Follow-up, Stratified by Study Visit
| Single new HPV type |
Multiple new HPV types |
|||
| Characteristic | Unadjusteda OR (95% CI) | Adjustedab OR (95% CI) | Unadjusteda OR (95% CI) | Adjustedab OR (95% CI) |
| Visit t−1 | ||||
| HIV-uninfected women | 1.0 | 1.0 | 1.0 | 1.0 |
| HIV-infected womenc | 2.4 (1.4–4.1) | 2.3 (1.4–3.9) | 2.0 (1.0–4.2) | 1.4 (.6–3.2) |
| Visit tindex | ||||
| HIV-uninfected women | 1.0 | 1.0 | 1.0 | 1.0 |
| HIV-infected women | 1.1 (.7–1.9) | 1.1 (.6–1.9) | 4.2 (2.5–7.2) | 4.0 (2.3–6.8) |
| Visit t+1 | ||||
| HIV-uninfected women | 1.0 | 1.0 | 1.0 | 1.0 |
| HIV-infected women | 1.9 (1.2–3.2) | 1.7 (1.0–2.9) | 3.3 (1.9–5.8) | 2.9 (1.6–5.3) |
| Visit t+2 | ||||
| HIV-uninfected women | 1.0 | 1.0 | 1.0 | 1.0 |
| HIV-infected women | 2.8 (1.4–5.5) | 2.5 (1.2–5.1) | 4.6 (2.3–9.3) | 4.5 (2.2–9.3) |
NOTE. CI, confidence interval; HPV, human papillomavirus; OR, odds ratio; t−1, last visit before that when human immunodeficiency virus (HIV) was detected; tindex, index visit when HIV was detected; t+1, first visit after that when HIV was detected; t+2, second visit after that when HIV was detected.
Multinomial regression.
Adjusted for lifetime number of sex partners and primary partner risk. Marital status, living with partner, contraceptive method, composite sexually transmitted infection variable, gonorrhea, bacterial vaginosis, and herpes simplex virus type 2 infection were evaluated and not included in the final model.
Women who would become infected with HIV.
DISCUSSION
We observed a sustained, ∼5-fold increased odds of new HPV detection with multiple types in HIV-infected women, beginning at the first HIV-positive sampling. The risk of new single-type HPV detection was elevated among HIV-infected women both before and after HIV acquisition compared with women who remained HIV-uninfected during the observational period. The findings support our hypothesis that HIV causes a profound and immediate disruption to the genital tract immunity, resulting in increased risk of new or reactivated HPV infections.
Although the rapid depletion of gut-associated CD4+ cells following HIV acquisition in humans has been well described [13,19,32–34], no studies have evaluated the immunologic effects of HIV acquisition on genital tract immunity. While we were unable to directly assess this, our data suggest that HIV acquisition may rapidly compromise local immune memory function. This explanation would be consistent with the observation that reactivation of Mycobacterium tuberculosis infection was associated with a selective depletion of M. tuberculosis–specific memory CD4+ T cells in early HIV infection [35, 36].
We are unable to determine whether the increase in multiple new HPV type detection results from an increased susceptibility to new HPV infections or re-infection, reactivation of existing infections [37–42], or both. While new infections or re-infections from the male sex partner cannot be ruled out, increasing evidence suggests that loss of HPV detection may not reflect complete virologic clearance. Strickler et al [11] reported a high proportion of newly detected HPV infections in HIV-positive women who remained sexually abstinent for up to 18 months (22% among women with CD4 cell counts of <200 cells/μL). More recently, a nontrivial incidence rate of redetection of apparently cleared HPV type-specific infections (∼8%) was reported among young women in the placebo arm of a HPV vaccine trial [43]. In addition, it is difficult to reconcile the rapid emergence (<12 weeks) of multiple new HPV infections with the estimated time required for HPV to sufficiently replicate in the differentiating epithelium and become detectable in exfoliated cells [44].
The observation that the risk of single new HPV detection among HIV-infected women was increased during the interval preceding HIV acquisition, compared with women who remained HIV-uninfected, is interesting. One interpretation is that the risk difference in single new HPV detection simply reflects the higher burden of penile HPV infection among the male HIV-infected sex partners (ie, confounding by shared sexual risk) [45, 46]. The fact that the number of multiple new HPV detections was not similarly elevated prior to HIV acquisition suggests that confounding by shared sexual risk factors is unlikely to completely explain our observations.
Recently, we and others have reported an increased risk of HIV acquisition among HPV-positive women, particularly among women with loss of HPV detection in the interval preceding HIV acquisition [47, 48]. The apparent association between loss of HPV just prior to HIV acquisition and new detection of multiple HPV types at and subsequent to HIV acquisition raise important questions regarding the interaction of these viral infections. One explanation is that the lost HPV preceding HIV acquisition is then redetected at a later visit; indeed, this may explain the similar odds ratio of a new single HPV detection at the index visit among HIV-uninfected and HIV-infected women. However, only 16% of all new HPV type-specific detections in this study were recurrent detections following a single intercurrent negative result; excluding these infections as incident events did not change our findings of an increased risk of new multiple-type HPV detection following HIV acquisition. Taken together, these data suggest a complex immunologic microenvironment in HPV and HIV co-infection, which should be evaluated in more detail.
Our study has a number of strengths. First, it is one of the few studies to include women with early HIV infection with known dates of HIV acquisition. These dates were precisely defined by PCR-based methods, rather than by serology. Second, this study could sensitively detect up to 37 HPV genotypes and subtypes and could restrict the analysis to newly detected (rather than prevalent) infections. In addition, the study was prospective, had follow-up visits every 2–3 months, and could better detect HPV and behavioral changes during shorter time intervals bounding HIV acquisition compared with previous studies.
This study also has some limitations. We matched HIV-uninfected women to women becoming HIV-infected on a composite STI variable (positive for chlamydia, gonorrhea, and/or bacterial vaginosis) in an attempt to control for risk of STI; however, we did not achieve comparable prevalence of the individual infections in the study groups. We therefore included the composite variable in our final models. Adjustment for the individual STIs comprising the composite STI variable yielded similar results (data not shown). We were limited in our ability to evaluate immunologic markers of HIV-related immune status. However, peripheral measures of CD4+ T cells and HIV RNA loads at 1 visit after HIV acquisition (t+1) were not significantly associated with prevalent HPV infections, single new HPV infections, or multiple new HPV infections, suggesting that more comprehensive peripheral measures of immunity would not contribute significantly to this analysis. Measures of HPV-specific memory CD4+ T cells in the genital tract mucosa will be needed to directly evaluate the relationship between newly detected HPV infections and a loss in memory CD4+ T cells. This may prove challenging in human studies, however, due to the type-specificity and the lack of well-characterized HPV epitopes required for characterization of the HPV-specific immune response. Furthermore, complete recovery of HPV-specific T cells is unlikely from exfoliated cell samples, and invasive biopsies would not be feasible in a prospective study of HIV acquisition.
In summary, our observation of increased detection of multiple new HPV types in early HIV infection supports the notion of an immediate disruption in genital tract immunity following HIV infection. An increased risk of new single-type HPV detection both before and after HIV acquisition also suggests possible shared sexual risk. Therefore, we believe that an increase in HPV infection risk in the earliest stages of HIV infection may reflect a combination of confounding by shared sexual risk and immunologic effects of HIV infection. Alternative explanations for these observational associations are not mutually exclusive, and these data, together with the increased risk of HIV acquisition in women with HPV clearance but not HPV persistence, suggest a complex immunologic response to HIV and HPV co-infections in the genital tract that should be more fully explored.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through a contract with Family Health International (contract number N01-HD-0-3310).
Acknowledgments
We thank Darcy Phelan, Morgan Marks, and Proma Paul for their assistance with the statistical analysis.
References
- 1.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC., Jr Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med. 1997;337:1343–9. doi: 10.1056/NEJM199711063371903. [DOI] [PubMed] [Google Scholar]
- 2.Levi JE, Fernandes S, Tateno AF, et al. Presence of multiple human papillomavirus types in cervical samples from HIV-infected women. Gynecol Oncol. 2004;92:225–31. doi: 10.1016/j.ygyno.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Jamieson DJ, Duerr A, Burk R, et al. Characterization of genital human papillomavirus infection in women who have or who are at risk of having HIV infection. Am J Obstet Gynecol. 2002;186:21–7. doi: 10.1067/mob.2002.119776. [DOI] [PubMed] [Google Scholar]
- 4.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis. 2001;184:682–90. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 5.Clifford GM, Goncalves MA, Franceschi S HPV and HIV Study Group. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS. 2006;20:2337–44. doi: 10.1097/01.aids.0000253361.63578.14. [DOI] [PubMed] [Google Scholar]
- 6.Minkoff H, Feldman J, DeHovitz J, Landesman S, Burk R. A longitudinal study of human papillomavirus carriage in human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. Am J Obstet Gynecol. 1998;178:982–6. doi: 10.1016/s0002-9378(98)70535-6. [DOI] [PubMed] [Google Scholar]
- 7.Palefsky JM. Cervical human papillomavirus infection and cervical intraepithelial neoplasia in women positive for human immunodeficiency virus in the era of highly active antiretroviral therapy. Curr Opin Oncol. 2003;15:382–8. doi: 10.1097/00001622-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Vermund SH, Kelley KF, Klein RS, et al. High risk of human papillomavirus infection and cervical squamous intraepithelial lesions among women with symptomatic human immunodeficiency virus infection. Am J Obstet Gynecol. 1991;165:392–400. doi: 10.1016/0002-9378(91)90101-v. [DOI] [PubMed] [Google Scholar]
- 9.Blossom DB, Beigi RH, Farrell JJ, et al. Human papillomavirus genotypes associated with cervical cytologic abnormalities and HIV infection in Ugandan women. J Med Virol. 2007;79:758–65. doi: 10.1002/jmv.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowhani-Rahbar A, Hawes SE, Sow PS, et al. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J Infect Dis. 2007;196:887–94. doi: 10.1086/520883. [DOI] [PubMed] [Google Scholar]
- 11.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 12.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J Infect Dis. 2003;187:769–76. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 13.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–7. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–7. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 16.Lim SG, Condez A, Lee CA, Johnson MA, Elia C, Poulter LW. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993;92:448–54. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quayle AJ, Kourtis AP, Cu-Uvin S, et al. T-lymphocyte profile and total and virus-specific immunoglobulin concentrations in the cervix of HIV-1-infected women. J Acquir Immune Defic Syndr. 2007;44:292–8. doi: 10.1097/QAI.0b013e31802c5b3a. [DOI] [PubMed] [Google Scholar]
- 18.Gupta P, Collins KB, Ratner D, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–76. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 20.Zhang ZQ, Wietgrefe SW, Li Q, et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 2004;101:5640–5. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malavige GN, Jones L, Black AP, Ogg GS. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin Exp Immunol. 2008;152:522–31. doi: 10.1111/j.1365-2249.2008.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol. 2008;197:83–96. doi: 10.1007/s00430-008-0082-5. [DOI] [PubMed] [Google Scholar]
- 23.Sedaghat AR, Siliciano RF, Wilke CO. Low-level HIV-1 replication and the dynamics of the resting CD4+ T cell reservoir for HIV-1 in the setting of HAART. BMC Infect Dis. 2008;8:2. doi: 10.1186/1471-2334-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borowski AB, Boesteanu AC, Mueller YM, et al. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179:6494–503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 25.Pourgheysari B, Piper KP, McLarnon A, et al. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43:853–61. doi: 10.1038/bmt.2008.403. [DOI] [PubMed] [Google Scholar]
- 26.Morrison CS, Richardson BA, Mmiro F, et al. Hormonal contraception and the risk of HIV acquisition. AIDS. 2007;21:85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 27.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal Gram stain for the diagnosis of bacterial vaginosis. Obstet Gynecol. 1996;88:573–6. doi: 10.1016/0029-7844(96)00233-5. [DOI] [PubMed] [Google Scholar]
- 29.Brown JM, Wald A, Hubbard A, et al. Incident and prevalent herpes simplex virus type 2 infection increases risk of HIV acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21:1515–23. doi: 10.1097/QAD.0b013e3282004929. [DOI] [PubMed] [Google Scholar]
- 30.Morrison CS, Wang J, Van Der Pol B, Padian N, Salata RA, Richardson BA. Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21:1027–34. doi: 10.1097/QAD.0b013e3280f00fc4. [DOI] [PubMed] [Google Scholar]
- 31.Xue X, Gange SJ, Zhong Y, et al. Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev. 2010;19:159–69. doi: 10.1158/1055-9965.EPI-09-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picker LJ. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol. 2006;18:399–405. doi: 10.1016/j.coi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–52. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 34.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufmann SH, McMichael AJ. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat Med. 2005;11:S33–44. doi: 10.1038/nm1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geldmacher C, Schuetz A, Ngwenyama N, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198:1590–8. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–36. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 38.Broker TR, Jin G, Croom-Rivers A, et al. Viral latency—the papillomavirus model. Dev Biol (Basel) 2001;106:443–51. discussion 452–3, 465–75. [PubMed] [Google Scholar]
- 39.Schuman P, Ohmit SE, Klein RS, et al. Longitudinal study of cervical squamous intraepithelial lesions in human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2003;188:128–36. doi: 10.1086/375783. [DOI] [PubMed] [Google Scholar]
- 40.Critchlow CW, Hawes SE, Kuypers JM, et al. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12:1177–84. doi: 10.1097/00002030-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–74. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Pineres AJ, Hildesheim A, Herrero R, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006;66:11070–6. doi: 10.1158/0008-5472.CAN-06-2034. [DOI] [PubMed] [Google Scholar]
- 43.Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev. 2007;16:709–15. doi: 10.1158/1055-9965.EPI-06-0846. [DOI] [PubMed] [Google Scholar]
- 44.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 45.Gray RH, Serwadda D, Kong X, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1455–62. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serwadda D, Wawer MJ, Makumbi F, et al. Circumcision of HIV-infected men: effects on high-risk human papillomavirus infections in a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1463–9. doi: 10.1086/652185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Averbach SH, Gravitt PE, Nowak RG, et al. The association between cervical human papillomavirus infection and HIV acquisition among women in Zimbabwe. AIDS. 2010;24:1035–42. doi: 10.1097/qad.0b013e3283377973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith-McCune KK, Shiboski S, Chirenje MZ, et al. Type-specific cervico-vaginal human papillomavirus infection increases risk of HIV acquisition independent of other sexually transmitted infections. PLoS One. 2010;5:e10094. doi: 10.1371/journal.pone.0010094. [DOI] [PMC free article] [PubMed] [Google Scholar]